Abstract
While oral anticoagulants are associated with greater hematoma expansion and higher mortality rates in patients with intracerebral hemorrhage (ICH), there is ongoing discussion whether pretreatment with antiplatelet drugs also worsens prognosis. Using an experimental model of ICH, we investigated the effects of antiplatelet pretreatment on hematoma volume and functional outcome. CD-1 mice were treated with acetyl-salicylic acid (ASA, 60 mg/kg per 24 hours), clopidogrel (22.5 mg/kg per 24 hours), or both (ASA+clopidogrel) through drinking water for 3 days (n=20 per group). Thereafter, platelet aggregation was found to be significantly reduced. Untreated mice and mice pretreated with warfarin served as controls. A stereotactic injection of collagenase into the right striatum was used to induce ICH. Twenty-four hours after ICH induction, hematoma volume was measured to be 15.0±4.4 μL in controls, 14.1±5.3 μL in ASA mice, 16.8±5.1 μL in clopidogrel mice, and 16.4±5.1 μL in ASA+clopidogrel animals. These differences were not statistically significant. However, mice pretreated with warfarin revealed largely increased hematoma volumes (25.0±7.4 μL versus controls, P=0.001). Neurologic outcome was not different between antiplatelet-pretreated animals and untreated controls. Our results suggest that plasmatic coagulation rather than platelet function is the most critical element for preventing hematoma expansion in acute ICH. Future therapeutic strategies may take these findings into account.
Keywords: aspirin, clopidogrel, intracerebral hemorrhage, mice, platelets
Introduction
At present, up to one-third of all intracerebral hemorrhage (ICH) occurs in patients taking antiplatelet drugs (Foerch et al, 2006; Stead et al, 2010). While oral anticoagulants are known to be associated with larger hematoma volumes and higher mortality rates, there is ongoing controversy whether pretreatment with antiplatelet agents also worsens prognosis in patients with ICH (Wijman, 2009).
With platelets having a key role in preventing blood loss in response to vascular injury, it is reasonable to assume that impaired platelet function might lead to prolonged hematoma expansion. Accordingly, observational studies have indicated that previous use of platelet inhibitors in ICH is associated with higher mortality rates (Roquer et al, 2005; Saloheimo et al, 2006). Furthermore, reduced platelet activity may also be related to an increased early hematoma growth and a worse functional outcome at 3 months (Naidech et al, 2009; Toyoda et al, 2005). In contrast, several investigations have failed to identify the previous usage of antiplatelet agents as an independent risk factor of hematoma expansion and functional outcome in patients with ICH (Foerch et al, 2006; Moussouttas et al, 2010; Sansing et al, 2009).
A critical drawback of all of these clinical studies is their inability to sufficiently adjust for confounding variables interfering with the prognosis of the disease. Patients taking antiplatelet drugs are likely to be older, have more severe comorbidity, and are more frequently disabled before ICH than are patients without antiplatelet pretreatment. Data obtained from a large German stroke registry revealed that adjusting for age and premorbid disability has a critical effect on whether the association between previous antiplatelet intake and functional outcome after ICH is significant (Foerch et al, 2006).
We have previously shown that a well-controlled mouse model of collagenase-induced ICH is able to detect the detrimental effects of warfarin on ICH volume (Foerch et al, 2008, 2009). In this study, we used a similar model to compare the effects of acetyl-salicylic acid (ASA) and clopidogrel on hematoma development and neurologic outcomes after ICH induction.
Materials and methods
Animals and Antiplatelet Pretreatment
All experiments conformed to an institutionally approved protocol in accordance with the National Institute of Health's guide for the care and use of laboratory animals. For the entire study, male CD-1 mice (Charles River Laboratories, Wilmington, MA, USA) aged 12 to 16 weeks with a mean body weight of 40±2 g were used. This mouse strain has been successfully implemented in similar experimental studies investigating the effects of warfarin pretreatment on ICH volume (Foerch et al, 2008, 2009).
Mice were randomly assigned to four groups receiving placebo (untreated controls), ASA (Bayer Health Care, Morristown, NJ, USA), clopidogrel (Sanofi, Paris, France), or ASA+clopidogrel through dissolution in their drinking water (concentration: ASA 0.4 mg/mL, clopidogrel 0.15 mg/mL). Medication was applied for 72 hours. A water consumption of 15 mL/100 g per 24 hours was assumed providing an estimated daily intake of 60 mg/kg ASA and 22.5 mg/kg clopidogrel per mouse (Foerch et al, 2008). These dosages were selected based on previous experimental studies (Momi et al, 2005) and after a thorough evaluation of the effects of various concentrations of the antiplatelet agents on antiplatelet activity in mice, revealing that a further increase in the respective dosage did not decrease platelet activity any more (Supplementary Figures 1 and 2). Mice pretreated with warfarin (2 mg/kg body weight per 24 hours) having an international normalized ratio of 3.5±0.9 (Foerch et al, 2008, 2009) served as positive controls. All experimental procedures and outcome assessments were performed in a blinded manner.
Platelet Aggregation Measurements
Ex vivo platelet aggregation testing was performed. Whole blood (0.675 mL) was collected from deeply anesthetized mice by cardiac puncture using a 19-G needle. Blood volume from 4 mice (2.7 mL) per group was pooled into 3-mL tubes (BD Vacutainer, Franklin Lakes, NJ, USA) containing 0.3 mL of 3.2% sodium citrate. Platelet-rich plasma was prepared from these pooled samples by centrifugation at 250 g for 10 minutes. The platelet-rich plasma was then transferred to fresh plastic tubes. Platelet-poor plasma was obtained by centrifuging the remaining blood at 2,000 g for 10 minutes. Platelet count was measured using an ADVIA 120 (Bayer Health Care). Aggregation was initiated by adding 25 μL adenosine 5′-diphosphate (ADP, Helena Laboratories, Beaumont, TX, USA; category no. 5366) to 225 μL platelet-rich plasma for a final concentration of 20 μmol/L, or by adding 25 μL arachidonic acid (Helena Laboratories; category no. 5364) to 225 μL platelet-rich plasma for a final concentration of 500 μg/mL. Aggregation was analyzed using an aggregometer (AGGRAM Aggregometer, Helena Laboratories) by recording light transmission. Specimens were continuously mixed with a stir bar and kept at 37°C during aggregation measurements. This procedure was repeated once per group (i.e., aggregation measurements rely on a total of eight animals per group).
Determination of Platelet Adhesion In Vivo
In all, 11 mice per group were anesthetized with isoflurane (1.5% to 2.0%) in a nitrous oxide/oxygen mixture with spontaneous respiration. The distal tip of the tail was cut at 2 mm using a scalpel blade. The tail was immediately inserted into a tube containing 3 mL prewarmed (37°C) phosphate-buffered saline. After 3 minutes, the tube was vortexed for homogenization, and ultrasound was applied for 1 minute to lyse erythrocyte cell membranes. The solution was centrifuged for 30 minutes at 13,000 r.p.m. (4°C). A volume of 250 μL of the supernatant was transferred to 1,000 μL of Drabkin's reagent. After 15 minutes of incubation, a photometer was used to determine absorption rates at 540 nm. Blood volumes were calculated using a standard curve.
Intracerebral Hemorrhage Induction
Overall, 20 mice per group (i.e., untreated controls, ASA, clopidogrel, ASA+clopidogrel) and 4 warfarin-treated animals (positive controls) were anesthetized with isoflurane (1.5% to 2.0%). To maintain similar circulation conditions, the deepness of anesthesia was adjusted by achieving a reduction of 50% in respiratory rate (i.e., 80 to 100 breaths per minute). Body temperature was maintained using a heating lamp. A small burr hole was drilled, and a 32-G 0.5-μL injection needle (Hamilton, 7000 series, Hamilton, Reno, NV, USA) was slowly lowered into the right striatum (0.0 mm anterior and 2 mm lateral to the bregma at a depth of 3.5 mm). In all, 0.5 μL of saline containing 0.3125 Units collagenase VII-S (Sigma-Aldrich, St Louis, MO, USA) was administered over a period of 5 minutes. The needle was left in place for the subsequent 10 minutes and then slowly removed over a period of 5 minutes. The burr hole was sealed with bone wax and the scalp closed (Foerch et al, 2008, 2009). The surgical procedure lasted ∼35 minutes for each animal. Thereafter, animals were allowed to recover in their cages until outcome assessment after 24 hours.
Outcome Assessment
Twenty-four hours after ICH induction, mice typically show gait disturbances (particularly ‘circling' to the right). Thus, neurologic outcomes were rated on a 5-point ordinal scale primarily focusing on evaluating gait performance (0: no apparent deficit, 1: slight instability while walking without circling, 2: circling towards the right with some straight movement, 3: heavy circling towards the right without straight movement or no movement at all, and 4: deceased) (Foerch et al, 2008, 2009).
In addition, to evaluate balance disturbance, mice were placed on a ‘hanging wire' until they had achieved a firm grip with their paws (Foerch et al, 2008, 2009; Li et al, 2004). The period of time to falloff was recorded. A maximum of 60 seconds of hanging was allowed. This test was repeated 3 times for each mouse that survived the first 24 hours after induction of ICH.
Quantifying Intracerebral Hemorrhage Volume
After outcome assessment, mice underwent transcardial perfusion with 30 mL phosphate-buffered saline under deep anesthesia. After removal, their brains were placed in glass tubes containing 3 mL phosphate-buffered saline. After 30 seconds of homogenization using a disperser, ultrasound was applied for 1 minute to lyse erythrocyte cell membranes. The solution was centrifuged for 30 minutes at 13,000 r.p.m. (4°C). A volume of 250 μL of the supernatant was transferred to 1,000 μL of Drabkin's reagent. After 15 minutes of incubation, a photometer was used to determine absorption rates at 540 nm. Hematoma volumes were calculated using a standard curve (Foerch et al, 2008, 2009). Mice that died within 24 hours of hemorrhage induction could not undergo transcardial perfusion before measurements. We determined 1.95±0.26 μL (mean±s.d.) to be the mean difference in blood volume between three unperfused and three perfused brains. Therefore, we subtracted 1.95 μL from the total hematoma volume that was calculated for these unperfused brains.
Statistical Analysis
We used SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis. Mean values in the platelet aggregation study were compared using the t-test. Tail-bleeding volume and ICH volume were compared between groups (control, ASA, clopidogrel, ASA+clopidogrel) by one-way ANOVA (analysis of variance) testing. The t-test was used for comparison of ICH volume between controls and warfarin-treated mice. Statistical analysis of nonparametric outcome data was performed by the Kruskal–Wallis test.
Results
Platelet Aggregation Testing
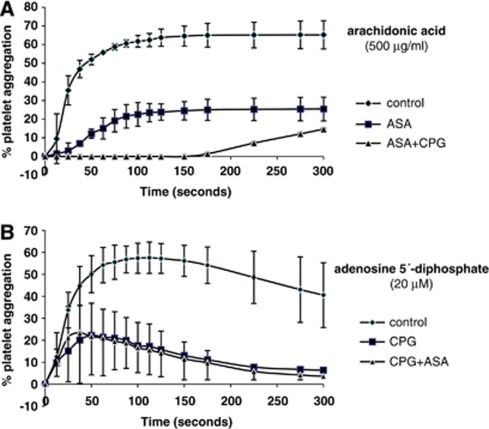
Pretreatment with ASA, clopidogrel, and ASA+clopidogrel reduced platelet aggregation (Figure 1). After 100 seconds, aggregation induced by arachidonic acid was 61.8%±3.1% (mean±s.d.) in controls, 22.5%±6.4% in ASA mice, and 0.1%±0.0% in ASA+clopidogrel mice (clopidogrel versus controls, P=0.016; ASA+clopidogrel versus controls, P=0.001). At the same time point, ADP-induced aggregation was 57.2%±6.9% in untreated controls, 17.6%±10.5% in clopidogrel mice, and 16.6%±11.2% in ASA+clopidogrel mice (clopidogrel versus controls, P=0.047; ASA+clopidogrel versus controls, P=0.048).
Figure 1.
Results of platelet aggregation testing. (A) Induction by arachidonic acid (500 μg/mL) for control mice, ASA mice, and ASA+CPG mice. (B) Induction by adenosine 5′-diphosphate (20 μmol/L) for control mice, CPG mice, and ASA+CPG mice (a total of 8 animals were tested per group). ASA, acetyl-salicylic acid; CPG, clopidogrel.
Bleeding volume after tail cutting was significantly different between groups (controls: 2.6±3.4 μL, ASA: 5.6±6.9 μL, clopidogrel: 10.8±5.9 μL, ASA+clopidogrel: 12.3±10.0 μL; one-way ANOVA, P=0.007; post hoc: ASA versus controls: P=1.000, clopidogrel versus controls: P=0.052, ASA+clopidogrel versus controls: P=0.013) (Figure 2).
Figure 2.
Tail-bleeding volumes measured by quantitative hemoglobin content determination in control mice, ASA mice, CPG mice, and ASA+CPG mice (n=11 per group). One-way ANOVA was used for comparison between treatment groups and controls. * Indicates significant difference. ANOVA, analysis of variance; ASA, acetyl-salicylic acid; CPG, clopidogrel.
Functional Outcome at 24 hours
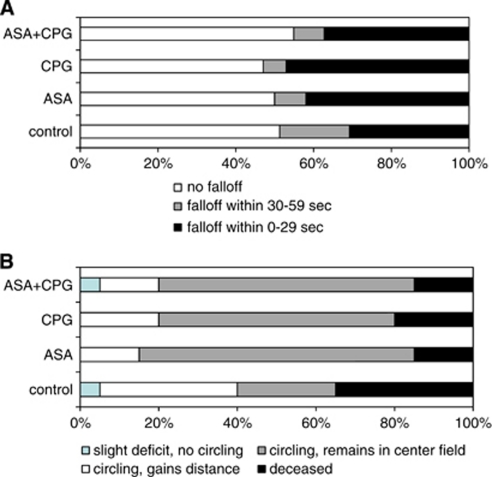
Mortality rate at 24 hours after ICH induction was 7 of 20 in controls, 3 of 20 in ASA mice, 4 of 20 in clopidogrel mice, and 3 of 20 in ASA+clopidogrel-treated animals. There were no statistically significant differences in both neurologic outcome and hanging wire performance between groups (Figure 3). One of four warfarin-treated mice (that served as positive controls) was found dead after 24 hours, and the other three animals were rated as ‘3' on the neurologic scale.
Figure 3.
Functional outcome. (A) Results of the hanging wire testing (% of attempts with three attempts per mouse). (B) Neurologic outcome as assessed on a 5-point scale for control mice, ASA mice, CPG mice, and ASA+CPG mice (% of animals, n=20 per group). Both for hanging wire testing and the neurologic scale, outcome was not different between groups. ASA, acetyl-salicylic acid; CPG, clopidogrel.
Hematoma Volume at 24 hours
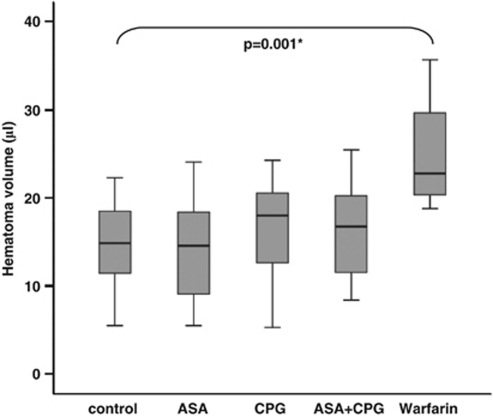
Twenty-four hours after ICH induction, the mean hematoma volume was 15.0±4.4 μL in controls, 14.1±5.3 μL in the ASA group, 16.8±5.1 μL in the clopidogrel group, and 16.4±5.2 μL in the group of animals that received both ASA and clopidogrel (n=20 per group). The between-group differences in hematoma volume were found to be not significant (one-way ANOVA, P=0.300; Figure 4). Excluding those animals that died within 24 hours did not change the results (P=0.428). In contrast, animals pretreated with warfarin as a positive control (n=4) had significantly larger bleeds than did untreated control mice (25.0±7.4 μL; t-test: controls versus warfarin, P=0.001). Correlation between hematoma volume and functional outcome at 24 hours was found to be significant (Spearman's rank, P=0.009).
Figure 4.
Hematoma volume determined 24 hours after hemorrhage induction by means of quantitative hemoglobin content determination for untreated control mice, ASA mice, CPG mice, and ASA+CPG mice (n=20 per group). Warfarin mice served as a positive control (n=4). One-way ANOVA revealed no differences between treatment groups (ASA, CPG, ASA+CPG) and controls. The t-test was used for comparison between warfarin mice and controls. * Indicates significant differences. ANOVA, analysis of variance; ASA, acetyl-salicylic acid; CPG, clopidogrel.
Discussion
The main finding of our study is that pretreatment with antiplatelet drugs (such as ASA, clopidogrel) does not increase hematoma volume and does not worsen functional outcome in this experimental model of ICH.
We have recently described an experimental model of warfarin-associated ICH (Foerch et al, 2008). Warfarin pretreatment with international normalized ratio (INR) values of 3.5±0.9 at the time point of hemorrhage induction provoked a significant, ∼2.5-fold increase in hematoma volume after 24 hours as compared with mice with normal coagulation. Furthermore, in a similar model, we showed that rapid reversal of warfarin anticoagulation prevented from extensive hematoma formation (Foerch et al, 2009). These experimental data are consistent with many clinical observations reporting increased hematoma volumes in patients with anticoagulation-associated ICH (Steiner et al, 2006). With regard to antiplatelet pretreatment, clinical studies revealed much more controversial results (Wijman, 2009). Some authors reported a significant influence of antiplatelet pretreatment on hematoma growth and outcome (Naidech et al, 2009; Roquer et al, 2005; Saloheimo et al, 2006; Toyoda et al, 2005), whereas others did not (Foerch et al, 2006; Moussouttas et al, 2010; Sansing et al, 2009). Interestingly, adjusting for baseline factors such as premorbid disability and concomitant diseases seems to be a crucial factor (Foerch et al, 2006). Our experimental animal model allowed us to address this unsolved question while controlling for confounding variables. Our data undoubtedly strengthen the hypothesis that pretreatment with antiplatelet drugs does not increase hematoma volume and does not worsen functional outcome in acute ICH.
In our model, we tested ASA and clopidogrel as two commonly prescribed antiplatelet drugs. Both agents mostly make an impact on the extension phase of primary hemostasis. Cyclooxygenase-1 is inactivated by ASA resulting in decreased thromboxane (TXA2) generation in platelets. Thromboxane-2 activates platelets through the TXA2 receptor (TP). One of the substances secreted from dense granules of platelets is ADP, which activates platelets through the G protein-coupled receptors, P2Y1 and P2Y12. The active metabolite derived from clopidogrel in the liver blocks P2Y12. Both TXA2 and ADP work as positive feedback mediators in platelet activation.
Platelet aggregation testing confirmed that platelet activity was significantly reduced by ASA and clopidogrel in our model. Interestingly, although receiving the same dose of ASA, animals treated with both ASA and clopidogrel had less response to arachidonic acid than did those receiving ASA only. This may be attributed to an ASA-synergistic effect of clopidogrel on arachidonic acid-induced aggregation, recently described by Hobson et al (2009). Besides aggregation, platelet granule release and platelet adhesion also represent important facets of platelet function. Therefore, we used an in vivo model to investigate the effects of ASA and clopidogrel on tail-bleeding volumes.
Interestingly, besides measurable effects on platelet aggregation and tail-bleeding volumes, pretreatment with antiplatelet agents did not result in larger ICH volumes. Tail bleeding is also prolonged in animals receiving warfarin, correlating with INR. In this setting, prolonged bleeding times are associated with larger intracerebral hematoma volumes (Illanes et al, 2010). How can these findings be explained? Tail-cut bleeding differs in some ways from collagenase-induced ICH. By removing the tail tip, mainly venous vessels are cut, where low-shear conditions dominate. In arteries, shear-induced binding to the von Willebrand factor leads to an adhesion interaction that may result in aggregation (Reininger, 2008). High shear also causes enrichment of platelets at the boundaries of the vessels' diameter. In contrast, their allocation is more equal under venous conditions (Ruggeri, 2009). An impairment of platelet recruitment owing to ASA and clopidogrel in the tail-bleeding tests might therefore lead to increased blood loss. In addition, by cutting the tail tip, the protective lining of the tissue factor located in the extracellular matrix around the vessel is breached. The openings of the cut vessels in the tail lead mostly into the saline. The unrestrained blood flow has a diluting effect on mediators of hemostasis. The percentage of blood getting into contact with the subendothelial matrix and, thus, with a thrombogenic surface, is lower in comparison with ICH. Under these circumstances, higher rates of P2Y12 and TP activation might lead to a faster clot growth and therefore less bleeding as compared with treated animals.
Similarly, collagen present in the subendothelial matrix is another very important platelet activator. Under conditions with high amounts of ADP, TXA2, and thrombin, these mediators can be sufficient to recruit platelets independently of the presence of collagen (Brass, 2003). With tail bleeding, less activation of TP or P2Y12 owing to ASA and clopidogrel in combination with less collagen-dependent activation might lead to an augmented effect of the antiplatelet agents. This does not apply in ICH, whereas it might have a role in subarachnoid hemorrhage.
There are other agonists on G protein-coupled receptors of platelets, such as epinephrine, prostaglandin E2, and serotonin. These mediators may be weaker as compared with TXA2 and ADP, but amplify platelet responses to other activators (Offermanns, 2006). Both ASA and clopidogrel only minimally inhibit platelet activation by these other agonists. The same is true for thrombin, which is the strongest known platelet activator (Offermanns, 2006) and very important for arresting bleeding after trauma (Jennings, 2009).
Tissue-specific reactions to vascular injury should be considered as well. A meta-analysis of GpIIb/IIIa inhibitors in patients undergoing percutaneous coronary intervention (PCI) revealed that GpIIb/IIIa inhibitors involved higher rates of major bleeding, except intracranial bleeding (Boersma et al, 2002). Although the statistical power of this finding has been questioned, it is known that platelet-dependent mechanisms and coagulation interdigitate. The platelet surface has a pivotal role in the promotion and regulation of thrombin generation (Monroe et al, 2002). After P2Y12 blockage in rabbits, thrombin generation and thrombus size is decreased in experimental vessel wall injury of mesenteric arterioles (van Gestel et al, 2003). However, brain pericytes are also capable of providing the surface for the prothrombinase complex and, given normal concentrations of prothrombin, deliver very high rates of thrombin generation (Bouchard et al, 1997).
Taken together, our findings suggest that plasmatic coagulation and antiplatelet pathways differentially affect ICH. However, there are several important caveats in our experimental studies. First, we did not quantify platelet function in animals used later for ICH induction, as the blood collection procedure may have had an impact on hemostasis and blood pressure by itself. Thus, we were unable to directly assess whether a relation exists between impaired platelet function and ICH volumes. However, administering the antiplatelet agents ASA and clopidogrel through drinking water for 72 hours leads to well-reproducible reductions in platelet activity. Second, the chosen modus of ASA and clopidogrel administration by drinking water is simple, but reflects antiplatelet ‘loading' rather than low-dose and long-term treatment. If compared with humans, the applied dosage is higher than the standard treatment used for prevention. It is known that higher concentrations of ASA inhibit cyclooxygenase-2 in endothelial cells, thereby reducing prostacyclin-2 generation. Having opposite effects on platelet function and vascular tone to TXA2, prostacyclin-2 reduction leads to a prothrombotic status at the vessel wall. We do not believe that the ASA dose in this study was excessive because 10 μg/mL collagen aggregation was only slightly reduced (Supplementary Figure 3), similar to what is consistently observed in human patients receiving antithrombotic ASA doses (81 to 325 mg). However, collagen aggregation is reduced by an excessive aspirin dose (500 mg) (Roehm, 1995). Momi et al (2005) observed that even higher doses of ASA further prolonged bleeding time after tail transection. Furthermore, in human studies, bleeding time increase after different doses of ASA has not been found to be related to changes in the urinary excretion of prostaglandin (Perneby et al, 2006). Third, although the coagulation and fibrinolytic systems of mice are in many ways very similar to those of humans, there are some differences in the cellular part of hemostasis. Mice have approximately a four times higher platelet count than do humans. Although platelet morphologies are alike, murine platelets are only about half of the volume and have approximately only one-half the life span of human platelets. In contrary to human platelets, response to epinephrine, serotonin, and platelet-activating factor (PAF) seems to be lower in mice, and ADP does not seem to trigger the release reaction of dense granula (Tsakiris et al, 1999). Fourth, collagenase disrupts brain vessels rather unselectively. Thus, it may not accurately replicate the local arteriolar rupture in human ICH. Whether these differences interfere with hemostasis remains to be determined. Furthermore, collagenase induces an inflammatory reaction (NINDS ICH Workshop Participants). As hemostasis and inflammation are linked to each other (Smyth et al, 2009), our results may theoretically be influenced by the collagenase-induced inflammatory reaction on the one hand and the use of mice lacking major histocompatibility complex-like molecules on the surface of antigen-presenting cells (CD-1) on the other. This might be of particular relevance for studies investigating inflammatory reaction and blood–brain barrier damage after experimental ICH. Fifth, it should be emphasized that ICH during antiplatelet treatment occurs typically in elderly and comorbid patients, whereas animals used in this study were young and healthy. Age has to be considered as a relevant confounder in this context, because several investigations showed an age-related increase in platelet aggregation even under treatment with ASA or ASA+clopidogrel (Franchini, 2006; Koltai et al, 2008). Finally, it is also important to mention that we determined hematoma volumes at one time point only, i.e., 24 hours after ICH induction. This time point was chosen as a frequently used end point in clinical and experimental studies of ICH (Foerch et al, 2008; Mayer et al, 2008). No conclusions can be derived from our study regarding the effects of antiplatelet pretreatment on hematoma expansion over time.
In conclusion, the current medical literature is unclear regarding the influence of antiplatelet medication in acute ICH. Our study is the first to provide experimental evidence that ASA and clopidogrel pretreatment does not increase hematoma volume and does not worsen functional outcome in this scenario. It has been suggested that reduced platelet activity in acute ICH (e.g., resulting from antiplatelet pretreatment) may be increased rapidly by platelet transfusion (Naidech et al, 2009)). This topic is currently investigated in the PATCH (Platelet Transfusion in Cerebral Hemorrhage) study (de Gans et al, 2010) However, our results imply that potential hemostatic approaches to prevent hematoma expansion should rather concentrate on the coagulation side of hemostasis (Mayer et al, 2008).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported in part by NIH grants R37-NS37074, R01-NS56458, P01-NS55104.
Supplementary Material
References
- Boersma E, Harrington RA, Moliterno DJ, White H, Simoons ML. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. Lancet. 2002;360:342–343. doi: 10.1016/s0140-6736(02)09532-6. [DOI] [PubMed] [Google Scholar]
- Bouchard BA, Shatos MA, Tracy PB. Human brain pericytes differentially regulate expression of procoagulant enzyme complexes comprising the extrinsic pathway of blood coagulation. Arterioscler Thromb Vasc Biol. 1997;17:1–9. doi: 10.1161/01.atv.17.1.1. [DOI] [PubMed] [Google Scholar]
- Brass LF. Thrombin and platelet activation. Chest. 2003;124:18S–25S. doi: 10.1378/chest.124.3_suppl.18s. [DOI] [PubMed] [Google Scholar]
- de Gans K, de Haan RJ, Majoie CB, Koopman MM, Brand A, Dijkgraaf MG, Vermeulen M, Roos YB. PATCH: Platelet Transfusion in Cerebral Haemorrhage: study protocol for a multicentre, randomised, controlled trial. BMC Neurol. 2010;10:19. doi: 10.1186/1471-2377-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerch C, Arai K, Jin G, Park KP, Pallast S, van Leyen K, Lo EH. Experimental model of warfarin-associated intracerebral hemorrhage. Stroke. 2008;39:3397–3404. doi: 10.1161/STROKEAHA.108.517482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerch C, Arai K, Van Cott EM, van Leyen K, Lo EH. Rapid reversal of anticoagulation reduces hemorrhage volume in a mouse model of warfarin-associated intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29:1015–1021. doi: 10.1038/jcbfm.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerch C, Sitzer M, Steinmetz H, Neumann-Haefelin T. Pretreatment with antiplatelet agents is not independently associated with unfavorable outcome in intracerebral hemorrhage. Stroke. 2006;37:2165–2167. doi: 10.1161/01.STR.0000231842.32153.74. [DOI] [PubMed] [Google Scholar]
- Franchini M. Hemostasis and aging. Crit Rev Oncol Hematol. 2006;60:144–151. doi: 10.1016/j.critrevonc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hobson AR, Qureshi Z, Banks P, Curzen NP. Effects of clopidogrel on ‘aspirin specific' pathways of platelet inhibition. Platelets. 2009;20:386–390. doi: 10.1080/09537100903003227. [DOI] [PubMed] [Google Scholar]
- Illanes S, Zhou W, Heiland S, Markus Z, Veltkamp R. Kinetics of hematoma expansion in murine warfarin-associated intracerebral hemorrhage. Brain Res. 2010;1320:135–142. doi: 10.1016/j.brainres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102:248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- Koltai K, Feher G, Kenyeres P, Lenart I, Alexy T, Horvath B, Marton Z, Kesmarky G, Toth K. Relation of platelet aggregation and fibrinogen levels to advancing age in aspirin- and thienopyridine-treated patients. Clin Hemorheol Microcirc. 2008;40:295–302. [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- Momi S, Pitchford SC, Alberti PF, Minuz P, Del Soldato P, Gresele P. Nitroaspirin plus clopidogrel versus aspirin plus clopidogrel against platelet thromboembolism and intimal thickening in mice. Thromb Haemost. 2005;93:535–543. doi: 10.1160/TH04-07-0464. [DOI] [PubMed] [Google Scholar]
- Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381–1389. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- Moussouttas M, Malhotra R, Fernandez L, Maltenfort M, Holowecki M, Delgado J, Lawson N, Badjatia N. Role of antiplatelet agents in hematoma expansion during the acute period of intracerebral hemorrhage. Neurocrit Care. 2010;12:24–29. doi: 10.1007/s12028-009-9290-0. [DOI] [PubMed] [Google Scholar]
- Naidech AM, Jovanovic B, Liebling S, Garg RK, Bassin SL, Bendok BR, Bernstein RA, Alberts MJ, Batjer HH. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. 2009;40:2398–2401. doi: 10.1161/STROKEAHA.109.550939. [DOI] [PubMed] [Google Scholar]
- NINDS ICH Workshop Participants Priorities for clinical research in intracerebral hemorrhage: Report from a National Institute of Neurological Disorders and Stroke workshop. Stroke. 2005;36:e23–e41. doi: 10.1161/01.STR.0000155685.77775.4c. [DOI] [PubMed] [Google Scholar]
- Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- Perneby C, Wallen NH, Rooney C, Fitzgerald D, Hjemdahl P. Dose- and time-dependent antiplatelet effects of aspirin. Thromb Haemost. 2006;95:652–658. [PubMed] [Google Scholar]
- Reininger AJ. VWF attributes–impact on thrombus formation. Thromb Res. 2008;122 (Suppl 4:S9–S13. doi: 10.1016/S0049-3848(08)70028-8. [DOI] [PubMed] [Google Scholar]
- Roehm E. Low-dose aspirin inhibits arachidonic acid-precipitated platelet aggregation but not collagen-precipitated platelet aggregation. Am J Cardiol. 1995;76:637–638. doi: 10.1016/s0002-9149(99)80177-5. [DOI] [PubMed] [Google Scholar]
- Roquer J, Rodriguez Campello A, Gomis M, Ois A, Puente V, Munteis E. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol. 2005;252:412–416. doi: 10.1007/s00415-005-0659-5. [DOI] [PubMed] [Google Scholar]
- Ruggeri ZM. Platelet adhesion under flow. Microcirculation. 2009;16:58–83. doi: 10.1080/10739680802651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen ER, Hillbom M. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke. 2006;37:129–133. doi: 10.1161/01.STR.0000196991.03618.31. [DOI] [PubMed] [Google Scholar]
- Sansing LH, Messe SR, Cucchiara BL, Cohen SN, Lyden PD, Kasner SE. Prior antiplatelet use does not affect hemorrhage growth or outcome after ICH. Neurology. 2009;72:1397–1402. doi: 10.1212/01.wnl.0000342709.31341.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- Stead LG, Jain A, Bellolio MF, Odufuye AO, Dhillon RK, Manivannan V, Gilmore RM, Rabinstein AA, Chandra R, Serrano LA, Yerragondu N, Palamari B, Decker WW. Effect of anticoagulant and antiplatelet therapy in patients with spontaneous intra-cerebral hemorrhage: does medication use predict worse outcome. Clin Neurol Neurosurg. 2010;112:275–281. doi: 10.1016/j.clineuro.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke. 2006;37:256–262. doi: 10.1161/01.STR.0000196989.09900.f8. [DOI] [PubMed] [Google Scholar]
- Toyoda K, Okada Y, Minematsu K, Kamouchi M, Fujimoto S, Ibayashi S, Inoue T. Antiplatelet therapy contributes to acute deterioration of intracerebral hemorrhage. Neurology. 2005;65:1000–1004. doi: 10.1212/01.wnl.0000179178.37713.69. [DOI] [PubMed] [Google Scholar]
- Tsakiris DA, Scudder L, Hodivala-Dilke K, Hynes RO, Coller BS. Hemostasis in the mouse (Mus musculus): a review. Thromb Haemost. 1999;81:177–188. [PubMed] [Google Scholar]
- van Gestel MA, Heemskerk JW, Slaaf DW, Heijnen VV, Reneman RS, oude Egbrink MG. In vivo blockade of platelet ADP receptor P2Y12 reduces embolus and thrombus formation but not thrombus stability. Arterioscler Thromb Vasc Biol. 2003;23:518–523. doi: 10.1161/01.ATV.0000057809.32354.22. [DOI] [PubMed] [Google Scholar]
- Wijman CA. Is platelet activity important in acute intracerebral hemorrhage. Neurocrit Care. 2009;11:305–306. doi: 10.1007/s12028-009-9271-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.