Abstract
Mechanisms underlying failure of novel 2009 H1N1 influenza vaccine-induced Ab responses in HIV-infected persons are poorly understood. This study prospectively evaluated 16 HIV-infected patients on combination antiretroviral therapy and eight healthy controls (HC) who received a single 15 μg dose of nonadjuvanted novel 2009 H1N1 influenza vaccine during the 2009 H1N1 epidemic. Peripheral blood was collected at baseline (T0) and at 7 d (T1) and 28 d (T2) postvaccination for evaluation of immune responses. Prevaccination hemagglutination inhibition Ab titer was <1:20 in all except one study participant. At T2, all HC and 8 out of 16 patients (50%) developed a vaccine-induced Ab titer of ≥1:40. Vaccine responder (R) and vaccine nonresponder patients were comparable at T0 in age, CD4 counts, virus load, and B cell immunophenotypic characteristics. At T2, HC and R patients developed an expansion of phenotypic and functional memory B cells and ex vivo H1N1-stimulated IgG Ab-secreting cells in an ELISPOT assay. The memory B cell response was preceded by a significant expansion of plasmablasts and spontaneous H1N1-specific Ab-secreting cells at T1. At T2, HC and R patients also exhibited significant increases in serum IL-21 levels and in the frequency and mean fluorescence intensity of IL-21R–expressing B cells, which correlated with serum H1N1 Ab titers. Vaccine nonresponder patients failed to develop the above-described vaccine-induced immunologic responses. The novel association of novel 2009 H1N1 vaccine-induced Ab responses with IL-21/IL-21R upregulation and with development of memory B cells and plasmablasts has implications for future research in vaccine design.
Vaccination is the most effective way to reduce the morbidity and mortality of influenza. In June of 2009, the World Health Organization declared a pandemic of a novel influenza A “novel 2009 H1N1” virus of swine origin, which resulted in an estimated 57 million cases in the United States from April 2009 to January 2010 (1). The 2009 influenza pandemic was considered to be especially dangerous because the influenza A novel 2009 H1N1 virus represented a novel strain caused by antigenic shift, unlike the yearly changes in the seasonal influenza virus resulting from antigenic drift. As this H1N1 strain was last associated with the 1918 influenza pandemic, people born after 1930 (i.e., in the age group <65 y) were most likely to lack pre-existing immunity (2–6) and to be more susceptible to the virus than people older than 65 y. The Centers for Disease Control recommended priority novel 2009 H1N1 influenza virus vaccination for selected populations that were considered to be at greatest risk of infection, including those with immunocompromised immune systems such as HIV-infected people (7).
Humoral immunity against influenza viruses is a good predictor of protection against infection (8). For the novel 2009 H1N1 influenza vaccine, an Ab titer of >1:40 hemagglutination inhibition units or a 4-fold increase from baseline is considered as being protective (9). Following the recommended single 15 μg dose of nonadjuvanted novel 2009 H1N1 vaccine, seroconversion with development of protective Ab titers has been reported to occur in 95% of the general population (6). Surprisingly, among HIV-infected individuals without pre-existing immunity to H1N1 virus, only 60% developed protective Ab titers following vaccination, even though they were well controlled for their HIV status (10). In this context, although greater expansion of memory B cells has been noted with adjuvanted H1N1 vaccines, their success in improving seroconversion rates has been variable (11–13). The mechanism underlying the poor serologic response to novel 2009 H1N1 vaccines in HIV-infected patients is not well understood.
In the generation of a primary Ab response to a vaccine, naive (N) B cells activated by the vaccine Ag are required to undergo a process of maturation, differentiation, and proliferation to generate Ab-secreting cells (ASC), memory B cells (14), and long-lived plasma cells (15). This process is regulated by cooperative interactions of B cell-intrinsic and extrinsic factors that are dependent upon a variety of accessory ligand–receptor interactions (16–18). IL-21 is a pleiotropic cytokine secreted almost exclusively by CD4 T cells and is known to play a major role in development of B cells and to promote Ab production (19–23). N human B cells are efficiently induced to secrete Ig via IL-21 following cognate T–B interaction (24). Recently, expression of the IL-21R on B cells was shown to be critical for development of memory B cells (25). HIV-infected persons are known to exhibit phenotypic and functional B cell defects, which are only partially corrected with potent combination antiretroviral therapy (26–28). Memory B cell deficiency may persist in these patients, resulting in poor responses to influenza virus vaccination (29–31), but the underlying mechanisms have not been elucidated. There is also accumulating evidence for impaired Ag-specific IL-21 secretion by CD4 T cells in progressive HIV infection (32–34). Based on the above observations and the high failure rates of the novel 2009 H1N1 vaccine in HIV-infected people, we hypothesized that defects in the IL-21/IL-21R pathway could prevent a successful serologic response to the vaccine. In the current study, we examined the relationship of the cytokine IL-21 and of its receptor expression on B cells to novel 2009 H1N1 vaccine responses in HIV-infected patients and healthy controls (HC). We observed that the HIV-infected vaccine responder (R) patients and HC were clearly distinguishable from vaccine nonresponder (NR) patients by their cellular immune responses and upregulation of IL-21/IL-21R expression. Notably, the findings imply that the IL-21/IL-21R pathway is likely to be playing an important role in generating serologic responses to the novel 2009 H1N1 vaccine.
Materials and Methods
Patient characteristics and response to vaccination
During the 2009–2010 influenza season, HIV-infected persons in the special immunology clinic at University of Miami who agreed to be vaccinated against novel 2009 H1N1 influenza virus as standard of care were invited to participate in a prospective study to evaluate immune responses to the vaccine. A single i.m. dose (15 μg; 0.5 ml) of inactivated monovalent A/California/07/2009 H1N1 vaccine (Novartis Vaccines and Diagnostics) was given to vaccine recipients. Data are presented for 16 patients who were on potent combination antiretroviral therapy (cART), had plasma virus load of <400 copies/ml, and completed follow-up visits on days 7 and 28 postvaccination. Eight HIV uninfected persons who were given the same vaccine served as HC. Characteristics of the study population are shown in Table I. Prevaccine H1N1 Ab titers were <1:20 in all study participants except one HC (identification number 027) who had a titer of 1:40. Postvaccination, H1N1 Ab titers of >1:40 on day 28 or a 4-fold increase from baseline were considered as protective (9). At 4 wk postvaccination, 8 of 16 (50%) patients developed Ab titers of >1:40 (median, 480; range, 80–2560) and were designated as H1N1 R patients. Eight patients had H1N1 Ab titers of ≤1:20 U at T2 and were designated as H1N1 vaccine nonresponders (NR). At T0, mean age and frequencies of CD20+ B cells were statistically equivalent among the patient groups and HC; CD4 and CD8 T cell counts and plasma HIV RNA were not different between the patient groups. All HC developed protective H1N1 Ab titers (median, 720; range, 80–20,480). The study was approved by the institutional review board of the University of Miami.
Table I.
Characteristics of study population and Ab responses
| ID | Age (y) | Gender | CD4+ T Cell (Cells/mm3) | CD8+ T Cell (Cells/mm3) | B Cells (%) | HIV RNA (Copies/ml) | H1N1 Ab Titer
|
|
|---|---|---|---|---|---|---|---|---|
| T0 | T2 | |||||||
| R patients | ||||||||
| 1 | 50.9 | Male | 744 | 871 | 19.1 | <50 | <20 | 1,280 |
| 5 | 50.1 | Male | 448 | 576 | 5.2 | <40 | <20 | 320 |
| 7 | 45 | Male | 818 | 494 | 16.8 | <50 | <20 | 160 |
| 12 | 29.8 | Male | 911 | 699 | 15.3 | <50 | <20 | 640 |
| 15 | 57.5 | Male | 1,655 | 470 | 12.7 | <50 | <20 | 320 |
| 17 | 49.8 | Female | 236 | 960 | 13.3 | <50 | <20 | 2,560 |
| 20 | 36.9 | Male | 373 | 338 | 12.4 | <40 | <20 | 640 |
| 21 | 53.9 | Male | 874 | 818 | 4.16 | <40 | <20 | 80 |
| Mean | 46.7 | 757.3 | 653.3 | 12.3 | 750 | |||
| SD | 9.2 | 441.1 | 218.7 | 5.3 | 823.5 | |||
| NR patients | ||||||||
| 4 | 59.2 | Male | 1,177 | 1,736 | 18.2 | 50 | <20 | <20 |
| 9 | 50.2 | Male | 341 | 806 | 21.3 | <50 | <20 | <20 |
| 13 | 27.4 | Male | 690 | 488 | 18.7 | <50 | <20 | 20 |
| 14 | 47.4 | Male | 350 | 622 | 15.7 | <50 | <20 | <20 |
| 16 | 61.9 | Male | 282 | 327 | 14.9 | <48 | <20 | <20 |
| 18 | 51.6 | Female | 689 | 855 | 22.1 | <48 | <20 | 20 |
| 23 | 22.6 | Male | 463 | 944 | 11.2 | 70 | <20 | 20 |
| 24 | 29.0 | Male | 867 | 727 | 10.9 | 369 | <20 | <20 |
| Mean | 43.7 | 607.4 | 813.1 | 16.6 | 163 | |||
| SD | 15.1 | 309.1 | 423.7 | 4.2 | 178.7 | |||
| HC | ||||||||
| 01 | 29 | Female | 18.7 | <20 | 20,480 | |||
| 02 | 31 | Female | 19.7 | <20 | 1,280 | |||
| 03 | 35 | Male | 14.3 | <20 | 80 | |||
| 04 | 28 | Female | 22.3 | <20 | 1,280 | |||
| 05 | 33 | Female | 18.4 | <20 | 10,240 | |||
| 027 | 52 | Male | 21.3 | 40 | 160 | |||
| 029 | 65 | Male | 20.4 | <20 | 160 | |||
| H42 | 46 | Female | 14.8 | <20 | 80 | |||
| Mean | 40 | 18.7 | 4,220 | |||||
| SD | 13.5 | 2.8 | 7,417.6 | |||||
ID, identification number.
mAbs
CD3 amcyan, CD20 allophycocyanin cyanin-7 (Cy7), CD20 allophycocyanin, CD10 PE-Cy7, CD21 PE-Cy5, CD27 Verizon 450, CD27 Alexa Fluor 700, Ki67 FITC, and IL-21R allophycocyanin were purchased from BD Biosciences (San Jose, CA). Isotype Abs used were IgG1-Alexa 700, IgG1–PE-Cy7, IgG1–PE-Cy5, and IgG1 allophycocyanin.
Reagents
Reagents used were 1× PBS, penicillin (100 U/ml), and streptomycin (100 mg/ml) (Cellgro, Manassas, VA); RPMI 1640 and L-glutamine (200 mM) (Life Technologies, Grand Island, NY); heat-inactivated FBS (Hyclone, Logan, UT); Ficoll Paque (GE Healthcare Biosciences, Uppsala, Sweden); BSA, DMSO, and Staphylococcus aureus Cowan strain (SAC) (Sigma-Aldrich, St. Louis, MO); Cytofix/Cytoperm, Perm Wash buffer, and 3-amino-9-ethylcarbazole (BD Biosciences, San Jose, CA); goat anti-human IgG, HRP-conjugated streptavidin, and biotinylated goat anti-human IgG, Fcγ fragment specific (Jackson ImmunoResearch Laboratories, West Grove, PA); multiscreen HA 96-well plate for ELISPOT (Millipore, Billerica, MA); violet amine reactive viability dye (Molecular Probes, Invitrogen, Eugene, OR); paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA); cell-culture plates (Costar, Corning, NY); recombinant human IL-21 (R&D Systems, Minneapolis, MN); and IL-21 ELISA kit (eBioscience, San Diego, CA).
Processing of blood samples
Peripheral venous blood, 30 ml, was collected immediately before vaccination (T0) and on days 7 (T1) and 28 (T2) postvaccination in heparinized vacutainer tubes after obtaining written informed consent from the participants. PBMC were isolated by density gradient isolation (35) using Ficoll Paque and processed fresh for immunophenotyping; remaining PBMC were cryopreserved in 10% DMSO and 90% FCS in liquid nitrogen. Serum samples were stored at −80°C. Hemagglutination inhibition Ab titers against H1N1 were performed at Bioqual (Rockville, MD) in T0 and T2 serum samples using virus and turkey RBC (10).
B cell characterization by flow cytometry
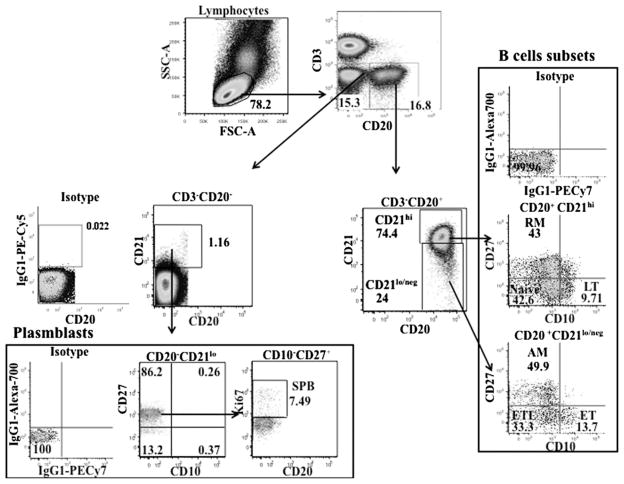
Total and maturational subsets of B cells were determined in HC and patients based on phenotypic characteristics described by Moir and Fauci (reviewed in Ref. 26). Freshly isolated PBMC (1 × 106 cells) obtained from blood samples at T0, T1, and T2 were stained with Abs against surface Ags using CD3, CD20, CD10, CD21, CD27, or isotype control Abs. Intracellular staining with Ki67 was performed in fixed permeabilized cells for evaluating cell proliferation. Samples were acquired on an LSRII flow cytometer (BD Biosciences) with collection of 200,000–500,000 events and were analyzed using FlowJo software (version 8.8.6; Tree Star). Flow cytometry analysis was performed after proper instrument setting, calibration, and compensation (36, 37). The gating scheme for identification of the B cell maturation subsets is shown in Fig. 1. Appropriate isotype control Abs were used at each step in gating of different B cell subsets to minimize effects due to nonspecific staining. The live cells (violet amine reactive viability dye negative) were first gated for CD3−CD20+ and CD3−CD20− subsets and sequentially on CD21, CD27, and CD10 to identify the B cell maturation subsets. The CD3−CD20+ B cells were gated into CD21hi and CD21lo/neg cells, each subset divided into CD27+ and CD27− subpopulation, and further characterized on the basis of CD10 expression. The CD3−CD20+CD21hi B cells were designated as resting memory (RM; CD27+CD10−), late transitional (LT; CD27−CD10+), and N (CD27−CD10−) subsets. The CD3−CD20+CD21lo/neg B cells were designated as activated mature differentiated (AM; CD27+CD10−), early transitional (ET; CD27−CD10+), and exhausted tissue-like (ETL; CD27− CD10−) subsets. The CD3−CD20− subset was divided into CD21− or CD21lo cells based on isotype Ab staining. The CD21lo cells were gated for CD27+CD10− cells and further characterized based on expression of Ki67 as short-lived plasmablasts (SPB; Ki67+) as described (26, 38). Frequencies of B cell subpopulations were calculated as percentages of the total B cells, identified in this study as CD3−CD20+ plus CD3−CD20− CD21+ lymphocytes. The various B cell populations were further investigated for frequency and median fluorescence intensity (MFI) for IL-21R based on isotype Ab staining (39).
FIGURE 1.
Characterization of B cell subsets and plasmablasts by flow cytometry. Fresh PBMC (1 × 106 cells) were stained with surface Abs to CD20, CD21, CD10, and CD27, and intracellular staining for Ki67 was performed on fixed permeabilized cells. Lymphocytes were gated based on forward and side scatter. CD3−CD20+ cells were gated into CD21hi and CD21lo/neg cells and further divided based on the expression of CD27 and CD10 as ET (CD21lo/negCD27−CD10+), LT (CD21hiCD27−CD10+), N (CD21hiCD27−CD10−), RM (CD21hiCD27+CD10−), AM (CD21lo/negCD27+CD10−), and ETL (CD21lo/negCD27−CD10−) subsets. Gated CD3−CD20−CD21lo cells were characterized as SPB (CD10−CD27+Ki67+).
ELISPOT assays for memory and effector H1N1 IgG ASC
Cryopreserved PBMC from different time points were thawed, rested overnight, and analyzed for ASC by ELISPOT using the method described by Kyu et al. (40). For analysis of memory B cells, 1 × 106 PBMC/ml from T0 and T2 were cultured for 5 d at 37°C in the presence or absence of 5 μg/ml H1N1 vaccine Ag. SAC, 1/10,000 dilution, was used as a positive control. In some experiments, exogenous cytokine IL-21, 50 ng/ml, was added to cultures together with H1N1 Ag at culture initiation. On day 5 of culture, cells were washed, and 100,000 cells/well were plated in triplicate for 5 h at 37°C in anti-IgG–coated ELISPOT plates. Thereafter, the plates were sequentially washed and incubated at room temperature for 1 h first with 100 μl/well biotinylated goat anti-human IgG (1:5000 dilution) followed by 100 μl/well streptavidin-HRP (1:5000 dilution). Plates were washed and developed using 3-amino-9-ethylcarbazole substrate (41, 42). Spots representing H1N1 Ag-stimulated IgG-secreting ASC were enumerated using an automated ELISPOT reader (Immunospot version 2.08; Cellular Technologies), and values in unstimulated wells were subtracted from those in test wells (41). IgG-secreting cells were expressed as ASC/106 B cells.
For analysis of effector cell responses, spontaneous H1N1-specific ASC (43) were determined in rested cryopreserved PBMC samples from T0, T1, and T2 without ex vivo Ag stimulation. Cells were washed and plated in triplicate for 6 h at 37°C at 100,000 cells/well in H1N1 Ag-coated (5 μg/ml) ELISPOT plates. Plates were washed and processed as described above for determining frequency of ASC/106 B cells.
ELISA assays for serum levels IL-21
The concentrations of IL-21 were determined in serum samples using a commercial ELISA kit (eBioscience) as previously described (44). Plates were coated with 100 μl capture Ab (1:250 dilution) for 12 h at 4°C, washed five times with wash buffer (PBS plus 0.05% Tween 20), and blocked with assay diluents (1:5 dilution) for 1 h. Thereafter, standards, 100 μl/well, and serum, 100 μl/well, were added in duplicate and plates left overnight at 4°C. Plates were washed five times, and 100 μl/well detection Ab (biotin-labeled, 1:250 dilution in assay diluent) was added at room temperature for 1 h. Plates were washed again followed by addition of 100 μl enzyme conjugate (avidin-GRP, 1:250 dilution in assay diluent) at room temperature for 30 min and 100 μl substrate for 15 min. The reaction was stopped by adding 50 μl stop solution (2 N H2SO4), and plates were read at 450 nm. Enzyme activities were determined at an OD of 450 nm. Mean values of serum IL-21 were reported as picograms per milliliter. Lower limit of detection of IL-21 was 31 pg/ml.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software (version 4.01; GraphPad). We used a general linear mixed-models procedure to perform a repeated-measures ANOVA with one between-subjects factor (groups) and one within-subjects factor (time). Contrasts were used to test for significant differences between groups and times. Correlation between variables was determined either by Pearson correlation or linear regression analysis. The p values <0.05 were considered significant.
Results
Expansions of memory B cells and plasmablasts occur post-novel 2009 H1N1 vaccination in seroconverters
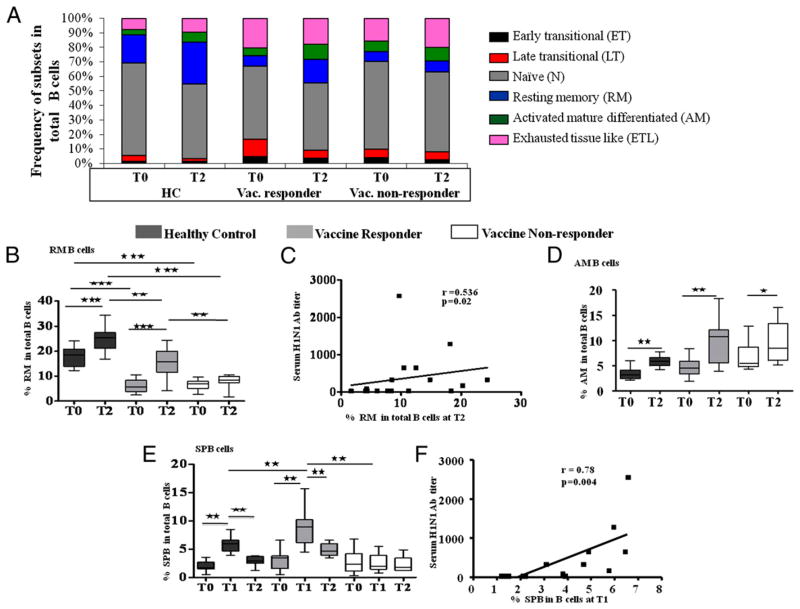
HIV-infected persons are known to manifest alterations in B cell maturation subsets, which are partially reversed when they are treated with potent cART (38, 45). Frequencies of B cell maturation subsets were determined in patients and HC in PBMC based on the gating scheme (Fig. 1) described in Materials and Methods and are depicted in Fig. 2 and Supplemental Fig. 1. A composite of all B cell maturation subsets at T0 and T2 is shown in Fig. 2A. Frequencies of B cell maturation subsets in patients differed from HC as has been previously described (38, 45), but there was no obvious difference between vaccine responder and nonresponder patients. At T0, frequencies of RM B cells were reduced in patients and were >2.5-fold higher in HC compared with R and NR patients (Fig. 2B).
FIGURE 2.
Changes in B cell subsets and plasmablasts in healthy controls and HIV-infected patients post-H1N1 vaccination: fresh PBMC (1 × 106 cells) was stained with Abs for B cell subset and plasmablast characterization (CD20, CD21, CD10, CD27, Ki67). The percentages of cells in each B cell subpopulation were determined in healthy controls and HIV-infected patients before (T0), 7 d (T1), and 28 d (T2) after H1N1 vaccination. Data are shown for T0 and T2, as there was no difference in the subpopulations at T1 compared with T0. A, Composite of frequency of all B cell subsets at T0 and T2 in eight HC, eight R, and eight NR patients. Frequencies of individual subsets are shown as box plots: RM (B), AM (D), and SPB (E). Box plots include median with 25th and 75th percentile borders, and error bars represent 10th and 90th percentiles. C, Correlation of RM B cells at T2. F, Correlation of SPB at T1 with H1N1 Ab titer in HIV-infected patients. Stars indicate the level of significance. ★p < 0.05, ★★p < 0.01, ★★★p < 0.001.
At T2, however, the R and NR patients had widely divergent cellular responses, with expansions of RM and SPB in R patients but not in NR patients. A significant expansion of RM B cells from baseline was noted at T2, and this occurred in HC and R patients but not in NR patients. Expansion of the RM B cells was correlated with the H1N1Ab titer in patients (Fig. 2C) and also in HC at T2 (r = 0.53, p = 0.02; not shown). Responses of R patients resembled HC but did not always exhibit the same magnitude of response. Frequencies of AM B cells were not significantly different among study groups at T0, and all showed expansions at T2 (Fig. 2D). A notable characteristic of B cells in influenza vaccinees is the rapid expansion of plasmablasts within 1 wk, and this has been primarily associated with the response of memory B cells (43, 46, 47). In the current study, frequencies of the SPB, which were equivalent at T0 among study groups, showed an increase of ≥3-fold at T1 (day 7 postvaccination) in HC and in R patients but not in NR patients, followed by a downward trend toward baseline by T2 in all (Fig. 2E). The H1N1 Ab titers at T2 correlated with frequency SPB at T1 in patients (Fig. 2F) and also in HC (r = −0.78, p = 0.004; data not shown). As expected, LT B cells (Supplemental Fig. 1C) were decreased at T2 postvaccination in HC and R patients, and N B cells (Supplemental Fig. 1D) showed a decrease in HC, as both these subsets constitute developmental intermediates for generation of human mature B cells (48). Patients had greater frequencies of ET (Supplemental Fig. 1B) and ETL B cells (Supplemental Fig. 1G) than HC at T0, and these subsets did not change following vaccination. Except for SPB, no other B cell subset was altered at T1 in relation to T0 (not shown).
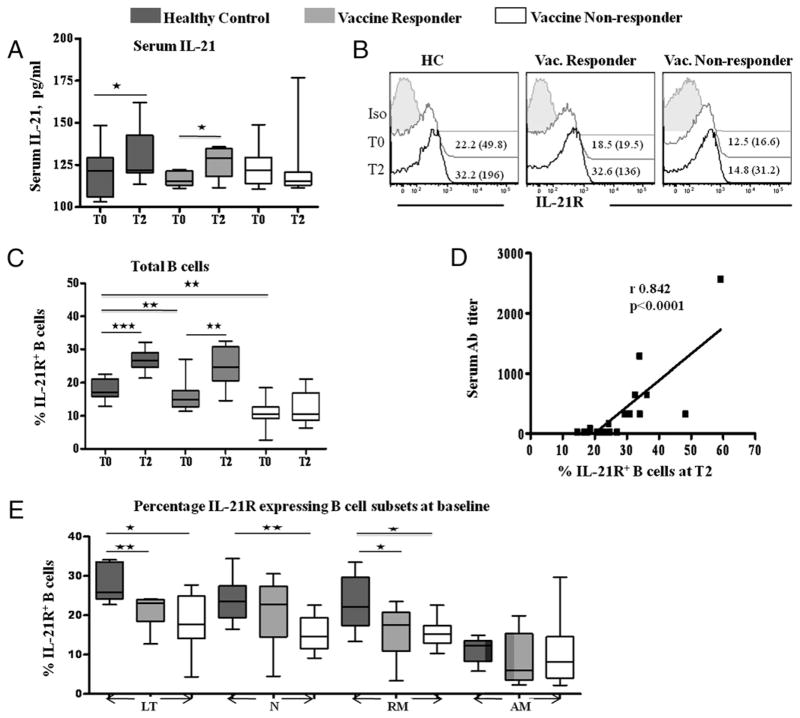
Serum IL-21 and frequency of IL-21R+ B cells are selectively increased at T2 in vaccine responders
In support of the hypothesis that the IL-21R/IL-21 axis was involved in the development of Ab responses to the novel 2009 H1N1 vaccine, we noted that serum IL-21 levels increased from T0 to T2 in HC and R but not in NR patients (Fig. 3A). To further ascertain the role of IL-21/IL-21R in vaccine responses, we examined the IL-21R expression on total B cells and in B cell maturation subsets. The pattern of IL-21R expression in B cells of HC and patients (Fig. 3B, 3C) suggests that an increase in IL-21R was an important correlate of H1N1 Ab response. At T0, frequencies of IL-21R in total B cells were comparable between R and NR, but were higher in HC than either patient group, whereas MFI of IL-21R was equivalent among HC (30.7 ± 10.3), R patients (36.2 ± 15.9), and NR patients (24.6 ± 14.3). At T2, there was an increase in frequencies of IL-21R+ B cells in HC and R patients with increases in MFI (156.9 ± 35.5 and 103.1 ± 32.5, respectively), but not in NR patients (MFI 36.9 ± 11.4). A direct correlation was observed between H1N1 Ab titer and IL21R+ B cells at T2 (Fig. 3D). Among B cell subsets at T0 (Fig. 3E), frequencies of IL-21R+ cells were highest in the LT subset and lowest in AM B cells and were significantly higher in HC in LT and RM subsets than both R and NR patients and also in N B cells of HC in comparison with NR patients. At T2, however, the IL-21R–expressing cells increased (Supplemental Fig. 2), and H1N1 Ab titer was correlated with IL-21R–expressing LT, N, and memory B cells in patients as well as HC (not shown). No differences were noted in IL-21R+ AM B cells among study groups, and no change occurred in frequencies of IL-21R+ B cells or their subsets at T1.
FIGURE 3.
Serum IL-21 levels and IL-21R expression in B lymphocyte subsets in HC and HIV-infected patients pre- and post-H1N1 vaccination: serum levels of IL-21 were measured by ELISA (A). Fresh PBMC (1 × 106 cells) were stained with surface markers for B cell subset characterization (CD20, CD21, CD10, CD27) and for IL-21R. Total B cells and various B cell subsets were analyzed at baseline (T0), 7 d (T1), and 28 d (T2) postvaccination for IL-21R by flow cytometry. Data are shown for T0 and T2 as no changes were noted at T1 compared with T0. B, Representative histograms showing frequencies and MFI (in parentheses) of IL-21R expression in total B cells of eight HC, eight HIV-infected H1N1 Ab responders, and eight HIV-infected H1N1 Ab nonresponders at T0 (gray) and T2 (black) compared with isotype controls (filled). C, Box plots are shown to illustrate frequency of IL21R expression in total B cells. D, Correlation of H1N1 Ab titer with percentage of IL21R+ B cells at T2 in HIV-infected patients. E, Frequency of IL21R expression B cell subsets at baseline. Box plots include median with 25th and 75th percentile borders, and error bars represent 10th and 90th percentiles. Stars indicate the level of significance. ★p < 0.05, ★★p < 0.01, ★★★p < 0.001.
H1N1 vaccine responders mount ASC responses at T1 and T2
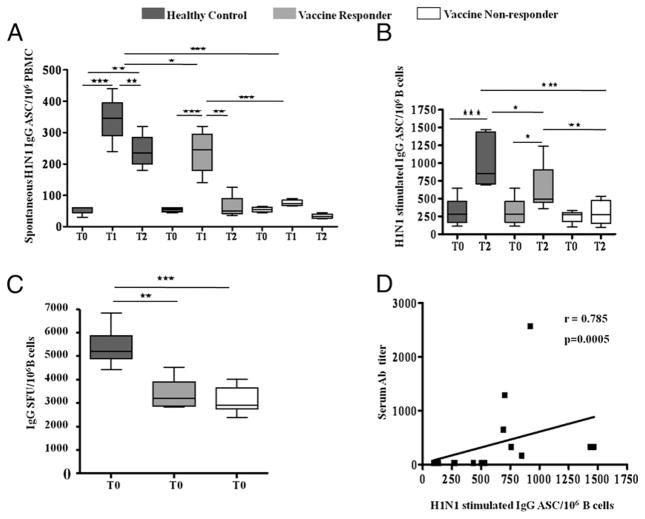
Next, we aimed to determine if the serum Ab responses were correlated with functional responses of memory B cells and plasmablasts in ELISPOT assays for ASC in cryopreserved PBMC. As a measure of functional plasmablasts, spontaneous (unstimulated ex vivo) H1N1-specific ASC responses were detected at T1 (Fig. 4A), in concurrence with increase in phenotypic SPB and were noted to occur only in HC and R patients, with 1.5-fold higher ASC in HC. These responses were short-lived and were close to baseline at T2. An expansion of spontaneous H1N1-specific ASC at T1 did not occur in NR patients.
FIGURE 4.
H1N1-specific ASC in healthy controls and HIV-infected patients pre- and post-H1N1 vaccination: ASC response was measured by ELI-SPOT in HC and HIV-infected patients. Box plots include median with 25th and 75th percentile borders, and error bars represent 10th and 90th percentiles. Spontaneous H1N1-specific ASC/106 B cells (A), IgG-producing ASC following ex vivo stimulation with H1N1 Ag (B), and IgG-producing ASC following ex vivo stimulation with SAC at baseline (C). D, Correlation between serum H1N1 Ab titer and H1N1-stimulated IgG ASC at T2 in PBMC of HIV-infected individuals. Stars indicate the level of significance. ★p < 0.05, ★★p < 0.01, ★★★p < 0.001.
Functional memory B cells were analyzed by determining IgG ASC after ex vivo stimulation of PBMC with H1N1 Ag for 5 d. The H1N1 Ag-stimulated IgG ASC response occurred at T2 in R patients and in HC but not in NR patients (Fig. 4B). The IgG ASC response at T2 in HC was greater than R patients. At T2, serum H1N1 Ab titers correlated with H1N1-stimulated IgG ASC in patients (Fig. 4C) and in HC (r = 0.53, p = 0.02, data not shown). ASC responses to SAC were equivalent at T0 in R and NR patients but significantly lower than in HC (Fig. 4D) and did not change from T0 at T1 or T2 (not shown).
H1N1-stimulated IgG-secreting cells are differentially influenced by exogenous addition of cytokines IL-21 to PBMC cultures
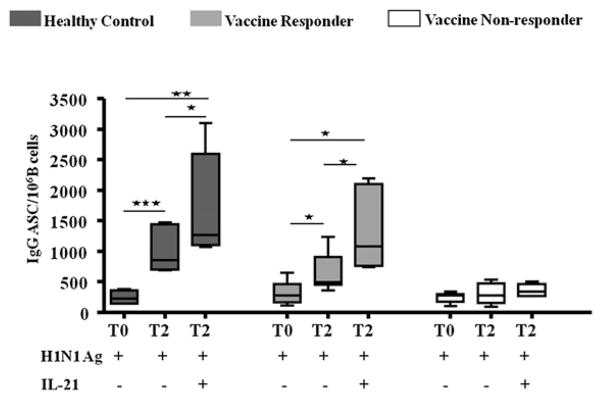
As described earlier, memory B cell responses determined by ex vivo H1N1 vaccine Ag-stimulated IgG ASC response failed to show an increase in NR patients from T0 to T2 (Fig. 4B). Based on the observed increase in the T2 serum IL-21 levels in R versus NR patients, we investigated if exogenous IL-21 supplementation at culture initiation would influence the H1N1-stimulated IgG ASC responses, particularly in NR patients at T2 (Fig. 5). Supplementation of cultures with exogenous IL-21 selectively enhanced Ag stimulated IgG-secreting cells only of HC and R patients, but failed to do so in NR patients.
FIGURE 5.
H1N1 Ag-stimulated IgG responses in HC and HIV-infected patients pre- and post-H1N1 vaccination: H1N1 vaccine Ag-stimulated IgG ASC with and without exogenous IL-21 at T0 and T2. Box plots include median with 25th and 75th percentile borders, and error bars represent 10th and 90th percentiles. Stars indicate the level of significance. ★p < 0.05, ★★p < 0.01, ★★★p < 0.001.
Discussion
Influenza is a serious threat to the HIV-infected population and can cause significant morbidity. During the 2009 H1N1 influenza pandemic, the HIV-infected populations in our clinics were offered priority vaccination with a nonadjuvanted inactive novel 2009 H1N1 vaccine. The 2009 H1N1 vaccine Ag was novel for the age bracket of our study population, and a majority of recipients were seronegative for this vaccine at the time of vaccination. In agreement with published data (10), a large proportion of patients on cART failed to seroconvert despite good virologic control and CD4 reconstitution. This study was conducted to understand why 2009 H1N1 vaccination did not always engage the immune system efficiently in this patient population. We made the novel observation that R patients uniquely upregulated IL-21R on B cells and increased their serum IL-21 levels following vaccination. Notably, serum H1N1 Ab titers were correlated with vaccine-induced changes in phenotypic and functional memory B cells, plasmablasts, and IL-21R expression on B cells.
Typically, Ag-induced activation, proliferation, and differentiation of N B cells at the site of immunization and draining lymph nodes generates plasmablasts in the germinal centers followed by their migration to bone marrow, where they develop into plasma cells for secretion of serum Ab. Migration of B cells to sites such as the respiratory tract where they secrete mucosal Abs including IgA has been described for parenteral influenza vaccines (17, 49–51). Memory B cells are generated, poised to respond by activation and proliferation following re-exposure to the same Ag. Indeed, following H1N1 vaccination, we noted phenotypic and functional evidence for expansions of peripheral memory B cells at T2 in R patients and HC but not in NR patients. In the current study, all patients were on cART, and the observed Ab or B cell responses did not correlate with CD4 T cell counts; thus, the deficiencies in memory B cells cannot be attributed to a quantitative loss of total CD4 T cells previously described in HIV-infected patients (30). There was, however, evidence for qualitative T cell dysfunction in vaccine NR based on analysis of serum IL-21 levels, which may have contributed to the failure of maturation and expansion of ASC.
Increases in serum IL-21 at T2 were noted selectively in the R patients and HC in this study and occurred concomitantly with increase in frequency and MFI of IL-21R+ B cells, suggesting that responsiveness to IL-21 was important for the Ab response. As IL-21 is produced mainly from CD4 cells, in particular from T follicular helper cells (52), reconstitution of this subset of CD4 T cells with cART in HIV-infected patients may be critically important for restoring B cell function. Morita et al. (24) recently reported that specific CXCR5+CD4+ T cell subsets in peripheral blood represent T follicular cell counterparts and induce Ig production by B cells through secretion of IL-21. We found that frequency of peripheral CD4+CXCR5+ cells increased from T0 to T2 in R patients and HC (S.Y. Silva, S. Pallikkuth, S. Kanthikeel, M. Fischl, R. Pahwa, and S. Pahwa, manuscript in preparation). Thus, it is quite possible that these CD4 T cells are involved in regulation of vaccine-induced Ab responses, and a deficiency in this subset is not readily apparent in total CD4 T cell analysis because of its low frequency in peripheral blood. A deficiency of HIV-specific IL-21–secreting CD4 T cells following ex vivo activation has in fact been described in HIV infection (32–34). The importance of IL-21 in HIV infection has been attributed mainly to its role in augmenting antiviral properties of CD8 T cells (34, 53), and recently, IL-21 was shown to be more highly expressed in CD4 T cells of HIV-infected elite controllers versus progressors (32, 33). Our data suggest that IL-21 may also play an important role in regulating B cell differentiation to generate novel 2009 H1N1 influenza vaccine-stimulated memory B cells and that failure to upregulate IL-21/IL-21R could constitute a critical defect, leading to impaired vaccine-induced Ab responses in HIV-infected patients.
Upregulation of IL-21R on B cells (including LT, N, and memory subsets) was strongly correlated with the ability to make a serologic response to novel 2009 H1N1 vaccine. Activation of B cells is known to upregulate IL-21R (54), which is highly expressed in immature and N B cells. Whether the activation-induced IL-21R upregulation occurred solely via a B cell-intrinsic mechanism or if IL-21 was involved in upregulation of IL-21R on B cells is not known. In CD4 T cells, IL-21 is clearly known to exercise amplification of its receptor (55). If a similar scenario were true for B cells, failure of IL-21R amplification by IL-21 could be a cause for the impaired IL-21R upregulation in B cells of NR patients. Preliminary observations made with PBMC of healthy donors suggest that ex vivo culture with IL-21 can result in increased frequencies and MFI of IL-21R expression in total B cells and in RM, LT, and N B cell subsets. Intrinsic B cell defects in IL-21R expression cannot be ruled out, as baseline frequencies of IL-21R–expressing total B cells and IL-21R+ LT and RM B cell subsets were reduced in patients despite equivalent serum IL-21 levels among patients and HC at T0. Regardless of the underlying mechanism, failure to upregulate IL-21R was a distinguishing feature of NR vaccine recipients. Collectively, the data for IL-21/IL-21R upregulation and the associated expansion of memory B cells in R patients suggest that this ligand–receptor pair was contributing to the regulation of B cell differentiation to generate novel 2009 H1N1 influenza vaccine-stimulated memory B cells and that failure to upregulate IL-21/IL-21R could be an underlying mechanism for impaired vaccine-induced Ab responses in HIV-infected patients. This contention is supported by other studies in which upregulation of IL-21/IL-21R were shown to be critical for memory B cell generation (20, 25, 56, 57). IL-21 signals through the common γ-chain receptor subunit of the IL-21R to phosphorylate STAT3 signaling molecules in a sustained manner in human B cells. STAT3 activation induces B cell maturation with expression of plasma cell genes, phenotypic plasma cell formation, and Ab secretion (20, 58, 59). Defects in IL-21 signaling can impair B cell responses to protein Ags, resulting in poor germinal center persistence and function, impaired transition into memory B cells, poor affinity maturation, and absence of Ig class switching (56, 57). Recently, it was shown that IL-21R knockout mice fail to develop Ag-specific memory B cells and plasma cells and have severely compromised secondary immune responses (25). The addition of exogenous IL-21 to cultures of H1N1-stimulated memory B cells resulted in significant enhancement of ASC responses only in R patients and HC. The inability of IL-21 to enhance memory B cell responses ex vivo in NR patients could partially be a consequence of the decreased frequency of IL-21R–expressing memory B cells in addition to a reduced memory pool size. Moreover, the increased frequency of B cells with the exhausted phenotype in NR patients may render them unresponsive to helper signals.
An expansion of plasmablasts on day 7 postvaccination was another benchmark of successful vaccination in this study. Integrated signals from a wide spectrum of sources regulate the ability of B cells to differentiate into pathways resulting in generation of SPB and memory cells (18, 59). A rapid expansion SPB originating from memory cells is regulated largely by innate immune factors (60, 61). On day 7 post-seasonal influenza vaccination, healthy donors have been shown to manifest an expanded functional plasmablast population originating largely from Ag-responsive memory B cells (30, 43, 46, 47) that constitute ~6% of peripheral B cells (43). Surprisingly, in R patients in our study, we observed a similar kinetic and frequency of SPB, the presumed source of spontaneous H1N1 ASC detected at T1 in the ELISPOT assay. Although we cannot exclude the contribution of cross-reactive memory B cells (3, 46) to the SPB pool, it is more likely that SPB resulted from expansion of unprimed B cells (62), rather than pre-existing Ag-specific memory cells. It is interesting that the IL-21R knockout mice who manifested severe deficits in memory B cells and recall Ab responses also had a poor early primary immune response (25). We contend that the IL-21R–IL-21 interaction contributes in some measure to the expansion of SPB from IL-21R+ immature and N B cells in a primary immune response, in addition to the well-established role of IL-21/IL-21R signaling in the development of long-lived memory cells.
The results of our studies point to the need to define the molecular basis of the observed immunologic defects in efforts toward optimization of vaccination strategies for influenza viruses. Vaccine-induced transcriptional regulation of B cell survival, proliferation, differentiation, class switch, and somatic mutation need further study. Levels of activation-induced cytidine de-aminase, important for regulating class switch, were recently found to be deficient in ex vivo influenza vaccine-stimulated B cells in aging humans (63) who are known to have poor Ab responses to influenza vaccines and have poor in vitro B cell differentiation responses (64). Given the prevailing notion that the immune system in HIV infection undergoes premature immune senescence (65–67), this line of investigation is worthwhile. We also recognize the need to conduct more precise studies in purified cell populations to address critical aspects of B cell maturation in the NR patients and to evaluate the role of innate factors such as BAFF and a proliferation-inducing ligand, which are important in B cell maturation and differentiation (16, 60, 68–70) (S. Pallikkuth, S. Pilaka Kanthikee, S.Y. Silva, M. Fischl, R. Pahwa, and S. Pahwa, manuscript in preparation). It is of interest that a combination of IL-21 and BAFF in the absence of further costimulation is capable of inducing differentiation of memory B cells that reside in the marginal zone region of the human spleen (71).
The major limitation of this pilot study is the small sample size and its effect on data interpretation. The small sample size may have masked greater differences between HC and patients and between the two patient groups. To maximize the impact of the study, we ensured that patients were virologically controlled on cART, randomly selected prior to vaccination before knowing the vaccination outcome, and studied prospectively. Despite the sample size and gender distribution limitations, differences between R and NR patients as indicated in Table I are quite remarkable. Not only did the proportion who failed to seroconvert closely match the published data (10, 12, 13), but also distinct and significant differences in immune responses were identified that set the NR apart from R patients and also revealed subtle differences between R patients and HC. The IL-21/IL-21R upregulation was associated with expansion of phenotypic and functional memory B cells, which, together with expansion of SPB with effector function, stood out as key indicators of successful vaccine-induced activation of the immune system. Future studies to evaluate IL-21/IL-21R screens to predict responsiveness to influenza vaccines at the level of individual patients may be worthwhile. These studies point to the need for continuing investigations to examine the cumulative signaling elements that control B cell differentiation in response to vaccines for insight into improving immunization strategies for immune deficient patients against influenza viruses.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant A1077501 (to S.P.) and a specialty laboratory grant from the International Maternal, Pediatric and Adolescent AIDS Clinical Trials Group. Overall support for International Maternal, Pediatric and Adolescent AIDS Clinical Trials Group was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health (AI068632).
We thank the Laboratory Sciences Core of the Developmental Center for AIDS Research for facilitating conduct of the flow cytometry studies and Dr. Kristopher L. Arheart of the Clinical Sciences Core for providing statistical input. We also thank Drs. Wasif Khan, Bonnie Blomberg, and Daniela Frasca of the Department of Microbiology and Immunology for helpful discussions and the patients for participation in this study.
Abbreviations used in this article
- AM
activated mature differentiated
- ASC
Ab-secreting cell
- cART
combination antiretroviral therapy
- Cy7
cyanin-7
- ET
early transitional
- ETL
exhausted tissue-like
- HC
healthy control
- LT
late transitional
- MFI
median fluorescence intensity
- N
naive
- NR
vaccine nonresponder
- R
vaccine responder
- RM
resting memory
- SAC
Staphylococcus aureus Cowan strain
- SPB
short-lived plasmablast
- T0
baseline
- T1
7 d postvaccination
- T2
28 d postvaccination
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.National Center for Immunization and Respiratory Diseases, CDCCenters for Disease Control and Prevention (CDC) Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-10):1–8. [PubMed] [Google Scholar]
- 2.Adalja AA, Henderson DA. Original antigenic sin and pandemic (H1N1) 2009. Emerg Infect Dis. 2010;16:1028–1029. doi: 10.3201/eid1606.091563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, Salimi N, Vita R, Ponomarenko J, Scheuermann RH, Sette A, Peters B. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci USA. 2009;106:20365–20370. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 5.Reichert T, Chowell G, Nishiura H, Christensen RA, McCullers JA. Does Glycosylation as a modifier of Original Antigenic Sin explain the case age distribution and unusual toxicity in pandemic novel H1N1 influenza? BMC Infect Dis. 2010;10:5. doi: 10.1186/1471-2334-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, Basser RL. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 7.Klein MB, Lu Y, DelBalso L, Coté S, Boivin G. Influenzavirus infection is a primary cause of febrile respiratory illness in HIV-infected adults, despite vaccination. Clin Infect Dis. 2007;45:234–240. doi: 10.1086/518986. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talbot HK, Keitel W, Cate TR, Treanor J, Campbell J, Brady RC, Graham I, Dekker CL, Ho D, Winokur P, et al. Immunogenicity, safety and consistency of new trivalent inactivated influenza vaccine. Vaccine. 2008;26:4057–4061. doi: 10.1016/j.vaccine.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, Thomas A, Kenney T, Kappes R, Wagner W, Maffei K, Sullivan K Center for AIDS Research and Clinical Trials Unit of the University of Pennsylvania. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 11.Kajaste-Rudnitski A, Galli L, Nozza S, Tambussi G, Di Pietro A, Pellicciotta G, Monti A, Mascagni P, Moro M, Vicenzi E. Induction of protective antibody response by MF59-adjuvanted 2009 pandemic A/H1N1v influenza vaccine in HIV-1-infected individuals. AIDS. 2011;25:177–183. doi: 10.1097/QAD.0b013e328341afa8. [DOI] [PubMed] [Google Scholar]
- 12.Ho J, Moir S, Wang W, Posada JG, Gu W, Rehman MT, Dewar R, Kovacs C, Sneller MC, Chun TW, et al. Enhancing effects of adjuvanted 2009 pandemic H1N1 influenza A vaccine on memory B-cell responses in HIV-infected individuals. AIDS. 2011;25:295–302. doi: 10.1097/QAD.0b013e328342328b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bickel M, Wieters I, Khaykin P, Nisius G, Haberl A, Stephan C, Von Hentig N, Herrmann E, Doerr HW, Brodt HR, Allwinn R. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS. 2010;24:F31–F35. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 14.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. 2010;185:3117–3125. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zúñiga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 16.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 17.Elgueta R, V, de Vries C, Noelle RJ. The immortality of humoral immunity. Immunol Rev. 2010;236:139–150. doi: 10.1111/j.1600-065X.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 19.Avery DT, Bryant VL, Ma CS, de Waal Malefyt R, Tangye SG. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J Immunol. 2008;181:1767–1779. doi: 10.4049/jimmunol.181.3.1767. [DOI] [PubMed] [Google Scholar]
- 20.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U, Bustamante J, Boisson-Dupuis S, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 23.Pène J, Gauchat JF, Lécart S, Drouet E, Guglielmi P, Boulay V, Delwail A, Foster D, Lecron JC, Yssel H. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172:5154–5157. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 24.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5(+) CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rankin AL, MacLeod H, Keegan S, Andreyeva T, Lowe L, Bloom L, Collins M, Nickerson-Nutter C, Young D, Guay H. IL-21 receptor is critical for the development of memory B cell responses. J Immunol. 2011;186:667–674. doi: 10.4049/jimmunol.0903207. [DOI] [PubMed] [Google Scholar]
- 26.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, Liu S, Adelsberger J, Lapointe R, Hwu P, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci USA. 2001;98:10362–10367. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroon FP, Rimmelzwaan GF, Roos MT, Osterhaus AD, Hamann D, Miedema F, van Dissel JT. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active anti-retroviral therapy. AIDS. 1998;12:F217–F223. doi: 10.1097/00002030-199817000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, Kottilil S, Gezmu M, Follmann D, Vodeiko GM, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 31.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grützmeier S, Atlas A, Hejdeman B, Kroon FP, Lopalco L, Nilsson A, Chiodi F. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 32.Chevalier MF, Jülg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol. 2011;85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, Zajac AJ, Goepfert PA. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol. 2011;85:2316–2324. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, Liu J, Song H, Jones RB, Sheth P, et al. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J Immunol. 2010;185:498–506. doi: 10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
- 35.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 36.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69:1037–1042. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 37.Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay P, Roederer M. Quality assurance for polychromatic flow cytometry. Nat Protoc. 2006;1:1522–1530. doi: 10.1038/nprot.2006.250. [DOI] [PubMed] [Google Scholar]
- 38.Moir S, Malaspina A, Ho J, Wang W, Dipoto AC, O’Shea MA, Roby G, Mican JM, Kottilil S, Chun TW, et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis. 2008;197:572–579. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 39.Good KL, V, Bryant L, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 40.Kyu SY, Kobie J, Yang H, Zand MS, Topham DJ, Quataert SA, Sanz I, Lee FE. Frequencies of human influenza-specific antibody secreting cells or plasmablasts post vaccination from fresh and frozen peripheral blood mononuclear cells. J Immunol Methods. 2009;340:42–47. doi: 10.1016/j.jim.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buseyne F, Catteau A, Scott-Algara D, Corre B, Porrot F, Rouzioux C, Blanche S, Rivière Y. A vaccinia-based elispot assay for detection of CD8+ T cells from HIV-1 infected children. J Immunol Methods. 2005;298:105–118. doi: 10.1016/j.jim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, Routy JP, Ahmad A. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol. 2010;184:114–126. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- 45.Malaspina A, Moir S, Ho J, Wang W, Howell ML, O’Shea MA, Roby GA, Rehm CA, Mican JM, Chun TW, Fauci AS. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci USA. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci USA. 2009;106:7962–7967. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, Kemble GW, Arvin AM, Greenberg HB. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81:215–228. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marie-Cardine A, Divay F, Dutot I, Green A, Perdrix A, Boyer O, Contentin N, Tilly H, Tron F, Vannier JP, Jacquot S. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol. 2008;127:14–25. doi: 10.1016/j.clim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171:198–203. doi: 10.1093/infdis/171.1.198. [DOI] [PubMed] [Google Scholar]
- 50.Brokstad KA, Cox RJ, Oxford JS, Haaheim LR. IgA, IgA subclasses, and secretory component levels in oral fluid collected from subjects after parental influenza vaccination. J Infect Dis. 1995;171:1072–1074. doi: 10.1093/infdis/171.4.1072-a. [DOI] [PubMed] [Google Scholar]
- 51.el-Madhun AS, Cox RJ, Søreide A, Olofsson J, Haaheim LR. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J Infect Dis. 1998;178:933–939. doi: 10.1086/515656. [DOI] [PubMed] [Google Scholar]
- 52.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 53.White L, Krishnan S, Strbo N, Liu H, Kolber MA, Lichtenheld MG, Pahwa RN, Pahwa S. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007;109:3873–3880. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 55.Caprioli F, Sarra M, Caruso R, Stolfi C, Fina D, Sica G, MacDonald TT, Pallone F, Monteleone G. Autocrine regulation of IL-21 production in human T lymphocytes. J Immunol. 2008;180:1800–1807. doi: 10.4049/jimmunol.180.3.1800. [DOI] [PubMed] [Google Scholar]
- 56.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diehl SA, Schmidlin H, Nagasawa M, van Haren SD, Kwakkenbos MJ, Yasuda E, Beaumont T, Scheeren FA, Spits H. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J Immunol. 2008;180:4805–4815. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurosaki T, Aiba Y, Kometani K, Moriyama S, Takahashi Y. Unique properties of memory B cells of different isotypes. Immunol Rev. 2010;237:104–116. doi: 10.1111/j.1600-065X.2010.00939.x. [DOI] [PubMed] [Google Scholar]
- 60.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, Brink R, Mackay F, Hodgkin PD, Tangye SG. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 62.Benson MJ, Erickson LD, Gleeson MW, Noelle RJ. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr Opin Immunol. 2007;19:275–280. doi: 10.1016/j.coi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frasca D, Diaz A, Romero M, Landin AM, Phillips M, Lechner SC, Ryan JG, Blomberg BB. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010;28:8077–8084. doi: 10.1016/j.vaccine.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pahwa SG, Pahwa RN, Good RA. Decreased in vitro humoral immune responses in aged humans. J Clin Invest. 1981;67:1094–1102. doi: 10.1172/JCI110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gerontol. 2007;42:432–437. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr. 2009;50:137–147. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE, Janoff EN, Justice AC, Kuritzkes D, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. Synthetic CpG oligodeoxynucleotides augment BAFF-and APRIL-mediated immunoglobulin secretion. Eur J Immunol. 2007;37:1785–1795. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 69.Mantchev GT, Cortesão CS, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol. 2007;179:2282–2288. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 70.Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, Choi YS. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int Immunol. 2005;17:779–788. doi: 10.1093/intimm/dxh259. [DOI] [PubMed] [Google Scholar]
- 71.Ettinger R, Sims GP, Robbins R, Withers D, Fischer RT, Grammer AC, Kuchen S, Lipsky PE. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J Immunol. 2007;178:2872–2882. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.