Abstract
The impact of the ovo-proteins ovalbumin and lysozyme—present in the first stage of egg shell formation—on the homogeneous formation of the liquid-amorphous calcium carbonate (LACC) precursor, was studied by a combination of complementing methods: in situ WAXS, SANS, XANES, TEM, and immunogold labeling. Lysozyme (pI = 9.3) destabilizes the LACC emulsion whereas the glycoprotein ovalbumin (pI = 4.7) extends the lifespan of the emulsified state remarkably. In the light of the presented data: (a) Ovalbumin is shown to behave commensurable to the ‘polymer-induced liquid precursor’ (PILP) process proposed by Gower et al. Ovalbumin can be assumed to take a key role during eggshell formation where it serves as an effective stabilization agent for transient precursors and prevents undirected mineralization of the eggshell. (b) It is further shown that the emulsified LACC carries a negative surface charge and is electrostatically stabilized. (c) We propose that the liquid amorphous calcium carbonate is affected by polymers by depletion stabilization and de-emulsification rather than ‘induced’ by acidic proteins and polymers during a polymer-induced liquid-precursor process. The original PILP coating effect, first reported by Gower et al., appears to be a result of a de-emulsification process of a stabilized LACC phase. The behavior of the liquid amorphous carbonate phase and the polymer-induced liquid-precursor phase itself can be well described by colloid chemical terms: electrostatic and depletion stabilization and de-emulsification by depletion destabilization.
Keywords: Biomineralisation, in situ synchrotron X-ray scattering, calcium carbonate, PILP, LACC
Introduction
Gaining control over morphogenesis and phase selection is a pivotal challenge for material science and technology since the entire characteristics of a material can be altered by modifying its shape, phase, size and mesoscale substructure. Biominerals show prominently that abundant minerals may exhibit superior properties in order to serve as sensors, skeletal support, or protection of soft tissues.1-5 By combination of inorganic strength and stiffness with organic elasticity,6 biomineral properties excel as compared to those of their purely inorganic counterparts. Intra-crystalline biomacromolecules (e.g. proteins, glycoproteins, polysaccharides, or proteoglycans), which are frequently very acidic,7 are known to take a crucial role in the formation of these tailored materials. They are assumed to control nucleation, phase, growth, size, and shape of the emerging biomineral but their definite role still remains vague.8 In recent years, intense examination of biomineralisation processes and their emulation in biomimetic/bioinspired crystallizations brought the awareness that the classical picture of nucleation and crystal growth is oversimplified and that, in fact, nonclassical crystallization via transient phases are the normal case rather than an exception—just as in case of the complex calcium carbonate system.6,9
A prominent example of nonclassical crystallization is the crystallization pathway of mesocrystallisation6, 10 which was recently validated by the identification of pre-nucleation clusters whose discovery puts classical nucleation into question.11 The second known nonclassical crystallization pathway of the calcium carbonate system involves a liquid/liquid phase separation leading to an emulsified state of a highly hydrated liquid amorphous calcium carbonate (LACC). This exceptional liquid phase forms during the homogenous formation of calcium carbonate from a pure, near neutral, and saturated calcium bicarbonate solution in absence of additives and seems to behave like a classical electrostatically-stabilized emulsion.9, 12, 13 Calcium carbonate—and later, its relatives9, 13—was the first inorganic mineral which was shown to undergo such a liquid/liquid phase separation, a well-known process for proteins and polymers14, 15 but unknown for minerals.
Gower et al. reported a related liquid intermediate calcium carbonate phase whose existence was ascribed to be generated by the addition of tiny amounts of small anionic polymers and was consequently termed ‘polymer-induced liquid-precursor’ (PILP).16, 17 The PILP phase has been employed for the synthesis of non-equilibrium morphologies (e.g. films and tablets composed of CaCO3 by sedimentation of the PILP).16-18 The first and main objective of this contribution is to link the two phenomena of LACC and PILP mechanistically. For this we have chosen a model system associated with a real biomineralisation system: two proteins of different pI from the egg shell of gallus gallus. This model system was already chosen and investigated by one of the founding fathers of biomineralization, Pieter Harting. He employed, among other organic substances (e.g mucus secreted from molluscs), a protein extract of hen egg white for his groundbreaking research on biomimetic morphosynthesis. His careful observations (e.g. his description of ‘calcospherites’) coincide very well with the observations presented in this article. Indeed, he already described morphologies which resemble PILP-like products prepared by the aid of the hen egg white extract.19, 20
The formation of domestic fowl egg shells is a common example of a fast but highly controlled extracellular biomineralisation: 5 g of calcium carbonate are deposited within 22 h.21 The biopolymers and proteinaceous constituents of the uterine secretion are assumed to strongly control the calcium carbonate deposition in the oviduct because the uterine fluid is 60- to 100-fold supersaturated with regard to the solubility product of calcite, but the eggshell is crystallographic highly orientated, permeable, and its fracture is crystallographically controlled.22, 23 Therefore, the components of the uterine fluid have to prevent unfocused precipitation in favor of a controlled, spatially restricted growth of the eggshell. Each phase of shell mineralization (i.e. nucleation, rapid crystal growth, and the completion of shell formation) is associated with a specific composition of the uterine secretion.24 Ovalbumin and lysozyme are two of the egg white proteins which are present during the initial stage of eggshell formation.24, 25 The acidic glycoprotein ovalbumin (pI = 4.7) is the most dominant protein during the initial stage, and comprises 54% of the hen egg white but its biological function is still unclear.26 Ovalbumin is singly N-glycosylated at Asn-292 by acidic sialyloligosaccharides27 and carries two phosphorylated sites at Ser-68 and Ser-344.28 Roughly one fourth of the amino acid residues are acidic whereas 15% of the amino acids are basic (16% Glu, 11% Asp, 12% Ser, 8% Lys, 7% Arg).28 The quite low isoelectric point of ovalbumin thus arises both from the primary sequence and the post-translational modifications. The alkaline and non-glycosylated lysozyme (3.5% of the hen egg white, pI = 9.3) fulfills antibacterial tasks and thus plays a chemical protective function during avian embryonic development.29, 30
Contrasting both proteins, this contribution studies the effect of ovo-proteins/biopolymers of different type and isoelectric point (pI) on the liquid amorphous calcium carbonate phase (LACC). The aim of this contribution is twofold: (a) to study the probable tasks of proteins of different pI in general and the ovo-proteins ovalbumin and (ovo-)lysozyme in particular during biomineralisation and (b) to analyze the mechanisms of stabilization/destabilization of the LACC phase and its link to so-called PILP processes. These aims were accomplished by a combination of methods of characterization: in situ synchrotron X-ray scattering experiments were performed at a micro-focus beam-line to monitor the mineral phase formation and different stages of the crystallization were characterized by transmission and scanning electron microscopy (TEM, SEM). Ex situ small angle neutron scattering and X-ray absorption spectroscopy near the Ca-K edge were employed to characterize the antecedent and intermediate states of protein/ion interaction. All crystallization experiments were conducted employing ultrasonic levitation in order to rule out any heterogeneous influences of foreign materials and their phase boundaries.12
Results and Discussion
The crystallization was carried out homogenously according to the Kitano method31 in which calcium carbonate is formed by slow evaporation of water and concomitant slow release of carbon dioxide from a saturated solution of calcium bicarbonate. The precipitation proceeds slowly at nearly neutral and constant pH because of the inherent bicarbonate buffer system (pH = 7.35–7.45) and is thus comparable to conditions in the uterine fluid. In a typical experiment, one droplet of 4 μl of solution was levitated which contained beside the respective ovo-proteins (7.5 mg·mL-1) calcium bicarbonate at a saturated concentration (~ 10 mmol·L-1), which is about 160-fold supersaturated with respect to calcite. The quite high concentration of proteins was chosen in order to roughly resemble the conditions in the uterine fluid. The precipitation was followed in situ by means of wide-angle X-ray scattering. The resulting X-ray diffraction patterns are shown in Fig. 1. The appearance of distinct Bragg reflections indicates the incipient crystallization—more precisely the formation of the first crystalline phase as the formation of amorphous phases might precede. The elapsed time until the appearance of first reflections was quantified by an analysis of the integral intensity of the corresponding reflections according to Avrami:32, 33 a Weibull function was fit to the normalized integral intensities of the (104) reflection, the derived inflexion point was used as a comparative value t104 which corresponds to the time when the calcite formation rate has reached its maximum and decreased afterwards (cf. Fig. 2). In absence of proteins, the first detectable reflection belongs to the {104} set of calcite lattice planes. Later, other reflections follow: (102), (110), and (202) appeared almost simultaneously whereas the weakest reflection (006) was detected last. A t104 value of 34.5 min was derived (Fig. 2). Evaluating the WAXS pattern of the finally dry sample, only the stable calcite phase was found (Fig. S1). The dry sample was investigated with scanning electron microscopy and revealed that spherical solid particles were present along with rhombohedral calcite crystals (Fig. 3a); the former particles consisted of solidified dry and presumably amorphous calcium carbonate, which did not transform into crystalline material. During evaporation, the levitated drop shrank continuously and by this the transient calcium carbonate precursors were accumulated near the droplets surface and formed this spherulites by aggregation.12 In the presence of lysozyme, the evolution of the WAXS patterns (Fig. 1) did not change significantly. A slow increase of intensity in the SAXS regime (q < 5 nm-1) indicates aggregation of lysozyme.34, 35 This scattering ceased when the sample had reached its final dry state. The kinetics of the precipitation process did not differ significantly from those of a protein-free mineralization (t104 = 31 min, cf. Fig. 2). Just like in absence of proteins, the final mineral phase is the thermodynamically stable phase calcite. In contrast to lysozyme, the presence of ovalbumin affected the precipitation of calcium carbonate considerably. The formation of crystalline calcium carbonate was strongly retarded; reflections appear quite abruptly after 53 min (Fig. 1 and 2). In contrast to the two other experiments with lysozyme and without protein, only the presence of ovalbumin led to a mixture of calcium carbonate phases (Fig. 1 and S1). A ratio of 80.16% vaterite vs. 19.83% calcite was determined based on Rietveld refinements.36 The intensity of the SAXS regime increased later than in the case of lysozyme. Ovalbumin is known to undergo aggregation in terms of a Gaussian chain with large segments if it experiences either thermally-37, 38 or calcium-induced39 denaturation. Transmission electron and standard light microscopy revealed that these protein chains aggregate further to form fibrils featuring a high aspect ratio (Fig. S2). In the finally dry sample, these protein fibrils fully dominated the appearance of the residue (Fig. 3b). Energy dispersive X-ray spectroscopy revealed that the prominent knobs at the residue’s outer surface consist of CaCO3 whereas the other remains are protein aggregates.
Figure 1.
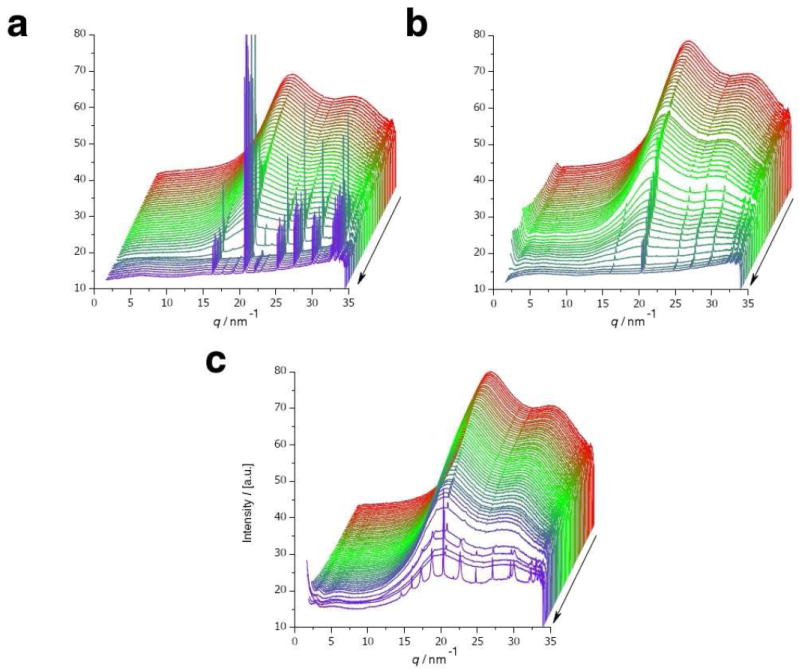
Evolution of scattering intensities during the in situ monitoring of the evaporation of a levitated calcium bicarbonate solution a) in absence of protein, and in presence of b) lysozyme and c) ovalbumin (7.5 mg·mL-1). The two shoulders at 20 nm-1 and 30 nm-1, which dominate all three scattering evolutions, originate from the diffuse scattering of water.
Figure 2.
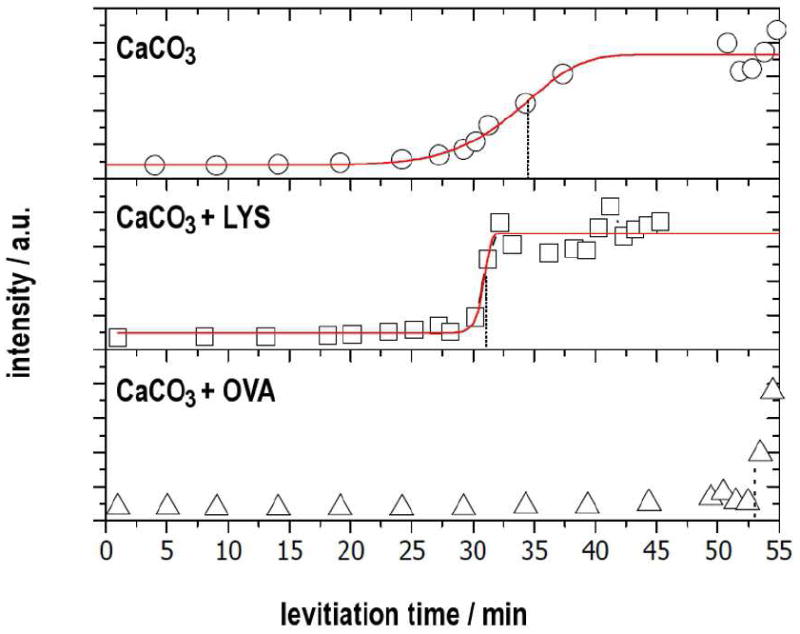
Integral intensity of the (104)- reflections as a function of time, fitted by a Weibull function. The corresponding inflexion points t104 are indicated by dotted anchor lines.
Figure 3.
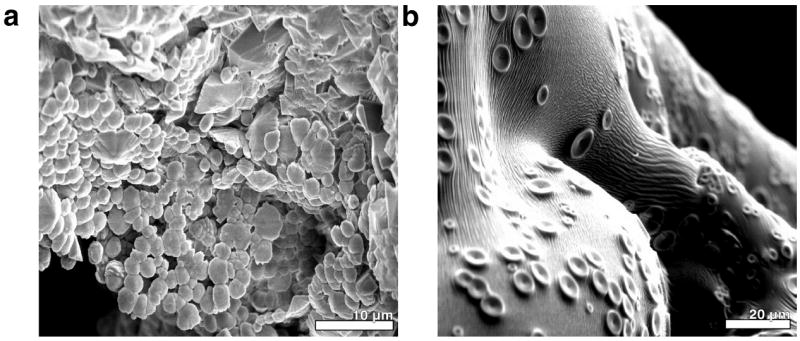
SEM micrographs of the final stages of precipitation of a) pure CaCO3, b) CaCO3 in presence of 7.5 mg·mL-1 ovalbumin.
As amorphous intermediates cannot be traced by X-ray diffraction techniques, the early stages of mineralization were investigated by TEM. In the case of the pure calcium bicarbonate solution, the liquid/liquid phase separation occurs at the outset of the precipitation prior formation of the crystalline calcite phase (Fig. 4a).12 The amorphous state of the emulsified droplets, which are formed during the early stages of precipitation, was ascertained by electron diffraction (ED, inset in Fig 4a). The low contrast variation of the droplets gives evidence of their liquid-like character as solid spherical particles would show a distinct increase in contrast from the particle boundary to their center. The droplets consist of highly hydrated calcium carbonate—their crystallization yielding calcite could be induced by increased irradiative stress during TEM analysis, which we attribute to a loss of water of hydration by co-action of the irradiative stress and ultrahigh vacuum.
Figure 4.
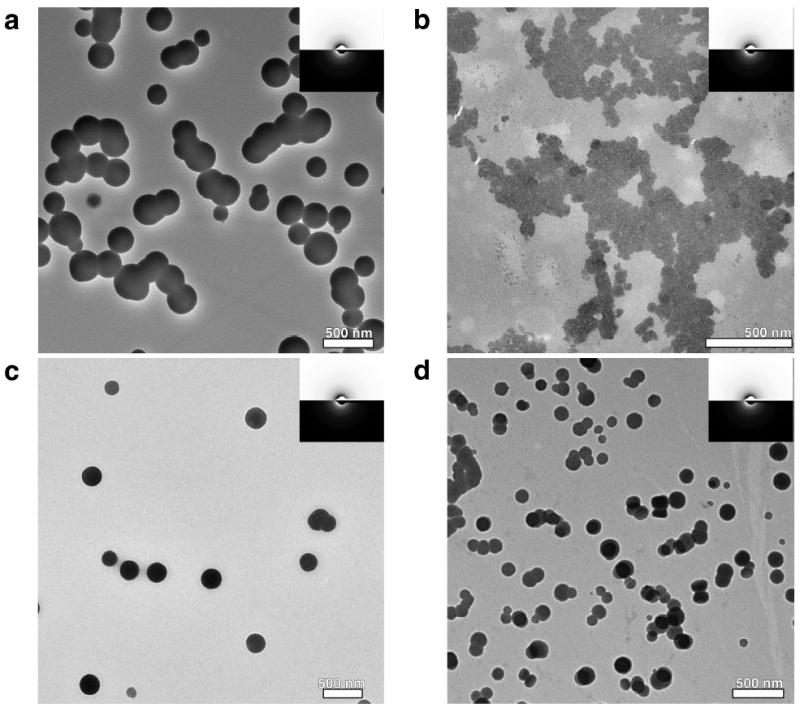
TEM micrographs of precipitations of CaCO3 (a) in absence of ovo-proteins, (b) in presence of 7.5 mg·mL-1 lysozyme, (c, d) in presence of 7.5 mg·mL-1 ovalbumin. The sampling was done at different times: (a,b) 400 s, (c) 500 s and (d) 1000 s. The respective electron diffraction patterns are shown in the insets and indicate the amorphous state of the droplets.
If lysozyme is present during the precipitation process, the salient emulsion-like appearance of the precursor vanished. Instead of individual droplets, an intense coalescence has occurred during the first 400 s (Fig. 4b). Electron diffraction showed these structures to be non-crystalline (inset of Fig. 4b). As implied by the deviation of the t104 value, the impact of ovalbumin on the precursor structures differed distinctly from the effect lysozyme induced. The liquid intermediate was considerably stabilized and its lifespan was greatly extended if ovalbumin was present during the precipitation whereas sporadic crystalline material could be found after ~ 500 s by TEM in absence of proteins, an emulsion-like state still existed after 500 s (Fig. 3c) and persisted up to ~ 1000 s in presence of ovalbumin (Fig. 4d). The appearance of the ovalbumin-stabilized mineral emulsion closely resembled the one formed in absence of protein (Fig. 4a vs. 4d) and the amorphous state of the droplets could be verified by ED (inset in Fig. 4d).
The localization of proteins is commonly accomplished by immunogold (IG) staining provided that antibodies of the respective protein are available, a method which is obviously not applicable for synthetic polymers. Following the above findings, ovalbumin represents the first protein which behaves commensurable to a so-called PILP process, so the assumed accumulation of polymer/protein in LACC droplets during a PILP process could be directly probed for the first time. Samples which were obtained after 300 s of levitation at a protein concentration of 0.5 mg·mL-1 were fixated by drying at 40° C for 48 h and studied by a polyclonal IG labeling in order to locate the ovalbumin. As shown in Fig. S3, the LACC droplets suffered from the rinsing steps, which are inevitable during IG labeling. Nevertheless, IG labeling revealed that ovalbumin is accumulated partially within the droplets and—to a lesser extent—randomly distributed as well. The number of nanoparticles per area associated with the LACC droplets was found to be higher by a factor of 6.78 compared to the number of nanoparticles randomly distributed. Thus, ovalbumin remains partially in solution but, in fact, a moiety is incorporated in the droplets of liquid calcium carbonate.
Recently, we reported a detailed small-angle neutron-scattering study on a biomimetic mineralization involving ovalbumin in this journal: its calcium-induced aggregation and its loading with calcium.39 The final ovalbumin aggregates can briefly be described as Gaussian linear chains with relatively large, rodlike segments. Under addition of calcium, the scattering length density of the acidic glycoprotein ovalbumin increased by 6.6% which is caused by an uptake of roughly 130 calcium ions per protein. We now complement this study with corresponding scattering experiments employing lysozyme. We compared the small-angle neutron scattering patterns of lysozyme at three different concentrations (2.5 mg·mL-1, 5 mg·mL-1 and 7.5 mg·mL-1) in different buffer solutions: pure protein, 10 mM·L-1 CaCl2, and 30 mM·L-1 NaCl (i.e. identical ionic strength as 10 mM·L-1CaCl2). At 2.5 mg·mL-1 lysozyme (cf. Fig. S4a), all three scattering curves resemble each other which means that no loading occurs: the scattering length density of the protein does not change and no structure factor is superimposed. At higher concentration of lysozyme (5 mg·mL-1 and 7.5 mg/mL, cf. Fig. S4b and S4c), the structure factor, which arises from protein-protein interaction, is screened only insufficiently at the given salt concentration, which was chosen to be equal to that in the saturated calcium bicarbonate solution for the sake of comparability. However, at higher q, evidence suggests that no loading of lysozyme with calcium occurs as there are no deviations of the three scattering patterns. Measurement of the calcium activity of the respective solution affirmed that the calcium activity does not significantly decrease in the presence of lysozyme whereas in presence of ovalbumin the calcium activity is considerably diminished.
X-ray absorption spectra near the Ca-K edge were taken in order to probe the interaction of calcium with the both proteins. A comparison of XANES spectra of a pure calcium chloride solution with calcite as a standard and with solutions which contain additionally 1 mg·mL-1 ovalbumin or 1 mg·mL-1 lysozyme is shown in Fig. 5. The spectra of the solutions are identical: each spectrum features a pronounced pre-edge 1s→3d transition which is formally forbidden in octahedral/inversion symmetry. Compared to crystalline calcite, a considerable decrease in features occurs which indicates a decrease in structural order:40 all solution spectra show one large peak around 4049 eV, which arises from Ca–O scattering in the first shell, whereas crystalline calcite shows two distinct peaks at 4047 eV and 4058 eV originating from diverse Ca–O first-shell scattering paths41 and the additional shoulder at 4043 eV from a 1s→4p transition. In pure calcium chloride solutions, the calcium ions are completely hydrated as chloride is a weak complex ligand.42 The well-pronounced 1s→3d pre-edge transition indicates a non-octahedral coordination sphere and thus accounts for a coordination number higher than six as it is formally forbidden in centrosymmetric (e.g. octahedral) coordination. Recent contributions reported a median hydration number of seven to eight.42, 43 Calcite has a trigonal symmetry (crystal symmetry 2/m), and, therefore, the pre-edge transition is present. Lysozyme does not bind calcium ions—as demonstrated by SANS—hence the pre-edge peak remains. Although calcium is bound to ovalbumin, the pre-edge signal remains unchanged and thus the high coordination number persists: it seems that calcium remains highly coordinated thus is probably still highly hydrated. No specific chelating binding pockets of ovalbumin seem to be involved; it seems merely to bind the proteins surface (including its post translational modifications). In fact, ovalbumin does not contain calcium-specific binding motives: no sequence similarities were found when comparing the primary sequence of ovalbumin with the calcium-specific binding motives of EF-hand, C2, S-100 and annexins.
Figure 5.
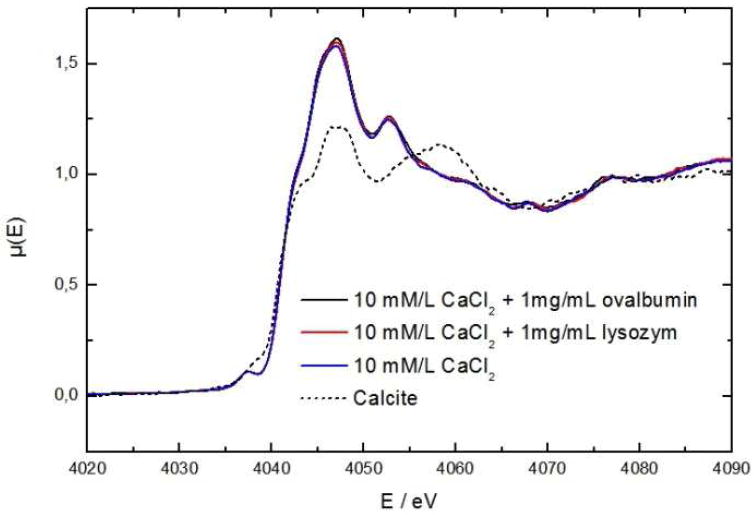
Comparison of the X-ray absorption spectrum near the Ca-K-edge of a pure calcium chloride solution with solutions with additionally contain 1 mg·mL-1 ovalbumin or 1 mg·mL-1 lysozyme and with solid calcite.
Conclusions
Ovalbumin is capable of extensively stabilizing the liquid precursor phase LACC of calcium carbonate whereas lysozyme destabilizes the emulsified transient phase and leads to a strong coalescence. In the light of the presented data, we assume ovalbumin to take a key role during eggshell formation. Ovalbumin may serve as (a) effective stabilization agent for a transient mineral precursor, (b) a storage protein (aggregate) of the inorganic eggshell components and as a (c) prevention of undirected mineralization in favor of a directed mineralization of the eggshell. Ovalbumin binds a considerable amount of calcium ions from solution as evidenced by SANS and calcium activity measurements although it does not possess a specific calcium binding site: it thus can be counted among the high-capacity low-affinity calcium binding proteins.
The effect of ovalbumin can be briefly summarized as follows: In the beginning, ovalbumin binds calcium and thus relieves the supersaturation which retards crystallization resp. phase separation. After phase separation, the IG labeling data indicates that the calcium-loaded ovalbumin is partially incorporated in the LAAC droplets and partially remains in solution. But the influences exerted by ovalbumin on the liquid mineral phase are multifold and have to be discussed in more detail.
The recent data from small-angle neutron-scattering (SANS) showed ovalbumin to act like a ‘cation sponge’;39 it accumulates calcium ions from solution which are complexated by the protein’s carboxylic groups. It decreases dramatically the calcium activity of the bulk solution to increase locally the calcium concentration next to the protein. The decrease in calcium activity due to complexation relieves the solution’s supersaturation and thus decelerates formation of crystalline material, whose formation would require higher supersaturation based on their solubility products, and thus extends the lifespan of the liquid and amorphous precursors.
If we approach the problem from a colloidochemical point of view, the LACC dispersed in the mother solution of calcium bicarbonate behaves like a classical emulsion: In absence of (bio)polymers the emulsified state is assumed to be electrostatically stabilized as no other of the classical colloidal stabilization mechanisms (i.e. sterical or depletion stabilization) can apply.9, 12 The basic and thus positively charged protein lysozyme (pI = 9.3) destabilizes the emulsified state whereas the negatively charged acidic protein ovalbumin (pI = 4.7) extends its lifespan. It is a well-known behavior of colloidal systems that if the additive is of opposite charge in respect to the emulsified moiety phase, it may destabilize the emulsion either by compensating the surface charges or interconnecting the emulsified droplets.44 Based on these findings, we deduce that the surface of the emulsified liquid calcium carbonate phase is actually negatively charged and the positively charged lysozyme induces coalescence via charge neutralization or interconnection. The effect of the negatively charged polymer one may call depletion stabilization. These findings are well corroborated by earlier results based on electrospray ionization mass spectrometry (ESI-MS) which revealed all the pre-critical carbonate-coordinates complexes to be of negative charge.13 The formation of the liquid amorphous state seems rather to be a characteristic of the carbonate-based systems than literally induced by the presence of polymers: the liquid phase appears also in absence of polymers which thus are no conditio sine qua non of the liquid/liquid phase separation of the calcium carbonate system.
In colloidal systems a destabilization is well-known which occurs at distinct lower concentrations of non-absorbing polymer: depletion flocculation—in this case depletion segregation, strictly speaking (this effect is shortly explained in footnote 45). It seems reasonable that one can describe the original PILP coating effect as a result of a de-emulsifying process: the moiety of the acidic, negatively charged polymer, which remains in solution and is not incorporated in the LACC droplets, cannot absorb on the LACC droplets surface as they carry the same charge. At distinct low polymer concentration as employed in PILP processes, the depletion effect initiates the breaking and de-emulsifying of the LACC emulsion and the incipient settlement of the LACC resp. “polymer-induced liquid precursor” yields the characteristic coating effect of PILP processes.
We turn now to the nucleation event itself. Ovalbumin, loaded with calcium ions, can be regarded as a ‘fluctuation in calcium concentration’. Such concentration fluctuations are the crucial point during formation of a new phase.6 In pure solutions, these fluctuations base on statistical processes and are thus seldom occurring. In the current case, they are actually provided by the presence of ovalbumin as it gathers calcium ions from solution and which may promote nucleation by lowering this main activation barrier of the upcoming phase separation. One may thus speculate that the presence of a calcium-loaded (bio)polymer may aid nucleation: it may switch the liquid/liquid phase separation from a spinodal, in which no concentration gradient is initially present, to a binodal process, in which crystallization starts at an initial concentration variation.12, 50 This rationalization is commensurable to the polymer-induced liquid-precursor (PILP) concept and would narrow down the meaning of the “induction” of the liquid precursor: the formation of the liquid mineral precursor is not induced in the sense of being generated because it occurs as well in absenco of polymers,17, 51, 52 but it is facilitated and accelerated as it provides an up-concentration of calcium ions. Then the initial uphill diffusion of spinodal decomposition would be rendered unnecessary or already partially provided.
In a later state of mineralization, ovalbumin or small anionic polymers in general which are incorporated in the amorphous mineral phase increase the disorder of the ‘glassy’ state by providing an additional multitude of different binding and bridging possibilities and by this stabilize the amorphous state. These findings re-emphasize once again the crucial task of acidic biopolymers in biomineralization: they prolong the life span of the moldable transient phase and stabilize it in such a manner that the mineralized tissue is able to retain it morphology in its later crystalline state.
In a concluding summary, ovalbumin was shown to stabilize the liquid calcium carbonate phase remarkably under neutral and diffusion-controlled conditions and thus behaves commensurable to the PILP process which corroborates recent suggestions of L. Gower that acidic biopolymers may generate a PILP route.53 The emulsified liquid amorphous calcium carbonate phase carries as negative surface charge and thus the pure emulsified liquid calcium carbonate precursor is stabilized electrostatically. Concerning the PILP model, we propose that the liquid amorphous calcium carbonate is affected by polymers in terms of depletion stabilization and demulsifying than literally ‘induced’ by acidic proteins and polymers during a polymer-induced liquid-precursor process. The behavior of the liquid amorphous carbonate phase and the polymer-induced liquid-precursor phase itself can apparently be well described in terms of colloidal chemical terms: electrostatic and depletion stabilization and segregation by depletion destabilization.
Supplementary Material
Acknowledgments
We thank Simone Rolf and Ralf Bienert (BAM) for technical support at the beam line. We are much obliged to Frédéric Marin and Ute Schloßmacher for assistance in IG labeling and fruitful discussions. We are grateful to the Deutsche Forschungsgemeinschaft (DFG) for support within the priority program No. 1415: „Kristalline Nichtgleichgewichtsphasen“. Stephan E. Wolf gratefully acknowledges the Konrad Adenauer-Foundation for a doctoral fellowship. We thank the BioCAT team at the Advanced Photon Source for excellent support during XAS experiments and we are grateful to Dr. David Gore for his careful revision of the English style.
Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. BioCAT is a National Institutes of Health-supported Research Center RR-08630.
Footnotes
Supporting Information Available Experimental section and the following additional figures: (S1) Wide-angle X-ray scattering patterns of pure CaCO3 and of CaCO3 in presence of lysozyme and ovalbumin, (S2) Micrographs of ovalbumin aggregates (S3) Transmission electron micrographs of IG labeling experiments, (S4) Small-angle neutron scattering patterns of lysozyme at three different concentrations in three different buffers. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Aizenberg J, Weiner S, Tkachenko A, Addadi L, Hendler G. Nature. 2001;412:819–22. doi: 10.1038/35090573. [DOI] [PubMed] [Google Scholar]
- 2.Oaki Y, Imai H. Small. 2006;2:66–70. doi: 10.1002/smll.200500246. [DOI] [PubMed] [Google Scholar]
- 3.Mann S. Biomineralization. Oxford University Press; Oxford, USA: 2002. [Google Scholar]
- 4.Lowenstam HA, Weiner S. On Biomineralization. Oxford University Press; Oxford, USA: 1989. [Google Scholar]
- 5.Bäuerlein E, editor. Handbook of Biomineralization. Wiley-VCH Verlag GmbH; Weinheim, Germany: 2007. [Google Scholar]
- 6.Cölfen H, Antonietti M. Mesocrystals and Nonclassical Crystallization. Wiley-VCH Verlag GmbH; 2008. [Google Scholar]
- 7.Marin F, Luquet G. In: Unusually Acidic Proteins in Biomineralization In: Handbook of Biomineralization: Biological Aspects and Structure Formation. Bäuerlein E, editor. Vol. 1. Wiley-VCH Verlag GmbH; Weinheim, Germany: 2007. pp. 273–290. [Google Scholar]
- 8.Gebauer D, Cölfen H, Verch A, Antonietti M. Adv Mater. 2009;21:435–439. [Google Scholar]
- 9.Wolf SE. Nonclassical Crystallization of Bivalent Metal Carbonates. SVH; Saarbrücken, Germany: 2011. [Google Scholar]
- 10.Cölfen H, Antonietti M. Angew Chemie Intern Ed. 2005;44:5576–91. doi: 10.1002/anie.200500496. [DOI] [PubMed] [Google Scholar]
- 11.Gebauer D, Völkel A, Cölfen H. Science. 2008;322:1819–22. doi: 10.1126/science.1164271. [DOI] [PubMed] [Google Scholar]
- 12.Wolf SE, Leiterer J, Kappl M, Emmerling F, Tremel W. J Am Chem Soc. 2008;130:12342–7. doi: 10.1021/ja800984y. [DOI] [PubMed] [Google Scholar]
- 13.Wolf SE, Müller L, Barrea R, Kampf CJ, Leiterer J, Panne U, Hoffmann T, Emmerling F, Tremel W. Nanoscale. 2011:19–21. doi: 10.1039/c0nr00761g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galkin O, Vekilov PG. Proc Nat Acad Sci U S A. 2000;97:6277–81. doi: 10.1073/pnas.110000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vekilov PG. Soft Matter. 2010;6:5254. [Google Scholar]
- 16.Gower LB, Tirell DA. J Cryst Growth. 1998;191:153–160. [Google Scholar]
- 17.Gower LB, Odom DJ. J Cryst Growth. 2000;210:719–734. [Google Scholar]
- 18.Kim Y-Y, Gower LB, Douglas EP. Langmuir. 2007;23:4862–4870. doi: 10.1021/la061975l. [DOI] [PubMed] [Google Scholar]
- 19.Harting P. Recherches de morphologie synthéthique Sur la production artificielle de quelques formations calcaires organiques. C. G. van der Post; Amsterdam, Netherlands: 1872. [Google Scholar]
- 20.Harting P. Quart J Microsc Sci. 1872;12:118–123. [Google Scholar]
- 21.Arias JL, Fink DJ, Xiao S-Q, Heuer AH, Caplan AI. Int Rev Cyt. 1993;145:217–250. doi: 10.1016/s0074-7696(08)60428-3. [DOI] [PubMed] [Google Scholar]
- 22.Heyn AJ. J Appl Phys. 1962;33:2658. [Google Scholar]
- 23.Sharp RM, Silyn-Roberts H. Biophysical journal. 1984;46:175–9. doi: 10.1016/S0006-3495(84)84010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautron J, Hincke MT, Nys Y. Connective Tissue Research. 1997;36:195–210. doi: 10.3109/03008209709160220. [DOI] [PubMed] [Google Scholar]
- 25.Dominguez-Vera JM, Gautron J, Garcia-Ruiz JM, Nys Y. Poultry Science. 2000;79:901–907. doi: 10.1093/ps/79.6.901. [DOI] [PubMed] [Google Scholar]
- 26.Kelly JF, Locke SJ, Ramaley L, Thibault P. Journal of Chromatography A. 1996;720:409–427. doi: 10.1016/0021-9673(94)01197-4. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita K, Tachibana Y, Hitoi A, Kobata A. Carbohydrate Research. 1984;130:271–288. doi: 10.1016/0008-6215(84)85285-4. [DOI] [PubMed] [Google Scholar]
- 28.Nisbet AD, Saundry RH, Moir AJ, Fothergill LA, Fothergill JE. Eur J Biochem. 1981;115:335–345. doi: 10.1111/j.1432-1033.1981.tb05243.x. [DOI] [PubMed] [Google Scholar]
- 29.Awad AC, Moreau S, Mollé D, Brulé G, Mauboisa J-L. J Chrom A. 1994;677:279–288. doi: 10.1016/0021-9673(94)80156-8. [DOI] [PubMed] [Google Scholar]
- 30.Arias JL, Fink DJ, Xiao SQ, Heuer AH, Caplan AI. Int Rev Cyt. 1993;145:217–250. doi: 10.1016/s0074-7696(08)60428-3. [DOI] [PubMed] [Google Scholar]
- 31.Kitano Y. Bull Chem Soc Japan. 1962;35:1973–1980. [Google Scholar]
- 32.Avrami M. J Chem Phys. 1939;7:1103–1112. [Google Scholar]
- 33.Avrami M. J Chem Phys. 1940;8:212–224. [Google Scholar]
- 34.Chodankar S, Aswal V, Hassan P, Wagh A. Physica B: Condensed Matter. 2007;398:112–117. [Google Scholar]
- 35.Chodankar S, Aswal V. Physical Review E. 2005;72 doi: 10.1103/PhysRevE.72.041931. [DOI] [PubMed] [Google Scholar]
- 36.Rietveld HM. Journal of Applied Crystallography. 1969;2:65–71. [Google Scholar]
- 37.Weijers M, Visschers RW, Nicolai T. Macromolecules. 2002;35:4753–4762. [Google Scholar]
- 38.Nemoto N, Koike A, Osaki K, Koseki T, Doi E. Biopolymers. 1993;33:551–9. doi: 10.1002/bip.360330405. [DOI] [PubMed] [Google Scholar]
- 39.Pipich V, Balz M, Wolf SE, Tremel W, Schwahn D. J Am Chem Soc. 2008;130:6879–6892. doi: 10.1021/ja801798h. [DOI] [PubMed] [Google Scholar]
- 40.Politi Y, Levi-Kalisman Y, Raz S, Wilt F, Addadi L, Weiner S, Sagi I. Adv Func Mater. 2006;16:1289–1298. [Google Scholar]
- 41.Fulton JL, Heald SM, Badyal YS, Simonson JM. J Phys Chem A. 2003;107:4688–4696. [Google Scholar]
- 42.Fulton JL, Heald SM, Badyal YS, Simonson JM. J Phys Chem A. 2003;107:4688–4696. [Google Scholar]
- 43.Benfatto M. Topics Curr Chem. 1988;145 [Google Scholar]
- 44.Somasundaran P. Encyclopedia of Surface and Colloid Science. 2. CRC Press; 2006. [Google Scholar]
- 45.Dispersed particles are surrounded by a layer of thickness Δ in which dissolved non-absorbing polymers experience a reduction in conformational entropy compared to those polymers outside of this layer. If the distance between two dispersed particles is considerably longer than Δ, then the solution is stable and the polymer concentration between the dispersed is equal to that outside. If the inter-particle distance is in the range of 2Δ, then polymers will be forced to leave the inter-particle space. Because of the resulting concentration gradient, solvent diffuse out of the inter-particle space: the particles start flocculate/segregate (depletion destabilization). For more details, see ref. 44 and 46-49.
- 46.Mondain-Monval O, Leal-Calderon F, Phillip J, Bibette J. Phys Rev Lett. 1995;75:3364–3367. doi: 10.1103/PhysRevLett.75.3364. [DOI] [PubMed] [Google Scholar]
- 47.Vincent B, Edwards J, Emmetta S, Jones A. Coll Surf. 1986;18(2-4):261–281. [Google Scholar]
- 48.Vincent B, Clarke1 J, Barnett KG. Coll Surf. 1986;17(1):51–65. [Google Scholar]
- 49.Vincent B, Edwards J, Emmetta S, Croot R. Coll Surf. 1988;31:267–298. [Google Scholar]
- 50.Rieger J, Frechen T, Cox G, Heckmann W, Schmidt C, Thieme J. Faraday Disc. 2007;136:265. doi: 10.1039/b701450c. [DOI] [PubMed] [Google Scholar]
- 51.Dai L, Douglas E, Gower LB. J Non-Cryst Sol. 2008;354:1845–1854. [Google Scholar]
- 52.Homeijer SJ, Barrett RA, Gower LB. Crystal Growth & Design. 2010;10:1040–1052. [Google Scholar]
- 53.Gower LB. Chem Rev. 2008;108:4551–4627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lakshminarayanan R, Loh XJ, Gayathri S, Sindhu S, Banerjee Y, Kini RM, Valiyaveettil S. Biomacromolecules. 2006;7:3202–3209. doi: 10.1021/bm0605412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


