Abstract
The ischemic tissue becomes acidic after initiation of anaerobic respiration, which may result in impaired tissue metabolism and, ultimately, in severe tissue damage. Although changes in the major cerebral metabolites can be studied using magnetic resonance (MR) spectroscopy (MRS)-based techniques, their spatiotemporal resolution is often not sufficient for routine examination of fast-evolving and heterogeneous acute stroke lesions. Recently, pH-weighted MR imaging (MRI) has been proposed as a means to assess tissue acidosis by probing the pH-dependent chemical exchange of amide protons from endogenous proteins and peptides. In this study, we characterized acute ischemic tissue damage using localized proton MRS and multiparametric imaging techniques that included perfusion, diffusion, pH, and relaxation MRI. Our study showed that pH-weighted MRI can detect ischemic lesions and strongly correlates with tissue lactate content measured by 1H MRS, indicating lactic acidosis. Our results also confirmed the correlation between apparent diffusion coefficient and lactate; however, no significant relationship was found for perfusion, T1, and T2. In summary, our study showed that optimized endogenous pH-weighted MRI, by sensitizing to local tissue pH, remains a promising tool for providing a surrogate imaging marker of lactic acidosis and altered tissue metabolism, and augments conventional techniques for stroke diagnosis.
Keywords: acute stroke, animal studies, brain ischemia, diffusion-weighted MRI, MRI
Introduction
The cerebral tissue undergoes a complex cascade of metabolic disturbances during acute ischemic stroke—among them are altered glucose and oxygen metabolism, tissue acidosis, adenosine triphosphate depletion, and ultimately, severe tissue damage (An et al, 2009; Astrup et al, 1981; Hossmann, 1994; Siesjo, 1992a). During ischemia, anaerobic respiration is initiated as an alternative to the disrupted oxidative metabolism pathway, which is inefficient for generating high-energy substrates and, because of the production of lactate as a byproduct, often leads to tissue acidosis (Paschen et al, 1992). Metabolic disruption may be further exacerbated by hypoperfusion and the reduced buffering capacity of biocarbonate at acidic pH, which may lead to further tissue acidification (Macdonald and Stoodley, 1998; Zauner et al, 2002). As sufficient energy metabolism is vital for cell viability and salvageability, it is important to characterize metabolic impairments by monitoring altered energy substrates and lactate concentration, neuronal biomarkers, and tissue pH/acidosis.
Magnetic resonance (MR) techniques including MR imaging (MRI) and MR spectroscopy (MRS) have greatly advanced our understanding of stroke pathophysiology (Guadagno et al, 2003; Kloska et al, 2010). In particular, perfusion- and diffusion-weighted MRI (PWI and DWI, respectively), two of the most widely used imaging techniques, can quantify tissue hemodynamic and microscopic structural changes. In addition, localized MRS (1H and 31P) can detect manifestations of altered tissue metabolism, such as lactate accumulation, glucose, N-acetylaspartate (NAA) and adenosine triphosphate depletion, and tissue acidosis. Nevertheless, conventional MR techniques are somewhat limited. In particular, PWI lesions often overestimate infarction volume, whereas early DWI lesions are reversible (Ringer et al, 2001). As such, the postulation that a PWI/DWI mismatch approximates the salvageable ischemic penumbra has to be carefully revised (Kidwell et al, 2003). Moreover, metabolic derangement in the DWI lesion has been found to be heterogeneous (Guadagno et al, 2006; Nicoli et al, 2003), although conversely, the sensitivity and spatiotemporal resolution of MRS or spectroscopy imaging are often not sufficient to routinely examine fast-evolving and heterogeneous ischemic lesions. Such limitations underscore the urgency for new techniques to rapidly and specifically demarcate the heterogeneous ischemic tissue's metabolic impairments and to augment conventional MRS/MRI techniques for stroke diagnosis.
As tissue acidosis is associated with anaerobic glycolysis, pH, as measured with biochemical analysis or 31P MRS has been shown as an indicator of altered tissue metabolism and helps to predict tissue outcome (Hata et al, 1998; Tomlinson et al, 1993). As such, it remains important to develop noninvasive in vivo pH MRI techniques to image tissue acidosis. As the chemical exchange between amide protons and bulk tissue water is pH dependent, it has been shown that amide proton chemical exchange saturation transfer (CEST) MRI is capable of assessing tissue pH (Jokivarsi et al, 2007; Sun et al, 2007a; Sun and Sorensen, 2008; Ward and Balaban, 2000; Zhou et al, 2003). We have shown that pH-weighted amide proton transfer (APT) MRI is capable of detecting an ischemic lesion, even before diffusion abnormality (Sun et al, 2007b). In this study, we investigated ischemic tissue damage using multiparametric MRI techniques, including perfusion, diffusion, pH and relaxation MRI, and localized 1H MRS. We evaluated whether pH-weighted MRI can serve as a surrogate metabolic imaging marker for lactic acidosis, complementing commonly used stroke MRI and MRS scans.
Materials and methods
Animal Model
Animal experiments were carried out in accordance with institutional guidelines, as approved by the SRAC, MGH (Subcommittee on Research Animal Care, Massachusetts General Hospital). Adult male Wistar rats (n=12, weighing 312±41 g, Charles River Laboratory, Wilmington, MA, USA) were anesthetized with 1% to 1.5% isoflurane during surgery and MRI. Overall, 2 animals died during the MRI scan and 10 rats (n=10) were included in data analysis. The animal's core temperature was maintained using a circulating warm water jacket positioned around its torso. We induced standard permanent middle cerebral artery occlusion (MCAO) by inserting a 4-0 nylon suture into the lumen of the internal carotid artery to block the origin of the MCA. We also monitored the animal's heart rate and blood oxygen saturation (SpO2) online during MRI (Nonin Pulse Oximeter 8600; Nonin, Plymouth, MN, USA). In addition, we measured plasma glucose levels immediately before MCAO (Bayer Contour Meter, Tarrytown, NY, USA). Animal body weight, glucose level, typical SpO2, and heart rate are listed in Table 1.
Table 1. A total of 12 animals received permanent filament MCAO surgery, and scanned for multiparametric MRI and 1H MRS.
| Animal counts | Body weight (g) | Glucose (mg/dL) | SpO2 (%) | Heart rate (beats/min) | Inclusion/exclusion |
|---|---|---|---|---|---|
| 1 | 340 | — | 97 | 463 | + |
| 2 | 334 | — | 94 | 480 | + |
| 3 | 282 | 148 | 90 | 425 | + |
| 4 | 342 | 179 | 93 | 478 | + |
| 5 | 380 | 158 | 91 | 460 | + |
| 6 | 348 | 161 | 93 | 497 | + |
| 7 | 276 | 136 | 96 | 462 | + |
| 8 | 278 | 150 | 91 | 220 | Deceased (−) |
| 9 | 350 | 179 | 97 | 493 | Deceased (−) |
| 10 | 272 | 158 | 93 | 468 | + |
| 11 | 258 | 133 | 94 | 396 | + |
| 12 | 282 | 179 | 91 | 462 | + |
| Mean±s.d. | 312±41 | 158±17 | 93±2 | 442±75 |
MCAO, middle cerebral artery occlusion; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy.
The body weight, pre-MCAO plasma glucose level, typical SpO2, and heart rate of the 12 animals (Nonin Pulse Oximeter 8600) were included herein.
Two animals deceased during the MRI scan, and were excluded from data analysis.
1H Magnetic Resonance Spectrometry and Magnetic Resonance Imaging
All data were acquired using a standard Bruker cross-coil (volume-coil excitation, surface-coil detection) after high-order Fastmap shimming to achieve a homogeneous magnetic field (B0 and B1) and a high signal-to-noise ratio at 4.7 T (Bruker Biospin, Billerica, MA, USA). We obtained in vivo MRS using point-resolved single voxel spectroscopy from a cubic region of interest of 3.5 × 3.5 × 3.5 mm3, positioned approximately in the striatum, which often displays ischemic lesions in the MCAO model; the total acquisition time was ∼17 minutes. Multislice MRI (5 slices, slice thickness=2 mm) was obtained with single-shot echo-planar imaging (field of view: 25 × 25 mm2, matrix: 64 × 64, bandwidth: 200 kHz). Gradient-echo readout was used for perfusion, pH-weighted APT, and T1 MRI (Sun et al, 2010b), whereas diffusion and T2 MRI were obtained with spin-echo readout. It is important to note that the gradient-echo echo time (TE) was 14.8 milliseconds, and that the image distortion between the gradient-echo and spin-echo readouts was minimal (Figure 1). In particular, T1 images were acquired using an inversion recovery sequence, with 7 inversion delays from 250 to 3,000 milliseconds (repetition time (TR)/TE=6,500/14.8 milliseconds, number of average=4). T2 images were obtained with two spin-echo images with TE of 30 and 100 milliseconds (TR=3,250 milliseconds, number of average=16). In addition, single-shot isotropic diffusion-weighted MRI was acquired with two b-values of 250 and 1,000 seconds/mm2 (TR/TE=3,250/54 milliseconds, number of average=16) (Mori and van Zijl, 1995). Moreover, perfusion imaging was obtained using a continuous arterial spin labeling technique (Alsop and Detre (1998)) (TR/TE=6,500/14.8 milliseconds, number of average=32, TS (time of saturation)=3,250 milliseconds). Finally, for pH-weighted MRI, we set the recovery time to be 5,000 milliseconds, as well as primary radio frequency (RF) saturation duration (TS1=4,500 milliseconds) and secondary RF saturation duration (TS2=500 milliseconds) for an RF irradiation power of 0.75 μT (±3.5 p.p.m., 700 Hz at 4.7 T) (Sun et al, 2010b). The control scan was signal averaged 8 times, whereas saturated images were averaged 32 times.
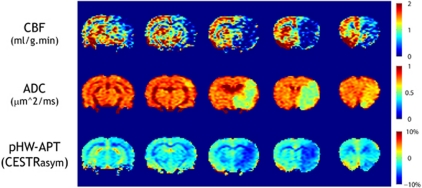
Figure 1.
Characterization of ischemic lesion with multiparametric perfusion, diffusion, and pH-weighted APT MRI of a representative acute stroke animal with MCAO. The animal showed a large perfusion and diffusion lesion mismatch, whereas the pH-weighted APT lesion agreed reasonably well with the diffusion deficit. ADC, apparent diffusion coefficient; APT, amide proton transfer; CBF, cerebral blood flow; CESTRasym, chemical exchange saturation transfer ratio asymmetry; MCAO, middle cerebral artery occlusion; MRI, magnetic resonance imaging.
Data Processing
We processed the MRS spectra with Java-based Magnetic Resonance User Interface (jMRUI v4.0, http://www.mrui.uab.es/mrui/), and MRI images with Matlab (Mathworks, Natick, MA, USA). In brief, the raw MRS data were first apodized using a 15-Hz Gaussian filter and phased corrected. Residual water removal was achieved by the Hackel-Lanczos Singular Value Decomposition algorithm (Pijnappel et al, 1992). The QUEST (Quantum estimation) method combined with subtraction approach for background modeling was used for measuring metabolite areas. Metabolite model signals of Cho (choline-containing compounds, Cr (total creatine), glutamate/glutamine, NAA, and lactate were quantum mechanically simulated in nuclear magnetic resonance spectra calculation using operators (nuclear magnetic resonance-SCOPE) (Graverondemilly et al, 1993). The numerical time-domain model functions of these metabolites were then used as previous knowledge in QUEST. The reliability of metabolite quantitation was assessed by errors in measurements as calculated by the Cramér–Rao lower bounds (Cudalbu et al, 2008). In addition, we obtained T1, T2, and apparent diffusion coefficient (ADC) maps using least-squares mono-exponential fitting of the signal intensities as a function of inversion time, TE, and b-value, respectively. It is important to point out that whereas a mono-exponential decay function seems overly simplistic to describe in vivo diffusion, it provides a reasonable delineation of ischemic diffusion lesion and has been widely used in acute ischemic stroke imaging. In addition, a cerebral blood flow (CBF) map was reconstructed using CBF = λ (Iref − Ilabel)/(2 ˙ Iref ˙ T1sat), in which λ is the brain–blood partition coefficient for water (0.63 mL/g) (Alsop and Detre, 1998; Williams et al, 1992). We used a representative T1sat of 0.86 seconds, similar to that used by Utting et al (2005). The pH-weighted image was derived as CEST ratio asymmetry (CESTRasym), which calculates the difference between label and reference images, normalized by the control scan (I0) per voxel as (CESTRasym) = (Iref − Ilabel)/I0).
Results
Figure 1 shows CBF, ADC, and pH-weighted APT MR images of a representative acute MCAO animal. The animal had a reasonably large hypoperfused ischemic lesion spanning all five slices, whereas the diffusion lesion was significantly smaller, covering mainly the third and fourth slices. In comparison, pH-weighted MRI showed an ischemic lesion similar to the diffusion lesion, and significantly smaller than the PWI deficit. As such, there was a sizeable mismatch between the perfusion, pH, and diffusion lesions, indicating the existence of a large viable ischemic tissue shortly after MCAO. In addition, pH-weighted MRI showed different levels of hypointensity, suggesting heterogeneous tissue acidification within PWI and DWI lesions (Bolas et al, 1988). It is important to note that whereas Figure 1 shows a reasonably good agreement between pH and diffusion MRI lesion size, we have also observed, in some cases, a sizeable pH and diffusion lesion mismatch. Such findings suggest that the mismatch among perfusion, pH, and diffusion MRI is complex and heterogeneous, and that further study is required to evaluate how pH MRI may augment the commonly used perfusion and diffusion MRI to elucidate ischemic tissue damage (Sun et al, 2007b).
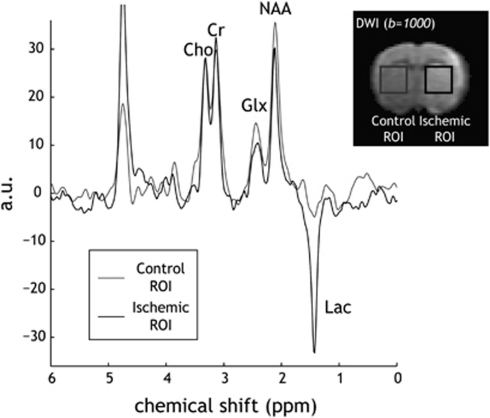
Representative localized MRS spectra are shown in Figure 2. Two regions of interest were positioned: one in the striatum, which often displays ischemic abnormality, and the other in the contralateral normal area, approximately symmetric to the ischemic region of interest. To improve the specificity of the lactate MRS signal, we used a long TE of 144 milliseconds, in which the lactate signal is inverted from other metabolite signals, whereas mobile lipid and macromolecular signals are significantly attenuated. Reliable quantitation of various metabolites was indicated by low Cramér–Rao lower bounds, which were calculated to be <10% in all metabolite estimations (Cudalbu et al, 2008). Figure 2 shows that several key metabolites including Cr, Cho, glutamate/glutamine, and NAA can be reasonably detected. It is important to note that choline peaks of the displayed spectra were normalized so that the changes in the spectra could be readily realized (Figure 2). We observed a subtle decrease in NAA signal, consistent with the notion that NAA is an early marker of neuronal damage. Moreover, we observed the inverted lactate signal for the ischemic region of interest, as expected given the chosen TE. The lactate peak was normalized to the sum of creatine and choline (i.e., Lactate/(Cr+Cho)) (Malisza et al, 1998), such that the subtle difference between coil loading and the distance between voxel and receiver coil can be normalized (Parsons et al, 2000). We also analyzed multiple MRI parameters using a paired two-tailed t-test (Table 2). In particular, the mean CBF decreased from 1.28±0.21 mL/g per min to 0.53±0.22 mL/g per min (P<0.0001), and ADC decreased from 0.76±0.01 μm2/milliseconds to 0.59±0.07 μm2/milliseconds (P<0.0001). In addition, pH-weighted CESTRasym decreased from −3.8%±0.7% to −5.9%±0.7% (P<0.0001). T1 increased slightly from 1.52±0.07 seconds to 1.67±0.07 seconds (P<0.0001), consistent with the observation made by Kaur et al (2009). Conversely, we observed no significant change in T2, which we measured at 55.4±2.2 milliseconds and at 55.8±2.7 milliseconds, respectively (P>0.05).
Figure 2.
Measurement of ischemic brain metabolites with PRESS MRS. Spectra were acquired (TR/TE=1,000/144 milliseconds, NA=1,024) from two ROIs: one in the ipsilateral ischemic lesion, which appeared as hyperintensive in DWI images (b=1,000 mm2/milliseconds), and the second one chosen in the contralateral normal area to serve as the reference ROI. The displayed spectra were normalized by choline signal, so that change in brain metabolites can be easily realized. Cho, choline-containing compound; Cr, total creatine; DWI, diffusion-weighted imaging; Glx, glutamate/glutamine; MRS, magnetic resonance spectroscopy; NA, number of average; NAA, N-acetylaspartate; PRESS, point-resolved single voxel spectroscopy; ROI, region of interest; TE, echo time; TR, repetition time.
Table 2. Comparison of multiparametric MRI and 1H MRS measurements during acute ischemic stroke.
| Contralateral normal area (mean±s.d.) | Ipsilateral ischemic lesion (mean±s.d.) | P-value | |
|---|---|---|---|
| Lactate/(Cho+Cr) | — | 0.90±0.35 | |
| NAA/(Cho+Cr) | 1.10±0.23 | 0.79±0.17* | 0.012 |
| T1 (seconds) | 1.52±0.07 | 1.67±0.07* | <0.0001 |
| T2 (milliseconds) | 55.4±2.2 | 55.8±2.7 | 0.45 |
| CBF (mL/g per min) | 1.28±0.21 | 0.53±0.22* | <0.0001 |
| ADC (μm2/milliseconds) | 0.76±0.01 | 0.59±0.07* | <0.0001 |
| CESTRasym (%) | −3.8±0.7 | −5.9±0.7* | <0.0001 |
| CESTRasym/T1 (%) | −2.5±0.5 | −3.5±0.5* | <0.0001 |
ADC, apparent diffusion coefficient; CBF, cerebral blood flow; CESTRasym, chemical exchange saturation transfer ratio asymmetry; Cho, choline-containing compound; Cr, total creatine; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NAA, N-acetylaspartate.
Ischemic lesions showed significant changes in T1, CBF, ADC, and pH-weighted amide proton CESTasym and the normalized NAA content, whereas no significant T2 change was observed. *P<0.05.
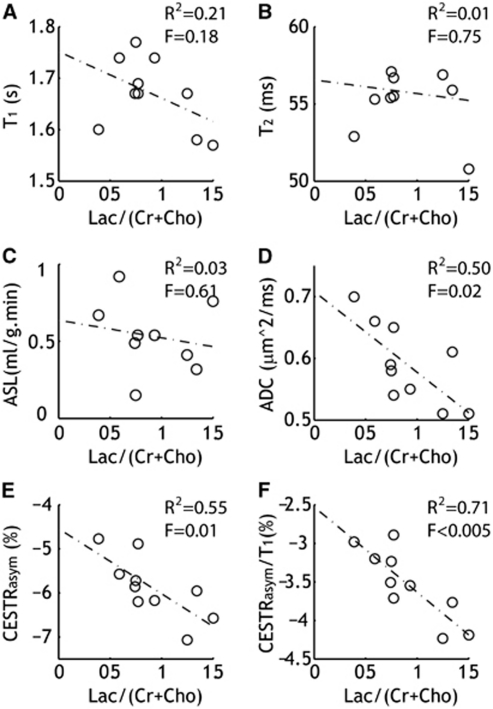
We evaluated the association between MRI and normalized lactate content (i.e., Lactate/(Cr+Cho)) with linear regression (Figure 3). No significant correlation was found between T1 (R2=0.21, significance F=0.18) and T2 (R2=0.01, significance F=0.75). In addition, the correlation between CBF and lactate was not significant (R2=0.03, significance F=0.61). Importantly, ADC (R2=0.50, significance F=0.02) and pH-weighted CESTRasym (R2=0.55, significance F=0.01) significantly correlated with lactate content, indicating that the severity of diffusion reduction and tissue acidosis is associated with lactate (Cvoro et al, 2009).
Figure 3.
Association between MRS measurement of brain lactate content and multiple MRI parameters (n=10). Linear regression test showed a significant correlation between ADC (D), pH-weighted APT MRI (E), and T1-normalized APT MRI (F) with normalized lactate content (Lac/(Cho+Cr)), whereas no such relationship was found for CBF (A) and relaxation measurements (B, C). ADC, apparent diffusion coefficient; APT, amide proton transfer; ASL, arterial spin labeling; CBF, cerebral blood flow; CESTRasym, chemical exchange saturation transfer ratio asymmetry; Cho, choline-containing compound; Cr, total creatine; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy.
Moreover, given that CEST contrast scales approximately with T1, T1-normalized CEST contrast should more faithfully capture the pH-induced contrast. In particular, CEST contrast can be described as 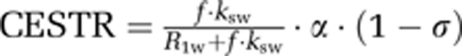 , where ksw is amide proton chemical exchange rate, f the amide proton concentration with regard to bulk water proton, R1w the bulk water longitudinal relaxation rate, α is labeling coefficient, and σ the spillover factor (Sun et al, 2005). Owing to the fact that endogenous amide proton concentration is relatively dilute and the exchange rate is relatively slow, we have
, where ksw is amide proton chemical exchange rate, f the amide proton concentration with regard to bulk water proton, R1w the bulk water longitudinal relaxation rate, α is labeling coefficient, and σ the spillover factor (Sun et al, 2005). Owing to the fact that endogenous amide proton concentration is relatively dilute and the exchange rate is relatively slow, we have  (Sun and Sorensen, 2008). As such, it is not surprising that the correlation between pH-weighted MRI and lactate was significantly improved when the T1 effect was taken into account (i.e., CESTRasym/T1), calculated as R2=0.71 and significance F<0.005.
(Sun and Sorensen, 2008). As such, it is not surprising that the correlation between pH-weighted MRI and lactate was significantly improved when the T1 effect was taken into account (i.e., CESTRasym/T1), calculated as R2=0.71 and significance F<0.005.
Discussion
Our study showed that T1-normalized pH-weighted APT/CEST MRI is capable of detecting ischemic lactic acidosis, consistent with previous findings (Jokivarsi et al, 2007; Katsura et al, 1991). In this study, we assessed lactic acidosis with an emerging endogenous pH-weighted MRI technique, the sensitivity and spatiotemporal resolution of which are significantly higher than those of conventional, 1H and 31P spectroscopy. Whereas it has been suggested that endogenous APT MRI contrast mainly reflects intracellular compartment because of its high concentration of mobile proteins/peptides in the cytoplasm (Zhou et al, 2003), Kintner et al (1999) showed that intracellular pH and extracellular pH quickly equilibrate after an initial brief buffering phase that delays the sudden change in intracellular pH. Therefore, it seems reasonable to assume that for the case of ischemic stroke, endogenous APT MRI should reflect local tissue pH. Indeed, our previous study has shown that there may be a sizeable CBF/pH/ADC mismatch in acute ischemic stroke animals, and that pH-weighted APT lesions can better predict tissue outcome (Sun et al, 2007b). In that study, we also postulated that pH-weighted MRI may help delineate the PWI/DWI mismatch into a benign oligemic region and metabolic penumbra, and thereby augment the commonly used PWI/DWI stroke MRI. As such, it remains interesting to further develop pH MRI as a surrogate imaging marker of impaired tissue metabolism and translate pH-weighted APT MRI to the clinic for investigation of patients with acute stroke (Sun et al, 2008, 2010a; Zhou et al, 2003). Towards this goal, we have recently proposed an unevenly segmented RF irradiation scheme, which enables multislice acquisition with improved sensitivity (Sun et al, 2010b). Such work should facilitate the validation of in vivo pH-weighted MRI, so that it may help delineate heterogeneous ischemic tissue damage based on the hemodynamic, metabolic, and structural status of the tissue, as estimated with multiparametric MRI and MRS, with the ultimate goal to guide individualized stroke treatment (Jacobs et al, 2000; Shen et al, 2005; Wu et al, 2001).
Whereas tissue pH correlates with lactate during acute ischemia, their relationship is complex upon reperfusion or for prolonged ischemia. For instance, pH may recover despite significantly elevated lactate, likely facilitated by alternative acid extrusion mechanisms (Allen et al, 1988; Pirttilä and Kauppinen, 1992). As such, it is important to monitor multiple parameters to better assess ischemic tissue metabolism for predicting tissue outcome and response to potential treatments, particularly in cases with significant residual/collateral flow or autolysis, towards which an imaging-based sensitive pH MRI technique is helpful. Nevertheless, commonly used in vivo APT MRI is somewhat oversimplified, and provides only pH-weighted information. Notably, the CEST asymmetry analysis is susceptible to concomitant RF irradiation effects, particularly, the slightly asymmetric magnetization transfer and nuclear overhauser effect (Pekar et al, 1996). For instance, there is a subtle baseline shift between the white matter and the gray matter in the brain CESTasym map (Figure 1). Fortunately, magnetization transfer and nuclear overhauser effect contrasts show little change with pH during acute ischemia, and thereby they only induce an asymmetry shift and the in vivo APT/CEST asymmetry analysis still provides pH-weighted contrast (Chen et al, 2006; Makela et al, 2002). In addition, confounding factors such as tissue temperature, edema, and relaxation time change may also affect the experimentally obtained pH MRI contrast. However, cerebral temperature change during focal ischemia is relatively small. In addition, the exchange rate decreases at lower temperature, which should enhance pH-weighted MRI contrast, and so does edema. Moreover, edema and T1 increase should partially offset the effects of each. This agrees with our finding that T1-normalized endogenous CEST asymmetry analysis provides stronger correlation with lactate content than that without T1 normalization. Nevertheless, additional work is urgently required to enhance the pH specificity of in vivo pH-weighted MRI and, ultimately, to develop quantitative tissue pH imaging.
Our study also measured plasma glucose levels immediately before filament occlusion, being 157±17 mg/dL (n=8). However, no significant correlation was found between preischemic glucose levels and lactate content after occlusion (R2=0.11, significance F=0.43). This suggested that confounding factors other than the preischemic glucose level may also affect lactic acidosis, for instance, collateral flow, oxygen delivery/consumption, and potentially the variance of MCAO model. In addition, the range of plasma glucose level was relatively narrow because of the use of young healthy animals, making it somewhat difficult to investigate the preischemic glucose effect on lactate and tissue acidification. Whereas conversely, it is expected that lactate levels will be elevated in cases of severe hyperglycemia such as diabetes mellitus, a disease for which it will be very interesting to test whether pH MRI can assess lactic acidosis and relevant pathophysiological changes (Parsons et al, 2002). In fact, it has been shown that preischemic hyperglycemia aggravates ischemic tissue damage, likely owing to worsened tissue acidosis (Siesjo, 1992b). As such, it is vitally important to elucidate the relationship between metabolic disruption, lactate, acidosis, and tissue damage. Finally, whereas our current study used a permanent stroke model to elucidate the correlation between tissue pH, diffusion, and lactic acidosis, it remains very promising to extend pH MRI to applications such as transient ischemic attack and cortical spreading depression in which the tissue pH change is dynamic and heterogeneous (Bisschops et al, 2002; Mutch and Hansen, 1984; Sukhotinsky et al, 2008).
Conclusions
Our study characterized heterogeneous ischemic tissue damage with multiparametric MRI of perfusion, pH, diffusion and relaxation imaging, and localized 1H MRS. We showed that T1-compensated pH-weighted endogenous amide proton CEST and diffusion MRI significantly correlated with lactate content, whereas no such correlation was found for perfusion and relaxation MRI. As such, our data show that pH-weighted MRI, by sensitizing to tissue acidification, provides a surrogate imaging marker of lactic acidosis and disrupted tissue metabolism, aiding the widely used MRI and MRS techniques for charactering heterogeneous ischemic tissue damage.
Acknowledgments
The authors thank Dr Risto Kauppinen for helpful discussion.
The authors declare no conflict of interest.
Footnotes
This study was supported in part by grants from AHA/SDG 0835384N, NIH/NIBIB 1K01EB009771-01, NIH/1R21NS061119, and NIH/NCRR-P41RR14075. This work has been presented at the Gordon Research Conference of in vivo MR in 2010.
References
- Allen K, Busza AL, Crockard HA, Frackowiak RSJ, Gadian DG, Proctor E, Ross Russell RW, Williams SR. Acute Cerebral Ischaemia: Concurrent Changes in Cerebral Blood Flow, Energy Metabolites, pH, and Lactate Measured with Hydrogen Clearance and 31P and1H Nuclear Magnetic Resonance Spectroscopy. III. Changes Following Ischaemia. J Cereb Blood Flow Metab. 1988;8:816–821. doi: 10.1038/jcbfm.1988.137. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208:410–416. doi: 10.1148/radiology.208.2.9680569. [DOI] [PubMed] [Google Scholar]
- An H, Liu Q, Chen Y, Lin W. Evaluation of MR-Derived Cerebral Oxygen Metabolic Index in Experimental Hyperoxic Hypercapnia, Hypoxia, and Ischemia. Stroke. 2009;40:2165–2172. doi: 10.1161/STROKEAHA.108.540864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Bisschops RHC, Kappelle LJ, Mali WPTM, van der Grond J. Hemodynamic and Metabolic Changes in Transient Ischemic Attack Patients: A Magnetic Resonance Angiography and 1H-Magnetic Resonance Spectroscopy Study Performed Within 3 Days of Onset of a Transient Ischemic Attack. Stroke. 2002;33:110–115. doi: 10.1161/hs0102.100879. [DOI] [PubMed] [Google Scholar]
- Bolas NM, Rajagopalan B, Mitsumori F, Radda GK. Metabolic changes during experimental cerebral ischemia in hyperglycemic rats, observed by 31P and 1H magnetic resonance spectroscopy. Stroke. 1988;19:608–614. doi: 10.1161/01.str.19.5.608. [DOI] [PubMed] [Google Scholar]
- Chen JH, Sambol EB, DeCarolis P, O'Connor R, Geha RC, Wu YV, Singer S. High-resolution MAS NMR spectroscopy detection of the spin magnetization exchange by cross-relaxation and chemical exchange in intact cell lines and human tissue specimens. Magn Reson Med. 2006;55:1246–1256. doi: 10.1002/mrm.20889. [DOI] [PubMed] [Google Scholar]
- Cudalbu C, Cavassila S, Rabeson H, van Ormondt D, Graveron-Demilly D. Influence of measured and simulated basis sets on metabolite concentration estimates. NMR Biomed. 2008;21:627–636. doi: 10.1002/nbm.1234. [DOI] [PubMed] [Google Scholar]
- Cvoro V, Wardlaw JM, Marshall I, Armitage PA, Rivers CS, Bastin ME, Carpenter TK, Wartolowska K, Farrall AJ, Dennis MS. Associations Between Diffusion and Perfusion Parameters, N-Acetyl Aspartate, and Lactate in Acute Ischemic Stroke. Stroke. 2009;40:767–772. doi: 10.1161/STROKEAHA.108.525626. [DOI] [PubMed] [Google Scholar]
- Graverondemilly D, Diop A, Briguet A, Fenet B. Product-Operator Algebra for Strongly Coupled Spin Systems. J Magn Reson, Series A. 1993;101:233–239. [Google Scholar]
- Guadagno JV, Calautti C, Baron JC. Progress in imaging stroke: emerging clinical applications. Br Med Bull. 2003;65:145–157. doi: 10.1093/bmb/65.1.145. [DOI] [PubMed] [Google Scholar]
- Guadagno JV, Jones PS, Fryer TD, Barret O, Aigbirhio FI, Carpenter TA, Price CJ, Gillard JH, Warburton EA, Baron J-C. Local Relationships Between Restricted Water Diffusion and Oxygen Consumption in the Ischemic Human Brain. Stroke. 2006;37:1741–1748. doi: 10.1161/01.STR.0000232437.00621.86. [DOI] [PubMed] [Google Scholar]
- Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann K-A. A Reproducible Model of Middle Cerebral Artery Occlusion in Mice: Hemodynamic, Biochemical, and Magnetic Resonance Imaging. J Cereb Blood Flow Metab. 1998;18:367–375. doi: 10.1097/00004647-199804000-00004. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Jacobs MA, Knight RA, Knight HS-Z, Zhang GZ, Goussev AV, Peck DJ, Windham JP, Chopp M. Unsupervised segmentation of multiparameter MRI in experimental cerebral ischemia with comparison to T2, diffusion, and ADC MRI parameters and histopathological validation. Stroke. 2000;11:425–437. doi: 10.1002/(sici)1522-2586(200004)11:4<425::aid-jmri11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jokivarsi KT, Gröhn HI, Gröhn OH, Kauppinen RA. Proton transfer ratio, lactate, and intracellular pH in acute cerebral ischemia. Magn Reson Med. 2007;57:647–653. doi: 10.1002/mrm.21181. [DOI] [PubMed] [Google Scholar]
- Katsura K, Ekholm A, Anders B, Siesjo BK. Extracellular pH in the Brain During Ischemia: Relationship to the Severity of Lactic Acidosis. J Cereb Blood Flow Metab. 1991;11:597–599. doi: 10.1038/jcbfm.1991.109. [DOI] [PubMed] [Google Scholar]
- Kaur J, Tuor UI, Zhao Z, Petersen J, Jin AY, Barber PA. Quantified T1 as an adjunct to apparent diffusion coefficient for early infarct detection: a high-field magnetic resonance study in a rat stroke model. Int J Stroke. 2009;4:159–168. doi: 10.1111/j.1747-4949.2009.00288.x. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Anderson ME, Sailor KA, Dienel G, Fitzpatrick JH, Jr, Gilboe DD. In vivo microdialysis of 2-deoxyglucose 6-phosphate into brain: a novel method for the measurement of interstitial pH using 31P-NMR. J Neurochem. 1999;72:405–412. doi: 10.1046/j.1471-4159.1999.0720405.x. [DOI] [PubMed] [Google Scholar]
- Kloska SP, Wintermark M, Engelhorn T, Fiebach JB. ) Acute stroke magnetic resonance imaging: current status and future perspective. Neuroradiology. 2010;52:189–201. doi: 10.1007/s00234-009-0637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R, Stoodley M. Pathophysiology of cerebral ischemia. Neurol Med Chir (Tokyo) 1998;38:1–11. doi: 10.2176/nmc.38.1. [DOI] [PubMed] [Google Scholar]
- Makela HI, Kettunen MI, Grohn OH, Kauppinen RA. Quantitative T1ρ and magnetic transfer magnetic resonance imaging of acute cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2002;22:547–558. doi: 10.1097/00004647-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Malisza K, Kozlowski P, Peeling J. A review of in vivo 1H magnetic resonance spectroscopy of cerebral ischemia in rats. Biochem Cell Biol. 1998;76:487–496. doi: 10.1139/bcb-76-2-3-487. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PCM. Diffusion weighting by the trace of the diffusion tensor within a single scan. Magn Reson Med. 1995;33:41–52. doi: 10.1002/mrm.1910330107. [DOI] [PubMed] [Google Scholar]
- Mutch WAC, Hansen AJ. Extracellular pH Changes During Spreading Depression and Cerebral Ischemia: Mechanisms of Brain pH Regulation. J Cereb Blood Flow Metab. 1984;4:17–27. doi: 10.1038/jcbfm.1984.3. [DOI] [PubMed] [Google Scholar]
- Nicoli F, Lefur Y, Denis B, Ranjeva JP, Confort-Gouny S, Cozzone PJ. Metabolic Counterpart of Decreased Apparent Diffusion Coefficient During Hyperacute Ischemic Stroke: A Brain Proton Magnetic Resonance Spectroscopic Imaging Study. Stroke. 2003;34:82–87. doi: 10.1161/01.STR.0000078659.43423.0A. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Li T, Barber PA, Yang Q, Darby DG, Desmond PM, Gerraty RP, Tress BM, Davis SM. Combined 1H MR spectroscopy and diffusion-weighted MRI improves the prediction of stroke outcome. Neurology. 2000;55:498–506. doi: 10.1212/wnl.55.4.498. [DOI] [PubMed] [Google Scholar]
- Paschen W, Mies G, Hossmann KA. Threshold relationship between cerebral blood flow, glucose utilization, and energy metabolites during development of stroke in gerbils. Exp Neurol. 1992;117:325–333. doi: 10.1016/0014-4886(92)90142-d. [DOI] [PubMed] [Google Scholar]
- Pekar J, Jezzard P, Roberts DA, Leigh JS, Frank JA, Mclaughlin AC. Perfusion imaging with compensation for asymmetric magnetization transfer effects. Magn Reson Med. 1996;35:70–79. doi: 10.1002/mrm.1910350110. [DOI] [PubMed] [Google Scholar]
- Pijnappel WWF, van den Boogaart A, de Beer R, van Ormondt D. SVD-based quantification of magnetic resonance signals. J Magn Reson, Series A. 1992;97:122–134. [Google Scholar]
- Pirttilä TRM, Kauppinen RA. Recovery of intracellular pH in cortical brain slices following anoxia studied by nuclear magnetic resonance spectroscopy: Role of lactate removal, extracellular sodium and sodium/hydrogen exchange. Neuroscience. 1992;47:155–164. doi: 10.1016/0306-4522(92)90128-o. [DOI] [PubMed] [Google Scholar]
- Ringer TM, Neumann-Haefelin T, Sobel RA, Moseley ME, Yenari MA. Reversal of early diffusion-weighted magnetic resonance imaging abnormalities does not necessarily reflect tissue salvage in experimental cerebral ischemia. Stroke. 2001;32:2362–2369. doi: 10.1161/hs1001.096058. [DOI] [PubMed] [Google Scholar]
- Shen Q, Ren H, Fisher M, Duong TQ. Statistical prediction of tissue fate in acute ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:1336–1345. doi: 10.1038/sj.jcbfm.9600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia: Part I: Pathophysiology. J Neurosurg. 1992a;77:169–184. doi: 10.3171/jns.1992.77.2.0169. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia: Part II: Mechanisms of damage and treatment. J Neurosurg. 1992b;77:337–354. doi: 10.3171/jns.1992.77.3.0337. [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I, Dilekoz E, Moskowitz MA, Ayata C. Hypoxia and hypotension transform the blood flow response to cortical spreading depression from hyperemia into hypoperfusion in the rat. J Cereb Blood Flow Metab. 2008;28:1369–1376. doi: 10.1038/jcbfm.2008.35. [DOI] [PubMed] [Google Scholar]
- Sun PZ, Benner T, Copen W, Sorensen A. Early experience of translating pH-weighted MRI to image human subjects at 3 Tesla. Stroke. 2010a;41:S147–S151. doi: 10.1161/STROKEAHA.110.595777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun PZ, Benner T, Kumar A, Sorensen AG. Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn Reson Med. 2008;60:834–841. doi: 10.1002/mrm.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun PZ, Cheung JS, Wang E, Benner T, Sorensen AG. Fast multi-slice pH-weighted chemical exchange saturation transfer (CEST) MRI with unevenly segmented RF irradiation. Magn Reson Med. 2010b;64:588–594. doi: 10.1002/mrm.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun PZ, Sorensen AG. Imaging pH using the Chemical Exchange Saturation Transfer (CEST) MRI: Correction of Concomitant RF Irradiation Effects to Quantify CEST MRI for Chemical Exchange Rate and pH. Magn Reson Med. 2008;60:390–397. doi: 10.1002/mrm.21653. [DOI] [PubMed] [Google Scholar]
- Sun PZ, van Zijl PCM, Zhou J. Optimization of the irradiation power in chemical exchange dependent saturation transfer experiments. J Magn Reson. 2005;175:193–200. doi: 10.1016/j.jmr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Sun PZ, Zhou J, Huang J, van Zijl P. Simplified Quantitative Description of Amide Proton Transfer (APT) Imaging During Acute Ischemia. Magn Reson Med. 2007a;57:405–410. doi: 10.1002/mrm.21151. [DOI] [PubMed] [Google Scholar]
- Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007b;27:1129–1136. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- Tomlinson FH, Anderson RE, Meyer FB. Acidic foci within the ischemic penumbra of the New Zealand white rabbit. Stroke. 1993;24:2030–2039. doi: 10.1161/01.str.24.12.2030. [DOI] [PubMed] [Google Scholar]
- Utting JF, Thomas DL, Gadian DG, Helliar RW, Lythgoe MF, Ordidge RJ. Understanding and optimizing the amplitude modulated control for multiple-slice continuous arterial spin labeling. Magn Reson Med. 2005;54:594–604. doi: 10.1002/mrm.20604. [DOI] [PubMed] [Google Scholar]
- Ward KM, Balaban RS. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST) Magn Reson Med. 2000;44:799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Williams D, Detre J, Leigh J, Koretsky A. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu O, Koroshetz WJ, Ostergaard L, Buonanno FS, Copen WA, Gonzalez RG, Rordorf G, Rosen BR, Schwamm LH, Weisskoff RM, Sorensen AG. Predicting Tissue Outcome in Acute Human Cerebral Ischemia Using Combined Diffusion- and Perfusion-Weighted MR Imaging. Stroke. 2001;32:933–942. doi: 10.1161/01.str.32.4.933. [DOI] [PubMed] [Google Scholar]
- Zauner A, Daugherty W, Bullock M, Warner D. Brain oxygenation and energy metabolism: part I-biological function and pathophysiology. Neurosurg. 2002;51:289–301. [PubMed] [Google Scholar]
- Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9:1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]