Abstract
Neuronal endoplasmic reticulum (ER), continuous from soma to dendritic spines, undergoes rapid fragmentation in response to N-methyl-D-aspartate (NMDA) receptor stimulation in hippocampal slices and neuronal primary cultures. Here, we show that ER fragments in the mouse brain following cardiac arrest (CA) induced brain ischemia. The ER structure was assessed in vivo in cortical pyramidal neurons in transgenic mice expressing ER-targeted GFP using two-photon laser scanning microscopy with fluorescence recovery after photobleaching (FRAP). Endoplasmic reticulum fragmentation occurred 1 to 2 minutes after CA and once induced, fragmentation was rapid (<15 seconds). We propose that acute ER fragmentation may be a protective response against severe ischemic stress.
Keywords: calcium, cardiac arrest, endoplasmic reticulum, fragmentation, in vivo, ischemia, two-photon
Introduction
The endoplasmic reticulum (ER) is involved in biosynthesis, protein quality control, cellular stress responses, and Ca2+ homeostasis. Neuronal ER is a key regulator of intracellular Ca2+ signaling, acting as a sink and store of releasable Ca2+, thereby important for synaptic transmission and plasticity (Verkhratsky, 2005). Three-dimensional reconstructions depict the neuronal ER as a complex, continuous network of interconnected cisternae and tubules extending from soma to apical dendrites (Terasaki et al, 1994). The continuous ER lumen permits an efficient diffusion and equilibration of ions from the soma to the dendrites (Choi et al, 2006; Dayel et al, 1999) and ER is often referred to as neuron-within-a-neuron where ER Ca2+ channels transmit a signal along its membrane through different neuronal compartments (Berridge, 1998).
We have previously shown that extracellular Ca2+ influx triggers rapid and reversible loss of ER continuity (fragmentation) in hippocampal slices or cultured neurons when transiently stimulated by NMDA receptor agonists or high potassium concentrations (Kucharz et al, 2009; Kucharz et al, 2011 (in press)). It is well established that during stroke, brain trauma and epilepsy neurons are transiently or permanently overloaded with Ca2+ that could be detrimental (Lau and Tymianski, 2010). Therefore, we asked whether ER dynamics could be monitored in the living brain, and if rapid ER fragmentation occurs in a condition of neuronal Ca2+ overload.
We utilized in vivo two-photon imaging techniques using transgenic mice expressing ER-targeted enhanced green fluorescent protein (EGFP) to assess ER dynamics in the brain of the anesthetized mouse. Using the global ischemia paradigm, we demonstrate for the first time the ER structure in the living mouse brain, and validate the occurrence of rapid ER fragmentation in situ.
Materials and methods
Generation of Thy1-Enhanced Green Fluorescent Protein-Endoplasmic Reticulum Transgenic Mice
Transgenic C57BL/6 mice were generated to express ER lumen-targeted soluble EGFP (EGFP-ER hereafter called GreenER) under Thy1 promoter. The transgene construct was created using EGFP gene containing calreticulin ER-targeting sequence and KDEL ER-retention sequence (gift from Thomas Oertner; Friedrich Miescher Institute) cloned into pThy1 vector (gift from Joshua R Sanes; Washington University). We assessed the fluorescence signal and the cellular expression patterns among three generated transgenic lines and chose the Thy1-EGFP-ER-7b line for further studies.
Surgery
All animal procedures were approved by the Malmö/Lund ethical committee. The surgery was performed according to Holtmaat et al (2009). Thy1-EGFP-ER mice were anesthetized (3.5% isoflurane in N2O/O2 (30:70)). During all the steps of surgery and imaging, the temperature of animal was monitored by a rectal thermistor probe (Linton Instrumentation, Norfolk, UK) and maintained at 37°C. The head was mounted onto a stereotactic frame, the eyes lubricated with Viscotears (Novartis, Basel, Switzerland), and the animals were treated with Temgesic (0.1 mg/kg, Schering-Plough, Kenilworth, NJ, USA). Isoflurane was decreased to 1.8% to 2%, the scalp and periosteum were removed, and Marcaine (2.5 mg/mL, bupivacain; AstraZeneca, London, UK) was applied to the scar, then covered with tissue adhesive (Vetbond, 3M, St Paul, MN, USA, 3 mol/L). Animals were treated with Rimadyl (5 mg/kg carprofen; Pfizer, Kent, UK) and Dexafort (2 mg/kg, dexamethasone; Intervet, Sollentuna, Sweden). The craniotomy (4 mm diameter) was performed 2 mm posterior to bregma and 2 mm right lateral to midline. To prevent excessive heating, the skull was flushed every 5 minutes with cold saline. The bone flap was removed and the intact dura was covered with a wetted gelatin sponge (Spongostan, Ferrosan, Denmark). Low melting agarose (Promega, Madison, WI, USA) was applied to the brain surface and a sterile 5 mm diameter coverglass (Karl Hecht GmbH and Co. KG ‘Assistent', Sondheim/Rhön, Germany) was positioned on top of the cranial opening with dental cement. A custom-made titanium bar was attached to stabilize the head during the imaging procedures.
For induction of cardiac arrest (CA), a femoral vein catheter was inserted. Thereafter, the animal was mounted to the imaging stage insert by the titanium bar. The animal was spontaneously breathing 1.8% to 2% isoflurane in N2O/O2 (30:70) gas mixture during imaging. Cardiac arrest was induced by intravenous injection of 0.5 mL 2 mol/L KCl.
In Vivo Imaging
The in vivo imaging was performed with a multiphoton Zeiss LSM 7MP upright laser scanning microscope using W-Plan Apochromat 20 × /1.0 DIC Vis-IR water immersion objective (Carl Zeiss, Jena, Germany). Enhanced green fluorescent protein was excited with Mai Tai DeepSee Ti:Sapphire pulse laser (Spectra-Physics, Irvine, CA, USA) tuned to 900 nm. For initial ER morphology analysis, nondescanned detector was used and the fluorescence was collected in range of 500 to 550 nm. For detailed and deep tissue analysis, we used Gallium–Arsenide–Phosphide detector. The 8-bit color depth Z-stack images were projected using ZEN 2010 (Carl Zeiss) imaging software with maximum intensity projection to assess ER continuity or semitransparent intensity projection to assess the details of ER morphology.
Fluorescence Recovery After Photobleaching Analysis
To assess the ER continuity, fluorescence recovery after photobleaching (FRAP) was used. From each mouse, 10 random dendrites with typical ER morphology were chosen. The GreenER signal was collected using Gallium–Arsenide–Phosphide detector from a 2 μm × 2 μm selected region of interest on a dendrite for 10 scanning cycles, bleached for 15 cycles with 900 nm Mai Tai laser, and the FRAP was recorded for 90 cycles. The parameters used for collecting FRAP were optimized for each animal used in the study. The data were normalized to the initial signal fluorescence intensity=1.
Results
The Morphology of Endoplasmic Reticulum In Vivo Before Cardiac Arrest
The GreenER was expressed under the control of Thy1 promoter in a subset of cortical neurons. The morphology of ER defined by GreenER signal did not differ from that observed in our previous studies in primary neuronal cultures and hippocampal slice cultures (Kucharz et al, 2009). The ER appeared continuous with no abnormalities in morphology such as blebbing or vacuolization (Figure 1A). For our studies, we selected structures in dendrites with typical ER morphology expressing GreenER in molecular layer I or external granular layer II.
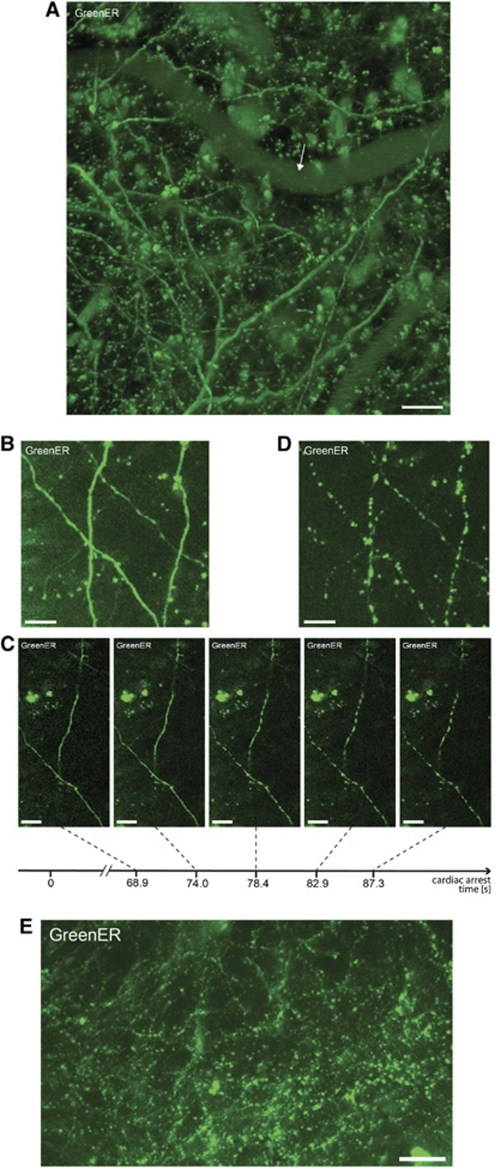
Figure 1.
Rapid dendritc endoplasmic reticulum (ER) fragmentation in cortical neurons following the cardiac arrest. (A) The projected Z-stack of dendrites of typical pyramidal neuron expressing GreenER in the outer layer of the cortex in Thy1-EGFP-ER-7b transgenic mouse line. The arrow indicates a blood vessel. Scale bar=100 μm. (B) The projected Z-stack of image scans show that dendritic ER in in vivo in its basal state is continous. Scale bar=20 μm. (C) Cessation of blood flow results in rapid reduction in ER continuity occuring over a period of 13 seconds starting 1 minute after cardiac arrest. Scale bar=20 μm. (D) Z-stack of image scans shows ER in its fragmented state, 3 minutes after cardiac arrest. Scale bar=20 μm. (E) The projected Z-stack of images of the fragmented state of dendritic ER in neurons of outer layer of cerebral cortex (dimensions of projected area: X=900 μm × Y=450 μm × Z=120 μm) following the cardiac arrest. Scale bar=100 μm. EGFP, enhanced green fluorescent protein.
Endoplasmic Reticulum Fission in the Mouse Brain
The analysis of ER morphology showed that under basal conditions in the anesthetized mouse, the dendritic ER in cortical neurons is continuous. The continuity of ER was verified by Z-series of image scans (Figure 1B; Supplementary Video 1). To assess the ER structural changes during ischemia, an intravenous bolus injection of 0.5 mL of 2 mol/L KCl via the femoral vein catheter was performed, which resulted in immediate CA. Within the first 1 to 2 minutes after CA, the ER remained continuous. Endoplasmic reticulum fragmentation occurred 1 to 2 minutes after injecting KCl, and loss of ER continuity was rapid, occurring within seconds (Figure 1C; Supplementary Video 2). The ER appeared as a string of small, disconnected beaded structures (Figure 1D; Supplementary Video 3). Endoplasmic reticulum fission after CA was a global process occurring in essentially all neurons expressing GreenER (Figure 1E).
Assessing Endoplasmic Reticulum Fragmentation by Fluorescence Recovery After Photobleaching Analysis
To confirm the ER fragmentation and compartmentalization, we performed FRAP analysis on dendritic ER of cortical neurons. Because of the secondary effects related to phototoxic damage occurring during the high-power multiphoton bleaching step and the high frame rate of time lapse recording of signal recovery, the parameters for collecting the FRAP signal (i.e., the time interval between scans, bleaching laser power, and iterations) were adjusted separately for each mouse used in the study.
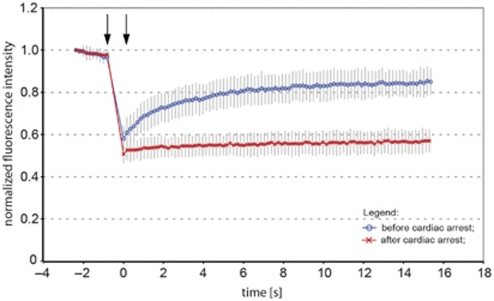
The dendritic signal from GreenER before CA displayed high degree of FRAP, reaching above 67% of initial signal strength, confirming the continuous structure of ER (Figure 2). Subsequently, CA was induced and 2 minutes thereafter the continuity of ER lumen was assessed by FRAP from neurons in the same brain area, as before CA. In all analyzed animals, the neurons exhibited loss of FRAP (Figure 2), confirming the reduction in ER lumen continuity. A similar loss of FRAP was seen in two additional investigated animals (data not shown).
Figure 2.
Fluorescence recovery after photobleaching (FRAP) analysis of dendritic endoplasmic reticulum (ER) fragmentation in cortical neurons following the cardiac arrest. Normalized average FRAP signal over time in dendrites of neurons in a mouse subjected to cardiac arrest. The graphs show FRAP signal recovery before (blue), and loss of FRAP (red) after the 2-minute of cardiac arrest. Photobleaching was performed in time between the arrows. Time=0 was set to when photobleaching ends and fluorescence starts to recover. The recovery of FRAP signal from dendritic ER before the cardiac arrest indicates ER continuity, while loss of FRAP demonstrates a fragmented structure of ER. Error bars are s.e.m., n=12 neurons.
Discussion
Multiphoton in vivo imaging of neurons has become increasingly useful to study dynamic events in the ischemic brain such as spine morphology and Ca2+ homeostasis (Misgeld and Kerschensteiner, 2006). Here, for the first time, we demonstrate the in vivo imaging of the neuronal ER and its dynamic structural changes in pyramidal cortical neurons in mice using two-photon laser scanning microscopy. The gross morphology of the dendrites in the living mouse was similar to that in vitro. Hence, under basal conditions, the neuronal ER lumen was continuous, displaying high degree of FRAP, as in cultured neurons and hippocampal slice cultures (Kucharz et al, 2009; Kucharz et al, 2011 (in press)).
The ER in primary cultures and organotypic slices undergoes rapid and reversible fragmentation upon NMDA receptor stimulation, while inhibition of NMDA receptors or removal of extracellular Ca2+ abolishes ER fragmentation (Kucharz et al, 2009; Kucharz et al, 2011 (in press)). Cardiac arrest results in an immediate loss of cerebral blood flow, followed by depletion of tissue glucose, ATP and PCr, and subsequently depolarization of neuronal plasma membranes (Hansen, 1985). The shortage of energy supply leads to inhibition of Ca2+ extrusion from neuronal cytosol and increase in intracellular Ca2+ levels (Silver and Erecinska, 1992). Noteworthy, the depolarization of cell membranes and intracellular Ca2+ increase is rapid and occurs 1 to 2 minutes after cessation of blood flow to the brain (Hansen, 1985; Xie et al, 1995). In our study, using potassium-induced CA we validated the occurrence of ER fragmentation in vivo in ischemic conditions. During the first 1 to 2 minutes after CA, the ER morphology appeared intact. Subsequently, ER fragmented rapidly (<15 seconds), which corresponded to the time at which Ca2+ enters the brain cells (Silver and Erecinska, 1992). This result strongly suggests that ER fragmentation during CA is regulated by the same mechanisms as in vitro, that is, Ca2+ entry to the cell (Kucharz et al, 2009; Kucharz et al, 2011 (in pres; Subramanian and Meyer, 1997). The rapid reduction in ER continuity in the brain was validated by loss of FRAP, and corresponded to the results from in vitro studies (Kucharz et al, 2009; Kucharz et al, 2011 (in pres). The ER fragmentation was not restricted to selected neurons, but was a global process, observed in all cortical neurons. Although we speculate that the influx of Ca2+ into neurons during ischemic conditions is responsible for the rapid fragmentation of ER, the injected potassium levels in the blood could potentially enter the brain causing ER fragmentation. This is unlikely, since the potassium must then cross the blood–brain barrier and enter the brain during the few cardiac strokes expected after KCl injection. If this occurred, an instantaneous fragmentation would be expected. Instead, ER fragmentation occurs >1 minute after the injection and coincides with the well-described effect of anoxic depolarization implicated in previous studies on ER structural rearrangements in the brain (Banno and Kohno, 1996).
The CA experiments are terminal; however, in the transient middle cerebral artery occlusion experimental model of stroke, the infarct core with irreversibly injured tissue is surrounded by penumbra area with cells that have the potential to recover and exhibit trains of transient membrane depolarizations. A transient ER fragmentation in these conditions may contribute to neuroprotection by limiting the diffusion of Ca2+ within ER, as proposed previously (Kucharz et al, 2009; Kucharz et al, 2011 (in press)). Whether the reversible ER fission occurs in penumbra and if it contributes to cell survival are of subject of future research.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by Swedish RC grants (#8466 and #60431501) and the EU 7th WP through the European Stroke Network (#201024), The Swedish Brain Fund, The Åhlen Foundation, and The Physiographic Society in Lund.
Supplementary Material
References
- Banno T, Kohno K. Conformational changes of smooth endoplasmic reticulum induced by brief anoxia in rat Purkinje cells. J Comp Neurol. 1996;369:462–471. doi: 10.1002/(SICI)1096-9861(19960603)369:3<462::AID-CNE10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Choi YM, Kim SH, Chung S, Uhm DY, Park MK. Regional interaction of endoplasmic reticulum Ca2+ signals between soma and dendrites through rapid luminal Ca2+ diffusion. J Neurosci. 2006;26:12127–12136. doi: 10.1523/JNEUROSCI.3158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayel MJ, Hom EF, Verkman AS. Diffusion of green fluorescent protein in the aqueous-phase lumen of endoplasmic reticulum. Biophys J. 1999;76:2843–2851. doi: 10.1016/S0006-3495(99)77438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65:101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hubener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009;4:1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharz K, Krogh M, Ng AN, Toresson H. NMDA receptor stimulation induces reversible fission of the neuronal endoplasmic reticulum. PLoS One. 2009;4:e5250. doi: 10.1371/journal.pone.0005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharz K, Wieloch T, Toresson H.2011Potassium-induced structural changes of the endoplasmic reticulum in pyramidal neurons in murine organotypic hippocampal slices J Neurosci Res(in press) [DOI] [PubMed]
- Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- Misgeld T, Kerschensteiner M. In vivo imaging of the diseased nervous system. Nat Rev Neurosci. 2006;7:449–463. doi: 10.1038/nrn1905. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Ion homeostasis in rat brain in vivo: intra- and extracellular [Ca2+] and [H+] in the hippocampus during recovery from short-term, transient ischemia. J Cereb Blood Flow Metab. 1992;12:759–772. doi: 10.1038/jcbfm.1992.107. [DOI] [PubMed] [Google Scholar]
- Subramanian K, Meyer T. Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell. 1997;89:963–971. doi: 10.1016/s0092-8674(00)80281-0. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Slater NT, Fein A, Schmidek A, Reese TS. Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc Natl Acad Sci USA. 1994;91:7510–7514. doi: 10.1073/pnas.91.16.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zacharias E, Hoff P, Tegtmeier F. Ion channel involvement in anoxic depolarization induced by cardiac arrest in rat brain. J Cereb Blood Flow Metab. 1995;15:587–594. doi: 10.1038/jcbfm.1995.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.