Abstract
Background and Aims
More than 2000 adult-to-adult living donor liver transplants (LDLT) have been performed in the U.S., yet the potential benefit to liver transplant candidates of undergoing LDLT compared to waiting for deceased donor liver transplant (DDLT) is unknown. The aim of this study was to determine if there is a survival benefit of adult LDLT
Methods
Adults with chronic liver disease who had a potential living donor evaluated from 1/98 to 2/03 at nine university-based hospitals were analyzed. Starting at the time of a potential donor’s evaluation, we compared mortality after LDLT to mortality among those who remained on the waitlist or received DDLT. Median follow-up was 4.4 years. Comparisons were made by hazard ratios (HR) adjusted for LDLT candidate characteristics at the time of donor evaluation.
Results
Among 807 potential living donor recipients, 389 received LDLT, 249 received DDLT, 99 died without transplant, and 70 were awaiting transplant at last follow-up. Receipt of LDLT was associated with an adjusted mortality HR of 0.56 (95% confidence interval [CI] 0.42–0.74; P<0.001) relative to candidates who did not receive LDLT. As centers gained greater experience (> 20 LDLT), LDLT benefit was magnified, with a mortality HR of 0.35 (CI 0.23–0.53; P<0.001).
Conclusions
Adult LDLT was associated with lower mortality than the alternative of waiting for DDLT. This reduction in mortality was magnified as centers gained experience with living donor liver transplantation. This reduction in transplant candidate mortality must be balanced against the risks undertaken by the living donors themselves.
INTRODUCTION
Demand for liver transplantation in the United States has grown faster than the availability of deceased donor organs. Consequently, waiting time for liver transplantation has increased more than four-fold, and death on the waitlist is common. Despite efforts to increase organ donation and changes to the organ allocation system that have reduced candidate mortality, about 2000 adults die yearly while awaiting liver transplantation in the U.S.1 An attractive but controversial approach to increasing availability of livers for transplantation is the use of live donors. First developed for pediatric recipients, utilizing the left lateral segment from adult living donors, the technique was later modified to use the larger right lobe from adult donors for transplantation into adult recipients.2 The challenge associated with living donor liver transplantation (LDLT) in adults is that almost 60% of the liver mass of the donor must be transplanted, and rapid liver regeneration is required in both donor and recipient.
Over 2000 LDLT have been performed in the U.S., but the benefits and risks to the recipients relative to deceased donor liver transplantation (DDLT) have not been fully assessed. LDLT permits more timely transplantation, eliminating the mortality associated with continued residence on the waitlist.3 In addition, elective receipt of a living donor allograft might permit the recipient to be healthier at the time of transplantation, and thus diminish post-transplant mortality. These theoretical benefits could be negated by the use of a partial liver and the increased surgical complexity of LDLT.4
A single report found a 50% reduction in waitlist mortality among liver transplant candidates who had a potential living donor compared to candidates who did not have a potential living donor. The overall rate of transplantation was markedly higher for candidates with a potential living donor.5 Markov models suggested a benefit of LDLT in comparison to waiting for DDLT, particularly for patients with hepatocellular carcinoma (HCC).6,7 However, reports of recipient outcomes following LDLT 8–13 and comparisons of outcomes after LDLT versus DDLT, starting from the date of transplant surgery, 14,15 have demonstrated higher allograft failure rates and trends toward lower patient survival in LDLT recipients.
Previous reports provide little guidance for patient decision-making as none specifically addressed whether the benefit of shortening the time until transplantation with LDLT outweighs the potential for poorer post-transplant outcome. In order to counsel patients regarding the potential benefits of LDLT, we analyzed the mortality experience for a large cohort of LDLT candidates from the time of evaluation of their first potential living donor. This design allowed comparison of LDLT recipients to the transplant candidates who remained on the waitlist without transplant or received DDLT.
METHODS
The primary objective of the Adult-to-Adult Living Donor Liver Transplantation (A2ALL) Retrospective Cohort Study was to determine the survival benefit, if any, of adult LDLT. Patient entry occurred at the date that each potential LDLT candidate’s first potential living donor presented for their initial donor history and physical examination.
Data sources
Candidate and donor data were provided by the nine participating A2ALL transplant centers based on a common protocol. Chart reviews were supplemented by additional ascertainment of deaths and transplants through October 2005 under a data use agreement with the Scientific Registry of Transplant Recipients.16 The A2ALL study included 819 patients who had at least one potential living donor evaluated between 1/1/98 and 2/28/03. Transplant candidates with fulminant hepatic failure (n=12) were excluded from the analyses. For the remaining 807 candidates, median follow-up time was 4.4 years.
Statistical methods
The cumulative incidence function was calculated using the SAS® macro “comprisk”.3 The Model for End-stage Liver Disease score (MELD) was calculated based on laboratory data only,17 and ignored exception MELD scores used in allocation. LDLT recipients were classified as having received their transplant when the center was less experienced (had performed ≤ 20 LDLT) or more experienced (had performed >20 LDLT).4 Eight centers each performed >20 LDLT during the study and one performed 20.
Survival analyses, starting at the time of evaluation of each subject’s first potential donor, were used to compare mortality following LDLT versus the standard strategy of continued waiting for possible DDLT. The non-LDLT group included those who received DDLT or domino liver transplant, those who remained on the waitlist at study end, and those who died without receiving a transplant. LDLT or DDLT procedures that were aborted intraoperatively due to recipient reasons were considered to be transplants.
We used two Cox regression methods to compare the effect of LDLT with not receiving an LDLT. Both methods address the following question: “Does the patient who undergoes an LDLT have lower mortality than a comparable patient who waits for a DDLT?” Modeling LDLT as a conventional time-dependent variable compared mortality following a given LDLT with mortality for all other patients alive at that point who had not yet received LDLT, including those who had already received a DDLT.18 These latter patients, although uncommon, are no longer truly LDLT candidates and thus should not be in the comparison group. The second approach, sequential stratification,19 provides a similar comparison but correctly excludes any recipients who have already received DDLT from the comparison group for a given LDLT. Specifically, for one or more LDLTs performed at a given number of days since first donor evaluation, a separate comparison group (stratum) was created that included all patients alive and without any transplant (either LDLT or DDLT) prior to the time of the index LDLT(s). The survival of the index LDLT patient(s) was compared to that of all other eligible patients in that stratum. Within each stratum, patients were censored at the earliest of the date of later receipt of an LDLT, the date of last known follow-up, or end of study (October 2005). The results across all LDLT strata were pooled in a stratified Cox regression. Because portions of the time at risk for a patient may appear in multiple strata, the standard errors of the hazard ratios were corrected with robust (sandwich estimator) variance estimates. Both time-dependent and sequentially stratified Cox models were adjusted for baseline covariates of age, HCC, and MELD score, all determined at the time of first donor evaluation. Multiplicative interactions (effect modification) between LDLT and the other variables in the final model were evaluated. An additional Cox regression model examined the risk of mortality by transplant type, starting from the time of transplant, adjusted for age, HCC, and MELD score at transplant.
For graphical representation, survival probabilities were calculated as follows. Survival without any transplant was estimated from a Cox regression censored at LDLT or DDLT. Survival following LDLT or DDLT was estimated based on a Cox model stratified by transplant type. Both models were adjusted for age, HCC, and MELD score. Probabilities of survival through the waitlist period followed by transplant were estimated by multiplying the waitlist survival probability at the respective median transplant time by the post-transplant (LDLT or DDLT) survival probability. All analyses were carried out using SAS 9.1 software (SAS Publishing, Cary, NC: SAS Institute Inc., 2004).
Human subjects protection
The Institutional Review Boards and Privacy Boards of the Data Coordinating Center and the nine participating transplant centers approved the study.
RESULTS
The 807 non-fulminant liver transplant candidates for whom at least one potential living donor was evaluated represented 9.9% of 8,176 liver transplant candidates listed for DDLT at the nine A2ALL centers during the study period. Compared with patients evaluated for LDLT (whose characteristics are shown in Table 1), this much larger group had higher mean body mass index (28.5 kg/m2; p<0.001), were less likely (p<0.05) to be female (39%), white (84%), have a diagnosis of hepatitis C (42%) or hepatocellular carcinoma (5%), or a history of variceal bleeding (5%).
Table 1.
Characteristics of potential LDLT recipients at time of donor evaluation
| Characteristic | Overall (n=807) * Mean±SD or Percent | LDLT (n=389) Mean±SD or Percent | Non-LDLT (n=418) † Mean±SD or Percent | LDLT vs. Non-LDLT P-Value |
|---|---|---|---|---|
| Age (years) | 50.3±10.1 | 49.3±10.7 | 51.3±9.5 | 0.006 |
| Sex | 0.55 | |||
| Male | 57% | 58% | 56% | |
| Female | 43% | 42% | 44% | |
| Race | 0.03 | |||
| White | 90% | 91% | 89% | |
| African-American | 5% | 3% | 7% | |
| Other | 5% | 6% | 4% | |
| Height (cm) | 171.1±10.3 | 171.4±10.8 | 170.8±9.8 | 0.45 |
| Weight (kg) | 79.6±18.0 | 78.6±18.0 | 80.5±18.0 | 0.15 |
| Body Mass Index (kg/m2) | 27.1±5.2 | 26.7±5.2 | 27.4±5.2 | 0.04 |
| Previous Transplant Diagnosis‡ | 2% | 3% | 1% | 0.25 |
| Hepatitis C | 47% | 48% | 47% | 0.79 |
| Hepatocellular Carcinoma (HCC) | 13% | 15% | 11% | 0.10 |
| Alcoholic liver disease | 14% | 14% | 15% | 0.62 |
| Cholestatic liver disease | 19% | 19% | 19% | 0.97 |
| Other non-cholestatic cirrhosis | 20% | 21% | 20% | 0.87 |
| Metabolic disease | 3% | 3% | 3% | 0.81 |
| Biliary atresia | 0.4% | 1% | 0% | 0.11 |
| Non-HCC malignancy | 2% | 3% | 2% | 0.29 |
| Other | 3% | 3% | 4% | 0.31 |
| Ascites | 65% | 61% | 68% | 0.01 |
| Encephalopathy | 48% | 40% | 55% | <0.001 |
| Variceal Bleed | 18% | 17% | 19% | 0.34 |
| Upper Abdominal Surgery | 20% | 20% | 19% | 0.51 |
| Spontaneous Bacterial Peritonitis | 7% | 8% | 6% | 0.27 |
| TIPSS§ | 11% | 8% | 12% | 0.14 |
| MELD¶ | 15.6±6.8 | 14.8±6.4 | 16.4±7.2 | 0.002 |
| MELD (categories) | 0.003 | |||
| 6–10 | 22% | 26% | 19% | |
| 11–20 | 56% | 56% | 55% | |
| 21–30 | 15% | 11% | 18% | |
| 31–40 | 5% | 3% | 6% | |
| Missing | 3% | 4% | 2% | |
| Recipient Medical Condition | 0.22 | |||
| ICU | 2% | 1% | 3% | |
| Hospitalization, no ICU | 7% | 6% | 8% | |
| Not hospitalized | 90% | 92% | 89% | |
| Diabetes Mellitus | 20% | 19% | 21% | 0.14 |
| Angina/Coronary Artery Disease | 4% | 3% | 5% | 0.50 |
| Drug Treated Systemic Hypertension | 13% | 12% | 13% | 0.19 |
| Functional Status | 0.003 | |||
| No activity limitations | 46% | 54% | 40% | |
| Activities of daily living with some assistance | 40% | 34% | 45% | |
| Activities of daily living with total assistance | 1% | 1% | 1% | |
| Hospitalized | 7% | 6% | 8% | |
| Unknown | 5% | 4% | 5% | |
| Employment Status | 0.03 | |||
| Working full time | 24% | 29% | 19% | |
| Working part time | 11% | 9% | 12% | |
| Not working | 50% | 48% | 53% | |
| Retired | 8% | 7% | 8% | |
| Unknown | 8% | 7% | 8% | |
| Year of Evaluation | <0.001 | |||
| 1998 | 4% | 6% | 3% | |
| 1999 | 14% | 17% | 10% | |
| 2000 | 22% | 23% | 21% | |
| 2001 to 2/27/2002 | 35% | 35% | 36% | |
| 2/28/2002 to 2003 (MELD era) | 25% | 19% | 30% | |
| Time from Candidate Listing to Donor Evaluation (days) | 225±344 | 192±319 | 256±362 | 0.009 |
Percent of values missing is less than 3% for each variable.
Non-LDLT group includes deceased donor transplant recipients and those who are still waitlisted.
Patients may have more than one diagnosis.
TIPSS: transjugular intrahepatic portosystemic shunt.
MELD: Model for End-stage Liver Disease score.
Eighty-two percent of the potential living donor recipients had one potential donor evaluated, 15% had two donors evaluated, and 3% had at least three donors evaluated (maximum of seven). Nearly one-half of the living donor recipient candidates (48% or 389) actually underwent LDLT (Figure 1). The LDLT recipients were similar to the other patients in regard to most features such as sex, body weight, diagnosis and various co-morbidities (Table 1). There were small but statistically significant differences between the two groups in mean age, race, MELD score, presence of ascites, encephalopathy, functional status, employment status, year of evaluation, and time from listing to evaluation. Among the 389 LDLT recipients, the median time from donor evaluation to LDLT was 1.8 months (range 4 days to 4.3 years); eight (1%) occurred beyond one year. The median number of LDLT cases performed at each center was 31 (range 20 to 71). For the 249 DDLT recipients, the median time from donor evaluation to transplant was 4.6 months (range 2 days to 5.0 years). The probability of receiving an LDLT, receiving a DDLT, or dying on the waitlist starting from the time of initial donor evaluation is shown in Figure 2. By one year after donor evaluation, 47.2% of patients had received a LDLT, 22.7% had received a DDLT, and 7.7% had died without a transplant. By 3.5 years after donor evaluation, 30.8%, of patients had received a DDLT and 12.1% had died without a transplant.
Figure 1.
Flow diagram of the cohort of A2ALL liver transplant candidates from the time of first living donor evaluation.
Figure 2. Cumulative probability of receiving a LDLT or DDLT or dying while awaiting transplantation among 807 liver transplant candidates.
The small circles mark the time of performance of the median number of LDLT (1.8 months) and DDLT (4.6 months). The probability of remaining alive while still awaiting transplantation is also shown.
In an unadjusted sequential stratification analysis of time from initial donor evaluation to death, patients who underwent LDLT had a hazard ratio [HR] of 0.62 (95% confidence interval [CI] 0.47 – 0.82; P<0.001), compared to patients who did not receive an LDLT. In the adjusted analyses that controlled for differences at donor evaluation, patients who received LDLT had a HR of 0.56 (95% CI 0.42 – 0.74; P<0.001) relative to patients who did not receive LDLT (Figure 3). Recipients of LDLT during the early experience at a center (≤20 cases) had marginally lower mortality (HR=0.83; P=0.27) than non-LDLT patients (Table 2). In contrast, once a center had performed 20 LDLT, the relative mortality risk for LDLT was only 35% of the risk for candidates who did not receive LDLT (P<0.001). There was a significant difference in the relative mortality risk of LDLT between the less experienced and the more experienced periods (P<0.001). Older recipient age, higher MELD score, and diagnosis of hepatocellular carcinoma were also associated with death. No effect modifications were identified by multiplicative interaction between LDLT and these other features. Neither the year of donor evaluation nor the introduction of MELD-based DDLT allocation was a significant predictor of death.
Figure 3. Cumulative risk of death after initial living donor evaluation for patients receiving LDLT versus not receiving LDLT.
Risk of death following LDLT diverges beginning at median time of LDLT following donor evaluation (1.8 months) (green line). Estimates are adjusted for age, MELD score, and HCC status, and apply to a patient with age=50, MELD=15, and no HCC.
Table 2.
Risk factors for death among transplant candidates following evaluation of a potential living donor. Results are based on a Cox regression model using sequential stratification
| Overall (n=807) (Donor evaluation 1/1/1998 to 2/28/2003) | MELD era (n=198) (Donor evaluation 2/28/2002 to 2/28/2003) | |||||
|---|---|---|---|---|---|---|
| Variable | Mortality Hazard Ratio | 95% Confidence Interval | P-Value | Mortality Hazard Ratio | 95% Confidence Interval | P-Value |
| Recipient age at enrollment (per 10 years) | 1.25 | (1.04, 1.50) | 0.02 | 1.39 | (0.97, 1.99) | 0.07 |
| MELD at enrollment (per unit MELD) | 1.08 | (1.04, 1.11) | <.001 | 1.05 | (0.99, 1.11) | 0.13 |
| Diagnosis of hepatocellular carcinoma | 2.20 | (1.40, 3.48) | <.001 | 1.46 | (0.54, 3.91) | 0.45 |
| LDLT when center had done ≤20 cases (vs. no LDLT) | 0.83 | (0.59, 1.16) | 0.27 | --* | -- | -- |
| LDLT when center had done >20 cases (vs. no LDLT) | 0.35 | (0.23, 0.53) | <.001 | 0.32 | (0.14, 0.71) | 0.005 |
All but 4 LDLT transplants in the MELD era occurred when the center had done >20 cases. There were no deaths among these 4 LDLT transplants.
LDLT = Living donor liver transplantation
MELD = Model for End-Stage Liver Disease
In models designed to look for a possible center effect, we did not find a significant association either as a main effect (p=0.51) or as an interaction with the LDLT effect (p=0.10). The addition of center variables also did not alter the LDLT effect in the main effects model compared to the original model without the center variables (LDLT less experienced HR 0.82 vs. 0.83; LDLT more experienced HR 0.37 vs. 0.35, respectively). In addition, no significant association was found between waiting time for DDLT (dividing the centers into three groups based on the 25th percentile of each center’s OPO’s waiting time) and LDLT survival benefit.
Waiting time as a main effect was not significant (p=0.52), and the effect of LDLT in this model remained virtually identical to the original model without the waiting time variables (less experienced 0.81 vs. 0.83; more experienced 0.35 vs. 0.35, respectively). We also looked for an interaction between the LDLT survival benefit and waiting time (i.e., does the relative risk of death with LDLT vs. without LDLT vary by waiting time). This interaction test was also not significant (p=0.35). However, as expected, the direction of the interaction did indicate that LDLT at centers with longer waiting times were associated with greater benefit.
A separate Cox regression analysis that modeled LDLT as a conventional time-varying covariate showed results similar to that of the adjusted sequential stratification method (LDLT more experience: HR=0.47; 95% CI 0.32 – 0.69; P<0.001; LDLT less experience: HR=1.08; 95% CI 0.79 – 1.49; P=0.62).
Figure 4 shows the cumulative probability of mortality from the time of initial living donor evaluation for patients remaining on the waitlist, for patients received DDLT at the median time to DDLT (4.6 months), and for patients who had LDLT at the median time to LDLT when centers had less experience (1.7 months) or more experience (2.0 months). Long-term transplant candidate mortality on the waitlist was higher than the mortality after either LDLT or DDLT. The survival advantage of LDLT appeared to be a result of removal from continued exposure to the risk of death on the waitlist.
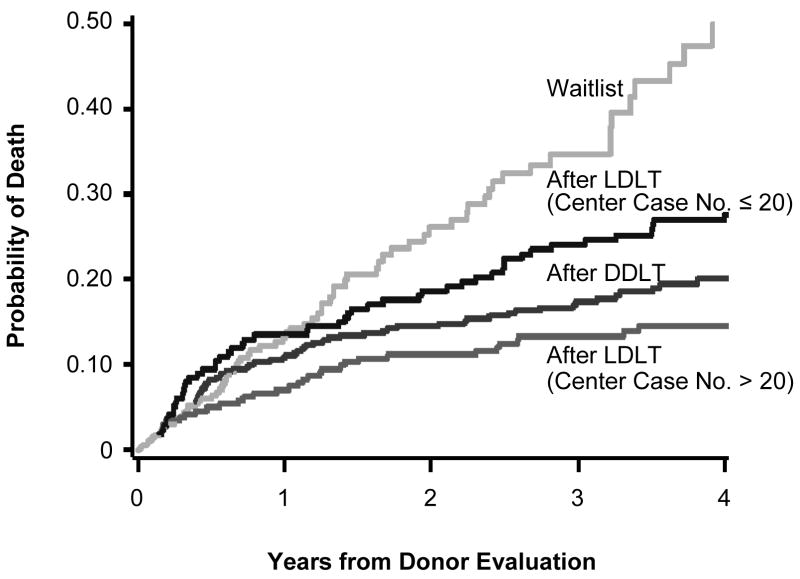
Figure 4. Cumulative risk of death after initial living donor evaluation for patients not transplanted (yellow), patients receiving DDLT (red), and patients receiving LDLT during earlier (blue) and later center experience (green).
Estimates are adjusted for age, MELD score, and HCC status, and apply to a patient with age=50, MELD=15, and no HCC. Risks of death following transplant diverge beginning at the median times for each type: LDLT case ≤20 (1.7 months); LDLT case >20 (2.0 months); and DDLT (4.6 months).
An analysis restricted to the 198 patients who had an initial living donor evaluation following the institution of the MELD liver allocation score (February 2002) had similar results as those of the entire cohort (Table 2). After introduction of MELD, mortality risk was 70% lower with LDLT overall (HR=0.30; 95% CI 0.14–0.67; P=0.0033), by which time all but four LDLT cases were performed after centers had performed more than 20 LDLT cases. The more favorable liver allocation for patients with higher MELD scores or hepatocellular cancer substantially decreased the mortality hazard ratios for each. An additional analysis of those with MELD score <15 at study entry (54%) showed an LDLT adjusted mortality hazard ratio of 0.94 (95% CI 0.59–1.52; p=0.81) during earlier center experience and 0.41 (95% CI 0.24–0.68; p<0.0001) with greater experience.
While not the primary focus of the study, analyses of survival were also performed beginning at the time of transplant (rather than at time of first donor evaluation) to compare mortality following the two different transplant procedures. In adjusted analyses, survival probabilities for DDLT recipients (with mean covariate values of age=50 years, MELD=15, and no HCC) were 92.1% at one year and 86.3% at three years. Corresponding post-transplant survival probabilities for LDLT recipients were 92.0% at one year and 84.8% at three years (P=0.36 compared with DDLT). Adjusted survival probabilities for LDLT performed while centers were less experienced were 89.4% at one year and 78.3% at three years (P=0.01 vs. DDLT). Post-LDLT survival increased after centers gained greater experience (94.0% at one year and 89.7% at three years; P=0.29 vs. DDLT; P<0.001 vs. LDLT with less experience). Early re-transplantation (<3 weeks) was performed in 1.1% of DDLT recipients, and 7.8% and 3.6% of LDLT recipients while centers were less and more experienced, respectively.
DISCUSSION
Adult-to-adult LDLT affords selected liver transplant candidates an alternative to waiting for a liver from a deceased donor. While the technique is practiced at numerous transplant centers around the world, analyses have not adequately assessed the potential benefits or risks of receipt of a living donor graft.20 The few published analyses that examined the outcomes of LDLT at individual U.S. liver transplant centers, or included the entire U.S. LDLT experience, have shown that LDLT is associated with higher mortality rates than DDLT. These analyses, however, were limited to the experience of candidates who survived to transplantation. Not considered by most of these reports was mortality while awaiting transplantation. As practiced in the U.S., pursuit of LDLT, which is initiated by the evaluation of a potential living donor, often results in a LDLT months or even years before DDLT would occur. We utilized a novel study design that accounted for the contribution of waitlist mortality in assessing the survival experience of individuals who did not receive an LDLT despite evaluation of a potential living donor. Results from this study are thus directly applicable to the counseling of liver transplant candidates who are contemplating pursuit of LDLT as an alternative to DDLT.
An advantage of the study design was that entry required evaluation of a potential donor without preconception of whether the recipient would actually receive an LDLT. As a result, LDLT recipients were drawn from the same pool of candidates as those who did not receive LDLT, reducing differences at time of study entry. Adjustment for any measurable differences between the two groups that might have affected survival had little effect on the estimated survival advantage of LDLT. It is possible that unmeasured confounders may have influenced the survival advantage of LDLT, despite the care taken to establish an appropriate comparison group and the apparent comparability of LDLT and non-LDLT patients in the study. However, given the substantial survival advantage of LDLT, the influence of unknown confounders would have had to have been large to eliminate this advantage.
Liver transplant candidates who received their LDLT after centers had gained considerable experience with the technique had markedly lower mortality than that of the candidates who were considered for, but did not receive, an LDLT. Conversely, the analysis did not find significantly better survival for LDLT recipients during the centers’ early LDLT experiences, when LDLT was associated with higher post-transplant mortality than either the later LDLT experience or DDLT. The benefit of greater experience resulted from lower post-transplant mortality, as was shown in our secondary analysis (Figure 4). Prior reports that described worse outcomes following LDLT compared to DDLT may have been heavily influenced by the early experiences of LDLT programs.14, 15 Our results suggest that even if waiting time for DDLT was only slightly longer than for LDLT, there would still be a survival benefit to choosing LDLT once centers are experienced. The benefit of LDLT is enhanced as waiting time for DDLT and its associated mortality increase.
The institution of deceased donor liver allocation using the MELD scoring system in 2002 has decreased the overall death rate on the waitlist by assigning priority to patients most likely to die in the short term. Thus, it is an important and relevant finding that an analysis restricted to patients eva luated for LDLT after the introduction of MELD demonstrated an LDLT survival advantage similar to that of the entire cohort. (Table 2). Furthermore, LDLT was even found to be beneficial for those with MELD <15, a group that was recently shown not to benefit from DDLT,21 and may reflect unmeasured differences between LDLT and DDLT candidates. Thus, the survival benefits observed in this study are directly applicable to the current MELD-based allocation environment.
Only 10% of liver transplant candidates at A2ALL centers had a donor evaluated for LDLT. As outlined, those who did not have a living donor evaluated were quite different from those who did. It cannot be assumed that the results would be similar to the current analysis if LDLT was available for a broader pool of transplant candidates with much higher MELD scores or comorbidities beyond those in our cohort. Rather, this study provides previously unavailable survival information to patients who are considering the possibility of LDLT and have characteristics similar to our study population. There is also the potential for selection bias on the recipient side within our study cohort. However, we have reported that progression to unsuitability as a living donor recipient after the initial donor history and physical examination accounted for only 21% of cases that did not result in completed living donor transplants.22 This supports the presumption of comparability between recipients of LDLT and those who did not receive LDLT in the current study.
LDLT poses significant ethical tension. It has the potential to substantially increase the number of livers available for transplantation and therefore decrease overall mortality for candidates awaiting transplantation. But this benefit must be weighed against the risks of morbidity and mortality borne by the healthy volunteer donor.23 The A2ALL group has reported on psychiatric morbidity after living liver donation24 and an overall rate of donor complications of 38% using a comprehensive inventory.25 This ethical issue can be informed by empirical information on whether the decision to perform LDLT improves survival for the recipient. Our results indicate that LDLT did reduce candidate mortality, and the observed benefit was greater after centers developed experience with the procedure. In fact, the magnitude of mortality reduction was among the largest observed with any form of transplant intervention.20 These findings should be useful for liver transplant candidates and potential donors as they attempt to balance the risks and benefits of the various routes to liver transplantation, as well as for transplant centers as they evaluate patients for LDLT or consider establishment of new LDLT programs.
Acknowledgments
This study was supported by National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (listed below). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS). The ASTS and HRSA played no specific role in the design and conduct of the study; collection, management and interpretation of the data; and preparation, review or approval of the manuscript. A representative of NIDDK (J.E.E.) contributed to the design of the study and supervised its conduct, actively participated in the interpretation of the data, and in the preparation, review and approval of the manuscript.
Dr. Berg and Dr. Gillespie had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning and conduct of the study and/or care of patients enrolled at each of the participating institutions as follows:
Columbia University Health Sciences, New York, NY (DK62483): PI: Jean C. Emond, MD; Study Coordinators: Rudina Odeh-Ramadan, PharmD; Taruna Chawla, MD
Northwestern University, Chicago, IL (DK62467): Co-PI: Andreas Blei, MD; Study Coordinator: Patrice Al-Saden, RN, CTCC
University of Pennsylvania Health System, Philadelphia, PA (DK62494): Co-PI: Kim M. Olthoff, MD; Study Coordinators: Mary Kaminski, PA-C; Mary Shaw, RN, BBA
University of Colorado Health Sciences Center, Denver, CO (DK62536): Co-PI: Igal Kam, MD; Study Coordinators: Scott Heese, BA; Carlos Garcia, BS
University of California Los Angeles, Los Angeles, CA (DK62496): Co-PI: Ronald W. Busuttil, MD, PhD; Study Coordinator: Lucy Artinian, RN, MN
University of California San Francisco, San Francisco, CA (DK62444): Co-PI: Norah A. Terrault, MD; Study Coordinator: Dulce MacLeod, RN
University of Michigan Medical Center, Ann Arbor, MI (DK62498): DCC Staff: Douglas R. Armstrong, RN, MS; Margaret Hill-Callahan, BS, LSW; Terese Howell, BS; Monique Lowe, MT(ASCP); Tempie H. Shearon, MS; Lan Tong, MS; Karen Wisniewski, MPH
University of North Carolina, Chapel Hill, NC (DK62505): Co-PI: Roshan Shrestha, M.D; Study Coordinator: Carrie A. Nielsen, MA
University of Virginia (DK62484): Co-PI: Timothy L. Pruett, MD; Study Coordinator: Jaye Davis, RN
Medical College of Virginia Hospitals, Virginia Commonwealth University, Richmond, VA (DK62531): Co-PI: Mitchell L. Shiffman, MD; Study Coordinators: Cheryl Rodgers, RN; Ede Fenick, RN; April Ashworth, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD:; Leonard B. Seeff, MD; Patricia R. Robuck, PhD; Jay H. Hoofnagle, MD
Supplemental data included here have been supplied by the Arbor Research Collaborative for Health as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
ABBREVIATIONS
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation
- ASTS
American Society of Transplant Surgeons
- DDLT
deceased donor liver transplant
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- HRSA
Health Resources and Services Administration
- LDLT
adult living donor liver transplants
- MELD
Model for End-stage Liver Disease score
- NIDDK
National Institute of Diabetes & Digestive & Kidney Diseases
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Supported in part by the National Institutes of Health (NIDDK grant numbers U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531), the American Society of Transplant Surgeons, and the US Department of Health and Human Services, Health Resources and Services Administration.
This is publication number 6 of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study.
CONFLICTS OF INTEREST
No Conflicts of Interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carl L. Berg, Department of Medicine, University of Virginia Health System, Charlottesville, VA.
Brenda W. Gillespie, Department of Biostatistics, University of Michigan, Ann Arbor, MI.
Robert M. Merion, Department of Surgery, University of Michigan, Ann Arbor, MI.
Robert S. Brown, Jr., Department of Medicine, Columbia University College of Physicians and Surgeons, New York, NY.
Michael M. Abecassis, Department of Surgery, Northwestern University, Chicago, IL.
James F. Trotter, Department of Surgery, University of Colorado, Denver, CO.
Robert A. Fisher, Department of Surgery, Medical College of Virginia Hospitals, Virginia Commonwealth University, Richmond, VA.
Chris E. Freise, Department of Surgery, University of California, San Francisco, CA.
R. Mark Ghobrial, Department of Surgery, University of California, Los Angeles, CA.
Abraham Shaked, Department of Surgery, University of Pennsylvania, Philadelphia, PA.
Jeffrey H. Fair, Department of Surgery, University of North Carolina, Chapel Hill, NC.
James E. Everhart, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD.
References
- 1.2005 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry for Transplant Recipients: Transplant Data 1995–2004. Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs, Division of Transplantation; Rockville, MD: United Network for Organ Sharing; Richmond, VA: University Renal Research and Education Association; Ann Arbor, MI: 2006. [Google Scholar]
- 2.Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–82. doi: 10.1056/NEJMra011629. [DOI] [PubMed] [Google Scholar]
- 3.Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatol. 2006;43:345–51. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]
- 4.Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242:314–23. doi: 10.1097/01.sla.0000179646.37145.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo MW, LaPointe-Rudow D, Kinkhabwala M, Emond J, Brown RS., Jr Impact of adult living donor liver transplantation on waiting time survival in candidates listed for liver transplantation. Am J Transplant. 2004;4:427–31. doi: 10.1111/j.1600-6143.2004.00336.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatol. 2001;33:1073–9. doi: 10.1053/jhep.2001.23311. [DOI] [PubMed] [Google Scholar]
- 7.Cheng SJ, Pratt DS, Freeman RB, Jr, Kaplan MM, Wong JB. Living-donor versus cadaveric liver transplantation for non-resectable small hepatocellular carcinoma and compensated cirrhosis: a decision analysis. Transplantation. 2001;72:861–8. doi: 10.1097/00007890-200109150-00021. [DOI] [PubMed] [Google Scholar]
- 8.Bak T, Wachs M, Trotter J, et al. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transpl. 2001;7:680–6. doi: 10.1053/jlts.2001.26509. [DOI] [PubMed] [Google Scholar]
- 9.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg. 2001;234:301–11. doi: 10.1097/00000658-200109000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inomata Y, Tanaka K, Uemoto S, et al. Living donor liver transplantation: an 8-year experience with 379 consecutive cases. Transplant Proc. 1999;31:381. doi: 10.1016/s0041-1345(98)01671-6. [DOI] [PubMed] [Google Scholar]
- 11.Marcos A, Ham JM, Fisher RA, Olzinski AT, Posner MP. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transpl. 2000;6:296–301. doi: 10.1053/lv.2000.6354. [DOI] [PubMed] [Google Scholar]
- 12.Ghobrial RM, Saab S, Lassman C, et al. Donor and recipient outcomes in right lobe adult living donor liver transplantation. Liver Transpl. 2002;8:901–9. doi: 10.1053/jlts.2002.35548. [DOI] [PubMed] [Google Scholar]
- 13.Suh KS, Kim SH, Kim SB, Lee HJ, Lee KU. Safety of right lobectomy in living donor liver transplantation. Liver Transpl. 2002;8:910–5. doi: 10.1053/jlts.2002.35665. [DOI] [PubMed] [Google Scholar]
- 14.Abt PL, Mange KC, Olthoff KM, Markmann JF, Reddy KR, Shaked A. Allograft survival following adult-to-adult living donor liver transplantation. Am J Transplant. 2004;4:1302–7. doi: 10.1111/j.1600-6143.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 15.Thuluvath PJ, Yoo HY. Graft and patient survival after adult live donor liver transplantation compared to a matched cohort who received a deceased donor transplantation. Liver Transpl. 2004;10:1263–8. doi: 10.1002/lt.20254. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson DM, Bryant PC, Williams MC, et al. Transplant data: sources, collection, and caveats. Am J Transplant. 2004;4 (Suppl 9):13–26. doi: 10.1111/j.1600-6135.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 17.Freeman RB, Jr, Wiesner RH, Roberts JP, McDiarmid S, Dykstra DM, Merion RM. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4 (Suppl 9):114–31. doi: 10.1111/j.1600-6135.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 18.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. Springer; New York: 2003. [Google Scholar]
- 19.Schaubel DE, Wolfe RA, Port FK. A sequential stratification method for estimating the effect of a time-dependent experimental treatment in observational studies. Biometrics. 2006;62 (3):910–17. doi: 10.1111/j.1541-0420.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 20.Calne R. Review of “Transplantation of the Liver”. N Engl J Med. 2006;354:881–2. [Google Scholar]
- 21.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–13. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 22.Trotter JF, Wisniewski KA, Terrault NA, et al. Outcomes of donor evaluation in adult-to-adult living donor liver transplantation. Hepatology. doi: 10.1002/hep.21845. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghobrial RM, Freise CE, Trotter JF, et al. Donor morbidity and mortality of adult living donors for liver transplantation. Am J Transplant. 2006;6 (Suppl 2):115. [Google Scholar]
- 24.Trotter JF, Hill-Callahan MM, Gillespie BW, et al. Severe psychiatric problems in right hepatic lobe donors for living donor liver transplantation. Transplantation. 2007;83:1506–1508. doi: 10.1097/01.tp.0000263343.21714.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghobrial RM, Freise CE, Trotter JF, et al. Donor morbidity and mortality of adult living donors for liver transplantation. Am J Transplant. 2006;6 (Suppl 2):115. [Google Scholar]