Abstract
Intra- and multicenter reproducibility of currently used arterial spin labeling (ASL) methods were assessed at three imaging centers in the Netherlands, equipped with Philips 3TMR scanners. Six healthy participants were scanned twice at each site. The imaging protocol consisted of continuous ASL (CASL), pseudo-continuous ASL (p-CASL) with and without background suppression, pulsed ASL (PASL) with single and multiple inversion times (TIs), and selective ASL for segmentation. Reproducibility was expressed in terms of the coefficient of repeatability and the repeatability index. Voxelwise analysis of variance was performed, yielding brain maps that reflected regional variability. Intra- and multicenter reproducibility were comparable for all methods, except for single TI PASL, with better intracenter reproducibility (F-test of equality of two variances, P<0.05). Pseudo-continuous ASL and multi TI PASL varied least between sites. Variability maps of all methods showed most variability near brain-feeding arteries within sessions and in gray matter between sessions. On the basis of the results of this study, one could consider the use of reference values in clinical routine, with whole-brain p-CASL perfusion varying <20% over repeated measurements within the same individuals considered to be normal. Knowledge on regional variability allows for the use of perfusion-weighted images in the assessment of local cerebral pathology.
Keywords: arterial spin labeling, multicenter, reference values, regional variability, reproducibility
Introduction
Cerebral perfusion imaging has an important role in the diagnosis and evaluation of different brain disorders, and in the examination of brain function. The distribution of perfusion throughout the brain reflects local metabolic demands and provides information about the delivery of metabolic substrates. Arterial spin labeling (ASL) is a relatively new and non-invasive perfusion imaging modality that can be used for visualization and quantification of cerebral blood flow (CBF). Arterial spin labeling uses magnetically labeled arterial blood water protons as an endogenous tracer of flow. Its non-invasive character makes ASL especially attractive for repeated CBF measurements in patient follow-up, longitudinal studies, pharmacological studies, and for measurements in the pediatric population. However, the clinical implementation of ASL remains challenging because of several difficulties. The method suffers from an intrinsic low signal-to-noise ratio (requiring longer scanning time periods to obtain sufficient perfusion signal), a difficult planning process, and uncertainties regarding cerebrovascular kinetics or blood equilibrium magnetization, directly affecting perfusion estimates. Because of these difficulties, ASL is being portrayed as a research tool that can only be used in highly specialized imaging centers. As many of these problems were solved by technical advances, ASL became more feasible for clinical use in the last few years (Golay et al, 2004; Petersen et al, 2010; Calamante et al, 1999; Wang and Licht, 2006; Detre et al, 2009; Donahue et al, 2010; Wang et al, 2008).
During the last decade, several single-center studies were performed to assess the robustness of whole-brain and flow territory perfusion estimates on the basis of either continuous or pulsed labeling schemes (Floyd et al, 2003; Gevers et al, 2009a; Hermes et al, 2007; Parkes et al, 2004; Jahng et al, 2005). The recently published quantitative star labeling of arterial regions (QUASAR), reproducibility study has been the first to describe the multicenter reproducibility of ASL. This study has shown that ASL is a reliable perfusion imaging technique that can be used in clinical routine without the need for special hardware or dedicated personnel (Petersen et al, 2010).
However, it is still uncertain whether comparable perfusion values would be obtained when scanning the same subjects multiple times at different imaging sites. The latter will determine whether reference values for CBF could be used in clinical decision making or whether each imaging center should first gauge its own perfusion values in healthy controls. In addition, reproducibility studies performed thus far focused on the quantitative assessment of reproducibility of perfusion estimates. The behavior of regional variability patterns in ASL perfusion imaging, however, is still unknown. Knowledge on regional variability patterns is valuable when ASL perfusion maps will be used in the diagnostic process in patients with local cerebrovascular pathology and can provide insight in the most important sources of variation for the different ASL techniques.
The aims of this study were twofold. The primary aim of this study was to assess whether ASL is a reliable method for perfusion imaging, enabling the use of reference values for cerebral perfusion in the evaluation of cerebral perfusion. The secondary aim of this study was to measure regional variability patterns in ASL perfusion imaging.
Materials and methods
Subject Recruitment and Study Design
Variability of continuous ASL (CASL), pulsed ASL (PASL), and pseudo-continuous ASL (p-ASL) sequences was assessed at three imaging centers in the Netherlands. Magnetic resonance (MR) investigations were performed on 3TMR scanners (Philips Healthcare, Best, the Netherlands) with the same implementation of ASL sequences. The local ethics committees of the participating imaging centers approved the study protocol. After obtaining written informed consent, six healthy volunteers (five men, one woman; aged 25 to 50 years) without known brain disease were scanned twice at each location, with 1 to 3 weeks between the sessions. No caffeine intake was allowed at the scanning days, before scanning sessions. Each session protocol consisted of a three-dimensional (3D) time-of-flight MR angiography, a CASL sequence, a PASL sequence with single and with multiple inversion time periods (single and multi TI PASL), a p-CASL sequence performed with and without additional background suppression pulses, a high-resolution 3D T1-weighted anatomical scan, and a selective ASL scan for registration and segmentation purposes. All scans were performed in a randomized manner. Magnetic resonance angiography and CASL scans were acquired with a transmit/receive head coil from the manufacturer (Philips Healthcare). Pulsed ASL, p-CASL, selective ASL, and anatomical scans were acquired with a SENSE-8-channel head coil and body coil transmission (Philips Healthcare). Total scan duration per session was 50 minutes.
ASL Sequences
Imaging parameters that were previously used in local studies at the participating imaging centers were used.
We used the amplitude-modulated CASL approach described by Alsop and Detre (1998), without violating specific absorption rate levels. Continuous ASL imaging parameters were as follows: sequence repetition time/echo time (TR/TE), 4,500 ms/32 ms; flip angle, 90° field of view (FOV), 210 × 210 mm2; matrix size, 64 × 45; 11 slices; thickness, 7 mm; no gap; spin-echo single-shot echo-planar imaging (EPI); number of dynamics, 40; labeling duration, 2.0 seconds; radio frequency pulse amplitude, 3.5 mT; gradient strength, 2.5 mT/m; modulation frequency, 250 Hz; post-labeling delay, 1.2 to 2.2 seconds, depending on the slice number; labeling gap between the center of the imaging volume and the labeling slab, 60 mm; and total scan duration, 7.6 minutes.
Our single TI PASL sequence was based on the PULSAR (pulsed star labeling of arterial regions) sequence developed by Golay et al (2005). Imaging parameters were as follows: TR/TE, 3,000 ms/20 ms; flip angle, 90° FOV, 240 × 240 mm2; matrix size, 80 × 79; 17 slices; thickness, 7 mm; no gap; gradient-echo single-shot EPI; SENSE 2.0; post-labeling delay, 1.2 to 2 seconds; number of dynamics, 40; labeling gap between the center of the imaging volume and the labeling slab, 25 mm; and total scan duration was 4.2 minutes. The multi TI PASL sequence that we used was based on the QUASAR sequence described by Petersen et al (2006) (van Osch et al, 2007). Imaging parameters were as follows: TR/TE, 3,000 ms/28 ms; flip angle, 30° FOV, 240 × 240 mm2; matrix size, 80 × 79; 5 slices; thickness, 7 mm; no gap; gradient-echo single-shot EPI; SENSE 1.2; number of dynamics, 40; labeling gap between the center of the imaging volume and the labeling slab, 25 mm; TIs ranging from 100 to 2700 ms with a 300 ms interval and a flip angle of 30° and total scan duration was 4.1 minutes.
The p-CASL sequence described by Dai et al (2008) was performed with and without additional background suppression pulses (Wu et al, 2007; Ye et al, 2000). Imaging parameters were as follows: TR/TE, 4,000 ms/14 ms; flip angle, 90° FOV, 240 × 240 mm2; matrix size, 80 × 79; 17 slices; thickness, 7 mm; no gap; gradient-echo single-shot EPI; SENSE 2.5; post-labeling delay, 1.525 to 2.1 seconds; number of dynamics, 40; and labeling gap between the center of the imaging volume and the labeling slab, 90 mm. Background suppression was achieved by applying a saturation pulse preceding labeling and by applying two inversion pulses, 1,680 and 2,830 ms, after the saturation pulse. Total scan duration was 5.3 minutes.
To determine the selective supply to the independent flow territories of the major brain-feeding arteries, we used planning-free vessel-encoded p-CASL, as implemented by Wong (2007). Imaging parameters were as follows: TR/TE, 4,000 ms/14 ms; flip angle, 90° FOV, 240 × 240 mm2; matrix size, 80 × 79; 17 slices; thickness, 7 mm; no gap; gradient-echo single-shot EPI; post-labeling delay, 1,525 ms; background suppression by a saturation pulse preceding the labeling and by two inversion pulses, 1,680 and 2,830 ms, after the saturation pulse; number of dynamics, 75; labeling gap between the center of the imaging volume and the labeling slab, 90 mm. To achieve selective labeling of major brain-feeding arteries, labeling efficiency was spatially manipulated within the labeling plane (Gevers et al, 2009b; Wong, 2007). Total scan duration was 5 minutes.
Planning Process
Labeling planes were positioned parallel to imaging volumes, at a level in which the relatively thin labeling planes used in CASL and p-CASL are more or less perpendicular to the distal ascending portions of the internal carotid and basilar arteries (ICAs and BA). Preceding each CASL scan, 3D time-of-flight MR angiography was performed to allow for careful planning of the CASL labeling plane, 10 to 20 mm below the circle of Willis. Planning of PASL- and p-CASL-based sequences was carried out once per imaging session using the same center and angulations of the imaging volume for all scans. Between sessions, screen dumps of the position of the labeling plane on sagittal 3D-T1 anatomicals were used as a guide for planning PASL and p-CASL sequences.
Post Processing
FSL (FMRIB Software Library, Functional Magnetic Resonance Imaging of the Brain Center, Department of Clinical Neurology, University of Oxford, Oxford, UK, http://www.fmrib.ox.ac.uk) and Matlab (The MathWorks, Natick, MA, USA; http://www.mathworks.com) were used for offline data processing.
Subtraction of labeled and control images yielded whole-brain perfusion-weighted images of all scans obtained from the 36 data sets (three sites, six volunteers, two repeated measurements).
The images obtained by planning-free vessel-encoded p-CASL were used for delineation of flow territories of the ICAs and the BA. Flow territories were defined by averaging the dynamic scans with equal spatial encoding of labeling, yielding four selectively or globally labeled perfusion-weighted images and one control image. The (selectively) labeled images were subtracted from the control image, resulting in one non-selective perfusion image and three selective perfusion maps. Relative labeling efficiency was calculated in all voxels and flow territories were identified using k-means clustering, as outlined by Wong and Kansagra (2008). Additionally, spatial voxel information was included as a feature in the clustering algorithm, in accordance with the K-Nearest Neighbor-based classification technique described by Anbeek et al (2004) (Gevers et al, 2009b).
Perfusion-weighted images and flow territories were transformed into anatomical space by 3D affine registration on gray matter masks of corresponding anatomical scans. After segmentation of perfusion-weighted images into flow territories, global cerebral perfusion and flow territory perfusion were quantified. Subsequently, all anatomical data were transformed into standard space. This yielded two transformation matrices per ASL scan, which were used to transform all 36 sets of individual dynamics into standard space to allow for voxelwise data comparison. Gaussian smoothing (full width at half maximum=6 mm) was used on the transformed dynamics to decrease the effect of registration mismatches.
Equilibrium Magnetization
Quantification of absolute blood flow by ASL requires an estimation of the equilibrium magnetization of arterial blood (M0a). In this study, we used the procedure outlined by Chalela et al (2000):
 |
in which Scsf is the signal intensity of cerebrospinal fluid in a manually defined ventricular region, λα is the mL of water per mL of arterial blood (0.76), TR is the sequence repetition time, T1csf is the relaxation rate of CSF (4.2 seconds) (Chalela et al, 2000). The M0a values were calculated for all individuals and for both head coils separately and scaled for relative voxel volumes. A mean M0a value per coil and imaging center was used in further perfusion estimates.
Quantification
We used the following models in the quantification of perfusion measured by the different ASL techniques:
- After calculation of M0a, perfusion measured by CASL and p-CASL was quantified using the equation outlined by Chalela et al (2000):
where f is the flow in mL per g per second, ΔM is the difference between the control and labeled image intensities, w is the post-labeling delay, T1a is the T1 of arterial blood (1.6 seconds), TE is the echo time, T2a is the T2 of arterial blood (130 ms for CASL), ρ is the density of brain tissue (1.05 g/mL) and α the labeling efficiency (0.68 for CASL and 0.85 for p-CASL). As p-CASL and PASL use gradient echo readout instead of spin echo readout, T2a was replaced by T2*a (T2* of arterial blood, 50 ms; St Lawrence and Wang, 2005; Uludag et al, 2009; Dai et al, 2008).
- For single TI PASL, a similar model was used.
with α, 1.0 for PASL and τ, the temporal bolus width (600 ms; Wong et al, 1998).
- For multi TI PASL iteratively fitted to the model described by Buxton et al (1998), with adaptations for multi TI PASL sequences proposed by Gunther et al (2001):

with θ, flip angle and  the longitudinal relaxation time of brain tissue, λ, the blood–brain partition coefficient (0.9), ΔTI, the time between two successive Look–Locker pulses, and Δt, the transit time.
the longitudinal relaxation time of brain tissue, λ, the blood–brain partition coefficient (0.9), ΔTI, the time between two successive Look–Locker pulses, and Δt, the transit time.
Statistical Analysis
Perfusion data: Boxplots and histograms were used to examine the distribution of perfusion data (not shown here). Data were normally distributed. The mean value (MV), standard deviation (s.d.), and corresponding coefficient of variability (CV), defined as CV=100% (s.d./MV), were calculated for mean whole-brain CBF measured at different sites using different ASL techniques. Differences between mean whole-brain CBF measured at different sites were tested using paired t-tests (SPSS 16.0.2 Statistics UK). Differences were regarded as significant if P<0.05.
Quantitative reproducibility analysis: Different ASL methods yielded different mean CBF values, as measured over all subjects and sessions. To be able to compare the reproducibility measures of the different ASL methods, the mean CBF differences were removed by scaling mean whole-brain CBF—as calculated over all subjects and sessions—to 30 mL per 100 g/min. This was carried out for all ASL techniques. Reproducibility analyses were performed for whole-brain perfusion measurements and for flow territory perfusion measurements. Reproducibility for intra- and multicenter perfusion measurements was analyzed using Bland–Altman plots, the coefficient of repeatability (CR), and the repeatability index. In Bland–Altman plots, the difference between two measurements was plotted against the mean from two measurements, showing the agreement between two measurements. From the difference between repeated acquisitions, the mean difference and the standard deviation of the difference (s.d.Δ) were calculated. Intra- and multicenter reproducibility were then expressed in terms of (Bland and Altman, 2010; Bland and Altman, 1996):
The CR, defined as the 95% confidence limits for the difference between repeated measurements is given by: CR=1.96s.d.Δ
The repeatability index (RI): RI=100% (CR/MV)
The difference between intra- and multicenter reproducibility measures was tested using the F-test of equality of two variances (s.d.Δ of intra- and multicenter repeated measurements).
Qualitative reproducibility analysis: For the evaluation of regional variability patterns, global perfusion differences were removed by scaling mean whole-brain perfusion for each ASL scan (after averaging over repeated measurements) to 30 mL per 100 g/min. Subsequently, a voxelwise one-way analysis of variance was performed, using analysis of variance (ANOVA). This analysis was performed for intra- and intersession perfusion data. Intrasession variability reflects perfusion variability in one voxel during one session. Intersession variability reflects perfusion variability in one voxel between sessions. To assess intrasession perfusion variability, the analysis was performed on the signal intensities in all voxels of 40 paired subtractions of labeled and control images (subtractions C−L). To assess intersession perfusion variability, the analysis was performed on the signal intensities in all voxels of 36 perfusion-weighted scans (means of subtractions C−L; Figure 1). The results of the voxelwise analyses were depicted in brain maps reflecting the standard deviations (s.d.s) of measured perfusion values in all voxels in the brain. Data from multi TI PASL were not used in this analysis, as no perfusion estimates can be obtained from single dynamics.
Figure 1.
Voxel-based analysis of variability. ANOVA, analysis of variance; CBF, cerebral blood flow; s.d, standard deviation.
Results
Perfusion Data
A representative example of the flow territories defined by selective ASL and of perfusion-weighted images gathered by the different ASL techniques is shown in Figure 2.
Figure 2.
Flow territories, as defined by planning-free selective arterial spin labeling (ASL; first row) and perfusion-weighted images obtained by pseudo-continuous ASL (p-CASL) with and without background suppression, single inversion time pulsed ASL (TI PASL), multi TI PASL and continuous ASL (CASL).
Figure 3 shows whole-brain perfusion values and corresponding s.d.s, measured by all ASL techniques. Mean whole-brain CBF was 23.6 to 40.5 mL per 100 g/min (25.3±4.2, 23.6±4.4, 30.7±10.0, 26.3±6.6, and 40.5±9.5 mL per 100 g/min for p-CASL with and without BS, CASL, single and multi TI PASL, respectively). For both CASL and single TI PASL, mean whole-brain CBF differed significantly between one of the imaging sites and the other two imaging sites. Pseudo-continuous ASL with background suppression and multi TI PASL perfusion data were less variable; however, for both a significant difference was found between values measured at two sites. Pseudo-continuous ASL without background suppression varied least between imaging sites. CVs for whole-brain CBF measurements by different ASL techniques at different sites are presented in Table 1. Lowest CVs were found for p-CASL with background suppression. CVs also indicated that data dispersion, especially of CASL and PASL, varied per imaging center. Highest CVs were found for CASL and single TI PASL data gathered at imaging center II. Mean CBF measured by CASL and single TI PASL also differed significantly between center II and the other two centers (Figure 3). Probably, this imaging center had an important role in CASL and single TI PASL reproducibility values reported in this study.
Figure 3.
Mean cerebral blood flow (CBF) per arterial spin labeling (ASL) method and imaging site. Error bars represent corresponding standard deviations (s.d.). A significant difference between perfusion values measured at one site and the other two sites was found for continuous ASL (CASL) and single inversion time pulsed ASL (TI PASL; as indicated by the double asterisk). A significant difference between perfusion values measured at one site and one other site was found for pseudo-continuous ASL (p-CASL) with background suppression and multi TI PASL (as indicated by the single asterisk).
Table 1. Coefficients of variability (CV) of perfusion measurements by different ASL techniques measured at three imaging centers.
| Center | p-CASL | p-CASL no background suppression | CASL | Single TI PASL | Multi TI PASL |
|---|---|---|---|---|---|
| I | 17.7 | 19.3 | 26.3 | 15.4 | 20.9 |
| II | 12.5 | 14.9 | 44.4 | 22.7 | 18.4 |
| III | 19.1 | 22.2 | 26.2 | 14.1 | 30.1 |
ASL, arterial spin labeling; CASL, continuous ASL; p-CASL, pseudo-continuous ASL; TI PASL, inversion time pulsed ASL.
Quantitative Reproducibility Analyses
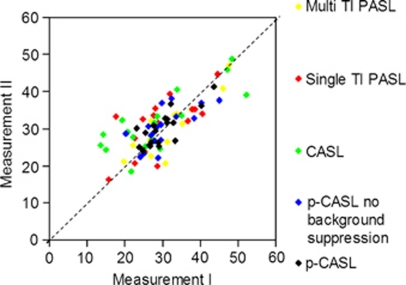
Intra- and multicenter perfusion differences plotted against mean perfusion were randomly distributed and showed no dependency on mean perfusion, as can be seen in the Bland–Altman plots shown in Figure 4. Test–retest values plotted in Figure 5 are around the line of equality of the repeated measurements, indicating that there was a good correlation between the first and the second measurement (Figure 5).
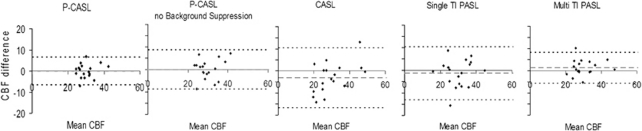
Figure 4.
Bland–Altman plots showing the difference between intracenter repeated cerebral blood flow (CBF) measurements and mean CBF (mL per 100 g/min). Dotted lines indicate the mean CBF difference to be ±1.96 s.d. of the difference between repeated measurements (i.e., the confidence interval for the difference between repeated measurements). Of the differences between repeated measurements, 95% will be between the dotted lines. CASL, continuous arterial spin labeling; p-CASL, pseudo-continuous arterial spin labeling; s.d., standard deviation; TI PASL, inversion time pulsed arterial spin labeling.
Figure 5.
Test–retest perfusion values measured using different arterial spin labeling (ASL) sequences (mL per 100 g/min). Values are centered around the line of equality, indicating good correlation between two measurements. CASL, continuous ASL; p-CASL, pseudo-continuous ASL; TI PASL, inversion time pulsed ASL.
The results of our quantitative reproducibility analysis are presented in Table 2. Pseudo-continuous ASL-based sequences showed less variability than CASL and single and multi TI PASL, and did clearly benefit from background suppression pulses. On the basis of the CRs and RIs presented here, one can be 95% sure that multicenter test–retest differences will be <20%. Intra- and multicenter reproducibility were comparable for all ASL methods, except for single TI PASL. For single TI PASL, intracenter reproducibility was significantly better than multicenter reproducibility for whole-brain measurements and measurements in the left ICA flow territory (P<0.05), whereas intra- and multicenter reproducibility were comparable for the flow territories of the right ICA and the BA.
Table 2. Coefficients of repeatability (CR, mL per 100 g/min) and repeatability indices (RI, %) for whole brain (WB) and flow territory data (left internal carotid artery (L-ICA) and right internal carotid artery (R-ICA) and basilar artery (BA)).
| p-CASL | p-CASL no background suppression | CASL | Single TI PASL | Multi TI PASL | |
|---|---|---|---|---|---|
| WB | |||||
| Intracenter | |||||
| CR | 6.6 | 8.8 | 13.6 | 11.8 | 6.4 |
| RI | 22.0 | 29.2 | 45.3 | 39.4 | 23.1 |
| Multicenter | |||||
| CR | 5.4 | 7.4 | 13.7 | 19.6 | 7.8 |
| RI | 18.0 | 24.9 | 45.7 | 65.5 | 25.9 |
| L-ICA | |||||
| Intracenter | |||||
| CR | 10.6 | 14.1 | 17.5 | 17.0 | 11.9 |
| RI | 22.7 | 30.0 | 42.1 | 41.4 | 26.7 |
| Multicenter | |||||
| CR | 8.3 | 10.2 | 18.7 | 36.1 | 13.0 |
| RI | 17.6 | 21.8 | 45.1 | 88.2 | 29.2 |
| R-ICA | |||||
| Intracenter | |||||
| CR | 10.2 | 12.7 | 20.1 | 22.2 | 9.2 |
| RI | 21.6 | 26.9 | 48.2 | 57.0 | 20.4 |
| Multicenter | |||||
| CR | 8.5 | 12.3 | 16.7 | 33.1 | 11.6 |
| RI | 18.0 | 26.1 | 40.0 | 84.9 | 25.9 |
| BA | |||||
| Intracenter | |||||
| CR | 11.1 | 16.4 | 19.4 | 34.6 | a |
| RI | 28.2 | 38.0 | 47.0 | 45.1 | a |
| Multicenter | |||||
| CR | 9.1 | 13.0 | 21.0 | 37.2 | a |
| RI | 23.3 | 30.4 | 51.0 | 48.4 | a |
CASL, continuous arterial spin labeling; p-CASL, pseudo-continuous arterial spin labeling; TI PASL, inversion time pulsed arterial spin labeling.
The posterior circulation was not included in the imaging volume used in multi TI PASL.
Qualitative Reproducibility Analysis
Mean perfusion-weighted images and s.d. maps reflecting regional variability patterns within and between imaging sessions, are shown in Figure 6. The mean perfusion-weighted images show apparent hyperperfusion of vascular regions in perfusion-weighted images obtained by all ASL methods. High signal intensity was also apparent in the posterior region imaged by single TI PASL, possibly because of the larger labeling volume for the posterior circulation and the fact that no QUIPSS pulses or vascular crushers were used in this study (Wong et al, 1998).
Figure 6.
Perfusion images registered to standard space with corresponding s.d. maps reflecting regional perfusion variability within and between imaging sessions. CASL, continuous arterial spin labeling; p-CASL, pseudo-continuous arterial spin labeling; s.d., standard deviation; TI PASL, inversion time pulsed arterial spin labeling.
Within-session s.d. maps showed that regional variability was smallest in p-CASL-based sequences. The use of background suppression further reduced regional variability. In single TI PASL s.d. maps, variability was largest in the brain region supplied by the BA. In CASL, obtained with a transmit/receive head coil, variability was largest in the frontal area. For all ASL techniques, most intrasession variability was seen in vascular regions.
Between-session s.d.s were smaller than within-session s.d.s. The s.d. maps for p-CASL-based sequences showed less variability than s.d. maps for single TI PASL and CASL. Again, in single TI PASL s.d. maps, variability was largest in the posterior region. In CASL, largest variability was seen in the frontal area and in vascular regions. For all ASL techniques, intersession variability was largest in gray matter.
Discussion
To our knowledge, this study is the first to assess intra- and multicenter reproducibility of main ASL methods for measuring cerebral perfusion, by scanning the same subjects multiple times and at multiple sites. This study was also the first to assess regional variability patterns of ASL methods.
The results of this study are twofold. First, perfusion values obtained by p-CASL with background suppression showed least data dispersion and best reproducibility measures for intra- and multicenter gathered data. Reproducibility analyses of p-CASL without background suppression and multi TI PASL yielded similar results. Reproducibility within and between imaging sites was comparable for all methods, except for single TI PASL, which showed better intracenter reproducibility. On the basis of the reproducibility measures presented in Table 2, the use of reference values could be considered, with within-subject test–retest values deviating by <20% considered to be normal.
Second, regional variability patterns showed that for all ASL methods, within-session variance is largest in vascular regions and between-session variance is largest in gray matter. Knowledge on regional variability patterns in ASL perfusion imaging methods could be of value in the diagnostic use of perfusion-weighted images, and could also reveal the origin of observed signal fluctuations.
Quantitative intra- and multicenter reproducibility data show a worse performance of CASL and single TI PASL sequences than previously published data on reproducibility of these techniques (Floyd et al, 2003; Gevers et al, 2009a; Hermes et al, 2007; Jahng et al, 2005; Parkes et al, 2004). Looking at mean CBF values and the amount of data dispersion, as indicated by the CVs, we noticed that CASL and single TI PASL data from one site were largely responsible for this discrepancy. This might illustrate that CASL and single TI PASL are more sensitive to site-specific differences, for example, scanner hardware instability and B0-homogeneity. Reproducibility measures of the multi TI PASL sequence were comparable with data described in the multicenter QUASAR reproducibility study performed by Petersen et al (2010), although the latter study was performed worldwide in a very large population. As the used single and multi TI PASL sequences use equal labeling modules, this implies that variations in labeling efficiency of single TI PASL probably cannot explain the observed differences in reproducibility. Our results underline the improved accuracy of multi TI PASL, being less dependent on model assumptions for the arterial transit time compared with single TI PASL (Petersen et al, 2006; van Osch et al, 2007).
The s.d. maps representing variability within imaging sessions showed most variability in regions of brain-feeding arteries. Small hotspots in these areas observed in single subject or in group analysis, should therefore not be considered as evidence for local cerebral pathology. Two different processes can explain these variations in ASL signal near the vessels. First, these variations can arise from the presence of residual label inside the arterial vasculature. The amount of residual label might vary, depending on, for example; the cardiac cycle, leading to the observed variations. Second, the signal intensity within the vessels can be large because of fresh inflow of spins, which will lead to relative larger subtraction errors and, therefore, more variance in the ASL data. The fact that the variance around the vessels is highly reduced in the p-CASL scan with background suppression seems to imply that fresh inflow of arterial blood has the most important role, as background suppression pulses will decrease the relative signal intensity of blood, thereby limiting the subtraction errors. The use of bipolar crushers could further reduce both types of vascular artifacts.
In p-CASL, the use of additional background suppression pulses clearly reduced regional variability within sessions by removing short-term physiologic noise. The effect on long-term noise turned out to be considerably smaller. Continuous ASL (CASL) showed a different variability pattern than all other ASL methods; this could be attributed to the different transmit B1 properties of the transmit–receive head coil, as compared with the body coil. Regional variability maps elucidated that CASL variability varied in the anterior–posterior direction. This pattern might be caused by inhomogeneity in the B1 field of the transmit–receive head coil, which resulted in a more stable ASL signal in the posterior region of the brain, positioned closest to the head coil.
The s.d. maps representing variability between imaging sessions, showed most variability in gray matter. This is explained by the physiologic variation in cerebral perfusion between sessions that were separated by a 1- to 3-week time interval. This is supported by the fact that all ASL methods showed similar patterns, and that the use of background suppression did not alter the observed pattern. However, single TI PASL showed much higher variability in the posterior flow territory. The fact that the higher variations are specific to the complete posterior territory is highly suggestive for fluctuations in the labeling efficiency of the blood in the BA and vertebral arteries between sessions. Such fluctuations could be explained by different planning of the labeling stack: Although differences with respect to the straight vessels of the carotid system will always result in a similar amount of labeled blood, similar changes with respect to the posterior circulation might result in a more drastic change of the amount of created label.
Stability of labeling efficiency could be responsible for the difference between regional variability in CASL and p-CASL. The small variability between sessions, which is remaining in p-CASL, is probably of physiologic origin. It clearly shows gray–white matter differentiation, leading to the assumption that this variability is purely caused by alterations in neuronal functioning at the time of imaging. Theoretically, the between-session variation would be zero if only a global perfusion difference was present on each occasion. We do not expect that this variation will decrease with better readout sequences, as background suppression has no effect, suggesting that most of this variation has a physiologic origin. For CASL and single TI PASL, however, one can assume that in the between-session variation, a large portion of variability is due to scanning variations (mostly variations in labeling efficiency). In general, the reported between-session s.d.s are smaller than the within session s.d.s, as global variability is removed by scaling of the data and because convergence of the measured signal causes large variability in the within-session data that can no longer be observed in the between-session data.
This study suffered from several limitations. First of all, this study was performed in a small subset of healthy volunteers. Future studies in patient populations and larger groups of healthy individuals are to be expected. A considerable amount of data sets (n=36) was acquired, however, by scanning all subjects multiple times. Therefore, the sample size was effectively larger as all calculations were based on 18 differences. In addition, no physiologic monitoring was performed that would have allowed for selection of a more homogenous population and conditions. The latter could have resulted in even better reproducibility of studied ASL sequences. Another limitation of this study was the use of a single field strength and scanner brand. 3T scanners are becoming more widely available and 3T is promising for clinical use of ASL. One might also assume a certain similarity of MR scanners provided by different vendors (Wong et al, 1998; Petersen et al, 2010). Imaging parameters were furthermore applied, as previously used in ongoing clinical studies within the participating imaging centers. Different parameter choices might of course have rendered different results (for instance, a relatively long echo time was used in the single TI PASL sequence and crusher gradients were not used). We indeed noticed that the within-session s.d. in a single subject improved only slightly if a shorter TE was used or if vascular crushers were added, underlining that the large differences found between p-CASL and single TI PASL are not solely attributable to our study design choices. Finally, we choose to evaluate frequently used ASL methods. We recognize the infinite range of ASL sequences; however, it was impossible to test all of them. Still, with the comparison of five ASL methods within and between different imaging centers, this study shows the possibilities and variability in CBF values when clinical multicenter studies are performed with similar or different ASL methods. In addition to previous ASL reproducibility studies, such as the worldwide QUASAR study that has been the first to overcome the limitation of small sample sizes, this study in which healthy subjects were scanned multiple times and at multiple sites gives a very strong support for the reliability of current state-of-the-art ASL techniques.
In conclusion, both intra- and multicenter reproducibility showed reasonable results for all ASL methods. Intra- and multicenter reproducibility were comparable for all methods but single TI PASL. On the basis of these results, one may expect that even multicenter differences between within-subject repeated measurements are within 20%, a finding that might enable the use of reference values in clinical routine, with perfusion data deviating <20% over repeated imaging sessions within the same subjects considered normal. Regional variability patterns showed that variability within sessions is mostly seen around brain-feeding vasculature and that variability between sessions mostly reflects neuronal activity. Knowledge on the distribution of variability allows for the use of perfusion-weighted images in the assessment of local cerebral pathology.
The authors declare no conflict of interest.
References
- Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208:410–416. doi: 10.1148/radiology.208.2.9680569. [DOI] [PubMed] [Google Scholar]
- Anbeek P, Vincken KL, van Osch MJ, Bisschops RH, van der Grond J. Probabilistic segmentation of white matter lesions in MR imaging. Neuroimage. 2004;21:1037–1044. doi: 10.1016/j.neuroimage.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud. 2010;47:931–936. [PubMed] [Google Scholar]
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab. 1999;19:701–735. doi: 10.1097/00004647-199907000-00001. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de BC, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53:392–398. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging. 2003;18:649–655. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- Gevers S, Majoie CB, van den Tweel X, Lavini C, Nederveen AJ. Acquisition time and reproducibility of continuous arterial spin-labeling perfusion imaging at 3T. AJNR Am J Neuroradiol. 2009a;30:968–971. doi: 10.3174/ajnr.A1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers S, Nederveen AJ, Hendrikse J, Bokkers RP, Kies DA, Teeuwisse WM, Majoie CB, van Osch MJ. Reproducibility of flow territories defined by plannings-free vessel encoded pseudo-continuous arterial spin labeling. Presented at ISMRM, 17th Scientific Meeting, Honolulu. 2009b. p. 1524.
- Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Top Magn Reson Imaging. 2004;15:10–27. doi: 10.1097/00002142-200402000-00003. [DOI] [PubMed] [Google Scholar]
- Golay X, Petersen ET, Hui F. Pulsed star labeling of arterial regions (PULSAR): a robust regional perfusion technique for high field imaging. Magn Reson Med. 2005;53:15–21. doi: 10.1002/mrm.20338. [DOI] [PubMed] [Google Scholar]
- Gunther M, Bock M, Schad LR. Arterial spin labeling in combination with a look-locker sampling strategy: inflow turbo-sampling EPI-FAIR (ITS-FAIR) Magn Reson Med. 2001;46:974–984. doi: 10.1002/mrm.1284. [DOI] [PubMed] [Google Scholar]
- Hermes M, Hagemann D, Britz P, Lieser S, Rock J, Naumann E, Walter C. Reproducibility of continuous arterial spin labeling perfusion MRI after 7 weeks. MAGMA. 2007;20:103–115. doi: 10.1007/s10334-007-0073-3. [DOI] [PubMed] [Google Scholar]
- Jahng GH, Song E, Zhu XP, Matson GB, Weiner MW, Schuff N. Human brain: reliability and reproducibility of pulsed arterial spin-labeling perfusion MR imaging. Radiology. 2005;234:909–916. doi: 10.1148/radiol.2343031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Petersen ET, Lim T, Golay X. Model-free arterial spin labeling quantification approach for perfusion MRI. Magn Reson Med. 2006;55:219–232. doi: 10.1002/mrm.20784. [DOI] [PubMed] [Google Scholar]
- Petersen ET, Mouridsen K, Golay X. The QUASAR Reproducibility Study, Part II: results from a multi-center arterial spin labeling test-retest study. Neuroimage. 2010;49:104–113. doi: 10.1016/j.neuroimage.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Lawrence KS, Wang J. Effects of the apparent transverse relaxation time on cerebral blood flow measurements obtained by arterial spin labeling. Magn Reson Med. 2005;53:425–433. doi: 10.1002/mrm.20364. [DOI] [PubMed] [Google Scholar]
- Uludag K, Muller-Bierl B, Ugurbil K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage. 2009;48:150–165. doi: 10.1016/j.neuroimage.2009.05.051. [DOI] [PubMed] [Google Scholar]
- van Osch MJ, Hendrikse J, van der Grond J. Sensitivity comparison of multiple versus single inversion time pulsed arterial spin labeling fMRI. J Magn Reson Imaging. 2007;25:215–221. doi: 10.1002/jmri.20823. [DOI] [PubMed] [Google Scholar]
- Wang J, Licht DJ.2006Pediatric perfusion MR imaging using arterial spin labeling Neuroimaging Clin N Am 16149–167.ix [DOI] [PubMed] [Google Scholar]
- Wang Z, Fernandez-Seara M, Alsop DC, Liu WC, Flax JF, Benasich AA, Detre JA. Assessment of functional development in normal infant brain using arterial spin labeled perfusion MRI. Neuroimage. 2008;39:973–978. doi: 10.1016/j.neuroimage.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magn Reson Med. 2007;58:1086–1091. doi: 10.1002/mrm.21293. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Wong EC, Kansagra AP. Mapping Middle Cerebral Artery Branch Territories with Vessel Encoded Pseudo-Continuous ASL: Sine/Cosine Tag Modulation and Data Clustering in Tagging Efficiency Space. Presented at ISMRM 16th Scientific Meeting, Toronto. 2008. p. 182.
- Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- Ye FQ, Frank JA, Weinberger DR, McLaughlin AC. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST) Magn Reson Med. 2000;44:92–100. doi: 10.1002/1522-2594(200007)44:1<92::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]