Abstract
In this communication, we reported another unique IspG-catalyzed transformation, the production of its substrate, MEcPP, from (2R,3R)-4-hydroxy-3-methyl-2,3-epoxybutanyl diphosphate (Epoxy-HMBPP) when reductants are excluded from the reaction mixture.
Isoprenoids, one of the largest class of natural products, are biosynthesized either through the classical mevalonate pathway (MVA pathway in animals, yeast, or archaea) or the recently identified deoxyxylulose phosphate pathway (DXP pathway in green algae, eubacteria, and plants).1–4 The penultimate step of the DXP pathway is an IspG-catalyzed reductive deoxygenation of methyl-D-erythritol-2,4-cyclodiphosphate (MEcPP, 1, Scheme 1) to (E)-4-hydroxy-3-methylbut-2-enyl diphosphate (HMBPP, 3). MEcPP was isolated almost a decade before its corresponding biosynthetic enzyme was identified and it accumulates to high concentrations (up to 50 mM) when bacteria are under oxidative stress conditions.5–7 Recently, the enzyme (GcpE or IspG) that utilizes MEcPP as the substrate was identified.8–11 Preliminary biochemical studies indicate that IspG is a unique iron site-containing [4Fe–4S] protein, and it catalyzes the reductive deoxygenation of MEcPP to HMBPP (Scheme 1).11–14
Scheme 1.
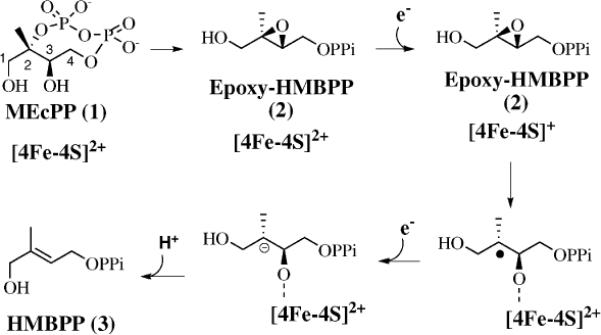
The epoxide model proposed by Rohdich et al. for IspG-catalyzed reductive deoxygenation of MEcPP to HMBPP.15
Several mechanistic models were proposed to account for the reductive deoxygenation of MEcPP to HMBPP, including the vitamin K epoxyquinone reductase model,12 the anaerobic ribonucleotide reductase (RNR) model,12,16 the deoxygenation model utilized in ascarylose biosynthesis,17 the cation model,14,18 and the epoxide model proposed by Rohdich et al. (Scheme 1).15 To date, several lines of evidence are consistent with the plausibility of the epoxide reductive deoxygenation model. First, it is known that synthetic [4Fe–4S] clusters catalyze reductive deoxygenation of epoxides to alkenes.19 Second, our initial characterization of (2R,3R)-4-hydroxy-3-methyl-2,3-epoxybutanyl diphosphate (Epoxy-HMBPP, 2) revealed that IspG catalyzes reductive deoxygenation of Epoxy-HMBPP to HMBPP with an efficiency comparable to that of MEcPP.20 Following our initial Epoxy-HMBPP studies, Oldfield and co-workers further suggested, on the basis of EPR studies, that a common organo-metallic transient species en route to HMBPP was generated from both MEcPP and Epoxy-HMBPP.21
The first step of the epoxide model (Scheme 1) is a nucleophilic attack by the C3 hydroxyl group at the C2 position, which leads to the ring opening of MEcPP to Epoxy-HMBPP. Reductive ring opening of Epoxy-HMBPP by the reduced iron-sulfur cluster ([4Fe–4S])1+ generates a substrate-based radical. Further reduction to a substrate-based carbanion followed by water elimination affords HMBPP. In our previous studies, we demonstrated that IspG indeed catalyzes the reductive deoxygenation of Epoxy-HMBPP to HMBPP. In addition, the kinetic parameters of IspG catalyzed reductive deoxygenations of both MEcPP and Epoxy-HMBPP are in close agreement.20 The similar kcat values (~20 min−1) for the IspG-catalyzed reductive deoxygenation of both MEcPP and Epoxy-HMBPP suggest that a step after Epoxy-HMBPP is the rate-limiting step if Epoxy-HMBPP is indeed an intermediate in the reductive deoxygenation of MEcPP (Scheme 1).20
Our previoulsy reported steady state kinetic analysis of the epoxide20 and Oldfield's recent report suggesting MEcPP and Epoxy-HMBPP proceed via a common downstream intermediate21 are consistent with the epoxide model. However, there is no other available information related to the Epoxy-HMBPP intermediacy in the IspG-catalyzed reductive deoxygenation of MEcPP, including the direct detection of the epoxide in this reaction. The overall reactions of MEcPP or Epoxy-HMBPP to HMBPP are reductive deoxygenation processes and two electrons are supplied by a reduction system. Under steady-state conditions and using dithionite and methyl viologen as the reduction system, we did not detect any accumulation of Epoxy-HMBPP during the reductive deoxygenation of MEcPP to HMBPP.13,20 However, according to the epoxide model (Scheme 1), the first step, MEcPP to Epoxy-HMBPP conversion, does not involve any redox chemistry. We hypothesized that if Epoxy-HMBPP is indeed an intermediate along the MEcPP reductive deoxygenation pathway, there might be a better chance of detecting an IspG-catalyzed inter-conversion between MEcPP and Epoxy-HMBPP by blocking the subsequent steps, which can be achieved by excluding the reduction system (e.g., NADPH–flavodoxin–flavodoxin reductase or dithionite/methyl viologen). Thus, we examined two scenarios regarding the relationship between MEcPP and Epoxy-HMBPP in IspG-catalyzed reductive deoxygenation reactions (Scheme 2A and B): First, production of Epoxy-HMBPP from MEcPP (Scheme 2A), and second, MEcPP as an intermediate in the reductive deoxygenation of Epoxy-HMBPP to HMBPP (Scheme 2B).
Scheme 2.
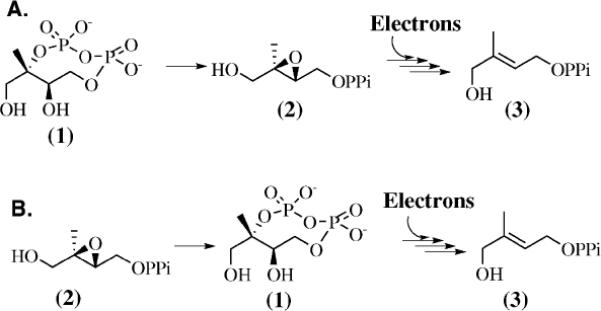
Two potential scenarios to account for IspG-catalyzed reductive deoxygenations of both MEcPP and Epoxy-HMBPP to HMBPP. (A) Epoxy-HMBPP as an intermediate in the reductive deoxygenation of MEcPP to HMBPP; (B) MEcPP as an intermediate in the reductive deoxygenation of Epoxy-HMBPP to HMBPP.
The inter-conversion between MEcPP and Epoxy-HMBPP reaction was monitored using our recently reported 1H-NMR assay.13,20 The C5 methyl group chemical shifts in the 1H-NMR spectra for MEcPP (1.32 ppm) and Epoxy-HMBPP (1.25 ppm) are well-resolved and can be used to monitor Epoxy-HMBPP formation (Fig. 1 and 2). The as-isolated [4Fe–4S]2+-containing IspG (5 μM) was incubated with MEcPP (1.7 mM) at 37 °C, and the reaction was monitored by 1H-NMR (Fig. 1). Over the course of 4 hours under these conditions, we did not detect the formation of Epoxy-HMBPP (Fig. 1B). For the 1H-NMR assay, Epoxy-HMBPP at 20 μM can be reliably detected. Our results from this study suggest that Epoxy-HMBPP is either not produced from MEcPP under these conditions or its production is at a level below our 1H-NMR assay detection limit.
Fig. 1.
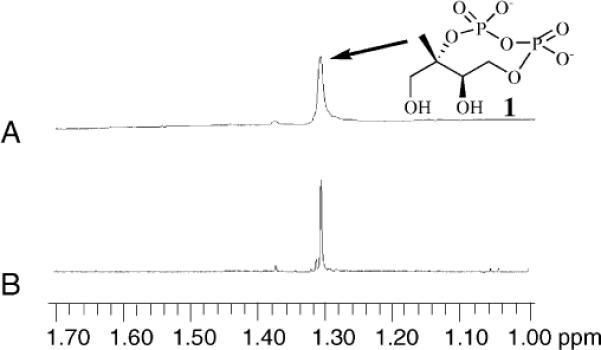
1H-NMR spectra of MEcPP (1) C5 methyl group region. The reaction mixture contained 5.0 μM of [4Fe–4S]2+-containing IspG, 1.7 mM MEcPP (1) at 37 °C in 100 mM Tris, pH 8.0 in the absence of extra reductants (e.g., dithionite/methyl viologen). (A) 5 min; (B) 4 hours.
Fig. 2.
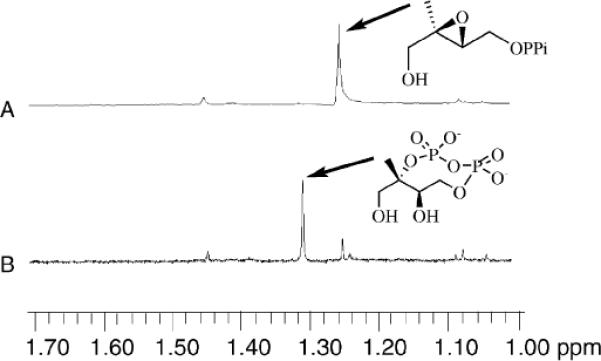
IspG-catalyzed production of MEcPP from Epoxy-HMBPP. 1H-NMR spectra of the C5 methyl group region. Spectrum A: Epoxy-HMBPP C5 methyl group region (in 100 mM Tris, pH 8.0); Spectrum B: IspG catalyzed MEcPP production from Epoxy-HMBPP (5.0 μM of [4Fe–4S]2+-containing IspG, 1.5 mM Epoxy-HMBPP in 100 mM Tris, pH 8.0 at 37 °C for 4 hours).
Because Epoxy-HMBPP is an IspG substrate, we also examined the reversibility of this reaction, MEcPP production from Epoxy-HMBPP (Scheme 2B, Fig. 2). Interestingly, when the [4Fe–4S]2+-containing IspG was incubated with Epoxy-HMBPP in the absence of reductants, a new species was observed in the 1H-NMR spectrum (1.32 ppm, Fig. 2B), downfield from the 1.25 ppm signal (C5 methyl group of Epoxy-HMBPP, Fig. 2A). The new species has the same chemical shift as that of the MEcPP methyl group. This new species was isolated and its spectroscopic properties are identical to that of authentic synthetic MEcPP (Fig. S1, ESI†). We further characterized the diphosphate-containing product using 1H–31P–31P COSY as previously reported.22 These results are also consistent with the IspG-catalyzed production of MEcPP from Epoxy-HMBPP (Scheme 2B and Fig. S2 in ESI†). In the absence of IspG, there is no MEcPP production from Epoxy-HMBPP, at least at our 1H-NMR assay detection level.
The kinetic parameters for the conversion of Epoxy-HMBPP to MEcPP were measured using the 1H-NMR assay with [4Fe–4S]2+-containing IspG as the catalyst (Fig. S3, ESI†). Under presumed saturating substrate conditions (1.0 mM of Epoxy-HMBPP) and at various [4Fe–4S]2+-containing IspG concentrations (2.5 μM–10.0 μM IspG, 100 mM Tris–HCl, pH 8.0 at 37 °C), the kcat value for this conversion (~2.0 min−1) was calculated using the average kcat at various IspG concentrations (Fig. S4, ESI†). Under these conditions, the kcat value (~2.0 min−1) is an order of magnitude lower than that of the reductive deoxygenation of Epoxy-HMBPP to HMBPP (20.1 min−1).
Previously, we demonstrated that IspG catalyzes a reductive deoxygenation of both MEcPP and Epoxy-HMBPP to HMBPP (Scheme 3).13,20 In this study, we have examined two mechanistic options that might explain the IspG-catalyzed reductive deoxygenation of both MEcPP and Epoxy-HMBPP to HMBPP (Scheme 2): (1) Epoxy-HMBPP as an intermediate in MEcPP reductive deoxygenation (Scheme 2A), and (2) MEcPP as an intermediate in the reductive deoxygenation of Epoxy-HMBPP (Scheme 2B). Our attempts to detect the formation of Epoxy-HMBPP from MEcPP under both reductive deoxygenation conditions and in the absence of excess reductants were not successful. Subjecting MEcPP to [4Fe–4S]2+-containing IspG did not result in the production of Epoxy-HMBPP to a level detectable in our 1H-NMR assay. Importantly, we detected an IspG-catalyzed conversion of Epoxy-HMBPP to MEcPP in the absence of reductants (Fig. 2 and Scheme 3). When chemical micro-reversibility is taken into consideration, the production of MEcPP from Epoxy-HMBPP implies that the inter-conversion to MEcPP from Epoxy-HMBPP might be chemically feasible. However, the steady-state kinetic analyses presented here are not sufficient to support the intermediacy of either MEcPP or Epoxy-HMBPP in IspG-catalyzed reductive deoxygenation reactions. Pre-steady state analysis in the future will shed light on the putative intermediacy of Epoxy-HMBPP in this reaction.
Scheme 3.
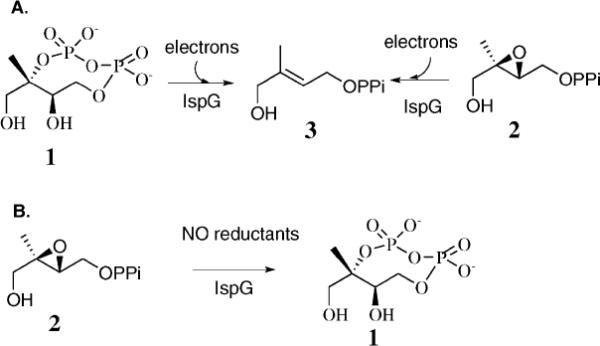
IspG-catalyzed reactions. (A) IspG-catalyzed reductive deoxygenation of MEcPP and Epoxy-HMBPP; (B) IspG-catalyzed MEcPP formation.
In our studies, we did not detect Epoxy-HMBPP formation from MEcPP. However, MEcPP is indeed produced from Epoxy-HMBPP, albeit at a steady state rate that is an order of magnitude lower than the kcats of reductive deoxygenation of both MEcPP and Epoxy-HMBPP. One way to interpret these results is that the slow steady state kcat does not support an epoxide intermediate as suggested in the epoxide model (Scheme 1). If that is the case, the reductive deoxygenation of MEcPP and Epoxy-HMBPP might be achieved through parallel pathways. Alternatively, it is possible that MEcPP is a transient, and/or tight binding intermediate during catalysis; it may not be released into solution at all or may dissociate from IspG active site at a very slow rate. Similar scenarios have been suggested in IspC catalysis, an early step of the DXP pathway,23,24 and in acetohydroxy acid isomeroreductase catalysis in the biosynthesis of branched-chain amino acids.25 The IspC-catalyzed reduction–isomerization reaction is believed to proceed via a transient aldehyde intermediate. However, the aldehyde intermediate has, to date, not been directly observed.
While this manuscript was in preparation, following our initial report on Epoxy-HMBPP studies,20 a report by Oldfield and co-workers suggested that a common organo-metallic transient species en route to HMBPP was generated from both MEcPP and Epoxy-HMBPP based on EPR studies.21 Observation of the same downstream intermediate from MEcPP and Epoxy-HMBPP could support a mechanism where MEcPP proceeds through Epoxy-HMBPP en route to HMBPP. However, as Epoxy-HMBPP has not been directly observed, it is still possible that MEcPP and Epoxy-HMBPP could produce a common downstream intermediate via parallel pathways.
Acknowledgments
This work was supported in part by funding to P.L. from NSF CAREER (CHE-0748504) and NIH (GM-093903). C.F.M. was supported by the Johns Hopkins Malaria Research Institute Pilot Grant. We thank Yan Sun for providing technical support in the H, P-NMR characterization of MEcPP.
Footnotes
Electronic supplementary information (ESI) available: Details of experimental procedure. See DOI: 10.1039/c0cc02594a
Notes and references
- 1.Eisenreich W, Bacher A, Arigoni D, Rohdich F. Cell. Mol. Life Sci. 2004;61:1401–1426. doi: 10.1007/s00018-004-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qureshi N, Porter JW. In: Biosynthesis of Isoprenoid Compounds. Porter JW, Spurgeon SL, editors. vol. 1. Wiley; New York: 1981. pp. 47–94. [Google Scholar]
- 3.Flores-Perez U, Perez-Gil J, Rodriguez-Villalon A, Gil M, Vera P, Rodriguez-Concepcion M. Biochem. Biophys. Res. Commun. 2008;371:510–514. doi: 10.1016/j.bbrc.2008.04.115. [DOI] [PubMed] [Google Scholar]
- 4.Rohdich F, Kis K, Bacher A, Eisenreich W. Curr. Opin. Chem. Biol. 2001;5:535–540. doi: 10.1016/s1367-5931(00)00240-4. [DOI] [PubMed] [Google Scholar]
- 5.Ostrovsky D, Shashkov A, Sviridov A. Biochem. J. 1993;295:901–902. doi: 10.1042/bj2950901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrovsky D, Diomina G, Lysak E, Matveeva E, Ogrel O, Trutko S. Arch. Microbiol. 1998;171:69–72. doi: 10.1007/s002030050680. [DOI] [PubMed] [Google Scholar]
- 7.Ostrovsky D, Shipanova I, Sibeldina L, Shashkov A, Kharatian E, Malyarova I, Tantsyrev G. FEBS Lett. 1992;298:159–161. doi: 10.1016/0014-5793(92)80045-i. [DOI] [PubMed] [Google Scholar]
- 8.Campos N, Rodriguez-Concepcion M, Seemann M, Rohmer M, Boronat A. FEBS Lett. 2001;488:170–173. doi: 10.1016/s0014-5793(00)02420-0. [DOI] [PubMed] [Google Scholar]
- 9.Querol J, Campos N, Imperial S, Boronat A, Rodriguez-Concepcion M. FEBS Lett. 2002;514:343–346. doi: 10.1016/s0014-5793(02)02402-x. [DOI] [PubMed] [Google Scholar]
- 10.Okada K, Hase T. J. Biol. Chem. 2005;280:20672–20679. doi: 10.1074/jbc.M500865200. [DOI] [PubMed] [Google Scholar]
- 11.Adedeji D, Hernandez H, Wiesner J, Kohler U, Jomaa H, Duin EC. FEBS Lett. 2007;581:279–283. doi: 10.1016/j.febslet.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Hecht S, Eisenreich W, Adam P, Amslinger S, Kis K, Bacher A, Arigoni D, Rohdich F. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14837–14842. doi: 10.1073/pnas.201399298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao Y, Zahariou G, Sanakis Y, Liu Y. Biochemistry. 2009;48:10483–10485. doi: 10.1021/bi901519q. [DOI] [PubMed] [Google Scholar]
- 14.Seemann M, Bui BTS, Wolff M, Tritsch D, Campos N, Boronat A, Marquet A, Rohmer M. Angew. Chem., Int. Ed. 2002;41:4337–4339. doi: 10.1002/1521-3773(20021115)41:22<4337::AID-ANIE4337>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Rohdich F, Zepeck F, Adam P, Hecht S, Kaiser J, Laupitz R, Grawert T, Amslinger S, Eisenreich W, Bacher A, Arigoni D. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1586–1591. doi: 10.1073/pnas.0337742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff M, Seemann M, Grosdemange-Billiard C, Tritsch D, Campos N, Rodriguez-Concepcion M, Boronat A, Rohmer M. Tetrahedron Lett. 2002;43:2555–2559. [Google Scholar]
- 17.He XM, Liu HW. Annu. Rev. Biochem. 2002;71:701–754. doi: 10.1146/annurev.biochem.71.110601.135339. [DOI] [PubMed] [Google Scholar]
- 18.Kollas AK, Duin EC, Eberl M, Altincicek B, Hintz M, Reichenberg A, Henschker D, Henne A, Steinbrecher I, Ostrovsky DN, Hedderich R, Beck E, Jomaa H, Wiesner J. FEBS Lett. 2002;532:432–436. doi: 10.1016/s0014-5793(02)03725-0. [DOI] [PubMed] [Google Scholar]
- 19.Itoh T, Nagano T, Sato M, Hirobe M. Tetrahedron Lett. 1989;30:6387–6388. [Google Scholar]
- 20.Nyland RL, II, Xiao Y, Liu P, Meyers CLF. J. Am. Chem. Soc. 2009;131:17734–17735. doi: 10.1021/ja907470n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Li J, Wang K, Huang C, Zhang Y, Oldfield E. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11189–11193. doi: 10.1073/pnas.1000264107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majumdar A, Shah M, Bitok JK, Hassis-LeBeau M, Meyers CLF. Mol. BioSyst. 2009;5:935–944. doi: 10.1039/b903513c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppisch AT, Fox DT, Blagg BS, Poulter CD. Biochemistry. 2002;41:236–243. doi: 10.1021/bi0118207. [DOI] [PubMed] [Google Scholar]
- 24.Hoeffler JF, Tritsch D, Grosdemange-Billiard C, Rohmer M. Eur. J. Biochem. 2002;269:4446–4457. doi: 10.1046/j.1432-1033.2002.03150.x. [DOI] [PubMed] [Google Scholar]
- 25.Dumas R, Biou V, Halgand F, Douce R, Duggleby RG. Acc. Chem. Res. 2001;34:399–408. doi: 10.1021/ar000082w. [DOI] [PubMed] [Google Scholar]


