Abstract
Astrocytes have various important roles in brain physiology. To further elucidate the details of astrocytic functions under normal and pathological states, astrocyte-specific measurements are mandatory. For studying brain energy metabolism, the use of the astrocyte-specific energy substrate acetate has proven to be of great value. Since the first applications of labeled acetate for brain studies about 50 years ago, numerous methodologies have been developed and employed in compartment-specific investigations of brain metabolism. Here, we provide an overview of these different methodological approaches and review studies employing acetate labeled with the most commonly used carbon isotopes.
Keywords: acetate, astrocytes, brain imaging, energy metabolism, kinetic modeling
Introduction
Labeled substrates have an important role in the investigation of brain energy metabolism. Most prominently, glucose analogs, such as radiolabeled 2-deoxyglucose, are used extensively for the study of cerebral glucose utilization in animals (Sokoloff et al, 1977). This approach gained an increase in popularity from the introduction of positron emission tomography (PET), which together with fluorodeoxyglucose, allowed noninvasive functional maps based on regional metabolic rates to be obtained in humans for the first time (Greenberg et al, 1981). Although excellent regional metabolic differentiation can be achieved with fluorodeoxyglucose PET, the spatial resolution does not yield direct cellular information. It is presently widely accepted that astrocytes have important roles in the neuron-glial metabolic interaction and exhibit distinct glycolytic and oxidative metabolism (Hertz et al, 2007; Magistretti, 2006). Astrocytes consume up to 30% of the total cerebral energy use (Hyder et al, 2006; Iadecola and Nedergaard, 2007) and account for about 15% to 30% of brain oxygen consumption (Hertz et al, 2007; Lebon et al, 2002). In this context, it is obvious that astrocyte-specific metabolic markers are highly desirable. Because the deoxyglucose method is not cell specific and reflects merely glycolytic rate rather than oxidative metabolism, it is not sufficient for the study of cerebral metabolism. Therefore, considerable attention has been attributed to acetate, which enters the metabolic pathway after the glycolytic branch point, as a tracer for astrocyte-specific oxidative metabolism. In this mini review, we first describe the molecule's characteristics and recapitulate the concept of metabolic compartmentation based on initial acetate experiments. Second, we focus on the different types of labeled acetate and introduce the related techniques used for their detection. The principal aim of this compilation is to concentrate on methodological aspects and to provide an overview of the different approaches highlighting their respective strengths and shortcomings.
‘Large' and ‘small' pool in brain energy metabolism
Cerebral energy metabolism is highly compartmentalized, and there is evidence for substrate trafficking between astrocytes and neurons. In the 1960s and 1970s, the concept of two separate metabolic pools was proposed based on predominant glutamine labeling following injection of certain [14C]-labeled substrates. In particular, data from studies using labeled acetate stimulated the notion of separate metabolic pools (Berl and Frigyesi, 1969; Van den Berg et al, 1969): a ‘small' and a ‘large' glutamate pool. Based on histochemical studies, the concept of cell specificity was introduced, thereafter assigning the large pool to neurons and the small pool to glial cells. In the intact brain, it was observed that glutamine synthetase, the enzyme converting glutamate to glutamine, was specifically localized in astrocytes (Martinez-Hernandez et al, 1977). Subsequently, [1-3H]-acetate autoradiography experiments further supported the concept of metabolic compartmentation by providing evidence for acetate accumulation chiefly in glial elements (Muir et al, 1986).
Why can acetate serve as a marker of astrocytic metabolism?
The aforementioned work demonstrated an entrapment of acetate mainly in astrocytes; however, the reason for this astrocytic preference was unclear. Many years after the first studies suggesting separate metabolic pools, Waniewski and Martin (1998) determined the biochemical basis for the preferential acetate uptake by astrocytes. They demonstrated a much higher [14C]-CO2 production in astrocytes compared with neurons following the application of [1-14C]-acetate, and showed that the high astrocytic turnover rate could be explained by its specific transport into astrocytes. The uptake properties resembled the ones reported for monocarboxylate transporters, that is it could be inhibited by compounds with high affinity for monocarboxylate transporters and was increased by preincubation with lactate. Recent work further supported monocarboxylate transporter-mediated acetate transport. Hosoi et al (2009) demonstrated pH-dependent acetate uptake in astrocytic cell cultures. In addition, they identified glutamate, Na+ and Ca2+ as important regulators. Glutamate directly stimulated astrocytic [14C]-acetate uptake. In contrast, [14C]-acetate uptake was reduced at increased intracellular Ca2+ and Na+ concentrations, which might be mediated by a transient mitochondrial dysfunction and by Na+/H+ exchangers or Na+-bicarbonate cotransporters affecting monocarboxylate transporter activity. Furthermore, concentration dependence of acetate uptake and utilization in astrocytes has been shown (Deelchand et al, 2009; Hosoi et al, 2009) and Patel et al (2010) demonstrated that cerebral acetate utilization in the cortex of anesthetized rats was saturated at considerably lower acetate levels than the transport capacity.
[14C], [13C], and [11C]: different labels, different methods, same target
The acetate molecule's cell specificity allows metabolism to be investigated on a cellular level despite the use of acquisition tools that do not reach cellular resolution. Acetate can be labeled with several carbon isotopes, including the stable [13C] and the radioisotopes [14C] and [11C] (Table 1). The most important methods used for the study of astrocyte metabolism are discussed here. An important aspect, which has to be taken into consideration when using labeled acetate, is the labeling position. As a consequence of its two-carbon structure, acetate can be labeled at the 1 or 2 position. When labeled at position 1 (1-C-acetate), the isotope already leaves during the second turn of the tricarboxylic acid (TCA) cycle, and a large fraction of the label is lost as CO2 (Figure 1A, left panel). When labeled at the 2 position (2-C-acetate), the isotope is lost only during the third and subsequent turns, and more label ends up in metabolites such as glutamate, which further delays the loss of label (Van den Berg et al, 1969; Van den Berg and Ronda, 1976). In accordance, 5 minutes after injection, the label is retained about 30% more with 2-C-acetate compared with 1-C-acetate (Dienel et al, 2001). Therefore, 2-C-acetate is the preferred tracer for autoradiographical use.
Table 1. Synopsis of labeled acetate techniques.
| Label | Detection method | Label position | Species | Tracer application | Label detection | Sensitivity |
Resolution |
Invasiveness | Further advantages | Example referencesa | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spatial | Temporal | ||||||||||
| C-14 | Autoradiography | C-2 | Rat | In vivo (awake possible) | Ex vivo | ++ | ++ | − | +++ | Quantitative studies possible | Dienel et al, 2001, 2007; Cruz et al, 2005; Hosoi et al, 2004; Muir et al, 1986 |
| C-14 | Microdialysis | C-2 | Rat | In vivo (awake possible) | In vivo (extracellular fluid) | ++ | + | + | + + | Possible in the freely moving animal | Zielke et al, 2009 |
| C-14 | Separation of metabolites Determination of uptake Measurement of oxidationb | C-1 | Rat | Ex vivo | Ex vivo | ++ | − | − | +++ | Hosoi et al, 2009; Waniewski and Martin, 1998 | |
| C-14 | Separation of metabolites Determination of uptake Measurement of oxidationb | C-1 | Cat, mouse | In vivo | Ex vivo | ++ | − | − | +++ | Berl and Frigyesi, 1969; Van den Berg et al, 1969; Van den Berg and Ronda, 1976 | |
| C-13 | NMR spectroscopy | C-2 | Rat | In vivo | Ex vivo | + | − | − | +++ | Molecular specificity; translational approach; no radiation burden; quantitative studies possible | Melo et al, 2007; Serres et al, 2008 |
| C-13 | NMR spectroscopy | C-2 | Human, rat | In vivo | In vivo | + | − to + | + | − | Molecular specificity; translational approach; no radiation burden; quantitative studies possible | Deelchand et al, 2009; Lebon et al, 2002; Patel et al, 2010 |
| C-11 | PET | C-1 | Human | In vivo | In vivo | +++ | + + | + + | − | Before-and-after type experiments possible; translational approach; no alteration of plasma levels; quantitative studies possible | Wyss et al, 2009 |
| C-11 | β Probe | C-1 | Rat | In vivo | In vivo | +++ | + | +++ | ++ | Before-and-after type experiments possible; translational approach; no alteration of plasma levels; quantitative studies possible | Wyss et al, 2009 |
NMR, nuclear magnetic resonance; PET, positron emission tomography.
Due to space restrictions, only references that were also included in the main text are listed.
Various methods of separation were described in literature (e.g., electrophoresis, chromatographic methods, etc.).
− denotes low; + denotes intermediate; ++ denotes high; and +++ denotes very high.
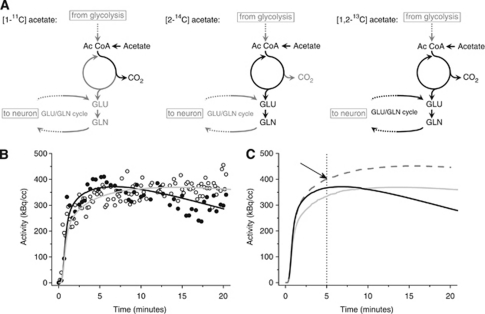
Figure 1.
(A) Most commonly used forms of labeled acetate and their predominant fates (in black) are schematically highlighted, for example, for [1-11C]-acetate it is the removal of label from tissue as labeled CO2, whereas for [2-14C]-acetate, the label largely enters several metabolites in astrocytes and neurons and label loss is delayed (Ac CoA, acetyl coenzyme A; GLN, glutamine; GLU, glutamate). (B) Infraorbital nerve stimulation increases the washout of 1-11C-acetate in rat cortex. Shown are tissue time–activity curves of a single experiment with one measurement acquired during baseline (open circles) and the other during stimulation conditions (black filled circles). In addition, the respective model fits are demonstrated (baseline: K1=0.078 mL per minute per mL tissue, k2=0.012 per minute (gray line); stimulation: K1=0.093 mL per minute per mL tissue, k2=0.030 per minute (black line)). (C) Data represent modeled time–activity curves based on the experimental data shown in (B). The gray line displays the situation during baseline conditions, the black line represents the kinetic changes during electrical infraorbital nerve stimulation. A higher increase and faster washout of radioactivity is evident. Modeling a curve assuming a sole increase of cerebral blood flow (K1=0.093 mL per minute per mL tissue, k2=0.012 per minute) yields the curve represented by the dashed gray line. Increased cerebral blood flow alone theoretically could lead to increased radioactivity in tissue after 5 minutes (arrow).
[14C]-Acetate: Turnover and Accumulation-Based Approaches
After initial studies, which mainly concentrated on rates of metabolism of [14C]-acetate in peripheral tissues (Busch and Baltrush, 1954), the ‘acetate era' for brain studies began about one decade later. Early work in the late 1960s and early 1970s concentrated on the chromatographic separation and subsequent scintillation counting of resulting fractions to study the build-up of labeled metabolites in brain tissue extracts (Berl and Frigyesi, 1969; Gonda and Quastel, 1966). This method offers the opportunity to follow the entry of the radiolabel ([14C]) into TCA cycle substrates and related metabolites (e.g., glutamate, glutamine, γ-aminobutyric acid, etc.). However, the methodology suffers from a poor spatial resolution, limiting regional metabolic information for exact localization of changes in astrocyte metabolism and inferences about acetate metabolism referring to areas with increased synaptic activity are difficult to make.
[14C]-labeled acetate can also be used in combination with microdialysis. This is applicable in the freely moving animal and allows conclusions about the relative compound oxidation (for review see Zielke et al, 2009). Following surgical implantation of microdialysis probes and the infusion of [14C]-labeled compounds into the interstitial fluid of the brain, the dialysate is collected and brain-generated [14C]-CO2 is analyzed. However, the insertion of the microdialysis probe is invasive and a calculation of absolute oxidation rate is not possible. Another disadvantage of this method is the limited number of measurements from a limited number of sites.
Somewhat later, an approach to measure the [14C]-acetate distribution in brain slices was introduced (Dienel et al, 2001; Muir et al, 1986). This accumulation-based autoradiographic method significantly increases spatial resolution and provides valuable information on the precise localization and spatial extent of acetate turnover in the brain. It allows correlation of, for example, stimulation-evoked changes in specific brain areas with astrocytic metabolism. However, intrinsic spatial resolution at a cellular level is still not reached. Quantitative autoradiography has been used in conscious animals to show that auditory and photic stimulation induced astrocyte activation in the acoustic and visual structures, respectively. In activated structures, acetate uptake was found to be increased between 14% and 20% compared with the contralateral hemisphere (Cruz et al, 2005; Dienel et al, 2007). However, the autoradiographic approach measures only total [14C] accumulation in tissue (i.e., [14C]-acetate and all its potential metabolites). To gain more detailed information, it has to be complemented with metabolite analysis, which, due to the long half-life of [14C], is straightforward. Another confounding factor is blood flow, which is also increased in activated areas. With a single measurement, it may not be easy to determine to what degree the increased blood flow enhances tracer accumulation. Dienel et al (2007) performed an autoradiographic study 5 minutes after injection of [2-14C]-acetate. Longer uptake periods resulted in a significant loss of radiolabeled products (either from decarboxylation reactions or from diffusion of labeled metabolites), causing an underestimation of utilization rates (Dienel et al, 2007). [14C]-Acetate is often injected into awake animals, but due to the low emitted energy, tissue needs to be harvested for the measurement of radioactivity. Thus, only one time point per animal can be evaluated, necessitating a high number of animals to obtain temporal information. We demonstrate below how temporal information may be helpful for accurate conclusions using radiotracers.
[13C]-Acetate: Nuclear Magnetic Resonance Spectroscopy Can Detect Labeled Metabolites
In comparison to the [14C]-based methods, the development of [13C] nuclear magnetic resonance (NMR) spectroscopy presented a substantial advance with respect to the identification of metabolic products. Initial in vivo studies were reported in the mid 1980s (Rothman et al, 1985). This powerful technique detects the label in different molecules and even at different positions in the same molecule following the injection of [13C]-labeled compounds, and thus allows brain metabolism to be observed in situ (Gruetter et al, 2003). Together with appropriate metabolic models, the measured dynamics of the [13C]-label in metabolic pathways permits the quantitative determination of metabolic rates in the brain and establishes the relationships between different cerebral metabolic fluxes. In comparison to [13C]-glucose studies, where individual metabolic fluxes in neurons and astrocytes require assumptions, the separation with the astrocyte-specific [13C]-acetate is more direct. As an example, using [2-13C]-acetate it was found that astroglial TCA cycle fluxes were 0.14±0.06 μmol per gram per minute; this value being about two times higher compared with the value obtained by the same group using [13C]-glucose (Lebon et al, 2002), thus reflecting the relative insensitivity of the glucose experiment due to entry into both compartments. Nevertheless, Sonnewald and Kondziella (2003) extensively used [13C] NMR spectroscopy to study the role of astrocytes in the metabolic interaction between neurons and astrocytes in normal brain and in numerous pathologic situations (Melo et al, 2007).
A problem of the NMR technique is its low sensitivity and as a consequence the need for a high concentration of label. This leads to high (unphysiological) acetate concentrations in blood and brain, which potentially interfere with the acetate kinetics (Deelchand et al, 2009; Dienel et al, 2007), an issue which is not present in studies where only tracer concentrations are used (radiotracer studies). Despite the recent technical advances of NMR spectroscopy, radioactivity-based methods remain superior with respect to this aspect.
[11C]-Acetate: Washout-Based Approach
Because of its applicability in humans, [1-11C]-acetate has previously been suggested for use as an astrocytic metabolism marker (Hosoi et al, 2004; Muir et al, 1986). It was first used in cardiac studies to measure cardiac oxygen consumption (Buck et al, 1991). Only recently, the feasibility of this approach for brain activation studies in vivo has been presented (Wyss et al, 2009). In an earlier ex vivo NMR spectroscopy study, it was suggested that in addition to close neurometabolic coupling, astrocytic metabolism is also closely linked to brain work (Serres et al, 2008). They found an increase in metabolic activity in both astrocytic and neuronal compartments in awake versus anesthetized rats and demonstrated a tight coupling between neuronal TCA cycle, Glu–Gln cycle, and glial TCA cycle activities, suggesting the importance of the oxidative compartment for glial energy production. In our study (Wyss et al, 2009), we showed for the first time that changes of [1-11C]-acetate kinetics can be used to estimate the activity of the astrocytic oxidative metabolism in animals and humans. By applying classical kinetic modeling, rate constants can be derived from the measured time–activity curves if an arterial input curve is acquired in parallel. We found an increase in the parameter k2 reflecting radiolabel washout upon stimulation of the cortical area under study (Figure 1B). We interpret this parameter to reflect oxidative metabolism. Prerequisites for this to be the case are (1) the removal of the [11C]-CO2 derived from acetate oxidation is not rate limiting and (2) that during the time of examination, no relevant amounts of other labeled TCA cycle metabolites, such as glutamate and glutamine, are transferred to neurons and oxidized therein. Both preconditions are fulfilled. First, [11C]-CO2 passes the blood–brain barrier without mentionable restrictions. Second, despite a highly active glutamate–glutamine cycle in the normal brain, it was found that during 20 minutes of acquisition, the labeled glutamine/glutamate mainly reflects astrocytic substrates (Lebon et al, 2002). In addition, the short physical half-life of [11C] (T1/2=20.4 minutes) allows several consecutive injections. Thus, refined study protocols are possible. For example, the kinetics at baseline and during an intervention can be assessed in the same subject.
One major advantage of [11C]-acetate compared with [14C]-acetate is the ability to inject tracer amounts and still acquire a strong signal that can be recorded with relatively high temporal resolution using β-probes or PET. Temporal information is valuable because it allows the separation of tracer delivery and metabolism. Using compartmental modeling, we demonstrated that both the delivery parameter K1 and the washout parameter k2 increased during stimulation (Figure 1B). With such a tracer, autoradiographic studies limited to only one time point are difficult to interpret. This is demonstrated in Figure 1, panel C. For instance, at 5 minutes, the increased tracer concentration can be purely the result of increased blood flow as demonstrated by the dashed gray curve. At later time points, the tissue concentration is even lower during stimulation than at baseline, due to the higher washout rate. Autoradiographic studies are therefore ideally performed with tracers that are trapped.
Future: how can the different methods profit from each other?
Each of the presented methods exhibits specific advantages and shortcomings. The latter can sometimes be overcome by a combination of modalities. For example, the kinetic radiotracer approach may benefit from biochemical knowledge obtained from NMR spectroscopy. The use of modified NMR-based models will enable fitting of PET/radiotracer data to extract specific glial metabolic fluxes (Lanz et al, in preparation). On the other hand, PET offers an alternative for faster measurements of astrocytic metabolism compared with NMR spectroscopy. Furthermore, glial functions are disturbed in the early stage of several neurologic disorders, such as temporal lobe epilepsy and hydrocephalus (Sonnewald and Kondziella, 2003). This finding highlights the clinical potential of an astrocytic metabolic marker.
Acknowledgments
MTW and BW are supported by the Swiss National Foundation (Grants 31003A-124739/1 and PP0033-110751).
The authors declare no conflict of interest.
References
- Berl S, Frigyesi TL. The turnover of glutamate, glutamine, aspartate and GABA labeled with [1-14C]acetate in caudate nucleus, thalamus and motor cortex (cat) Brain Res. 1969;12:444–455. doi: 10.1016/0006-8993(69)90012-2. [DOI] [PubMed] [Google Scholar]
- Buck A, Wolpers HG, Hutchins GD, Savas V, Mangner TJ, Nguyen N, Schwaiger M. Effect of carbon-11-acetate recirculation on estimates of myocardial oxygen consumption by PET. J Nucl Med. 1991;32:1950–1957. [PubMed] [Google Scholar]
- Busch H, Baltrush HA. Rates of metabolism of acetate-1-C14 in tissues in vivo. Cancer Res. 1954;14:448–455. [PubMed] [Google Scholar]
- Cruz NF, Lasater A, Zielke HR, Dienel GA. Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. J Neurochem. 2005;92:934–947. doi: 10.1111/j.1471-4159.2004.02935.x. [DOI] [PubMed] [Google Scholar]
- Deelchand DK, Shestov AA, Koski DM, Ugurbil K, Henry PG. Acetate transport and utilization in the rat brain. J Neurochem. 2009;109 (Suppl 1:46–54. doi: 10.1111/j.1471-4159.2009.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Liu K, Cruz NF. Local uptake of (14)C-labeled acetate and butyrate in rat brain in vivo during spreading cortical depression. J Neurosci Res. 2001;66:812–820. doi: 10.1002/jnr.10063. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Schmidt KC, Cruz NF. Astrocyte activation in vivo during graded photic stimulation. J Neurochem. 2007;103:1506–1522. doi: 10.1111/j.1471-4159.2007.04859.x. [DOI] [PubMed] [Google Scholar]
- Gonda O, Quastel JH. Transport and metabolism of acetate in rat brain cortex in vitro. Biochem J. 1966;100:83–94. doi: 10.1042/bj1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JH, Reivich M, Alavi A, Hand P, Rosenquist A, Rintelmann W, Stein A, Tusa R, Dann R, Christman D, Fowler J, MacGregor B, Wolf A. Metabolic mapping of functional activity in human subjects with the [18F]fluorodeoxyglucose technique. Science. 1981;212:678–680. doi: 10.1126/science.6971492. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Adriany G, Choi IY, Henry PG, Lei H, Oz G. Localized in vivo13C NMR spectroscopy of the brain. NMR Biomed. 2003;16:313–338. doi: 10.1002/nbm.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hosoi R, Okada M, Hatazawa J, Gee A, Inoue O. Effect of astrocytic energy metabolism depressant on 14C-acetate uptake in intact rat brain. J Cereb Blood Flow Metab. 2004;24:188–190. doi: 10.1097/01.WCB.0000098606.42140.02. [DOI] [PubMed] [Google Scholar]
- Hosoi R, Matsuyama Y, Hirose SI, Koyama Y, Matsuda T, Gee A, Inoue O. Characterization of (14)C-acetate uptake in cultured rat astrocytes. Brain Res. 2009;1253:69–73. doi: 10.1016/j.brainres.2008.11.068. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–1531. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Melo TM, Sonnewald U, Bastholm IA, Nehlig A. Astrocytes may play a role in the etiology of absence epilepsy: a comparison between immature GAERS not yet expressing seizures and adults. Neurobiol Dis. 2007;28:227–235. doi: 10.1016/j.nbd.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Muir D, Berl S, Clarke DD. Acetate and fluoroacetate as possible markers for glial metabolism in vivo. Brain Res. 1986;380:336–340. doi: 10.1016/0006-8993(86)90231-3. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Rothman DL, Behar KL, Mason GF. Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR. J Cereb Blood Flow Metab. 2010;30:1200–1213. doi: 10.1038/jcbfm.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Hetherington HP, den Hollander JA, Bendall MR, Petroff OA, Shulman RG. 1H-Observe/13C-decouple spectroscopic measurements of lactate and glutamate in the rat brain in vivo. Proc Natl Acad Sci USA. 1985;82:1633–1637. doi: 10.1073/pnas.82.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres S, Raffard G, Franconi JM, Merle M. Close coupling between astrocytic and neuronal metabolisms to fulfill anaplerotic and energy needs in the rat brain. J Cereb Blood Flow Metab. 2008;28:712–724. doi: 10.1038/sj.jcbfm.9600568. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Kondziella D. Neuronal glial interaction in different neurological diseases studied by ex vivo13C NMR spectroscopy. NMR Biomed. 2003;16:424–429. doi: 10.1002/nbm.837. [DOI] [PubMed] [Google Scholar]
- Van den Berg CJ, Krzalic L, Mela P, Waelsch H. Compartmentation of glutamate metabolism in brain. Evidence for the existence of two different tricarboxylic acid cycles in brain. Biochem J. 1969;113:281–290. doi: 10.1042/bj1130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg CJ, Ronda G. The incorporation of double-labelled acetate into glutamate ad related amino acids from adult mouse brain: compartmentation of amino acid metabolism in brain. J Neurochem. 1976;27:1443–1448. doi: 10.1111/j.1471-4159.1976.tb02627.x. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss MT, Weber B, Treyer V, Heer S, Pellerin L, Magistretti PJ, Buck A. Stimulation-induced increases of astrocytic oxidative metabolism in rats and humans investigated with 1-11C-acetate. J Cereb Blood Flow Metab. 2009;29:44–56. doi: 10.1038/jcbfm.2008.86. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ. Direct measurement of oxidative metabolism in the living brain by microdialysis: a review. J Neurochem. 2009;109 (Suppl 1:24–29. doi: 10.1111/j.1471-4159.2009.05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]