Abstract
Delayed hypothermia salvages CA1 neurons from global ischemic injury. However, the effects of this potent neuroprotectant on endogenous repair mechanisms, such as neurogenesis, have not been clearly examined. In this study, we quantified and phenotyped newly generated cells within the hippocampus following untreated and hypothermia-treated ischemia. We first show that CA1 pyramidal neurons did not spontaneously regenerate after ischemia. We then compared the level of neuroprotection when hypothermia was initiated either during or after ischemia. Treatment efficacy decreased with longer delays, but hypothermia delayed for up to 12 hours was neuroprotective. Although bromodeoxyuridine (BrdU) incorporation was elevated in ischemic groups, CA1 neurogenesis did not occur as the BrdU label did not colocalize with neuronal nuclei (NeuN) in any of the groups. Instead, the majority of BrdU-labeled cells were Iba-positive microglia, and neuroprotective hypothermia decreased the delayed generation of microglia during the third postischemic week. Conversely, hypothermia delayed for 12 hours significantly increased the survival of newly generated dentate granule cells at 4 weeks after ischemia. Thus, our findings show that CA1 neurogenesis does not contribute to hypothermic neuroprotection. Importantly, we also show that prolonged hypothermia positively interacts with postischemic repair processes, such as neurogenesis, resulting in improved functional outcome.
Keywords: CA1, microglia, neurogenesis, stroke, temperature
Introduction
Patients resuscitated from cardiac arrest, and those who undergo cardiac surgery, are often left with permanent memory impairments (Newman et al, 2001). These deficits arise in part from damage to the CA1 layer of the hippocampus, a structure highly vulnerable to ischemia (Pulsinelli et al, 1982; Colbourne et al, 1999a). Animal models of global ischemia show that CA1 neurons typically die 2 to 4 days later (Pulsinelli et al, 1982), leaving animals with impaired memory and poorer spatial navigation ability (Auer et al, 1989). Both the neuronal death and functional deficits are preventable with therapeutic hypothermia (MacLellan et al, 2009), but efficacy declines with delays to cooling (Colbourne et al, 1999b), an unfortunate clinical inevitability. This is markedly offset by increasing the duration of cooling. Indeed, brief cooling (e.g., 3 hours) provides little or transient benefit whereas prolonged hypothermia (e.g., 24 to 48 hours) provides robust, persistent protection even at greater intervention delays (Colbourne and Corbett, 1994; Colbourne et al, 1999b). Importantly, clinical trials used similar hypothermia treatments (e.g., 24 hours at 33°C) to significantly improve survival and neurologic outcome in out-of-hospital cardiac arrest patients (Bernard et al, 2002; HACA Group, 2002). Furthermore, hypothermia is the only neuroprotectant to be successfully translated from animal ischemia models.
Despite successfully translating therapeutic hypothermia for cardiac arrest and hypoxic-ischemic encephalopathy (Shankaran et al, 2005), there is a risk of central nervous system side effects (e.g., impaired plasticity), especially with prolonged treatment. Indeed, many stroke targets have a biphasic role in pathophysiology and recovery (Lo, 2008). For example, N-methyl--aspartate receptor activation is detrimental in the acute phase of ischemia, but delayed activation is required for enrichment-induced plasticity and neurogenesis (Lo, 2008). Accordingly, broad-spectrum treatments may then fail to improve clinical outcome.
Given the current clinical indications for therapeutic hypothermia and the considerable interest in using cooling for ischemic and hemorrhagic stroke, traumatic brain injury and spinal cord damage, we sought to evaluate whether cooling affects postischemic repair mechanisms, such as neurogenesis within the hippocampus (Bendel et al, 2005; Salazar-Colocho et al, 2008). We first hypothesized that cooling would attenuate the level of neurogenesis when significant neuroprotection occurred. Second, we considered the possibility that neurogenesis partly contributes to the ‘neuroprotection' found in the CA1 sector, as can be the case for other neuroprotective strategies (Zhao et al, 2006). In other words, hypothermia may rescue some neurons while simultaneously promoting neurogenesis within the CA1 zone. Postischemic hypothermia may influence CA1 cell genesis through several mechanism. First, inflammation inhibits neurogenesis (Monje et al, 2003); therefore, the antiinflammatory properties of hypothermia may enhance it. Second, postischemic hypothermia increases hippocampal levels of the neurotrophin brain-derived neurotrophic factor (BDNF) and its receptor TrkB (Boris-Möller et al, 1998), which promotes adult neurogenesis when activated (Scharfman et al, 2005). Interestingly, some (Schmidt and Reymann, 2002; Bendel et al, 2005; Salazar-Colocho et al, 2008), but not all studies (Colbourne and Corbett, 1995; Colbourne et al, 1999a; Tonchev and Yamashima, 2006; Yamashima et al, 2007; Langdon et al, 2008), observe spontaneous postischemic regeneration of CA1 pyramidal neurons via the migration of progenitor cells from the lateral ventricles. Other reports showed that greater regeneration is possible with intracerebral growth factor infusions during the first postischemic week (Nakatomi et al, 2002), further suggesting that treatment-induced regeneration of CA1 neurons is possible.
Ischemia also induces proliferation within the subgranular zone of the dentate gyrus (Liu et al, 1998; Kee et al, 2001; Sharp et al, 2002), which likely contributes to spontaneous behavioral recovery. Interestingly, this effect occurs even when CA1 cell death is prevented by preconditioning (Liu et al, 1998), indicating that large-scale cell death is not required for inducing neurogenesis. Pharmacological neuroprotectants, such as cilostazol (Lee et al, 2009), can also alter postischemic neurogenesis. Despite such evidence, the influence of hypothermia on postischemic neurogenesis has been largely ignored. One exception is a recent study, showing that postischemic hypothermia did not alter neurogenesis in the dentate gyrus (Lasarzik et al, 2009); however, rats were cooled for only 45 minutes, which was insufficient to provide neuroprotection and therefore has little preclinical relevance.
Currently, we quantified cell genesis in CA1 and dentate gyrus of rats that received global ischemia followed by prolonged (48 hours), systemic hypothermia. We chose the two-vessel occlusion (2VO) model of forebrain ischemia because it consistently damages CA1 and causes measurable behavioral dysfunction (Langdon et al, 2008). We used a proven cooling protocol that provides long-term neuroprotection (Colbourne and Corbett, 1995; Colbourne et al, 1999b). In experiment 1, we quantified the level of spontaneous regeneration in the 2VO model. In experiment 2, we evaluated neuroprotection and functional outcome when treatment was systematically delayed up to 24 hours after ischemia. We then used bromodeoxyuridine (BrdU) labeling to quantify cell genesis in the hippocampus during the third (experiment 2) and second (experiment 3) postischemic weeks.
Materials and methods
Subjects
Experiments were performed on 147 male Sprague-Dawley rats (Biosciences breeding colony, University of Alberta) weighing ∼300 g at the time of surgery. The animals were group housed in cages of four (except during temperature regulation, explained below). Water and food were available ad lib. Surgical procedures were performed aseptically, and all procedures were in accordance with the Canadian Council on Animal Care and were approved by the Biosciences Animal Care and Use Committee at the University of Alberta.
Rats were assigned randomly to experimental groups before receiving a 2VO or sham surgery. Rats in experiment 1 (Figure 1A) received 2VO ischemia and were killed either 14 (n=8) or 90 days (n=12) later. Experiment 2 contained eight groups: sham+normothermic (n=8), sham+hypothermic (n=8), 2VO+normothermic (n=10), 2VO+intraischemic hypothermic (n=12), 2VO+1 hour delayed hypothermic (n=11), 2VO+4 hours delayed hypothermic (n=12), 2VO+12 hours delayed hypothermic (n=11), and 2VO+24 hours delayed hypothermic (n=10). Experiment 3 contained four groups: sham+normothermic (n=4), sham+hypothermic (n=4), 2VO+12 hours delayed hypothermic (n=24), and 2VO+normothermic (n=16). All portions of these experiments, including behavioral assessment, histology, and surgeries (except for intraischemic hypothermia) were performed with the experimenter blinded to treatment condition.
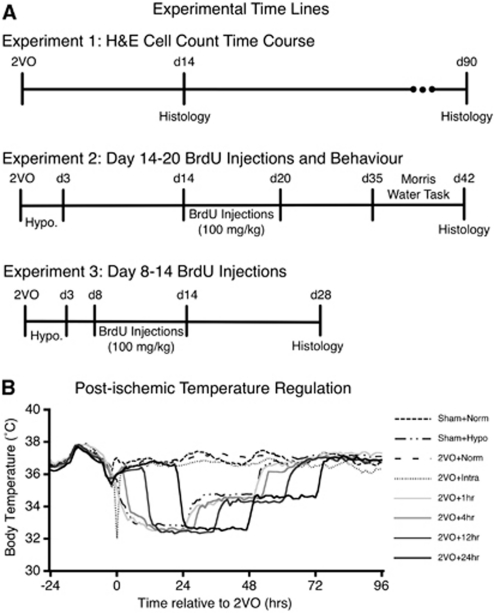
Figure 1.
Experimental timelines and body temperature measurements recorded by telemetry core probes. (A) Experiment 1 examined spontaneous regeneration of CA1 neurons after two-vessel occlusion (2VO) ischemia. Experiments 2 and 3 examined cell genesis when BrdU was injected during the third and second postischemic weeks, respectively. (B) Baseline temperature was recorded during the 24 hours preceding 2VO, whereas postischemic temperature was precisely regulated (within 0.5 °C) according to group allocation. Temperature recordings were collected continually every 30 seconds, and averaged over 60 minutes, to generate each data point. BrdU, bromodeoxyuridine; H&E, hematoxylin and eosin.
Temperature Measurement and Control
Core temperature was continually measured in rats by implanting a telemetry core probe (model TAT10TA-F40, Transoma Medical, St Paul, MN, USA) 4 days before ischemia as previously described. Temperature was recorded through a receiver underneath the rat's home cage and the data from 24 hours preceding the 2VO surgery served as baseline. Postoperative temperature was precisely controlled within 0.5°C of target via a servo-regulated computer system controlling a series of fans and water misters for cooling, and an infrared heating lamp for warming. Rats were housed in individual cages for this portion of the experiment, and each cage was equipped with a temperature regulation system. We used an established cooling protocol (Colbourne et al, 1999b) of lowering core temperature to 33°C over 1 hour, and maintaining that temperature for 24 hours, followed by another 24 hours at 35°C before finally being rewarmed to 36°C (rewarmed over 1 hour and maintained for an additional 24 hours). This cooling protocol was initiated with a 1-, 4-, 12-, or 24-hour delay after the induction of 2VO ischemia (depending on group allocation), and in all cases the animals were maintained normothermic for the delay period (Figure 1B). Rats in the intraoperative hypothermia condition were placed on a water blanket through which chilled water was circulated, thus lowering body temperature. Ischemia was initiated when the desired temperature of 33°C was reached. Following the completion of all temperature regulation, the rats were returned to group cages (four per cage) for the remainder of the experiment.
Global Ischemia Surgical Procedures
Forebrain ischemia was induced using the 2VO model (Smith et al, 1984). Rats were fasted for ∼18 hours before surgery in order to lower blood glucose levels into a consistent range (∼6 to 10 mmol/L). Anesthesia was induced with 4% isoflurane (mixed in 60% N2O, balanced O2) and maintained at 2% during surgery. Core temperature was maintained at 37°C through a rectal temperature probe connected to a warm water blanket (Gaymar TP3E, Orchard Park, NY, USA). To avoid a drop in brain temperature during ischemia, skull temperature was regulated through a subcutaneous thermocouple probe (model: HYPO-33-1-T-G-60-SMG-M, Omega, Stanford, CT, USA) connected to an overhead infrared lamp (150 W) that was directed toward the head of the animal. The tail artery was cannulated for continuous measurement of mean arterial blood pressure (PressureMAT, Pendotech, Princeton, NJ, USA) and to collect small blood samples (100 μL) to be analyzed with a blood gas machine (Radiometer ABL 810, Radiometer, Copenhagen, Denmark; see Supplementary Table 1). The common carotid arteries were isolated bilaterally, and the right jugular vein was isolated and cannulated with Silastic tubing connected to a heparinized syringe. Ischemia was induced by withdrawing blood into this syringe until mean arterial blood pressure (MABP) reached ∼35 mm Hg, at which point both common carotid arteries were occluded for 8 minutes using vascular clamps (00400-03, Fine Science Tools, Vancouver, Canada). Blood pressure was maintained at a target of 35 mm Hg for the ischemic period, at the end of which the exsanguinated blood was slowly reinfused, the catheters were withdrawn, and the neck and tail incisions were sutured. The sham procedure consisted of isolating all vessels as stated above, without withdrawing blood from the jugular vein or applying the vascular clips to the carotid arteries. Immediately after surgery, rats were returned to the temperature regulation station where core temperature was recorded and regulated as stated above.
Bromodeoxyuridine Administration
Rats received daily intraperitoneal injections of BrdU (100 mg/kg; Sigma, Oakville, ON, Canada) for 6 days starting either on postischemic day 14 (experiment 2) or postischemic day 8 (experiment 3). The BrdU was dissolved in warm, sterile saline at a concentration of 20 mg/mL, and the solution was prepared fresh before injection.
Behavioral Assessment
In experiment 2, spatial learning and memory was assessed in the Morris Water Task (Auer et al, 1989). A black swimming pool (1.5 m in diameter, 60 cm deep) was filled with water (21°C to 22°C) to 20 cm below the top of the wall. A clear Plexiglas platform was submerged 1.5 cm below the surface of the water, thus rendering it invisible from the rat's position. Rats were given four swim trials per day, with each trial starting from one of the four cardinal compass points along the edge of the pool. The first 4 testing days were performed with the platform maintained in the same location for each day. On the fifth day, a probe trial was administered where the platform was removed and rats could search the pool for 30 seconds. The rats were then tested for 4 consecutive days on a moving platform version of the task where the platform was moved to a novel location within the pool on each testing day. All trials lasted a maximum of 90 seconds and the rats were allowed to stay on the platform for an additional 10 seconds. In cases when a rat did not find the platform, it was placed on it. Performance (including latency, distance traveled, and swim speed) was recorded and analyzed through an overhead camera connected to a tracking system (Water 2020; HVS Image, Hampton, UK).
Histology
At the end of each experiment, rats were transcardially perfused with phosphate-buffered saline followed by 10% formalin. Extracted brains were postfixed in formalin for ∼24 hours, embedded in paraffin and sectioned at 6 μm on a microtome. Sections stained with hematoxylin and eosin were used to count remaining CA1 neurons. As routinely done (Colbourne and Corbett, 1995; Colbourne et al, 1999b; Langdon et al, 2008), the number of viable-looking neurons in the medial, middle, and lateral sectors of CA1 (each 0.2 mm long) were counted on a light microscope at 3.7 mm posterior of Bregma. Cell numbers were summed across each region and brain hemisphere. Neighboring sections were used for immunolabeling in order to visualize cell genesis and to determine the phenotype of newly generated cells. Before incubation with the primary antibody, antigen retrieval was performed by boiling the sections in 0.1 mol/L citrate buffer (pH 6.3) for 15 minutes in a microwave. The primary antibodies used were rat anti-BrdU (1:1,000; AbD Serotec, Cedarlane, Burlington, Ontario, Canada; product: OBT0030), mouse anti-GFAP (1:400; Sigma, product: G 3893), rabbit anti-Iba-1 (1:1,000; Wako, Richmond, VA, USA; product: 019-19741), mouse anti-neuronal nuclei (NeuN) (1:500; Chemicon, Billerica, MA, USA; product: MAB377), and rabbit anti-Ki67 (1:500; Vector, Burlington, Ontario, Canada; product: VP-K451). The secondary antibodies used were from Jackson Laboratories (West Grove, PA, USA), and were applied at the following concentrations: donkey anti-rat Cy3 (1:500), donkey anti-mouse 488 (1:500), and donkey anti-rabbit 594 (1:500). All incubations were at room temperature, and DAPI (4′,6-diamidino-2-phenylindole) (1:500; Sigma) was added during the final step of each procedure to visualize cell nuclei. Images were captured using an Olympus epifluorescent microscope (BX51) equipped with a CCD camera (Infinity 3; Ottawa, ON, Canada). When performing cell counts, the DAPI signal was used to confirm that the BrdU label colocalized with a cell nucleus.
Statistical Analyses
Comparisons were performed using either a one-way or repeated-measures analysis of variance (SPSS v18 Mac). To identify group differences, the Dunnett post hoc test was performed where P<0.05 was considered significant. As there were no significant differences between the sham+hypo and sham+normo groups on any measures, subjects were collapsed into a single sham group.
Results
CA1 Neurons Did not Spontaneously Regenerate After Ischemia
Previous studies suggest that CA1 neurons may spontaneously regenerate following global ischemia (Bendel et al, 2005; Salazar-Colocho et al, 2008). Therefore, we determined whether the neuronal depletion was permanent in our 2VO model. Rats were killed 14 or 90 days after ischemia and the remaining intact-looking neurons were counted in hematoxylin and eosin-stained sections. Compared with sham animals (353±7 cells; mean±s.e.m.), the number of CA1 neurons was significantly decreased in rats killed 14 (14±4) or 90 days (33±15) after ischemia. While both ischemia groups had far fewer cells than sham animals (P<0.0001), the difference between the ischemia groups was not significant (P=0.529).
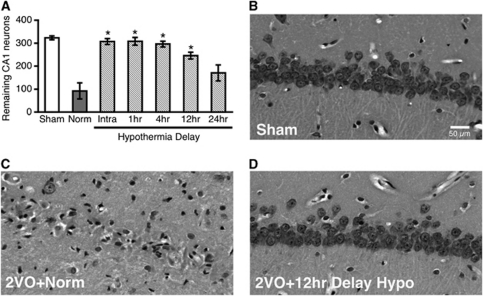
Hypothermia Delayed up to 12 hours After Ischemia Reduces CA1 Injury
We systematically varied the delay of hypothermia initiation in a single experiment and assessed CA1 injury at a 6-week survival. Hypothermia initiated either during ischemia or after a delay of 1, 4, or 12 hours significantly reduced CA1 injury relative to normothermia (Figure 2A; P<0.0001). The level of protection in the 2VO+24 hours delay hypo group was not significant, although there was a trend (P=0.053). Gross morphological characteristics of neurons from hypothermia-treated rats were normal in terms of size and density (Figures 2B and 2D), whereas in nontreated rats, there was a near-complete absence of the CA1 cell layer (Figure 2C).
Figure 2.
Neuroprotective efficacy diminishes with hypothermia delay (experiment 2). (A) Normothermic ischemia significantly decreased the number of remaining neurons 6 weeks after ischemia, relative to sham surgery. Hypothermia initiated either intraischemically or at a delay of 1, 4, or 12 hours was significantly neuroprotective. The two-vessel occlusion (2VO)+24 hours delay hypo group showed a trend for benefit, but was not significantly different from 2VO+norm (P=0.053). Hematoxylin and eosin (H&E) staining showed a severe depletion of CA1 pyramidal neurons in the 2VO+norm group (C) relative to shams (B). The general morphology of the pyramidal cell layer in the hypothermia-treated groups (D) was not different from shams (B). *Significantly different from 2VO+norm group; mean±s.e.m.
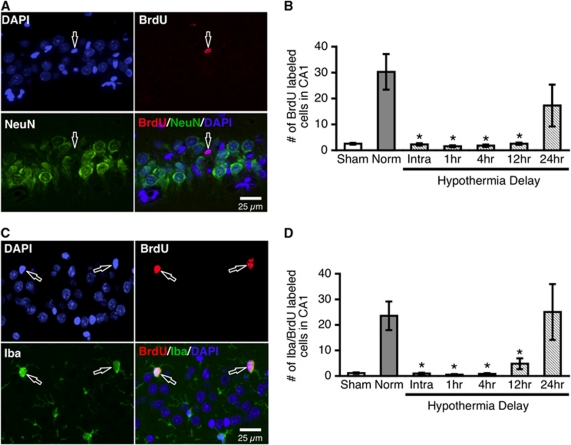
Neuroprotective Hypothermia Did not Induce CA1 Neurogenesis
Other neuroprotectants stimulate CA1 neurogenesis (Zhao et al, 2006); therefore, we investigated whether neurogenesis contributes to the apparent neuroprotective effect of hypothermia. We found that NeuN-positive neurons in the CA1 region do not colabel with BrdU when it is administered during the third week after ischemia (Figure 3A), thus indicating that neurogenesis does not occur during this period. Furthermore, we did not find any doublecortin-positive cells migrating into CA1. However, the number of BrdU-labeled nuclei was significantly increased in the 2VO+norm group relative to shams (P<0.0001). This increase was mitigated by neuroprotective hypothermia (intraischemic, 1-, 4-, 12-hour delay), as all of these groups had significantly fewer BrdU-labeled nuclei in CA1 relative to the 2VO+norm group (P<0.0001; Figure 3B).
Figure 3.
Neuroprotective hypothermia does not induce CA1 neurogenesis, but decreases chronic microglial proliferation (experiment 2). Normothermic ischemia significantly increased the number of BrdU-labeled nuclei, while neuroprotective hypothermia (intraischemic, 1-, 4-, and 12-hour delay) mitigated this effect (B). BrdU did not colocalize with the neuronal marker NeuN in any of the groups (A), indicating that neurogenesis does not contribute to the neuroprotective effect of hypothermia. The majority of BrdU-labeled CA1 cells colabeled with the microglial marker Iba (C), and neuroprotective hypothermia decreased the number of BrdU/Iba-colabeled cells (D). *Significantly different from two-vessel occlusion (2VO)+norm group; mean±s.e.m. BrdU, bromodeoxyuridine; NeuN, neuronal nuclei.
Neuroprotective Hypothermia Mitigates Chronic, but not Early Generation of Microglia in the CA1 Zone
We next determined the phenotype of the newly generated cells after ischemia. Almost all of the BrdU-positive nuclei colocalized with the microglial marker Iba (Figure 3C). Quantification of the number of BrdU/Iba-colabeled cells showed that in groups with significant CA1 neuronal loss (2VO+norm, and 2VO+24 hours delayed hypo), there were significantly more BrdU-labeled microglial cells relative to sham animals (P=0.004, P=0.002, respectively). The intraischemic, 1-, 4-, and 12-hour delayed hypothermia groups were all significantly lower than the 2VO+norm group (P<0.027; Figure 3D). Thus, following CA1 damage, there is ongoing generation of microglial cells in the third postoperative week. Furthermore, hypothermia initiated up to 12 hours after the injury mitigates both the neuronal loss and chronic microglial proliferation.
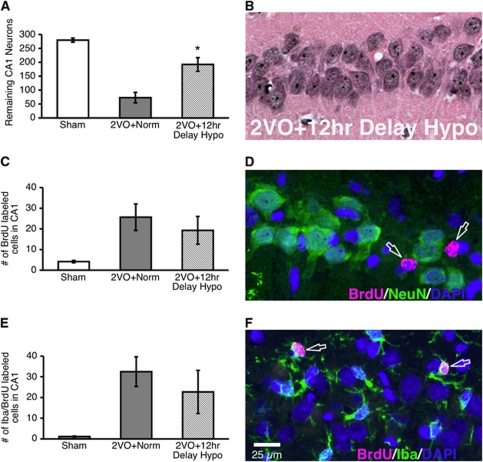
To determine whether hypothermia treatment has a similar effect on cellular proliferation earlier after ischemic injury, we performed an additional experiment where rats were injected with BrdU during the second postoperative week (experiment 3). Based on our findings from experiment 2, we restricted our analyses to a single hypothermia delay (12 hours), as this was both neuroprotective and it significantly reduced microglial proliferation. Hypothermia delayed for 12 hours was neuroprotective relative to normothermia (P<0.0001; Figures 4A and 4B), replicating our results from experiment 2. There were no doublecortin-positive neurons migrating into the CA1 region and we did not find any BrdU/NeuN-colabeled cells in the CA1 (Figure 4D). In addition, neither the number of BrdU-labeled cells (P=0.994; Figure 4C) nor the number of BrdU/Iba-colabeled cells (P=0.638; Figures 4E and 4F) was significantly decreased by hypothermia. Taken together, our results show that hypothermia delayed for 12 hours after 2VO ischemia decreases the proliferation of microglial cells generated in the third, but not the second week after ischemic injury.
Figure 4.
Neuroprotective hypothermia does not decrease microglial proliferation during the second postischemic week (experiment 3). Hematoxylin and eosin (H&E) cell counts confirmed that hypothermia delayed for 12 hours was neuroprotective (A, B). The number of BrdU-labeled cells in the CA1 was not influenced by hypothermia (C), and BrdU-labeled cells did not colocalize with the neuronal marker NeuN (D; arrows). There was also no difference in the number of proliferating microglia between the two-vessel occlusion (2VO)+norm and 2VO+12 hours delayed hypo groups (E, F). Arrows indicate BrdU/Iba-colabeled cells from a rat in the 2VO+12 hours delayed hypo group. *Significantly different from 2VO+norm group; mean±s.e.m. BrdU, bromodeoxyuridine; NeuN, neuronal nuclei.
Hypothermia Increased Survival of Newly Generated Neurons in the Dentate Gyrus
Following ischemia, neurogenesis in the dentate gyrus is upregulated for the first 14 days after the injury; however, most newly formed cells die or differentiate into glial cells (Liu et al, 1998). Importantly for our current experiment, ischemia-induced neurogenesis in the dentate gyrus occurs even when CA1 neurons are salvaged (Liu et al, 1998). Thus, we determined whether hypothermia altered the survival of newly generated cells. To do this, we quantified BrdU labeling in the dentate gyrus of rats receiving BrdU injections during the second postoperative week (experiment 3). Our results show that 4 weeks after ischemic injury there was a 60% increase in the number of BrdU/NeuN-positive dentate gyrus neurons of rats in the 2VO+12 hours delayed hypo group relative to the 2VO+norm group (P<0.0001; Figures 5A and 5C). The numerical decrease in the 2VO+norm group (relative to sham) was not significant (P=0.061). Ki67 labeling at the 4-week survival time indicated that the rate of cell genesis was not different among groups (P=0.414; Figure 5B), suggesting that the increase in BrdU/NeuN-colabeled cells is not due to a prolonged increase in the rate of cell division.
Figure 5.
Neuroprotective hypothermia increases the survival of newly generated neurons in the dentate gyrus (experiment 3). The two-vessel occlusion (2VO)+12 hours delayed hypo group had significantly more BrdU/NeuN-colabeled cells 4 weeks after ischemia relative to the 2VO+norm group (A). The difference between the 2VO+norm and sham groups was not significant. Ki67 labeling at 4 weeks after ischemia indicated that the rate of cell division was not different among groups (B). Arrows indicate BrdU/NeuN-colabeled cells in a rat from the 2VO+12 hours delayed hypo group (C). *Significantly different from 2VO+norm group; mean±s.e.m. BrdU, bromodeoxyuridine; NeuN, neuronal nuclei.
Hypothermia Improves Functional Outcome Following Global Ischemia
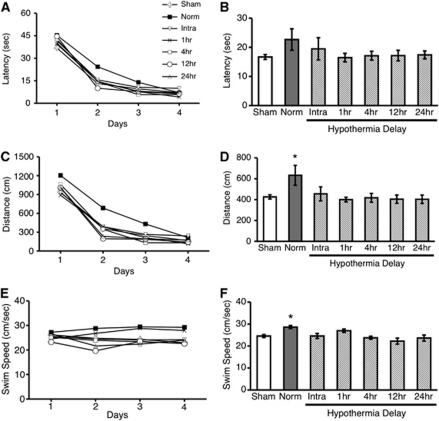
Rats were tested in the Morris Water Task starting 5 weeks after ischemia to determine whether hypothermia influenced functional performance (experiment 2). During acquisition learning (fixed platform location), all groups learned the task and improved across testing days on measures of latency (Figure 6A), and distance traveled (Figure 6C) when finding the platform (P<0.0001). Averaging across all four training days, however, showed that the 2VO+norm group traveled farther (P=0.009; Figure 6D), and also swam faster relative to the sham group (P=0.009; Figure 6F), whereas the latency to find the platform was not different among groups (P=0.150; Figure 6B). These results demonstrate a quantifiable but modest deficit in the nontreated group, as their behavioral pattern in solving the task was different from shams. In contrast, all hypothermia-treated groups were indistinguishable from sham performance (P=0.976), indicating that the stroke-induced deficit was ameliorated by the treatment. Furthermore, all hypothermia-treated groups, except for the 2VO+intraischemic hypo group (P=0.222) were also significantly better than the 2VO+norm group (P<0.032). Additional analyses of performance on the probe trial as well as reversal learning (moving platform location) did not reveal any further group differences (data not shown).
Figure 6.
Untreated two-vessel occlusion (2VO) ischemia produces a slight behavioral impairment in the Morris Water Task (experiment 2). During the 4 days of testing, all groups learned the task, as both the latency (A) and distance traveled (C) decreased across testing days when finding a hidden platform. Swim speed did not differ across days (E). The mean distance traveled across all 4 testing days was significantly longer in the 2VO+norm group relative to shams. Furthermore, all hypothermia-treated groups, except for the 2VO+intraischemic hypo group were significantly better than the 2VO+norm group (D). All other hypothermia-treated groups performed at sham levels. The 2VO+norm group also swam significantly faster than the sham group (F); therefore, mean latency was not significantly different from shams (B). *Significantly different from sham group mean±s.e.m.
Discussion
Our study, using the 2VO ischemia model, shows that prolonged mild hypothermia provides unparalleled CA1 protection with a broad therapeutic window. Contrary to our hypothesis, neurogenesis did not contribute to this neuroprotective effect in CA1. Thus, delayed hypothermia is a true and permanent neuroprotectant, at least when the treatment is prolonged. Furthermore, CA1 neurons did not spontaneously regenerate after untreated 2VO ischemia contrary to some previous work (Schmidt and Reymann, 2002; Bendel et al, 2005; Salazar-Colocho et al, 2008). Interestingly, hypothermia did increase the survival of newly formed neurons in the dentate gyrus, a mechanism possibly contributing to reduced functional impairments. Conversely, chronic microglial proliferation was attenuated by hypothermia possibly due to decreased neuronal death, which likely also contributes to improved behavioral performance. These findings strongly support the assertion that hypothermia, at least with the protocols tested, potently reduces ischemic brain injury while facilitating postischemic neuroplastic events such as neurogenesis.
Previous studies assume that neuroprotection is the primary mechanism by which hypothermia improves behavioral recovery after global ischemia. Our study, which is the first to examine hippocampal neurogenesis in the setting of varying levels of hypothermic neuroprotection, strongly supports this assumption within CA1. However, ischemia stimulates neurogenesis in two regions neighboring CA1, the dentate gyrus of the hippocampus and the subventricular zone of the lateral ventricles. Further, it is the cell migration from the lateral ventricles that is believed to underlie the limited spontaneous CA1 regeneration reported in some studies (Bendel et al, 2005). Our results show that CA1 neurons do not spontaneously regenerate even when we used survival times matching studies showing regeneration (experiment 1; Bendel et al, 2005). In addition, we found that regardless of treatment delay, hypothermia does not induce CA1 neurogenesis because we did not observe any BrdU-labeled neurons, or doublecortin-positive cells migrating into the CA1 region. Based on these observations, we conclude that neuroprotection is indeed the only mechanism by which the CA1 layer is kept intact. We cannot explain why some studies report spontaneous regeneration whereas others do not, but it is important to note that the long-term outcome is similar across studies with newly generated CA1 cells degenerating ∼125 days after injury (Bueters et al, 2008). In contrast, hypothermia offers permanent protection for CA1 neurons (Colbourne and Corbett, 1995; MacLellan et al, 2009).
Our investigation of neurogenesis in the dentate gyrus indicates that hypothermia increases the number of newly generated neurons that survive until 4 weeks after ischemia. Many forms of injury, including global ischemia, transiently increase proliferation in the hippocampal subgranular zone (Kernie and Parent, 2010). Indeed, BrdU incorporation is increased for ∼14 days after global ischemia. However, most of these newly formed cells differentiate into astrocytes or die within the next 4 weeks (Kee et al, 2001; Sharp et al, 2002). Enhancing neuronal differentiation and survival through voluntary wheel running improves functional recovery after ischemia in rats (Luo et al, 2007). Similarly, hypothermia treatment promotes increased survival of newly generated dentate gyrus neurons and improves functional performance. Perhaps this results from hypothermia preserving synaptic integrity within the hippocampus thereby allowing more of the newly generated neurons to establish synaptic connections and integrate into existing circuitry, resulting in increased survival. This interpretation also accounts for the numerical decrease in survival of BrdU-labeled neurons in the 2VO+norm group (relative to shams), as the hostile environment following untreated ischemia does not promote neuronal maturation and survival. Unfortunately, we are unable to determine whether the increased granule cell survival directly contributes to the improved functional outcome, as we would need to selectively ablate a portion of the newly formed cells to determine their contributions.
The behavioral performance of hypothermia-treated rats, studied previously using more sensitive tests (Colbourne and Corbett, 1995), further suggests that salvaging CA1 neurons preserves the functional circuitry of the hippocampus, thus eliminating any stroke-induced impairment. We found that untreated ischemic rats traveled significantly longer when locating a hidden platform in the Morris Water Task, whereas even the 2VO+12 hours delayed hypo group did not have such a deficit. Interestingly, the 24-hour delayed hypothermia provided only a trend for neuroprotection but significant functional benefit. Perhaps, the fact that ∼50% of CA1 neurons remained in the 24-hour delayed hypothermia group was sufficient for successful spatial learning (Auer et al, 1989). Unfortunately, the water maze protocol used is not sufficiently sensitive to hippocampal injury to conclude that variations in level of CA1 protection observed among hypothermia groups were equally beneficial. Extensive testing would be required to tease apart such differences, but this might have affected neurogenesis levels by acting as a rehabilitation treatment.
Our study is the first to show that microglial proliferation continues beyond 3 weeks after untreated global ischemia. Previous studies show that microglia in the hippocampus activate within minutes of reperfusion (Morioka et al, 1991), and persist in CA1 for up to 9 months following global ischemia (Langdon et al, 2008). The source of these microglia is still debated, as immune cells from the periphery (bone marrow) may infiltrate the brain and express microglial markers (Hanisch and Kettenmann, 2007), thus contributing to the delayed microglial reaction. Indeed, studies of focal ischemia show that the resident microglia, and not the infiltrating cells, are responsible for the initial activation and proliferation at the site of injury (Denes et al, 2007). Furthermore, selectively ablating only the proliferating microglia increased infarct volume and neuronal death, suggesting that the initial (local) microglial response may be neuroprotective (Denes et al, 2007). In experiment 2 we found that in all groups where hypothermia was neuroprotective, the treatment also decreased chronic microglial proliferation during the third postischemic week. In contrast, microglial proliferation during the second postischemic week was not decreased, although the treatment was still neuroprotective (experiment 3). Therefore, our results demonstrate that delayed hypothermia is neuroprotective and also mitigates the prolonged generation of microglia after global ischemia. However, the relationship between these two phenomena is not clear because hypothermia may independently and directly alter the immune response in addition to modifying it through decreased neuronal death. We suggest that hypothermia does not directly inhibit microglial proliferation, but rather, due to its neuroprotective properties, alters the cellular signaling that normally leads to prolonged microglial proliferation.
In sum, we demonstrate that CA1 neurogenesis does not contribute to the neuroprotective effect of hypothermia following global ischemia. However, cooling does attenuate the delayed generation of microglial cells in CA1, while enhancing the survival of newly generated neurons in the dentate gyrus. These finding emphasize the need to determine the effects of hypothermia beyond neuroprotection in additional stroke models in order to better understand the effects of the treatment on endogenous repair processes.
Acknowledgments
The authors thank Jon Epp for helpful advice about immunolabeling.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by a grant from the Canadian Institutes of Health Research (CIHR; MOP-97781) to FC, who is a senior medical scholar from Alberta Innovates-Health Solutions. GS was supported by a Focus on Stroke doctoral research award from the Heart and Stroke Foundation of Canada, the Canadian Stroke Network and CIHR.
Supplementary Material
References
- Auer RN, Jensen ML, Whishaw IQ. Neurobehavioral deficit due to ischemic brain damage limited to half of the CA1 sector of the hippocampus. J Neurosci. 1989;9:1641–1647. doi: 10.1523/JNEUROSCI.09-05-01641.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendel O, Bueters T, von Euler M, Ove Ogren S, Sandin J, von Euler G. Reappearance of hippocampal CA1 neurons after ischemia is associated with recovery of learning and memory. J Cereb Blood Flow Metab. 2005;25:1586–1595. doi: 10.1038/sj.jcbfm.9600153. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Boris-Möller F, Kamme F, Wieloch T. The effect of hypothermia on the expression of neurotrophin mRNA in the hippocampus following transient cerebral ischemia in the rat. Brain Res Mol Brain Res. 1998;63:163–173. doi: 10.1016/s0169-328x(98)00286-1. [DOI] [PubMed] [Google Scholar]
- Bueters T, von Euler M, Bendel O, von Euler G. Degeneration of newly formed CA1 neurons following global ischemia in the rat. Exp Neurol. 2008;209:114–124. doi: 10.1016/j.expneurol.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654:265–272. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F, Li H, Buchan AM. Continuing postischemic neuronal death in CA1: influence of ischemia duration and cytoprotective doses of NBQX and SNX-111 in rats. Stroke. 1999a;30:662–668. doi: 10.1161/01.str.30.3.662. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Sutherland GR, Auer RN. Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia. J Neurosci. 1999b;19:4200–4210. doi: 10.1523/JNEUROSCI.19-11-04200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Vidyasagar R, Feng J, Narvainen J, Mccoll BW, Kauppinen RA, Allan SM. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–1953. doi: 10.1038/sj.jcbfm.9600495. [DOI] [PubMed] [Google Scholar]
- HACA Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Hanisch U, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Kee NJ, Preston E, Wojtowicz JM. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp Brain Res. 2001;136:313–320. doi: 10.1007/s002210000591. [DOI] [PubMed] [Google Scholar]
- Kernie S, Parent JM. Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KD, Granter-Button S, Corbett D. Persistent behavioral impairments and neuroinflammation following global ischemia in the rat. Eur J Neurosci. 2008;28:2310–2318. doi: 10.1111/j.1460-9568.2008.06513.x. [DOI] [PubMed] [Google Scholar]
- Lasarzik I, Winkelheide U, Thal SC, Benz N, Lörscher M, Jahn-Eimermacher A, Werner C, Engelhard K. Mild hypothermia has no long-term impact on postischemic neurogenesis in rats. Anesth Analg. 2009;109:1632–1639. doi: 10.1213/ANE.0b013e3181bab451. [DOI] [PubMed] [Google Scholar]
- Lee JH, Shin HK, Park SY, Kim CD, Lee WS, Hong KW. Cilostazol preserves CA1 hippocampus and enhances generation of immature neuroblasts in dentate gyrus after transient forebrain ischemia in rats. Exp Neurol. 2009;215:87–94. doi: 10.1016/j.expneurol.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Luo CX, Jiang J, Zhou QG, Zhu XJ, Wang W, Zhang ZJ, Han X, Zhu DY. Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J Neurosci Res. 2007;85:1637–1646. doi: 10.1002/jnr.21317. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Clark DL, Silasi G, Colbourne F. Use of prolonged hypothermia to treat ischemic and hemorrhagic stroke. J Neurotrauma. 2009;26:313–323. doi: 10.1089/neu.2008.0580. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1991;11:966–973. doi: 10.1038/jcbfm.1991.162. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Salazar-Colocho P, Lanciego JL, Del Rio J, Frechilla D. Ischemia induces cell proliferation and neurogenesis in the gerbil hippocampus in response to neuronal death. Neurosci Res. 2008;61:27–37. doi: 10.1016/j.neures.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Reymann KG. Proliferating cells differentiate into neurons in the hippocampal CA1 region of gerbils after global cerebral ischemia. Neurosci Lett. 2002;334:153–156. doi: 10.1016/s0304-3940(02)01072-8. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Liu J, Bernabeu R. Neurogenesis following brain ischemia. Brain Res Dev Brain Res. 2002;134:23–30. doi: 10.1016/s0165-3806(01)00286-3. [DOI] [PubMed] [Google Scholar]
- Smith ML, Auer RN, Siesjö BK. The density and distribution of ischemic brain injury in the rat following 2-10 minutes of forebrain ischemia. Acta Neuropathol. 1984;64:319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T. Differential neurogenic potential of progenitor cells in dentate gyrus and CA1 sector of the postischemic adult monkey hippocampus. Exp Neurol. 2006;198:101–113. doi: 10.1016/j.expneurol.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Yamashima T, Tonchev AB, Borlongan CV. Differential response to ischemia in adjacent hippocampalsectors: neuronal death in CA1 versus neurogenesis in dentate gyrus. Biotechnol J. 2007;2:596–607. doi: 10.1002/biot.200600219. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Sun P, Chauhan N, Kaur J, Hill MD, Papadakis M, Buchan AM. Neuroprotection and neurogenesis: modulation of cornus ammonis 1 neuronal survival after transient forebrain ischemia by prior fimbria-fornix deafferentation. Neuroscience. 2006;140:219–226. doi: 10.1016/j.neuroscience.2006.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.