Summary
The Lyme disease agent, Borrelia burgdorferi, is primarily transmitted to vertebrates by Ixodes ticks. The classical and alternative complement pathways are important in Borrelia eradication by the vertebrate host. We recently identified a tick salivary protein, designated P8 that reduced complement-mediated killing of Borrelia. We now discover that P8 interferes with the human lectin complement cascade resulting in impaired neutrophil phagocytosis and chemotaxis, and diminished Borrelia lysis. Therefore, P8 was renamed the lectin complement pathway inhibitor (TSLPI). TSLPI-silenced ticks, or ticks exposed to TSLPI-immune mice, were hampered in Borrelia transmission. Moreover, Borrelia acquisition and persistence in tick midguts was impaired in ticks feeding on TSLPI-immunized B. burgdorferi-infected mice. Together, our findings suggest an essential role for the lectin complement cascade in Borrelia eradication and demonstrate how a vector-borne pathogen co-opts a vector protein to facilitate early mammalian infection and vector colonization.
Keywords: MBL, lectin, ficolin, tick immunity, Borrelia burgdorferi, complement, vaccine
Introduction
During the blood meal, Ixodes ticks can transmit various pathogens to the vertebrate host such as Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Babesia species and selected flaviviruses, among other agents (Estrada-Pena and Jongejan, 1999). Ticks introduce salivary proteins at the tick-bite site to suppress host defense mechanisms, in order to successfully obtain a blood meal. Several anticoagulant, anti-inflammatory, anti-complement and immunosuppressive tick salivary proteins have been identified (Hovius et al., 2008), which could not only facilitate tick feeding, but also enhance Borrelia transmission to the mammalian host and Borrelia acquisition by the tick vector (Schuijt et al., 2011a).
The complement cascade is a major part of the mammalian innate immune response consisting of the classical, the alternative and lectin pathway and serves as a functional bridge between innate and adaptive immune responses (Ricklin et al., 2010). The most prominent role of the complement system is to recognize and clear invading pathogens. Activation of the complement cascade results in opsonisation of the invading microorganism leading to enhanced phagocytosis, leukocyte chemotaxis and direct killing of pathogens by formation of the membrane attack complex (MAC). Initiation of the classical pathway relies on C1q binding to pathogens, either directly or to antibody-opsonized pathogens. The lectin pathway is activated upon binding of mannose-binding lectin (MBL) or ficolins (FCNs) to highly glycosylated pathogen associated molecular patterns (PAMPs) on the surface of pathogens (Ip et al., 2009; Matsushita, 2009). Both MBL and FCNs form a complex with pro-enzymes of MBL-associated serine proteases (MASPs) in serum, which are activated upon binding of MBL and FCNs to pathogens. Activated MASP-2 activates the complement cascade by cleaving C4 and C2 to generate the C4b2a complex. The alternative pathway spontaneously and continuously converts C3 and its predominant role is amplification of complement activation initially triggered by the classical and/or lectin pathway (Kishore and Reid, 2000).
Several tick salivary proteins from different tick species inhibit the host complement system. A well-characterized tick complement inhibitor is the C5 activation inhibitor from the soft tick Ornithodoros moubata OMCI that inhibits C5 activation by binding C5 and preventing its activation by C5 convertase (Fredslund et al., 2008) and inhibited complement-mediated haemolytic activity and the development of pathological features in a rodent model for myasthenia gravis (Hepburn et al., 2007). Other tick complement inhibitors, including Isac, Irac-1 and -2 and Salp20, belong to the Isac protein family and inhibit the alternative complement pathway by binding and displacing properdin and thereby inhibiting the formation of C3 convertase (Tyson et al., 2007; Valenzuela et al., 2000). Also for B. burgdorferi sensu lato the complement cascade is an important obstacle to overcome in order to establish an infection in the mammalian host. B. burgdorferi sensu lato isolates differ in their susceptibility to complement-dependent killing (van Dam et al., 1997) by expressing proteins on their outer membrane, named Erps and CRASPs, also known as Csp’s, which can bind host complement regulators such as factor H (FH) and factor H-like proteins (FHL-1) (Brissette et al., 2008) and by expressing a CD59-like protein on their outer membrane (Pausa et al., 2003). Interestingly, we have previously shown that inhibition of the complement system by tick salivary proteins might be instrumental for survival of B. burgdorferi sensu lato strains that do not have the ability to evade complement-dependent killing by themselves (Schuijt et al., 2008).
By probing an I. scapularis salivary gland yeast surface display library with tick-immune rabbit sera we have identified an I. scapularis salivary protein with a predicted molecular weight of 8 kDa, designated P8, and demonstrated that this protein has anti-complement activity (Schuijt et al., 2011b). We here report the molecular mechanism by which P8 – from here on referred to as Tick Salivary Lectin Pathway Inhibitor (TSLPI) – inhibits the human lectin complement pathway and reveals a crucial role of the lectin pathway in the host immune response against Borrelia.
Results
Characterization of the Ixodes scapularis salivary protein TSLPI
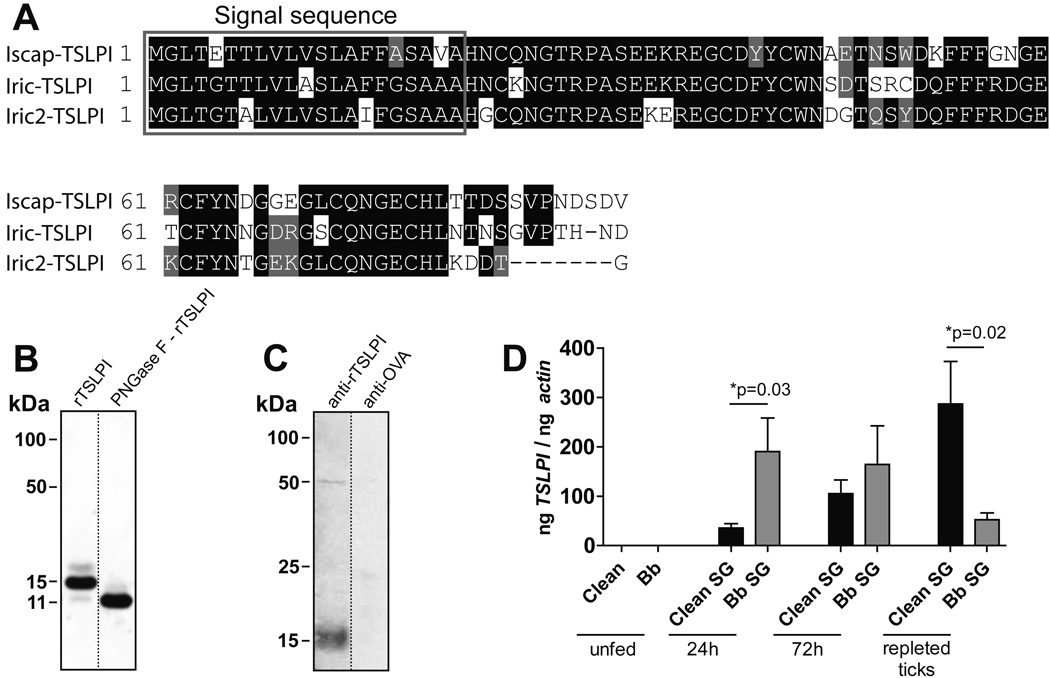
Recently, we identified an I. scapularis salivary protein - previously designated as P8 (GenBank: AEE89466.1) according to its molecular weight, but from here on referred to as Tick Salivary Lectin Pathway Inhibitor (TSLPI) based on its function - by probing a yeast surface display library with tick immune rabbit sera (Schuijt et al., 2011b). I. scapularis TSLPI has several homologues in I. ricinus (GenBank: AAS68352.1 and GenBank: AAP93884.1) indicating that this protein is conserved between these closely related Ixodes species, that both transmit Borrelia. Nucleotide homology between the genes was 83–86% and amino acid similarity between the proteins was 84%, with 73–75% identity (Figure 1A). Although TSLPI is a member of a previously described group of anti-coagulant proteins (Narasimhan et al., 2002), it did not show anti-coagulant activity (Schuijt et al., 2011b). Drosophila-expressed recombinant TSLPI was glycosylated and removing N-glycans from the protein backbone using N-glycosidase (PGNase) F reduced the molecular weight by 4 kDa (Figure 1B). Since TSLPI was antigenic in tick-immune rabbits, it is likely a secreted salivary protein (Schuijt et al., 2011b). Indeed, a signal peptide cleavage site was predicted, using the web-based software SignalP, between position 21 and 22 (Figure 1A) and native TSLPI was present in I. scapularis saliva when probed with rTSLPI antiserum (Figure 1C). In line with this, Ribeiro et al identified and annotated several proteins with high amino acid sequence identity to TSLPI (91–99%) as secreted salivary proteins (Ribeiro et al., 2006). In Borrelia-free nymphs TSLPI mRNA was upregulated during the course of the tick blood meal (Figure 1D). In the early phases of nymphal engorgement, B. burgdorferi infection of ticks resulted in significantly higher TSLPI mRNA levels, whereas after blood feeding lower TSLPI mRNA levels were found in Borrelia-infected ticks compared to Borrelia-free ticks (Figure 1D). TSLPI mRNA was not detected in midguts of either Borrelia-free or Borrelia-infected nymphs at all tested time points of feeding (data not shown), indicating that TSLPI is preferentially expressed in I. scapularis salivary glands.
Figure 1. Characterization, protein sequence and expression of TSLPI.
(A) Multiple sequence alignment of I. scapularis TSLPI aligned with two homologues of TSLPI in I. ricinus. Amino acids in white on a black background are identical; residues in white on a grey background are similar. Region inside the grey box shows the predicted signal peptide sequence. (B) Coomassie staining of rTSLPI on SDS-PAGE before and after deglycosylation with PNGase F. (C) I. scapularis saliva probed with rTSLPI-rabbit antiserum or OVA-rabbit antiserum. (D) Expression profile of TSLPI in salivary glands (SG) of pathogen-free (clean) nymphs and Borrelia infected (Bb) nymphs. TSLPI expression in unfed nymphs, after 24 and 72 h of feeding and repleted nymphs. Results represent mean ± SEM of values.
TSLPI impairs direct killing of B. burgdorferi sensu lato isolates by the complement system
We have previously shown that rTSLPI protects serum-sensitive B. garinii A87S against complement-mediated killing (Schuijt et al., 2011b). We here demonstrate that B. garinii A87S is protected from complement-dependent killing by rTSLPI in a dose-dependent manner, utilizing a B. garinii killing assay with 12.5% normal human serum (Figure 2A; left panel). A growth inhibition assay confirmed the results of actual killing and the protective effect of rTSLPI against complement mediated killing of Borrelia (Figure S1A). B. burgdorferi N40, which is resistant to normal human serum (NHS) was killed by the complement system in the presence of Borrelia-opsonizing antibodies using 12.5% Borrelia-immune human serum (IHS), (Figure 2A; right panel). Strikingly, recombinant TSLPI also protected B. burgdorferi N40 against antibody-mediated complement-dependent killing in a dose-dependent manner (Figure 2A; right panel). Heat-inactivated rTSLPI-rabbit antiserum completely abrogated the anti-complement activity of rTSLPI (Figure 2B), demonstrating the neutralizing capacity of these antibodies. In addition, heat-inactivated rTSLPI-rabbit antiserum substantially reduced the complement inhibitory activity of I. scapularis salivary gland extracts (Figure 2C), showing that TSLPI is a dominant complement inhibitor in tick saliva. To confirm that spirochetal killing by the complement system was induced by the formation of MAC complexes, deposition of C5b-9 was examined on the spirochete outer membrane. The amount of MAC complexes on the outer membrane of B. garinii A87S was greatly reduced when incubated with 12.5% NHS for up to 30 min in the presence of 0.25 µg/µl rTSLPI (Figure 2D). Scanning electron microscopy demonstrated that the complement inhibitory effect of rTSLPI resulted in less bleb formation on the outer surface of Borrelia spirochetes (Figure S1B,C).
Figure 2. Recombinant protein TSLPI inhibits complement-mediated killing of Borrelia in a dose-dependent manner and inhibits in vitro chemotaxis and phagocytosis of Borrelia by PMNs.
(A) B. garinii strain A87S was incubated with 12.5% NHS (left panel) and B. burgdorferi strain N40 was incubated with 12.5% IHS (right panel), in the presence of increasing concentrations of BSA (control), control tick salivary protein rP19 or rTSLPI. As a control, spirochetes were also incubated with heat-inactivated (HI) NHS or IHS. rTSLPI (B) or nymphal SGE (C) was pre-incubated with heat-inactivated OVA-rabbit antiserum (a-OVA) or rTSLPI-rabbit antiserum (a-rTSLPI). After 1.5 h of incubation with serum, the percentages of immotile spirochetes were determined. Asterisks indicate a statistically significant difference with the BSA control (*p<0.04), (**p<0.004) and (***p<0.0004). (D) C5b-9 deposition (red) on B. garinii A87S (green) incubated with 12.5% NHS in the presence of 0.25 µg/µl BSA or rTSLPI for 15 min (upper panel) and for 30 min (lower panel). Right panel: Percentage of spirochetes with C5b-9 complexes. Results based on triplicate countings of three independent experiments. Phagocytosis of CFSE-labeled viable B. garinii A87S (E) or B. burgdorferi N40 (F) by human PMNs in the presence of 1% or 2.5% NHS or 5% IHS preincubated with 0.25 µg/µl BSA or rTSLPI at 37°C. Cells were subjected to FACS analysis after 2,5,10 and 30 min of incubation. Heat inactivated IHS and NHS were used as controls. Asterisks indicate a statistically significant difference with the BSA control (*p<0.04), (** p<0.004) and (***p<0.0008). (G) The chemoattractant capacity of NHS preincubated with 0.5 µg/µl BSA (control) or rTSLPI and 2.5 × 107 B. garinii A87S was assessed in a Transwell in vitro migration assay with human isolated PMNs. Fluorescence (fluorescent units; FU) measured at 1 min intervals at the bottom side of the well is shown and represents the number of PMNs migrated in time. Values represent the mean ± SEM. See also Figure S1.
TSLPI inhibits phagocytosis of B. burgdorferi sensu lato strains by human neutrophils and Borrelia-induced complement-mediated chemotaxis
Activation of the complement system is important for recruitment of immune cells and phagocytosis (Ricklin et al., 2010) we therefore assessed whether rTSLPI impaired phagocytosis of Borrelia burgdorferi sensu lato isolates by human neutrophils. Flow cytometry showed that neutrophils were hampered in their ability to phagocytize B. garinii A87S in the presence of 0.25 µg/µl rTSLPI and either 1% or 2.5% NHS (Figure 2E). Human neutrophils also poorly phagocytized B. burgdorferi N40 in the presence of 0.25 µg/µl rTSLPI and 5% IHS (Figure 2F).
Complement split products C5a and C3a, generated by the action of serine proteases, are potent chemoattractants for leukocytes (Fernandez et al., 1978). Hence, next, the chemoattractant capacity of NHS pre-incubated with B. garinii A87S in the presence or absence of 0.5 µg/µl rTSLPI was assessed in a Transwell in vitro migration assay with human PMNs (Figure 2G). As shown in figure 2G fewer PMNs migrated towards NHS incubated with Borrelia and rTSLPI. Collectively, these data show that rTSLPI inhibits complement activation on Borrelia, resulting in impaired in vitro phagocytosis of Borrelia and impaired Borrelia-induced complement-mediated chemotaxis.
TSLPI directly inhibits activation of the MBL complement pathway
To understand how rTSLPI protected Borrelia against complement activation we next assessed whether complement inhibition by rTSLPI was Borrelia dependent or independent. We demonstrated that recombinant TSLPI did not bind Borrelia, as shown by a pull-down assay, suggesting that rTSLPI has a direct effect on the complement system (Figure 3A). Moreover, when rTSLPI was preincubated with 12.5% NHS for 30 min, rather than added simultaneously with the spirochetes, B. garinii A87S was significantly more protected against complement-mediated killing (Figure 3B). A similar effect was observed when 12.5% IHS was preincubated with rTSLPI before it was added to B. burgdorferi (Figure 3B). To further elucidate the mechanism by which rTSLPI inhibited complement activation, we investigated the effect of rTSLPI on the classical, alternative and lectin pathway. Thus far, the classical and alternative complement pathways have shown to be important in Borrelia eradication by the host. Surprisingly, recombinant TSLPI did not inhibit lysis of antibody-sensitized sheep erythrocytes in a CH50 hemolytic assay, demonstrating that the classical complement pathway was not affected by rTSLPI (Figure 3C). Furthermore, the alternative pathway (AP50) was not inhibited by rTSLPI (Figure 3D). Since rTSLPI clearly inhibited complement mediated killing of Borrelia, the above negative results prompted us to investigate the effect of rTSLPI on the MBL complement pathway. Pre-incubation of serum with rTSLPI resulted in a pronounced dose-dependent inhibition of C4 deposition using mannan as a ligand, evidently showing that the MBL complement pathway was inhibited by rTSLPI (Figure 3E).
Figure 3. Direct effect of rTSLPI on the complement system by inhibiting the lectin pathway.
(A) Binding of B. burgdorferi N40 to rTSLPI as analysed by Western blot as mentioned in text. Salp15 was used as a positive control. (B) Serum was pre-incubated (pre) with BSA, rP19 or rTSLPI for 30 min at 33°C or added without pre-incubation (direct) to Borrelia. Experiments with B. garinii were performed with 12.5% NHS and B. burgdorferi N40 with 12.5% IHS. Asterisks indicate a statistically significant difference (**p<0.01) and (***p<0.0001). The effect of rTSLPI on classical (C) and alternative (D) complement pathway-mediated haemolysis was performed by a CH50 and AP50 assay, respectively. Neutralizing C3 and C1q antibodies were used as controls. (E) The C4 cleaving activity of the MBL lectin pathway was measured in an ELISA format using mannan-coated microtiter plates in the presence of BSA, anti-MBL antibody or several concentrations of rTSLPI (0.03–0.5 µg/µl). Results are representative of at least two independent assays. Results represent mean ± SEM of values.
The molecular mechanism by which TSLPI inhibits the MBL complement pathway
We next set out to define the molecular mechanism by which rTSLPI inhibited the MBL complement pathway and assessed whether rTSLPI prevented binding of MBL to its ligands or inhibited complement activation through MASP-2 complement activation. Reduced MBL-ligand binding was observed when serum was pre-incubated with rTSLPI (Figure 4A, left panel) and increasing concentrations of rTSLPI dose-dependently decreased MBL binding to mannan (Figure 4A, right panel). In line with these observations MBL clearly bound to rTSLPI coated plates (Figure 4B, left panel), which did not result in activation of the MBL pathway (Figure 4B, right panel). Post-translational modifications, i.e. N-linked glycosylation, of recombinant Drosophila-expressed TSLPI, were shown to be crucial for its effect, since deglycosylation of rTSLPI by PGNase F largely abrogated the complement inhibitory effect of rTSLPI (Figure 4C), suggesting that TSLPI binds the carbohydrate recognition domains of MBL through its N-glycans. To rule out the possibility that rTSLPI could also impair MASP-2 activity, we investigated the effect of TSLPI on C4 deposition in a MBL/MASP complex activity assay. rTSLPI was incubated with bound MBL/MASP-2 complexes to determine the ability of activated MASP-2 to cleave complement C4. With comparable amounts of MBL/MASP2 complexes (Figure S2) bound to mannan-coated plates, equal amounts of C4 deposition were measured after incubation with NHS in the presence of rTSLPI or controls (Figure 4D), demonstrating that rTSLPI does not inhibit MASP-2 activity. Heat inactivated rTSLPI-rabbit antiserum reduced the lectin pathway inhibitory activity of I. scapularis salivary gland extracts (Figure 4E), showing that native TSLPI is a major inhibitor of complement activation through the lectin pathway.
Figure 4. Mechanism of the inhibiting effect of rTSLPI on the MBL-pathway.
(A) Serum MBL-binding to mannan coated plates was measured in the presence of rTSLPI. Left: serum was pre-incubated with, 0.25 µg/µl rTSLPI or BSA, or a neutralizing MBL antibody and MBL deficient serum as a control. Right: MBL binding to mannan coated plates after preincubation of 1:50 NHS with several concentrations of BSA or rTSLPI. (B) Serum MBL-binding to rTSLPI or rP19 (control) coated plates (left panel) and complement activation was detected (right panel). (C) C4 cleaving activity of the MBL lectin pathway in the presence of PGNase F treated and untreated 0.25 µg/µl BSA or rTSLPI was determined using 1:40 NHS. (D) The effect of rTSLPI on MASP-2 activity was determined by capturing MBL/MASP-2 complexes on mannan coated plates by incubation of 1:50, 1:100 and 1:300 diluted serum in MBL-binding buffer. C4 deposition was measured in the presence of 0.25 µg/µl BSA, rTSLPI or C1 inhibitor. (E) Nymphal SGE was pre-incubated with heat-inactivated OVA-rabbit antiserum or rTSLPI-rabbit antiserum and was subjected to 1:40 NHS and complement activation was measured on mannan coated plates. Data are representative of two independent assays. Results are means ± SEM. See also Figure S2.
Since the MBL-lectin pathway was specifically inhibited by rTSLPI, the effect of rTSLPI on complement-dependent killing was determined in MBL-deficient serum (<0.02 µg/ml MBL). Unexpectedly, rTSLPI significantly reduced complement-dependent killing of B. garinii A87S in MBL deficient serum (54.7 ± 0.88% in the BSA control group to 31 ± 2.3% in the rTSLPI treated group; p=0.0007) (figure 5A, right panel). In addition, when MBL activity was abrogated in NHS (MBL+/+) using a neutralizing MBL antibody, the percentage of non-motile spirochetes decreased from 81 ± 2.3% in the control group to 34 ± 1.8% in the MBL antibody-treated group (p<0.0001) (Figure 5A, left panel). Interestingly, when the MBL antibody was combined with 0.25 µg/µl rTSLPI, spirochetal killing (20 ± 0.88%) was significantly reduced compared to MBL antibody alone (p=0.002) (figure 5A, left panel). These data suggest that rTSLPI inhibits additional factors besides MBL in the lectin pathway. It has been well-established that also ficolins are able to initiate the lectin pathway similarly to MBL. Therefore, we next assessed whether rTSLPI also influenced ficolin-induced C4 deposition. Faro et al. previously described an assay to specifically measure L-ficolin binding and lectin pathway activation using acetylated low-density lipoprotein (Ac-LDL) as a ligand (Faro et al., 2008). Indeed, rTSLPI dose-dependently impaired L-ficolin binding to Ac-LDL and thereby inhibited complement activation (Figure 5B).
Figure 5. L-ficolin activity is inhibited by rTSLPI.
(A) Serum-sensitive B. garinii strain A87S was incubated with 12.5% normal human MBL positive (1.16 µg/ml; left panel) or MBL deficient (0.01 µg/ml; right panel) serum in the presence of BSA (control), 0.25 µg/µl rTSLPI, 15 µg/ml neutralizing MBL antibody (anti-MBL) single or combined. As a control, spirochetes were also incubated with heat-inactivated (HI) NHS. Data represent mean ± SEM of values. Asterisks indicate a statistically significant difference with the control (***p<0.0001). (B) Acetylated-LDL coated plates were incubated with serum preincubated with 0.25 µg/µl rP19 or several concentrations of rTSLPI (0.03–0.5 µg/µl). Results are representative of two independent assays. See also Figure S3.
RNAi silencing of TSLPI and TSLPI immunization impairs Borrelia survival
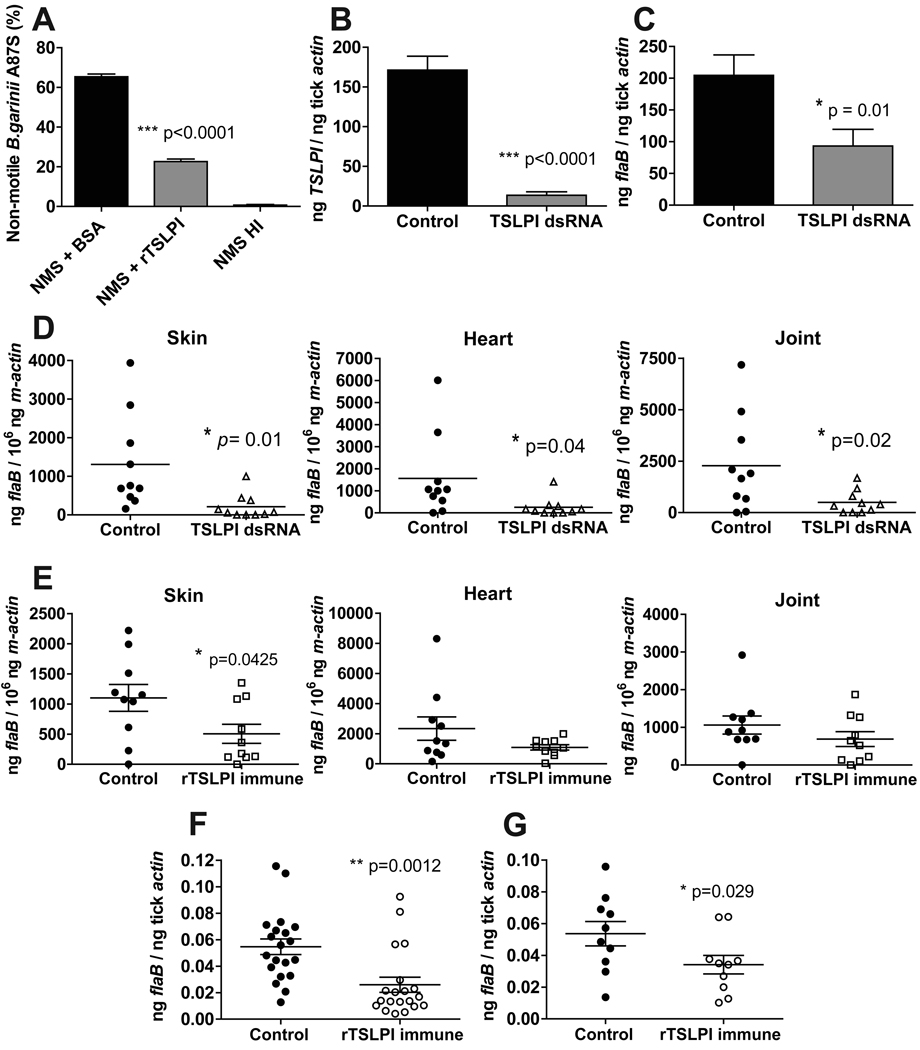
Having established that TSLPI impaired the lectin pathway of complement activation and thereby impaired clearance of Borrelia in vitro, we set out to identify the role of TSLPI in vivo. We first demonstrated that rTSLPI also protected Borrelia against complement-dependent killing by normal mouse serum from C3H mice (Figure 6A). To assess the role of TSLPI in the transmission of B. burgdorferi from the tick to the host we injected B. burgdorferi-infected nymphs with dsRNA directed against TSLPI (or mock injection as a control). Silencing resulted in a ~13 fold reduction of TSLPI mRNA (Figure 6B). The Borrelia loads in nymphs (Figure 6C) 72 h post feeding were significantly reduced in TSLPI-silenced Borrelia-infected nymphs, showing the importance of TSLPI for Borrelia persistence in the tick midguts during feeding. Moreover, transmission of Borrelia was impaired from TSLPI-silenced nymphs to mice, reflected by a significantly lower spirochete burden in skin after 7 days of infection, and in hearts and joints after 21 days of infection (Figure 6D). To further study the role of TSLPI in Borrelia transmission, mice were passively administered rTSLPI rabbit antiserum. The Borrelia load in skin from rTSLPI-immunized mice 7 days post infection was significantly lower and showed a trend towards lower Borrelia numbers in hearts and joints 21 days post infection (Figure 6E).
Figure 6. Decreased Borrelia survival after RNAi silencing TSLPI and in rTSLPI antiserum immunized mice.
(A) Borrelia was incubated with 12.5% normal mouse serum (NMS) or with heat-inactivated (HI) NMS for 1.5 h. (B) TSLPI expression in B. burgdorferi N40 infected nymphs after microinjection of TSLPI dsRNA or mock injection after 72 h of feeding on mice. (C) Borrelia flaB levels in nymphs in TSLPI silenced ticks and mock injected ticks post feeding on mice. (D) Borrelia transmission from TSLPI silenced or mock injected Borrelia infected nymphs was determined by measuring flaB levels in mouse skin 7 days, and in hearts and joint tissue, 21 days post feeding. (E) Borrelia loads in murine skin 7 days and hearts or joints 21 days post feeding on mice passively immunized with rabbit rTSLPI-antiserum. (F) Borrelia flaB levels in pooled larvae after feeding on mice passively immunized with rTSLPI-antiserum or control rabbit serum. (G) A second group of larvae were allowed to molt to the nymphal stage and Borrelia flaB levels were determined. Horizontal bars represent the mean ± SEM. Asterisks indicate a statistically significant difference.
A strong innate immune response at the tick-bite site, initiated by the host complement system, could also impair successful acquisition of Borrelia by I. scapularis from Borrelia-infected hosts. Therefore, we next assessed the role of TSLPI in Borrelia acquisition. The acquisition of Borrelia by larval I. scapularis ticks was impaired from rTSLPI-immunized B. burgdorferi-infected mice (Figure 6F). In line with this observation, larvae that molted to nymphs after feeding on rTSLPI-immunized Borrelia-infected mice had lower spirochetal loads (Figure 6G).
Together, these data show that TSLPI plays a significant role in both transmission of Borrelia from the arthropod vector to the naive mammalian host and acquisition of Borrelia from the infected mammalian host by larval ticks.
Discussion
We here show that the lectin complement pathway is of paramount importance in the eradication of the causative agent of Lyme disease. Furthermore, we demonstrate how Borrelia co-opts a tick protein to inhibit the host complement system to facilitate early mammalian infection as well as vector colonization. Our findings add to the understanding of the molecular mechanisms exploited by B. burgdorferi s.l. to survive in its enzootic life cycle encompassing the mammalian host and the arthropod vector.
Until now, it has been shown that both the classical and alternative complement pathways are involved in complement-dependent killing of Borrelia (Kurtenbach et al., 2002; van Dam et al., 1997). Interestingly, rTSLPI impaired complement-dependent killing of B. burgdorferi s.l. through specific inhibition of the lectin pathway, both in the absence or presence of specific antibodies, revealing a major role for the lectin pathway in eradication of Borrelia. MBL, the major player in the lectin complement pathway, is a C-type lectin that forms a non-covalent complex with MASPs and mainly circulates as a serum protein (Ip et al., 2009). Via its carbohydrate recognition domains (CRDs), MBL recognizes and binds polysaccharide structures on various pathogens resulting in a conformational change causing auto activation of MASP-2 which subsequently initiates complement activation by cleavage of C4 and C2 (Ip et al., 2009). We showed that rTSLPI did not affect MASP-2 activity, but prevented MBL binding to its ligand. Removing N-glycans from the protein backbone using PGNase F largely impaired the complement inhibitory effect of rTSLPI, suggesting that the glycosylated rTSLPI binds to the CRDs of MBL blocking downstream activation of the lectin pathway. Although activation of the lectin pathway is not antibody-dependent, rTSLPI protected serum resistant B. burgdorferi N40 when incubated with immune human serum. Anti-Borrelia antibodies play a crucial role in effective MAC formation in complement resistant strains by altering the outer membrane of Borrelia (Kochi et al., 1991), which could explain the protective effect of rTSLPI in the presence of Borrelia antibodies. The importance of the MBL complement pathway in the direct lysis of Borrelia was underscored by the observation that a neutralizing MBL antibody greatly abrogated immobilization of B. garinii by MBL sufficient human serum. Approximately 25% of the general population is MBL deficient, defined as a MBL serum level < 0.5 µg/ml (Eisen et al., 2008), which could, based on our observations, make these individuals more susceptible for Lyme disease. Until now MBL deficiency has been linked to several infectious diseases, such as malaria, HIV, meningococcal and pneumococcal disease among other diseases (Ram et al., 2010). Complement-dependent killing of Borrelia in both NHS and MBL-deficient serum was completely abrogated by C1 esterase inhibitor (Figure S3), a serpin that inhibits both the classical pathway and lectin pathway of complement activation (Ricklin et al., 2010; Wouters et al., 2008), indicating that both the classical and lectin pathway are crucially important in initiating complement activation on Borrelia. In line with this finding, Borrelia complement-dependent killing was almost entirely ablated upon combined inhibition of the classical pathway and the lectin pathway by both neutralizing C1q antibody and rTSLPI (Figure S3). Irrespective of whether initiated by the lectin or classical complement pathway, C3 convertases bound to the surface of B. burgdorferi s.l. can induce amplification of the alternative pathway by enhancing C3 activation (Ricklin et al., 2010; Tyson et al., 2007), which could be detrimental for Borrelia survival. Recently, a constitutional role for MBL-associated serine protease 1 (MASP-1) in activation of the alternative pathway by cleaving factor D was demonstrated (Takahashi et al., 2010), showing that MASP-1 activation upon MBL and ficolin binding results in a direct activation of the alternative pathway as well. Thus, although the three complement routes are closely intertwined, our data demonstrate that the lectin pathway is of crucial importance in the eradication of Borrelia spirochetes.
Since rTSLPI also significantly reduced killing of Borrelia in MBL deficient serum and rTSLPI further reduced complement killing in normal serum incubated with neutralizing MBL antibodies, we assessed whether rTSLPI inhibited other components of the lectin pathway besides MBL. Apart from MBL, three types of ficolins, M-ficolin (also known as Ficolin-1), L-ficolin (also known as Ficolin-2) and H-ficolin (also known as Ficolin-3) are identified in humans and are able to activate the lectin pathway through the associated protease MASP-2 (Matsushita, 2009) and rTSLPI also showed to dose-dependently impair L-ficolin activity.
In addition to direct lysis of B. burgdorferi s.l. cells after MAC complex formation, complement activation also results in opsonisation of invading microorganisms and thereby enhancing phagocytosis by host immune cells (Fernandez et al., 1978; Ricklin et al., 2010). Indeed, opsonisation by complement factors was essential for phagocytosis of both B. garinii A87S and B. burgdorferi N40 by human PMNs, since these cells did not phagocytize Borrelia in the absence of a functional complement system, even in the presence of Borrelia-specific antibodies. Importantly, we observed that human PMNs were significantly impaired in their capacity to phagocytize B. garinii A87S and B. burgdorferi N40 (data not shown) when normal human serum was preincubated with rTSLPI. Selected Borrelia strains are able to partially evade complement activation through the alternative pathway by interacting with host complement regulator proteins, such as factor H (FH) and factor H-like (FHL-1) through expression of Erps and CRASP proteins on their outer membrane (Brissette et al., 2008) and by expressing a CD59-like protein that inhibits the assembly of (sub)lytic complexes (Pausa et al., 2003). However, others have shown that there was no difference in C3 deposition between serum sensitive strains and serum resistant strains when incubated in human serum (Breitner-Ruddock et al., 1997; Kochi et al., 1991). Since C3b deposition and its degradation products iC3b, C3c and C3d play a major role in complement receptor-induced phagocytosis (Ricklin et al., 2010), this indicates that, although serum resistant spirochetes evade direct lysis through the alternative complement route, they are equally susceptible to phagocytosis compared to serum sensitive Borrelia. In contrast, inhibition of complement initiating mechanisms on Borrelia, such as inhibition of the lectin pathway by TSLPI, is likely to result in decreased C3 opsonisation. This could explain the observed impaired phagocytosis of Borrelia by neutrophils incubated with normal human serum in the presence of rTSLPI. Interestingly, apart from initiating complement activation, MBL and ficolins also act as opsonins in the absence of C3 through several collectin receptors on phagocytic cells (Ip et al., 2009). In addition, MBL has been shown to cooperate with TLR-2/TLR-6 to enhance phagosome signaling in response to bacteria (Ip et al., 2008), further underscoring the relevance of inhibition of the lectin pathway by TSLPI for B. burgdorferi.
Although rTSLPI inhibited Borrelia-induced MBL complement pathway-mediated chemotaxis in vitro, preliminary experiments indicate that rTSLPI did not impair influx of phagocytes in mice intradermally injected with 104 B. garinii strain A87S (data not shown). It is possible that other tick proteins are necessary to impair innate immune responses towards Borrelia. Indeed, during tick feeding other salivary proteins are able to inhibit additional parts of the innate immune system such as dendritic cells, neutralizing chemokines, inhibition of inflammatory cytokines among other reported anti-inflammatory proteins (Hovius et al., 2008), which could also play a role in protecting Borrelia from clearance by the host immune system.
TSLPI was differentially expressed during the course of tick feeding. Interestingly, TSLPI was upregulated in Borrelia-infected ticks during the early stages of tick feeding. Furthermore, the anti-complement effect of native TSLPI in tick SGE was inhibited in both the Borrelia killing assay and lectin pathway assay in the presence of anti-rTSLPI antiserum, indicating that TSLPI is a major inhibitor of complement activation though the lectin pathway on Borrelia in Ixodes tick saliva and underscoring its biologic significance. Indeed, after silencing TSLPI in ticks we showed that Borrelia transmission to mice was significantly impaired after 72 h of tick feeding. Moreover, Borrelia loads in ticks after feeding were also more than 2-fold reduced. Others have shown that complement is still active in midguts of Ixodes ticks post-engorgement (Papatheodorou and Brossard, 1987). Thus, our data suggest that Borrelia is protected against complement activation by TSLPI in midguts of ticks. We next extended our studies by passively transferring rTSLPI rabbit antiserum to mice. After 72 h of feeding on rTSLPI immunized mice spirochete transmission from Borrelia-infected ticks to mice was significantly reduced as well. Since immunization against rTSLPI did not result in total abrogation of Borrelia transmission from the tick to the host, a future tick antigen-based vaccine to prevent Lyme borreliosis should probably be based on a combination of tick proteins expressed during the course of tick feeding and important for Borrelia transmission (Schuijt et al., 2011a). Together, our findings implicate that TSLPI is crucially important for Borrelia survival both in the tick vector and in the host during early infection. While TSLPI directly and specifically inhibited the lectin pathway of complement activation, this could facilitate transmission of other pathogens as well. Although TSLPI is specifically upregulated in tick salivary glands upon infection with B. burgdorferi, and not Anaplasma phagocytophilum, preliminary data show impaired A. phagocytophilum transmission by nymphs to rTSLPI-immune mice, using a mouse model for A. phagocytophilum infection (data not shown).
We also assessed the role of TSLPI in survival and migration of Borrelia from the mammalian host to ticks, a process referred to as acquisition. These experiments were performed with larval I. scapularis ticks since larvae naturally acquire B. burgdorferi s.l. and we have previously shown that TSLPI is expressed in engorging larvae (Schuijt et al., 2011b). Borrelia acquisition from Borrelia-infected mice by larval ticks was impaired when mice were immunized with rTSLPI, indicating that TSLPI activity is crucial at the tick-bite site for Borrelia in order to survive and migrate into the tick midgut as well. Thus, TSLPI is a tick salivary protein that plays a significant role in both Borrelia transmission to the mammalian host as well as Borrelia acquisition and persistence in ticks.
In conclusion, we have shown a crucial role for the lectin complement pathway in the eradication of the causative agent of Lyme disease. Furthermore, we have identified and characterized a tick salivary lectin pathway inhibitor (TSLPI) that abates complement activation on B. burgdorferi s.l. by impairing activation of the lectin complement pathway, which leads to impaired clearance of Borrelia. TSLPI silencing in ticks and TSLPI immunization studies demonstrated diminished Borrelia transmission and acquisition by ticks in which TSLPI activity was impaired, implying a crucial role for TSLPI in the enzootic life cycle of B. burgdorferi and revealing the potential of TSLPI as a component in a multivalent tick protein-based vaccine to prevent Lyme disease.
Experimental Procedures
Ticks and animals
Ixodes scapularis nymphs and larvae were obtained from a tick colony at the Connecticut Agricultural Experiment Station in New Haven CT, USA. Ticks were maintained at 23°C and 85% relative humidity under a 14 h light, 10 h dark photoperiod. Borrelia infected nymphs were generated by placing larvae on B. burgdorferi infected C3H mice and fed larvae were molted to nymphs. For active immunization studies, 6 week old inbred New Zealand white rabbits (Charles River Laboratories) were obtained and immunized as described before (Schuijt et al., 2011b). Preparation of I. scapularis nymphal salivary gland extract (SGE) and adult saliva are described in Supplemental Experimental Procedures. For the passive immunization experiments 4–6 weeks old female C3H/HeJ mice (Jackson Laboratory) were used. The protocol for the use of mice and rabbits was reviewed and approved by the Yale Animal Care and Use Committee. The Animal Care and Use Committee of the University of Amsterdam approved all animal experiments performed in Amsterdam and experiments have been conducted according to national guidelines.
Recombinant salivary proteins
Cloning and expression of TSLPI and p19 in the Drosophila Expression System (Invitrogen) and purification of recombinant protein was performed as described before (Schuijt et al., 2011b). See the Supplemental Experimental Procedures for more details. Deglycosylation of recombinant TSLPI with N-Glycosidase (PNGase) F (Sigma) was performed according to the manufacturer’s instructions.
Tick RNA isolation and quantitative RT-PCR
Ticks were allowed to feed for 24, 72 h or to repletion (between 80 and 96 h after initiation of tick feeding, ticks were monitored two times a day and recovered after detachment from the hosts) on experimental and control animals. Nymphs were dissected and salivary glands and midguts were pooled (3 ticks), homogenized and RNA was extracted using the RNeasy minikit (Qiagen, CA). DNA was removed by on-column DNase digestion. The same procedure was performed with unfed ticks. Total RNA was quantified (5–6 ng of total RNA) and the quality of the RNA was assessed (Nanodrop 2000c). cDNAwas synthesized using the iScript RT-PCR kit (Biorad, CA). According to the manufacturer’s instructions 2 µl of cDNA synthesis reaction was analyzed by quantitative PCR for the expression of tick actin and TSLPI, using the iQ Syber Green Supermix (Biorad, CA) on a MJ cycler (MJ Research, CA) and previously described primers (Schuijt et al., 2011b).
Assay for detection of complement-mediated killing of Borrelia spirochetes
The serum-sensitive Borrelia garinii isolate A87S was used (107 spirochetes ml−1) to determine complement-mediated killing in normal human serum (NHS) as described earlier (Schuijt et al., 2008). The serum resistant Borrelia burgdorferi N40 was used (107 spirochetes ml−1) to determine antibody dependent complement-mediated killing in Borrelia immune human serum (IHS). See Supplemental Experimental Procedures for more details.
Deposition of terminal C5b-9 complement complexes
For detection of the terminal C5b-9 complement complexes on the borrelial surface, a previously described C5b-9 immunofluorescence assay was used (Schuijt et al., 2008). See Supplemental Experimental Procedures for more details.
Phagocytosis assay
Borrelia phagocytosis assay was performed as described before (Hovius et al., 2009). Experiments with human PMNs were performed with minor modifications described in Supplemental Experimental Procedures.
Neutrophil Migration Assay
Human PMNs were isolated from blood with Polymorphprep (Axis-Shield) according to the manufacturer’s instructions. PMN migration was assessed as previously described (Hovius et al., 2009). See Supplemental Experimental Procedures for more details. 12.5% NHS was preincubated with 0.5 µg/µl BSA or rTSLPI for 30 min. Subsequently, 2.5 × 107 B. garinii A87S was added and incubated for 30 min at 37°C. Spirochetes were pelleted (30 min, 20,000g) and the supernatant was checked for the absence of Borrelia using dark field microscopy. Supernatant was used to dilute the NHS to a final concentration of 2.5% and was added to the bottom wells after which cell migration was assessed.
Borrelia binding assay
B. burgdorferi N40 (2.5 × 107) was incubated with 0.1 ng/µl recombinant Salp15 or TSLPI in PBS/0.1% BSA for 1 h at room temperature. Borrelia was pelleted and the pellet and supernatant were separated. The pellet was washed two times in 1.5 ml PBS/0.1% BSA and was resuspended in the same volume as the supernatant. Equal volumes of supernatant and pellet was used to run on a SDS gel and transferred to nitrocellulose membranes. The membranes were blocked with PBS/5% milk and immunoblots were probed and visualized with an HRP conjugated anti-V5 antibody (Invitrogen) and the enhanced chemiluminescence Western Blotting Detection System (GE Healthcare, NJ).
Classical and alternative complement pathway assays
Complement hemolytic activity of the classical pathway (CH50) was determined by using antibody coated sheep red blood cells (SRBCs). Opsonized SRCBs were incubated for 1 h with several dilutions of NHS preincubated with either 0.25 µg/µl neutralizing C3 antibody (antiC3-2, Sanquin), 50 µg/µl neutralizing C1q antibody (anti-C1q-85, Sanquin), 0.25 µg/µl BSA or 0.25 µg/µl rTSLPI in GVBS (VBS with 5.8% (w/v) sucrose, 0.5% (w/v) human serum albumin, 10 mM CaCl2, and 2 mM MgCl2, pH 7.4) in a shaker at 37°C. Complement hemolytic activity of the alternative pathway (AP50) was performed by using rabbit red blood cells (RRBCs). RRBCs were incubated for 1 h in a shaker at 37°C with several dilutions of serum preincubated with 0.25 µg/µl neutralizing C3 antibody, BSA or rTSLPI in GVBS containing 10 mM MgEGTA. For positive control of the CH50 and AP50 red blood cells were incubated in distilled water (100% lysis of cells) and for the negative control the red blood cells were incubated in GVBS or GVBS with 10 mM EDTA, respectively (0% lysis of cells). Plates were centrifuged at 1000g for 5 min and hemolysis was determined by measuring OD at 414 nm using the iMark Microplate Reader (Biorad).
Lectin pathway of complement C4 activation
High binding microtiter plates (Microlon, Greiner) were coated with 10 µg/ml mannan (Sigma) or acetylated LDL (A-LDL) (Biomedical Technologies Inc.) in a moist chamber overnight at 4°C. A-LDL is a useful ligand to specifically determine L-ficolin activity (Faro et al., 2008). Wells were blocked with blocking buffer (1:10 dilution of Starting Block (Pierce, IL, US) in TBS with 0.05% Tween20) at room temperature for 1h. After washing with TBS/0.05%Tween20/5 mM CaCl2, several NHS dilutions preincubated for 30 min with either BSA, rP19 or rTSLPI were incubated in the wells in C4 activation buffer (4 mM Na-diethyl-barbiturate, 145 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 2% (v/v) Starting Block, 0.02% (v/v) Tween20) for 1 h at room temperature. Wells were washed and were incubated with biotinylated monoclonal mouse anti-human C4 IgG (0.25 µg/ml). After washing, avidin conjugated HRP in TBS/Tween20/Ca was incubated for 30 min at room temperature. Wells were washed, TMB substrate was added and the absorbance at 450 nm was measured using the iMark Microplate Reader (Biorad).
MBL binding
Wells (Microlon, HB, Greiner) were coated with 10 µg/ml mannan overnight at 4°C, blocked with a 1:10 dilution of Starting Block (Pierce) in TBS with 0.05% Tween20 and washed with TBS/Tween20/Ca. Several serum dilutions were incubated with BSA or rTSLPI for 30 min in MBL binding buffer (20 mM Tris-HCL, 1 M NaCl, 10 mM CaCl2, 1 mg/ml BSA, 0.05% (v/v) Triton X-100) and serum MBL was allowed to bind the mannan coated wells overnight at 4°C. Wells were washed with TBS/Tween20/Ca and incubated with monoclonal mouse anti-human 10 µg/ml MBL antibody (Sanquin) in TBS/Tween20/Ca for 1 h at room temperature. After washing HRP-conjugated anti-mouse IgG was added and was incubated for 45 min at room temperature. The amount of MBL bound to the plates was determined by reading the absorbance at 450 nm using the iMark Microplate Reader (Biorad).
MBL/MASP-2 complex activity assay
The MBL/MASP-2 complex activity assay was used to determine C4 cleavage activity of MASP-2 as previously described (Gadjeva et al., 2004). Serum MBL/MASP-2 complexes were bound to mannan-coated plates as described above by using several serum dilutions (1:50, 1:100 and 1:300) in MBL binding buffer overnight at 4°C. After washing the plates MBL deficient serum was used as a C4 source in a final dilution of 1:100 and incubated with 0.25 µg/µl rTSLPI, BSA or C1 inhibitor (Sanquin) in C4 dilution buffer for 1 h at room temperature. C4 activation and deposition on the plates as well as the amount of MBL bound on the plates after incubation of BSA or rTSLPI was determined as described above.
Passive immunization and B. burgdorferi transmission
In experiments to address Borrelia transmission to mice, 6 B. burgdorferi N40 infected nymphs were placed on each mice passively immunized by intraperitoneal inoculation with 200 µl serum obtained from rTSLPI or OVA immunized rabbits. Nymphs were allowed to feed to repletion. Salivary glands and midguts were dissected for mRNA purification as described above. Transmission was assessed by quantitative PCR of DNA isolated from skin at 7 days and hearts and joints at 21 days post engorgement. To address Borrelia acquisition, at least 30 pathogen free I. scapularis larvae were placed on each B. burgdorferi-infected rTSLPI immune mouse and allowed to feed to repletion. At least three mice were used in each experiment. Larvae were analyzed in pools of 5.
RNAi silencing of TSLPI in Borrelia infected I. scapularis nymphs
Primers were designed by addition of a T7 promoter site (TAATACGACTCACTATAGGGAGA) at the 5’ end of the forward (AGGCTGCGACTATTACTGCTG) and reverse (TTGAATCGGTTGTCAAATGG) primers. dsRNA complementary to the TSLPI gene was synthesized by using the MEGAscript RNAi kit (Ambion). The dsRNA was purified and quantified spectroscopically. dsRNA (5 nl, 1×1012 molecules per µl) was injected into the body of Borrelia infected nymphs using 10 µl microdispensers (Drummond Scientific, Broomall, PA) drawn to fine-point needles by using a micropipette puller (Sutter Instruments, Novato) or with the same volume of dsRNA elution buffer (mock), provided by the RNAi kit (Ambion) alone. The needles were loaded onto a micromanipulator (Narishige, Tokyo) connected to a Nanojet microinjector (Drummond Scientific). At least 50 nymphs were used in each group. The ticks were allowed to rest for 3 h before placement on mice. Ticks were allowed to feed for 72 h, were weighted and dissected for mRNA isolation and quantitative RT-PCR as described above. Borrelia transmission was assessed by quantitative PCR of DNA isolated from skin at 7 days and hearts and joints at 21 days post engorgement.
Statistical analysis
The significance of the difference between the mean values of the groups was analyzed using a two-tailed student t test with Prism 5.0 software (GraphPad Software, USA) and the p value is indicated by asterisks in the figures.
Highlights.
Tick salivary protein TSLPI impairs complement-mediated killing of B. burgdorferi
Recombinant TSLPI inhibits neutrophil chemotaxis and phagocytosis of Borrelia
TSLPI directly inhibits mannose-binding lectin complement pathway activation
TSLPI silencing in ticks or TSLPI immunization of mice impairs Borrelia transmission
Supplementary Material
Acknowledgements
We would like to thank Henk van Veen for excellent assistance in SEM photography. This study was supported by grants 41440, 49200 and 32947 from the National Institutes of Health. T.J.Schuijt is supported by a grant from the OLVG research fund. J.W. Hovius is a recipient of a VENI stipend (91611065) from the Netherlands Organization for health research and development (ZonMw). Erol Fikrig is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breitner-Ruddock S, Wurzner R, Schulze J, Brade V. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med Microbiol Immunol. 1997;185:253–260. doi: 10.1007/s004300050038. [DOI] [PubMed] [Google Scholar]
- Brissette CA, Cooley AE, Burns LH, Riley SP, Verma A, Woodman ME, Bykowski T, Stevenson B. Lyme borreliosis spirochete Erp proteins, their known host ligands, and potential roles in mammalian infection. Int J Med Microbiol. 2008;298 Suppl 1:257–267. doi: 10.1016/j.ijmm.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen DP, Dean MM, Boermeester MA, Fidler KJ, Gordon AC, Kronborg G, Kun JF, Lau YL, Payeras A, Valdimarsson H, et al. Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clin Infect Dis. 2008;47:510–516. doi: 10.1086/590006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Pena A, Jongejan F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol. 1999;23:685–715. doi: 10.1023/a:1006241108739. [DOI] [PubMed] [Google Scholar]
- Faro J, Chen Y, Jhaveri P, Oza P, Spear GT, Lint TF, Gewurz H. L-ficolin binding and lectin pathway activation by acetylated low-density lipoprotein. Clin Exp Immunol. 2008;151:275–283. doi: 10.1111/j.1365-2249.2007.03538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez HN, Henson PM, Otani A, Hugli TE. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978;120:109–115. [PubMed] [Google Scholar]
- Fredslund F, Laursen NS, Roversi P, Jenner L, Oliveira CL, Pedersen JS, Nunn MA, Lea SM, Discipio R, Sottrup-Jensen L, et al. Structure of and influence of a tick complement inhibitor on human complement component 5. Nat Immunol. 2008;9:753–760. doi: 10.1038/ni.1625. [DOI] [PubMed] [Google Scholar]
- Gadjeva M, Thiel S, Jensenius JC. Assays for the mannan-binding lectin pathway. Curr Protoc Immunol. 2004;Chapter 13(Unit 13 16) doi: 10.1002/0471142735.im1306s58. [DOI] [PubMed] [Google Scholar]
- Hepburn NJ, Williams AS, Nunn MA, Chamberlain-Banoub JC, Hamer J, Morgan BP, Harris CL. In vivo characterization and therapeutic efficacy of a C5-specific inhibitor from the soft tick Ornithodoros moubata. J Biol Chem. 2007;282:8292–8299. doi: 10.1074/jbc.M609858200. [DOI] [PubMed] [Google Scholar]
- Hovius JW, Bijlsma MF, van der Windt GJ, Wiersinga WJ, Boukens BJ, Coumou J, Oei A, de Beer R, de Vos AF, van 't Veer C, et al. The urokinase receptor (uPAR) facilitates clearance of Borrelia burgdorferi. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000447. e1000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius JW, Levi M, Fikrig E. Salivating for knowledge: potential pharmacological agents in tick saliva. PLoS Med. 2008;5:e43. doi: 10.1371/journal.pmed.0050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RA. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med. 2008;205:169–181. doi: 10.1084/jem.20071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore U, Reid KB. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–170. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- Kochi SK, Johnson RC, Dalmasso AP. Complement-mediated killing of the Lyme disease spirochete Borrelia burgdorferi. Role of antibody in formation of an effective membrane attack complex. J Immunol. 1991;146:3964–3970. [PubMed] [Google Scholar]
- Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, Kraiczy P. Host association of Borrelia burgdorferi sensu lato--the key role of host complement. Trends Microbiol. 2002;10:74–79. doi: 10.1016/s0966-842x(01)02298-3. [DOI] [PubMed] [Google Scholar]
- Matsushita M. Ficolins: complement-activating lectins involved in innate immunity. J Innate Immun. 2009;2:24–32. doi: 10.1159/000228160. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Koski RA, Beaulieu B, Anderson JF, Ramamoorthi N, Kantor F, Cappello M, Fikrig E. A novel family of anticoagulants from the saliva of Ixodes scapularis. Insect Mol Biol. 2002;11:641–650. doi: 10.1046/j.1365-2583.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- Papatheodorou V, Brossard M. C3 levels in the sera of rabbits infested and reinfested with Ixodes ricinus L. and in midguts of fed ticks. Exp Appl Acarol. 1987;3:53–59. doi: 10.1007/BF01200413. [DOI] [PubMed] [Google Scholar]
- Pausa M, Pellis V, Cinco M, Giulianini PG, Presani G, Perticarari S, Murgia R, Tedesco F. Serum-resistant strains of Borrelia burgdorferi evade complement-mediated killing by expressing a CD59-like complement inhibitory molecule. J Immunol. 2003;170:3214–3222. doi: 10.4049/jimmunol.170.6.3214. [DOI] [PubMed] [Google Scholar]
- Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijt TJ, Hovius JW, van Burgel ND, Ramamoorthi N, Fikrig E, van Dam AP. The tick salivary protein Salp15 inhibits the killing of serum-sensitive Borrelia burgdorferi sensu lato isolates. Infect Immun. 2008;76:2888–2894. doi: 10.1128/IAI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijt TJ, Hovius JW, van der Poll T, van Dam AP, Fikrig E. Lyme borreliosis vaccination: the facts, the challenge, the future. Trends Parasitol. 2011a;27:40–47. doi: 10.1016/j.pt.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Schuijt TJ, Narasimhan S, Daffre S, Deponte K, Hovius JW, Veer CV, van der Poll T, Bakhtiari K, Meijers JC, Boder ET, et al. Identification and Characterization of Ixodes scapularis Antigens That Elicit Tick Immunity Using Yeast Surface Display. PLoS One. 2011b;6:e15926. doi: 10.1371/journal.pone.0015926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Ishida Y, Iwaki D, Kanno K, Suzuki T, Endo Y, Homma Y, Fujita T. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J Exp Med. 2010;207:29–37. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson K, Elkins C, Patterson H, Fikrig E, de Silva A. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol Biol. 2007;16:469–479. doi: 10.1111/j.1365-2583.2007.00742.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Charlab R, Mather TN, Ribeiro JM. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem. 2000;275:18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- van Dam AP, Oei A, Jaspars R, Fijen C, Wilske B, Spanjaard L, Dankert J. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect Immun. 1997;65:1228–1236. doi: 10.1128/iai.65.4.1228-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters D, Wagenaar-Bos I, van Ham M, Zeerleder S. C1 inhibitor: just a serine protease inhibitor? New and old considerations on therapeutic applications of C1 inhibitor. Expert Opin Biol Ther. 2008;8:1225–1240. doi: 10.1517/14712598.8.8.1225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.