Abstract
Degradation of mitotic cyclins on exit from M phase occurs by ubiquitin-mediated proteolysis. The ubiquitination of mitotic cyclins is regulated by the anaphase-promoting complex (APC) or cyclosome. Xe-p9, the Xenopus homolog of the Suc1/Cks protein, is required for some step in mitotic cyclin destruction in Xenopus egg extracts. Specifically, if p9 is removed from interphase egg extracts, these p9-depleted extracts are unable to carry out the proteolysis of cyclin B after entry into mitosis and thus remain arrested in M phase. To explore the molecular basis of this defect, we depleted p9 from extracts that had already entered M phase and thus contained an active APC. We found that ubiquitin-mediated proteolysis of cyclin B was not compromised under these circumstances, suggesting that p9 is not directly required for ubiquitination or proteolysis. Further analysis of extracts from which p9 had been removed during interphase showed that, at the beginning of mitosis, these extracts are unable to carry out the hyperphosphorylation of the Cdc27 component of the APC, which coincides with the initial activation of the APC. p9 can be found in a complex with a small fraction of the Cdc27 protein during M phase but not interphase. The phosphorylation of the Cdc27 protein (either associated with the APC or in an isolated, bacterially expressed form) by recombinant Cdc2/cyclin B is strongly enhanced by p9. Our results indicate that p9 directly regulates the phosphorylation of the APC by Cdc2/cyclin B. These studies indicate that the Suc1/Cks protein modulates substrate recognition by a cyclin-dependent kinase.
Keywords: Xe-p9, APC, mitosis, cyclin degradation, phosphorylation, Suc1
The transitions of the cell cycle are controlled by the cyclin-dependent kinases (Cdks) and numerous Cdk-regulatory factors (for review, see Coleman and Dunphy 1994; King et al. 1994; Morgan 1997). The Cdks consist of a catalytic subunit and a positive regulatory partner called cyclin. An important member of the Cdk family is maturation-promoting factor (MPF), which contains the protein kinase Cdc2 and a B-type cyclin (Dunphy et al. 1988; Gautier et al. 1988, 1990; Murray and Kirschner 1989; Murray et al. 1989; Gautier et al. 1990; Milarski et al. 1991). At the G2/M transition, the action of MPF results in the phosphorylation of numerous structural and regulatory proteins that are responsible for the progression of mitosis.
A third protein component of Cdk/cyclin complexes belongs to the Suc1/Cks family of proteins. This protein has been implicated in Cdk regulation through both genetic and biochemical methods. Suc1 (in fission yeast) and Cks1 (in budding yeast) were both identified on the basis of their ability to suppress certain temperature-sensitive mutations of Cdks (Hayles et al. 1986a,b; Brizuela et al. 1987; Hindley et al. 1987; Hadwiger et al. 1989; Moreno et al. 1989; Tang and Reed 1993; Basi and Draetta 1995). In human cells, two homologs of the Cks1 protein have been isolated (Richardson et al. 1990).
Xe-p9, the Xenopus homolog of the Suc1/Cks protein, has also been identified, and its biochemical functions have been analyzed in Xenopus egg extracts (Patra and Dunphy 1996). These studies have revealed that Xe-p9 (hereafter referred to simply as p9) has a role in multiple cell cycle transitions. Immunodepletion of p9 from interphase egg extracts prevents entry into mitosis, suggesting that p9 regulates the activation of Cdc2/cyclin B at the G2/M boundary. Because p9-depleted interphase extracts can be induced to enter mitosis by addition of the recombinant Cdc2-AF mutant, which cannot serve as a substrate for the inhibitory phosphorylations on Thr-14 and Tyr-15 (Solomon et al. 1992; Kumagai and Dunphy 1995), p9 appears to be required, directly or indirectly, for the activation of Cdc2 by the Cdc25-catalyzed dephosphorylation of Thr-14 and Tyr-15 (Dunphy and Kumagai 1991; Gautier et al. 1991). Strikingly, p9-depleted extracts that have been induced to enter mitosis by the Cdc2-AF mutant are unable to degrade mitotic cyclins. Consequently, these p9-depleted mitotic extracts are unable to re-enter interphase and the next round of the cell cycle. The ability of p9 to regulate inactivation of MPF through proteolysis of the cyclin B protein suggests that p9, besides binding to Cdc2/cyclin B, might also interact with other molecules.
The anaphase-promoting complex (APC) or cyclosome plays a pivotal role in the completion of mitosis (Lamb et al. 1994; Irniger et al. 1995; King et al. 1995; Tugendreich et al. 1995). The APC has been shown to be essential for the degradation of mitotic cyclins (King et al. 1995; Peters et al. 1996; Kotani et al. 1998) as well as regulators of chromosome transmission such as Pds1, Cut2, and Ase1 (Cohen-Fix et al. 1996; Funabiki et al. 1996; Juang et al. 1997). In addition, the Cdc20 and Hct1/Cdh1 family of proteins appears to regulate the APC in a substrate-specific manner (Schwab et al. 1997; Visintin et al. 1997). Destruction of mitotic cyclins occurs through ubiquitin-mediated proteolysis, which includes components called E1 (the ubiquitin-activating enzyme), E2 (the ubiquitin-conjugating enzyme), and E3 (the ubiquitin-ligase activity). The concerted action of these three components results in the ubiquitination of a protein and its targeting for destruction by the proteasome (for review, see Ciechanover 1994; Murray 1995; King et al. 1996; Hoyt 1997). In Xenopus, the E1 and E2 components are constitutively active, but the ubiquitin-ligase activity of E3 is activated only at M phase. This activity is associated with the large, multimeric 20S complex called the APC or cyclosome (King et al. 1995; Sudakin et al. 1995). Recently, many subunits of the APC, such as BIME (APC1), RSI1 (APC2), Cdc27 (APC3), and Cdc16 (APC6) have been identified in several organisms, thus establishing the APC as a key, evolutionarily conserved regulator of ubiquitin-mediated proteolysis (Irniger et al. 1995; King et al. 1995; Tugendreich et al. 1995; Peters et al. 1996; Yamashita et al. 1996; Zachariae et al. 1996, 1998; Kramer et al. 1998; Yu et al. 1998).
Several subunits of the APC are phosphorylated after entry into M phase (King et al. 1995; Peters et al. 1996; Yamada et al. 1997). In particular, APC1–BIME and APC3–Cdc27 undergo extensive hyperphosphorylation that results in a substantial reduction in electrophoretic mobility. Phosphorylation of the APC appears to be functionally important, as treatment of the APC with phosphatase abolishes its ubiquitin-ligase activity (King et al. 1995; Lahav-Baratz et al. 1995; Peters et al. 1996). In a cell-free system from clam oocytes, the inactive, interphase cyclosome can be activated by incubation with Cdc2/cyclin B, while the active mitotic form can be inactivated by treatment with an okadaic acid-sensitive phosphatase (Lahav-Baratz et al. 1995). Biochemical characterization of the APC in Xenopus suggests that the activation of the APC may depend on multiple kinases, including Cdc2/cyclin B (King et al. 1995).
In this report, we describe our investigation of the mechanism by which the absence of p9 compromises the ability of mitotic extracts to degrade cyclin B. In principle, the lack of p9 could result in a failure in the activation of the APC or in some other step in APC regulation, the inability of an active APC to mediate ubiquitination of cyclin B in the absence of p9, or a defect in the degradation of polyubiquitinated cyclin B by the proteasome when p9 is missing. Our results indicate that the mitotic phosphorylation of the APC, which coincides closely with its activation, does not occur normally in the absence of p9. In experiments with recombinant proteins, we find that p9 strongly enhances the phosphorylation of the Cdc27 component of the APC by the Cdc2/cyclin B complex.
Results
Depletion of p9 from extracts containing an active APC has no effect on cyclin B destruction
Previously, we reported that depletion of p9 from interphase extracts of Xenopus eggs abolished the ability of these extracts to degrade cyclin B after entry into mitosis (Patra and Dunphy 1996). In this study, we have investigated further the molecular basis of the inability of these p9-depleted extracts to carry out ubiquitin-mediated proteolysis of cyclin B. In principle, this defect could involve a failure in the activation of the APC, the inability of an active APC to mediate ubiquitination of a Cdc2–cyclin B complex lacking p9, or a defect in the recognition of ubiquitinated cyclin B by the proteasome in the absence of p9.
To distinguish between these possibilities, we first depleted p9 from a mitotic extract in which the APC is already present in its active, hyperphosphorylated state (Fig. 1). For this purpose, we added an amino-terminally truncated version of cyclin B1 (Δcyclin B) to interphase egg extracts containing cycloheximide. Egg extracts to which Δcyclin B has been added rapidly enter mitosis, but cannot exit mitosis because this truncated cyclin lacks a destruction box and thus cannot undergo ubiquitination (Glotzer et al. 1991). Importantly, however, Δcyclin B is fully competent for activation of the cyclin destruction machinery (Glotzer et al. 1991; Luca et al. 1991).
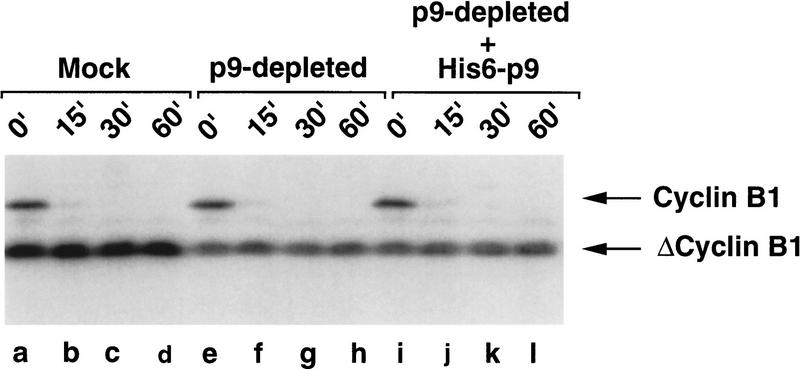
Figure 1.
Depletion of p9 from a mitotic extract has no effect on the degradation of cyclins. Demembranated sperm nuclei were added to a cycloheximide-containing interphase extract. After nuclear assembly (50 min), Δcyclin B (80 nm) was added to drive the extract into mitosis and arrest it at mitosis. This mitotic extract, in which cyclin degradation is activated, was then subjected to immunodepletion with anti-p9 antibodies (p9-depleted) or control rabbit anti-mouse IgG antibodies (mock). For the add-back experiment, His6–p9 (5 ng/μl) was added to a portion of the p9-depleted extract. A Cdc2–AF/cyclin B1 complex (10 nm) was added to these extracts. The destruction of cyclin B1 was monitored by processing of aliquots (2 μl) for immunoblotting with anti-cyclin B1 antibodies.
Next, we treated the mitotic extracts containing Δcyclin B with either control or anti-p9 antibodies bound to protein A beads. As described previously, treatment of egg extracts with anti-p9 antibodies resulted in the quantitative removal of p9. As expected, there was no reduction in the amount of p9 in mock-depleted extracts treated with control antibodies (data not shown; see Patra and Dunphy 1996). Then, we added full-length cyclin B1 (which contains a destruction box) to mock-depleted extracts, p9-depleted extracts, or p9-depleted extracts to which recombinant His6-p9 had been restored after the immunodepletion procedure.
We observed that mock-depleted mitotic extracts were able to degrade the full-length cyclin B rapidly (within 15 min), indicating that activation of the APC had occurred normally (Fig. 1, lanes a–d). Significantly, cyclin B1 destruction occurred with similar kinetics in p9-depleted extracts (Fig. 1, lanes e–h). Similar results were obtained with p9-depleted extracts containing exogenously added His6–p9 (Fig. 1, lanes i–l). Taken together, these observations suggest that p9 is not required for the recognition of Cdc2/cyclin B by the APC or the proteasome, as both ubiquitination and proteolysis of cyclin B can proceed normally in p9-depleted extracts in which the APC is already active.
p9 is required for the hyperphosphorylation of the Cdc27 component of the APC
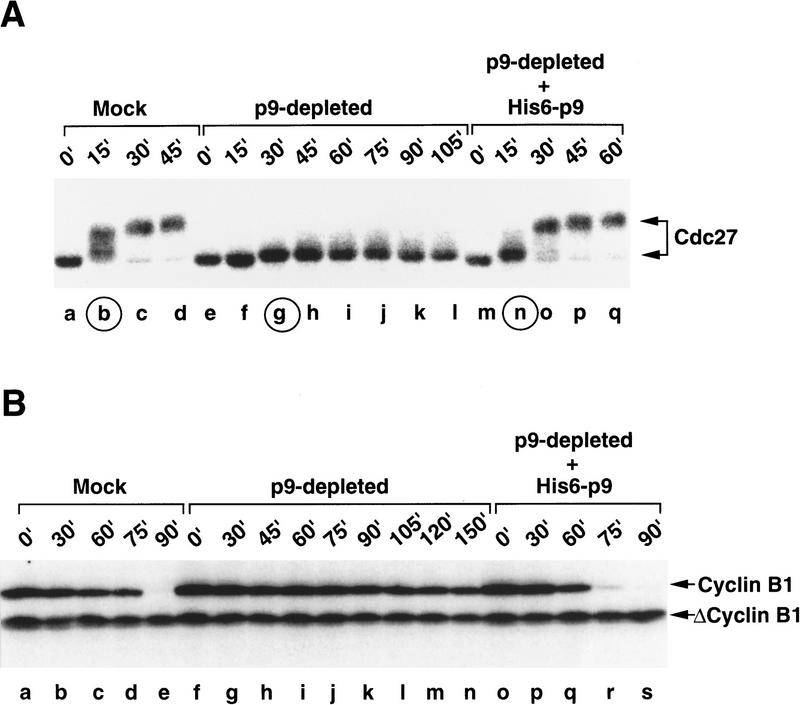
The activation of the APC on entry into mitosis closely correlates with the hyperphosphorylation of several of its subunits. In the case of Cdc27 (APC3), this phosphorylation results in a substantial reduction in the mobility of Cdc27 in SDS gels (King et al. 1995; Peters et al. 1996; Yamada et al. 1997). To assess whether p9 might be required for the mitotic hyperphosphorylation of the APC, we depleted p9 from interphase extracts. As described previously, p9-depleted extracts are unable to enter mitosis because of a failure to activate Cdc2 by dephosphorylation of its inhibitory Tyr-15 and Thr-14 residues (Patra and Dunphy 1996). However, p9-depleted interphase extracts can be induced to enter mitosis by the addition of the Cdc2-AF/Δcyclin B complex, which contains a mutant Cdc2 subunit that cannot undergo inhibitory phosphorylation. As described previously (Patra and Dunphy 1996), p9-depleted extracts driven into mitosis with Cdc2-AF/Δcyclin B cannot carry out degradation of full-length cyclin B1 (Fig. 2B, lanes f–n). In contrast, mock-depleted extracts or p9-depleted extracts containing exogenously added His6–p9 can degrade full-length cyclin B1 when triggered to enter mitosis with Cdc2-AF/Δcyclin B (Fig. 2B, lanes a–e and o–s, respectively). Strikingly, when we examined the phosphorylation state of Cdc27 in these various extracts, we observed that the hyperphosphorylation of Cdc27 was strongly compromised in the absence of p9. In particular, there was only a slight retardation in the electrophoretic mobility of Cdc27 in p9-depleted extracts on induction of mitosis with Cdc2-AF/Δcyclin B (Fig. 2A, lanes e–l), indicating that most, but not all, of the mitotic phosphorylation of Cdc27 depends on the presence of p9. In contrast, Cdc27 underwent extensive phosphorylation at mitosis in either mock-depleted extracts or p9-depleted extracts containing His6–p9, as indicated by a substantial decrease in the electrophoretic mobility of Cdc27 in SDS gels (Fig. 2A, lanes a–d and m–q, respectively).
Figure 2.
Lack of hyperphosphorylation of Cdc27 correlates with the inability of extracts to degrade cyclins when p9 is depleted prior to mitosis. (A) Interphase egg extracts containing cycloheximide were immunodepleted with control and anti-p9 antibodies. Demembranated sperm nuclei were added to a mock-depleted extract (lanes a–d), a p9-depleted extract (lanes e–l), and a p9-depleted extract containing 5 ng/μl of His6–p9 (lanes m–q). After a 50-min incubation, a Cdc2-AF/Δcyclin B complex (10 nm) was added to drive the extracts into mitosis. At the indicated times after addition of complex, aliquots (2 μl) were removed for immunoblotting with anti-Cdc27 antibodies. The time at which the extracts entered mitosis is indicated by the circled letters. (B) In the same experiment as in A, after the extracts had entered mitosis, full-length human cyclin B1 was added, and its destruction was monitored by immunoblotting with antibodies against human cyclin B1. (Lanes a–e) Mock-depleted extract; (lanes f–n), p9-depleted extract; (lanes o–s) p9-depleted extract to which recombinant His6–p9 (5 ng/μl) was added.
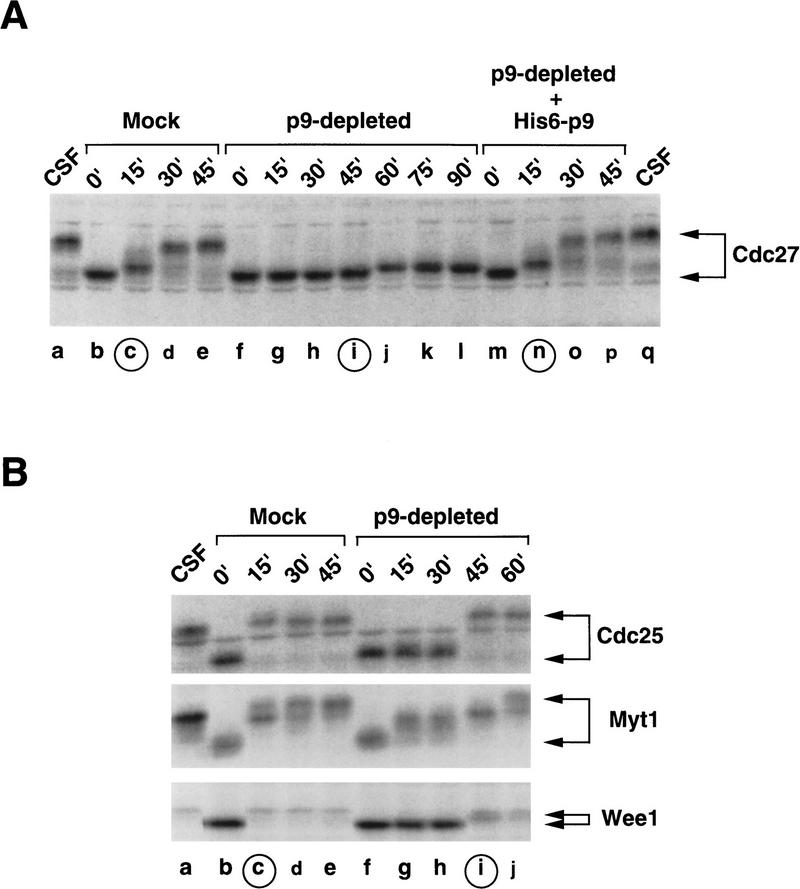
In addition to Cdc27, other regulators of mitosis not associated with the APC also undergo phosphorylation during M phase. Such proteins include Cdc25 (Izumi et al. 1992; Kumagai and Dunphy 1992), Wee1 (Parker and Piwnica-Worms 1992; McGowan and Russell 1993; Mueller et al. 1995a; Watanabe et al. 1995), and Myt1 (Mueller et al. 1995b; Liu et al. 1997), which collectively regulate the inhibitory phosphorylation of Cdc2. To assess whether the phosphorylation of these components might also be compromised in the absence of p9, we subjected mock-depleted and p9-depleted extracts to immunoblotting with anti-Cdc25, anti-Wee1, and anti-Myt1 antibodies (Fig. 3). Under conditions where the absence of p9 largely abolished mitotic hyperphosphorylation of Cdc27 (Fig. 3A), the phosphorylation of Cdc25, Wee1, and Myt1 did occur on entry of p9-depleted extracts into mitosis (Fig. 3B). However, the phosphorylation of these proteins, especially Cdc25 and Wee1, was delayed by about 30 min relative to the phosphorylation that occurred in control extracts (Fig. 3B). In other experiments, we treated p9-depleted interphase extracts with okadaic acid, a phosphatase inhibitor that induces premature mitosis in Xenopus egg extracts and other experimental systems (Félix et al. 1990a; Jessus et al. 1991; Picard et al. 1991). Although Cdc25, Wee1, and Myt1 all became phosphorylated on addition of okadaic acid, the hyperphosphorylation of Cdc27 did not occur in these p9-depleted, okadaic acid-treated extracts (data not shown). Taken together, these results indicate that p9 is required at mitosis for the phosphorylation of Cdc27 component of the APC but is less important for the phosphorylation of several other mitotic phosphoproteins such as Cdc25, Wee1, and Myt1. Furthermore, the defect in phosphorylation of Cdc27 in the absence of p9 cannot be bypassed by the addition of phosphatase inhibitor (e.g., okadaic acid) that strongly inhibits anti-mitotic phosphatase activity.
Figure 3.
Comparison between the phosphorylation of Cdc27 versus that of Cdc25, Wee1, and Myt1 during the transition from interphase to mitosis in the absence of p9. (A) Interphase egg extracts containing cycloheximide were immunodepleted with control and anti-p9 antibodies as in Fig. 2A. At the indicated times after addition of the Cdc2-AF/Δcyclin B complex, aliquots (2 μl) were removed for immunoblotting with anti-Cdc27 antibodies. The time of mitosis is indicated by the circled letters. (Lane a) 2 μl of CSF extract; (lanes b–e) mock-depleted extract; (lanes f–l) p9-depleted extract; (lanes m–q) p9-depleted extract containing 5 ng/μl of His6–p9. (B) In the same experiment as in A, the phosphorylation states of the Cdc25, Myt1, and Wee1 proteins were investigated. Aliquots (2 μl) were taken at 15-min intervals and processed for immunoblotting with antibodies against the Xenopus Cdc25 (top), Myt1 (middle), and Wee1 (bottom). (Lane a) 2 μl of CSF extract; (lanes b–e) mock-depleted extract; (lanes f–j) p9-depleted extract.
p9 and Cdc27 can be coimmunoprecipitated during mitosis
To explore the relationship between p9 and the APC in greater detail, we asked whether these components could associate physically with one another during the course of the cell cycle. For this purpose, we immunoprecipitated both interphase and M-phase extracts with anti-p9 or control antibodies and then immunoblotted the immunoprecipitates with anti-Cdc27 antibodies (Fig. 4A). We observed that the hyperphosphorylated Cdc27 present in M-phase extracts could clearly be immunoprecipitated with anti-p9 antibodies (Fig. 4A, lane d), whereas little, if any, of the hypophosphorylated Cdc27 in interphase extracts could be coimmunoprecipitated specifically with p9 (Fig. 4A, lane f). To pursue this observation further, we performed immunoprecipitation of M phase and interphase extracts with anti-Cdc27 antibodies and then processed these immunoprecipitates for immunoblotting with anti-p9 antibodies (Fig. 4B). Consistent with the results described above, we found that p9 could be detected in the anti-Cdc27 immunoprecipitates from M-phase but not interphase egg extracts. In quantitation experiments, we estimate that ∼2% of the p9 and Cdc27 can be coimmunoprecipitated during M phase (data not shown). By immunoblotting the anti-Cdc27 immunoprecipitates with antibodies against Xenopus Cdc2 and cyclin B2, we could also detect Cdc2 and cyclin B2 in association with a complex containing Cdc27 during M-phase. During interphase, neither Cdc2 nor cyclin B2 (which is absent in these cycloheximide-containing interphase extracts) could be found in anti-Cdc27 immunoprecipitates (Fig. 4B). In conclusion, p9, Cdc2, and cyclin B2 can be found in a complex with Cdc27 during M phase.
Figure 4.
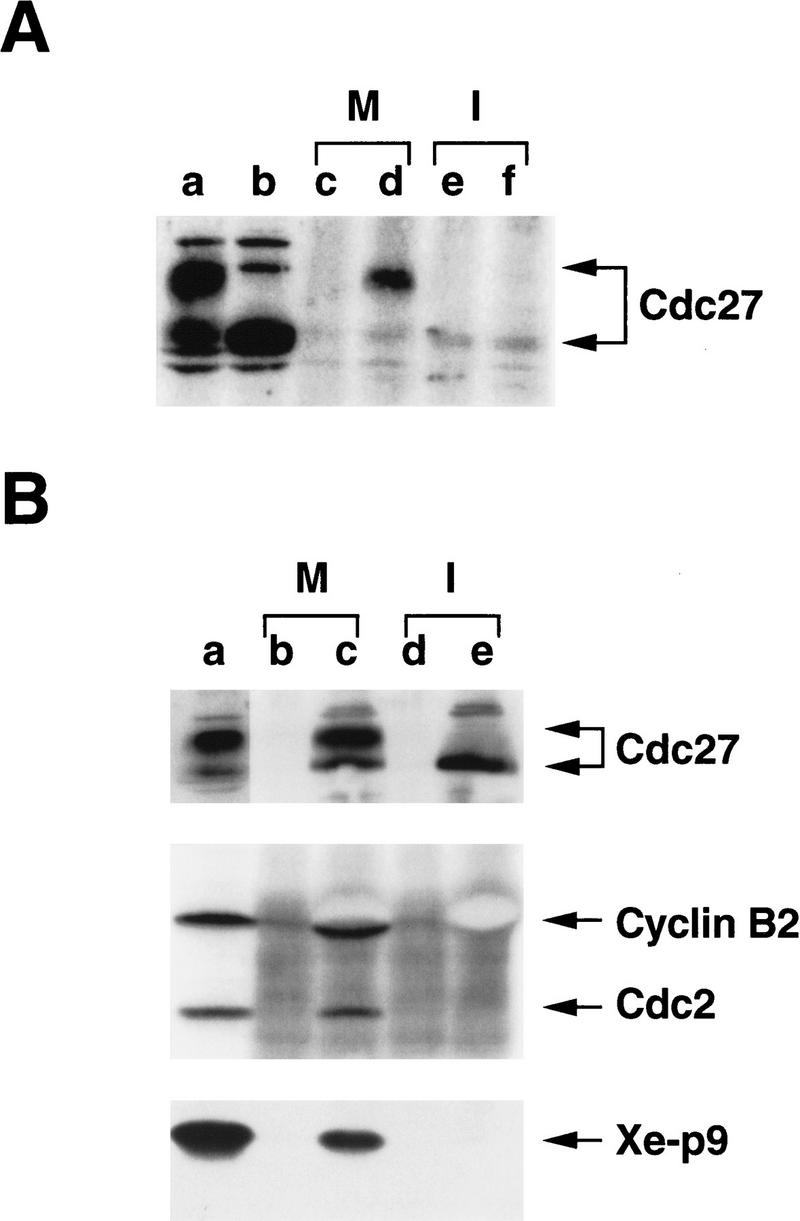
Cdc27 coimmunoprecipitates with p9 during M phase. (A) p9 was immunoprecipitated with anti-p9 antibodies from cycloheximide-containing CSF extracts (M) or interphase extracts (I). The antibodies (100 μg/ml) were recovered by use of protein A–Sepharose beads (100 μl per ml of extract). The beads were washed four times with EB buffer (80 mm β-glycerolphosphate, 20 mm EGTA, 15 mm MgCl2 at pH 7.3) containing 2 mm sodium orthovanadate and 25 mm sodium fluoride, followed by two washes with HBS. The beads were then subjected to SDS-PAGE in a 10% polyacrylamide gel and processed for immunoblotting with anti-Cdc27 antibodies. (Lane a) 2 μl of CSF extract; (lane b) 2 μl of interphase extract; (lanes c, e) protein A beads containing control antibodies that were incubated in CSF and interphase extracts, respectively; (lanes d, f) protein A beads containing anti-p9 antibodies that were incubated in CSF and interphase extracts, respectively. The panel depicts an immunoblot with anti-Cdc27 antibodies. (B) The experiment was repeated as in part A except that anti-Cdc27 antibodies were used for the immunoprecipitation. The panels depict immunoblots with anti-Cdc27, anti-Xenopus cyclin B2, anti-Cdc2, and anti-p9 antibodies, as indicated. (Lane a) Two microliters of CSF extract; (lanes b, d) protein A beads containing control antibodies that were incubated in CSF and interphase extracts, respectively; (lanes c, e) protein A beads containing anti-Cdc27 antibodies that were incubated in CSF and interphase extracts, respectively.
The phosphorylation of APC components by recombinant Cdc2/cyclin B is strongly enhanced by p9
In principle, the association of p9 with the Cdc27 during M phase could reflect a targeting of the p9/Cdc2/cyclin B complex for ubiquitination by the APC. An alternative, but not mutually exclusive, explanation is that because phosphorylation and activation of the APC depend on active Cdc2/cyclin B, p9 could direct this Cdk to APC components such as Cdc27 to facilitate their phosphorylation. To address this notion, we tested the ability of a recombinant Cdc2/cyclin B complex to phosphorylate APC components in the absence and presence of p9 in vitro. For these experiments, we utilized two different preparations of the APC: one that had been immunoisolated from egg extracts with anti-Cdc27 antibodies and another that had been partially purified by ion-exchange chromatography. Furthermore, we utilized bacterially expressed His6–Cdc27 as a substrate in these studies.
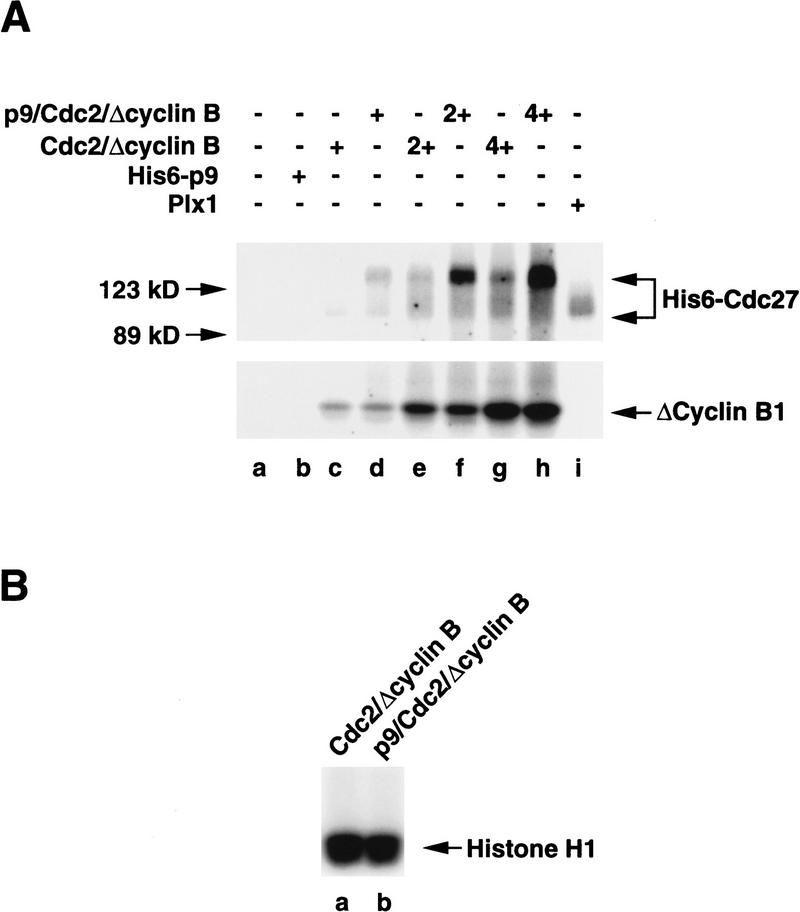
In the first set of experiments, we isolated the hypophosphorylated APC from interphase egg extracts with anti-Cdc27 antibodies (Fig. 5A). Then, we incubated the immunoprecipitated APC with either a dimeric Cdc2/Δcyclin B complex or a trimeric p9/Cdc2/Δcyclin B complex in the presence of [γ-32P]ATP. As assessed by silver staining, we determined that these two preparations contained equivalent amounts of Cdc2 and Δcyclin B (data not shown). As shown in Figure 5A (lane f), we observed that p9/Cdc2/Δcyclin B elicited substantial phosphorylation of two polypeptides that correspond to the hyperphosphorylated forms of the Cdc27 and BIME proteins, as determined by immunoblotting with anti-Cdc27 and anti-BIME antibodies (data not shown). In contrast, the phosphorylation of Cdc27 and BIME was much lower in the presence of a Cdc2/Δcyclin B complex lacking p9 (Fig. 5A, lane e). p9 does not appear to increase the intrinsic catalytic activity of the Cdc2/Δcyclin B complex in these assays because the autophosphorylation of Δcyclin B is similar in the presence and absence of p9 (Fig. 5A, cf. lanes e and f), and both complexes display similar histone H1 kinase activity (see Fig. 6).
Figure 5.
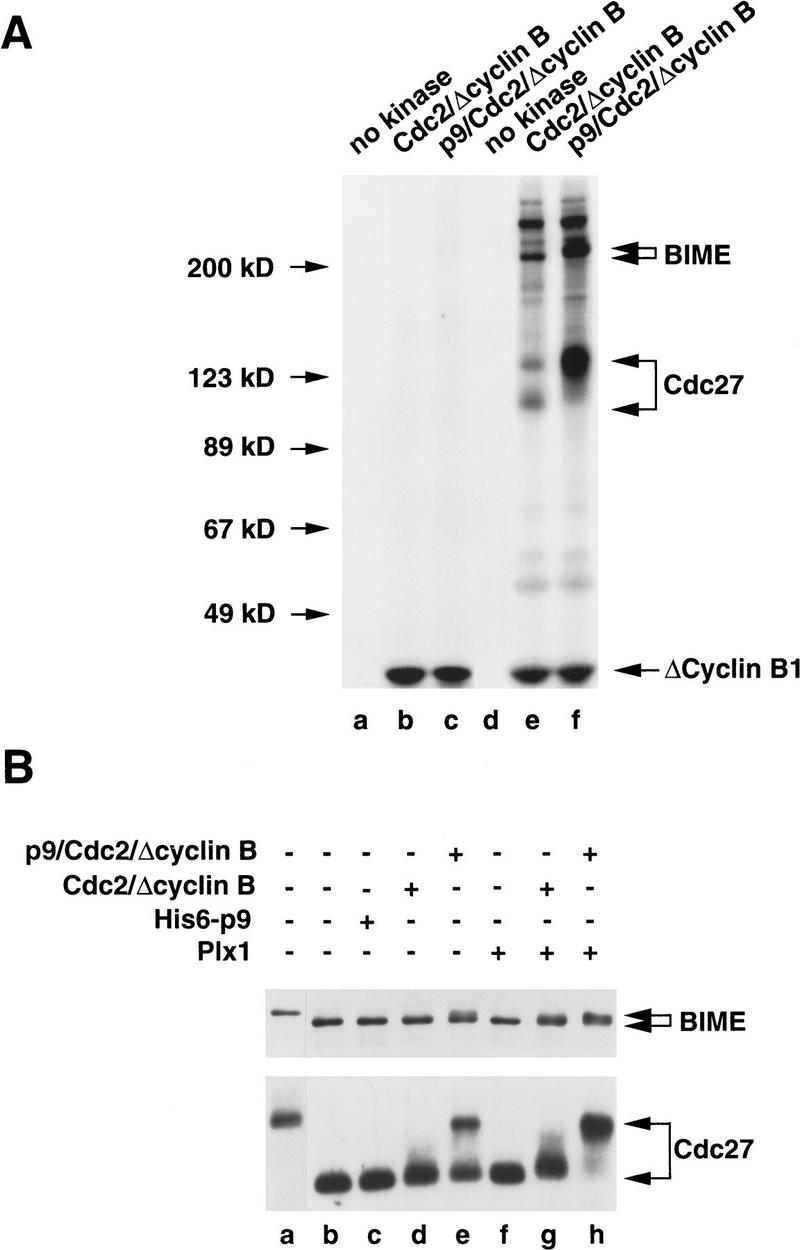
Phosphorylation of the Cdc27 and BIME subunits of the APC by recombinant Cdc2/cyclin B is strongly stimulated by p9. (A) The Xenopus APC was immunoprecipitated from interphase extracts with anti-Cdc27 antibodies bound to Affi-prep protein A beads (lanes d–f). In parallel, immunoprecipitations with control antibodies were also carried out (lanes a–c). The immunoprecipitates were washed as described in Materials and Methods. These washed immunoprecipitates were used as substrates for a Cdc2/Δcyclin B complex (lanes b,e) or a p9/Cdc2/Δcyclin B complex (lanes c,f). Lanes a and d were used as controls in which kinase buffer (5 mm Tris-HCl at pH 7.5, 10 mm MgCl2, 1 mm DTT, 0.1 mg/ml ovalbumin, 1 μm okadaic acid, and 100 μm ATP containing 5 μCi of [γ-32P]ATP) only was added. Phosphorylation was analyzed by SDS-PAGE in a 8% polyacrylamide gel. (B) The interphase form of the Xenopus APC was partially purified from egg extracts by ion-exchange chromatography as described in Materials and Methods and used as a substrate for the Cdc2/Δcyclin B complex (lanes d,g), the p9/Cdc2/Δcyclin B complex (lane e,h), and Plx1 (lane f–h). Following the kinase reactions, the samples were processed for immunoblotting with anti-Tsg24 antibodies (BIME; top) and anti-Cdc27 antibodies (bottom). Lane a depicts the partially purified APC from mitotic egg extracts; lane b shows a control containing a kinase buffer only; lane c contains no kinase but recombinant His6–p9 (10 ng/μl).
Figure 6.
p9 strongly enhances the phosphorylation of the isolated, recombinant His6–Cdc27 protein by Cdc2/Δcyclin B. (A) The human Cdc27 protein with a histidine-tag at its amino-terminal end (His6–Cdc27) was expressed in bacteria and tested as a substrate for the Cdc2/Δcyclin B complex (lanes c,e,g), the p9/Cdc2/Δcyclin B complex (lanes d,f,h), and Plx1 (lane i) in the presence of [γ-32P]ATP. Lanes e and f contain twice as much kinase as lanes c and d, and lanes g and h contain four times more kinase. Lane a is control to which kinase buffer only has been added. Lane b contains no kinase but recombinant His6–p9 (10 ng/μl). (B) The Cdc2/Δcyclin B (lane a) and p9/Cdc2/Δcyclin B complexes (lane b) exhibit very similar histone H1 kinase activity.
In parallel experiments, we treated an APC fraction from interphase egg extracts that had been partially purified by ion-exchange chromatography with Cdc2/Δcyclin B or p9/Cdc2/Δcyclin B (Fig. 5B). In addition, we incubated this fraction with the Xenopus Polo-like kinase Plx1, either alone or in combination with Cdc2/Δcyclin B or p9/Cdc2/Δcyclin B. To assess phosphorylation of the APC, we performed immunoblotting with anti-Cdc27 or anti-Tsg24 (BIME) antibodies. In the case of Cdc27, we observed that p9/Cdc2/Δcyclin B elicited the appearance of hyperphosphorylated Cdc27 whereas Cdc2/Δcyclin B alone had little effect (Fig. 5B, bottom, cf. lanes d and e). Active Plx1 alone did not cause hyperphosphorylation of Cdc27 under these conditions. However, Plx1 appeared to enhance the phosphorylation of Cdc27 in the presence of both p9/Cdc2/Δcyclin B and, to a lesser extent, Cdc2/Δcyclin B (Fig. 5B, lanes f–h). In the same samples, the BIME protein also displayed a subtle but reproducible reduction in electrophoretic mobility when treated with p9/Cdc2/Δcyclin B but not Cdc2/Δcyclin B (Fig. 5B, top, lanes d, e).
To pursue these observations further, we utilized a bacterially expressed form of the human His6–Cdc27 protein as a substrate (Fig. 6). These experiments were aimed at addressing the issue of whether p9 affects the phosphorylation of Cdc27 by Cdc2/Δcyclin B directly or acts indirectly through some other component in the APC preparations. We incubated His6–Cdc27 with varying concentrations of either Cdc2/Δcyclin B or p9/Cdc2/Δcyclin B in the presence of [γ-32P]ATP. Consistent with the results described above, p9 strongly stimulated the phosphorylation of His6–Cdc27 by recombinant Cdc2/Δcyclin B so that His6–Cdc27 migrated at its hyperphosphorylated position in SDS gels (Fig. 6A, top, lanes c–h). Although Plx1 did phosphorylate His6–Cdc27, it did not elicit hyperphosphorylation of this substrate under these conditions (Fig. 6A, lane i). In strong contrast to His6–Cdc27, the phosphorylation of Δcyclin B was similar in the absence and presence of p9 (Fig. 6A, bottom, lanes c–h). Finally, both the Cdc2/Δcyclin B and p9/Cdc2/Δcyclin B complexes phosphorylated the general Cdk substrate histone H1 to similar extents (Fig. 6B). Thus, it appears that p9 acts by facilitating the recognition of His6–Cdc27 by Cdc2/Δcyclin B rather than increasing the inherent catalytic activity of Cdc2/Δcyclin B.
Discussion
In this study we have analyzed the role of p9, a Xenopus homolog of the Cdk-associated Suc1/Cks protein, at mitosis in Xenopus egg extracts. As described previously, egg extracts that are induced to enter M phase in the absence of p9 are unable to carry out proteolysis of the mitotic B-type cyclins (Patra and Dunphy 1996). However, the molecular basis of this defect was not resolved previously. Similarly, fission yeast cells lacking the Suc1 protein become arrested in M phase by an unknown mechanism (Moreno et al. 1989; Basi and Draetta 1995). The proteolysis of cyclin B after entry into mitosis is achieved by the ubiquitin-dependent, APC-mediated proteasomal pathway (for review, see Ciechanover 1994; Murray 1995; King et al. 1996; Hoyt 1997). This pathway involves a series of concerted biochemical steps, one or more of which apparently requires the involvement of p9. Although the E1 and E2 components of this pathway are constitutively active throughout the cell cycle, the E3 component or ubiquitin ligase is activated only on entry into mitosis (King et al. 1995; Lahav-Baratz et al. 1995; Peters et al. 1996). The APC is essential for E3 activity, but other proteins such as those in the Cdc20 and Cdh1/Hct1 family of proteins, may also be necessary, even though apparently they are not permanent constituents of the APC (Schwab et al. 1997; Visintin et al. 1997). Once cyclin B undergoes polyubiquitination, it is delivered to the proteasome, where it is subjected to rapid and processive proteolysis. Cdc2 remains stably bound to polyubiquitinated cyclin B (Amon et al. 1994; Brandeis and Hunt 1996; Yamano et al. 1996), but both Cdc2 and p9 are spared from destruction by the proteasome.
In principle, p9 could be required for ubiquitin-mediated degradation of cyclin B at any one of several steps. The activation of the APC at mitosis is known to be dependent on active Cdc2/cyclin B (Luca and Ruderman 1989; Félix et al. 1990b; Hershko et al. 1994; Lahav-Baratz et al. 1995; Sudakin et al. 1995). The mechanism by which Cdc2/cyclin B triggers activation of the APC has been unresolved, but the critical target(s) of Cdc2/cyclin B in this pathway could require p9 for properly controlled phosphorylation. Another explanation would be that Cdc2/cyclin B cannot serve as a substrate for APC-dependent ubiquitin ligase activity when p9 is not associated with the Cdk complex. In this scenario, p9 would act as a specificity factor in targeting cyclin B for polyubiquitination by the active APC. Finally, another model would be that the complex of Cdc2 and polyubiquitinated cyclin B could not interact properly with the proteasome in the absence of p9.
To distinguish among these possibilities, we depleted p9 from M-phase extracts in which the APC is already active. If p9 were required directly for the ubiquitination and/or proteolysis of cyclin B, then removal of p9 at this time should abolish cyclin B destruction. Alternatively, if p9 plays a regulatory role in switching on or allowing the manifestation of ubiquitin-ligase (E3) activity, then the immunodepletion of p9 from extracts in which the cyclin destruction machinery has already been activated should not have any effect on cyclin B degradation. Our results indicate that p9 acts by controlling E3 activity, as depletion of p9 from extracts already in M phase has no effect on the ability of these extracts to degrade cyclin B. This observation is clearly not consistent with the notion that p9 is required directly for recognition of cyclin B by either the E3-ubiquitin ligase or the proteasome. Instead, our results strongly suggest that activation of the APC cannot occur in the absence of p9.
To explore how p9 might affect regulation of the APC, we have examined the phosphorylation of the Cdc27 component of the APC in extracts that have been induced to enter mitosis in the absence of p9. Normally, Cdc27 and a number of other subunits of the APC (e.g., APC1-BIME) undergo phosphorylation at M phase as the cyclin destruction machinery is switched on. Phosphorylation of the APC appears to be important for its activity at mitosis, because treatment of the APC with phosphatase abolishes ubiquitin–ligase activity (King et al. 1995; Lahav-Baratz et al. 1995; Peters et al. 1996). Significantly, we find that the mitotic hyperphosphorylation of the Cdc27 protein is abolished in extracts that have entered M phase in the absence of p9. These same extracts are totally incapable of carrying out the destruction of cyclin B. Taken together, our results strongly support a model in which p9 is required for activation of the cyclin destruction machinery.
In an effort to investigate how p9 might be regulating the destruction machinery, we examined whether p9 could associate physically with components of the APC and/or direct phosphorylation of the APC by the Cdc2/cyclin B complex. We found that some p9 can be found in a complex with the Cdc27 component of the APC at mitosis, but not during interphase. Similar results have been obtained in the clam oocyte system (Sudakin et al. 1997). However, only a small proportion of the Cdc27 appears to be associated with p9 at M phase in Xenopus egg extracts, suggesting that this is a weak or transient interaction.
In another series of experiments, we investigated whether p9 could direct the phosphorylation of the APC by the Cdc2/cyclin B complex. It is clear that activation of Cdc2/cyclin B at mitosis turns on the cyclin destruction machinery, but the molecular mechanism of this phenomenon has not been elucidated. The APC can be depleted from Xenopus egg extracts by the MPM-2 antibody, which recognizes a phosphopeptide epitope on mitotic phosphoproteins (King et al. 1995). However, although Cdc2 can create the MPM-2 epitope on substrate proteins, other kinases such as Plx1, the Xenopus Polo-like kinase, also clearly possess this capacity (Kuang and Ashorn 1993; Kumagai and Dunphy 1996). There is a decided lag between the appearance of active Cdc2/cyclin B and the phosphorylation of Cdc27 and activation of the APC (Hershko et al. 1994; Lahav-Baratz et al. 1995; Sudakin et al. 1995). This observation might suggest that there is an intermediary step(s) or modulating event(s) between activation of Cdc2/cyclin B and the APC. Consistent with this possibility, it has been reported that the murine Polo-like kinase (Plk) and Cdc2/cyclin B can collaborate to activate the APC in vitro (Kotani et al. 1998). Kotani et al. observed that Cdc2/cyclin B did not phosphorylate the APC directly but acted indirectly by activating Plk. However, it is not known whether Cdc2/cyclin B is the physiological activator of Plk in vivo. Similarly, Descombes and Nigg (1998) have implicated Plx1 in the control of cyclin degradation in cytostatic factor (CSF)-arrested Xenopus egg extracts. Furthermore, proteins in the Cdc20 and Cdh1/Hct1 family are essential for APC functions (Dawson et al. 1995; Sigrist et al. 1995; Schwab et al. 1997; Visintin et al. 1997; Shirayama et al. 1998). Collectively, these findings suggest that Cdc2/cyclin B is necessary but not sufficient for activation of the APC.
We find that recombinant Cdc2/cyclin B can bring about phosphorylation of APC components such as the Cdc27 and BIME proteins, but this reaction occurs efficiently only in the presence of p9. This effect is most obvious in the case of the Cdc27 component of the APC, but the Cdc2-dependent phosphorylation of BIME is also enhanced by p9. In the case of Cdc27, the p9-stimulated phosphorylation was observed with several distinct Cdc27-containing preparations. In particular, we utilized an APC fraction that had been partially purified by ion-exchange chromatography, an APC preparation that had been immunopurified with anti-Cdc27 antibodies, and recombinant His6–Cdc27 from bacteria. In all three cases, the presence of p9 in the Cdc2/cyclin B complex strongly stimulated the phosphorylation of Cdc27. Because this effect could be observed even with bacterially expressed His6–Cdc27, it appears that p9 directly enhances the ability of Cdc2/cyclin B to phosphorylate the Cdc27 protein. Although there is one report that the fission yeast Suc1 protein can suppress the phosphorylation of intermediate filament proteins by Cdc2 (Kusubata et al. 1992), our studies represent the first example in which a Suc1/Cks protein can be shown to modulate the functional properties of a Cdk by promoting phosphorylation of a Cdk substrate. This observation rationalizes why the Suc1/Cks protein is needed for cell cycle progression. As discussed above, the APC is most likely regulated by an additional kinase(s) besides Cdc2/cyclin B in vivo. In the future, it will be important to assess the relative contributions of Cdc2/cyclin B and other kinases such as the Polo-like kinases under conditions where Cdc2/cyclin B contains an associated p9 subunit. For example, Kotani et al. (1998) did not observe that baculovirus-expressed Cdc2/cyclin B could phosphorylate the APC, but this Cdk did not contain a recombinant Suc1/Cks protein.
The rationale for why Cdc2/cyclin B should require an accessory subunit to phosphorylate Cdc27 efficiently remains to be resolved. In structural studies, it has been found that the human Cks1 protein contains a potential anion binding pocket that could interact with a phosphate group (Arvai et al. 1995; Bourne et al. 1996). Human Cks1 is located on the same face of the Cdk as the catalytic site. The orientation of the anion-binding pocket suggests that it would most likely bind to a phosphate moiety in a ligand that interacts with the Cdk/cyclin complex (e.g., a substrate) rather than a phosphate in the Cdk/cyclin complex itself. Interestingly, Cdc27 is multiply phosphorylated at mitosis, which raises the possibility that the extent of phosphorylation of Cdc27 could influence its recognition by p9/Cdc2/cyclin B. In this scenario, p9/Cdc2/cyclin B would have a low affinity for unphosphorylated or hypophosphorylated Cdc27 and a higher affinity for more extensively phosphorylated Cdc27. In principle, this action of p9 as a docking factor might help to explain some characteristic features in the kinetics of cyclin B destruction, such as the requirement for a threshold concentration of Cdc2/cyclin B and the lag between the activation of Cdc2/cyclin B and the activation of the APC.
In conclusion, we have demonstrated that p9 directly regulates phosphorylation of the APC at mitosis. This observation should aid in unraveling the complex series of events that result in the metaphase–anaphase transition in eukaryotic cells. Moreover, it will be important to assess whether p9 controls the phosphorylation of Cdk substrates involved in other cell cycle processes.
Materials and methods
Preparation of recombinant, untagged Xe-p9 in Sf9 insect cells
To prepare a full-length recombinant p9 lacking a histidine tag, we subcloned the NdeI–EcoRI fragment of pVL1393N–His6–p9 (Patra and Dunphy 1996) into pVL1393 that had been digested with NdeI and EcoRI. The resulting vector was used for the production of untagged p9 in Sf9 insect cells (Kumagai and Dunphy 1995).
Preparation of Cdc2/cyclin B complexes
A Cdc2/Δcyclin B complex was prepared as described in Kumagai and Dunphy (1995). Because high concentrations of p9 inhibit activation of Cdc2 by the Cdk-activating kinase (D. Patra and W.G. Dunphy, unpubl.), a p9/Cdc2/Δcyclin B complex containing equimolar amounts of all three proteins was prepared as follows. Nickel-agarose beads containing Δcyclin B (25 μl) were incubated with 500 μl of a Cdc2-containing Sf9 cell lysate for 20 min at 22°C in the presence of 0.5 mm ATP and 10 mm MgCl2. The beads were washed three times with HEPES-buffered saline (HBS; 10 mm HEPES-KOH at pH 7.5, 150 mm NaCl) and then incubated with 500 μl of a p9-containing Sf9 cell lysate at 4°C for 30 min. The beads were isolated by centrifugation and then incubated further with a fresh 500-μl aliquot of p9-containing lysate for 30 min. The beads were then washed three times with HBS and eluted with 250 mm imidazole in HBS. The kinase complexes were then used at a final dilution of 1 : 10 in HBS (1 : 100 for assessing histone H1 kinase activity). Complexes between the Cdc2–AF mutant and either truncated Δcyclin B1 or the full-length cyclin B1 were prepared by gel filtration chromatography with a SMART system (Pharmacia; Kumagai and Dunphy 1995).
Preparation of Xenopus egg extracts
CSF-arrested extracts from unactivated Xenopus eggs were prepared as described (Murray 1991). Activation of these egg extracts by the addition of CaCl2 (0.4 mm) and visual monitoring of the assembly and disassembly of nuclei formed around sperm chromatin (500 demembranated Xenopus sperm nuclei per microliter of extract) were described previously (Kumagai and Dunphy 1995; Mueller et al. 1995a). In some cases, cycloheximide (100 μg/ml) was added as described in the legends to Figures 1–4.
Processing of anti-Cdc27 immunoprecipitates for treatment with kinases
To immunoprecipitate the Xenopus APC from interphase extracts to investigate the phosphorylation of APC subunits by the Cdc2/Δcyclin B complex or the p9/Cdc2/Δcyclin B complex, anti-Cdc27 antibodies (Tugendreich et al. 1995; King et al. 1995) were first bound to Affi-prep protein A support beads (Bio-Rad) at 4°C for an hour. After washing the beads with HBS, interphase extracts containing cycloheximide (100 μg/ml) were added, mixed thoroughly, and incubated at 4°C for 2 hr. The beads were then recovered by centrifugation and washed according to the protocol of King et al. (1995) with minor modifications. The beads were first washed six times with 20 mm Tris-HCl (pH 7.7), 500 mm KCl, 0.1 mm CaCl2, 1 mm MgCl2, 1 mm DTT, 0.5% NP-40 and then washed twice with 20 mm Tris-HCl (pH 7.7), 100 mm KCl, 0.1 mm CaCl2, 1 mm MgCl2, 1 mm DTT. Finally, the beads were washed twice with HBS.
Partial purification of the APC from Xenopus egg extracts
The Xenopus APC was prepared from high-speed supernatants of both CSF-arrested (M phase) and interphase egg extracts as described in King et al. (1995). Fractionations of the high-speed supernatants were performed with a 6-ml Source 15 Q column (Pharmacia) that was eluted with six column volumes of a linear salt gradient from 100 to 500 mm KCl in buffer Q (20 mm Tris-HCl at pH 7.7, 0.1 mm CaCl2, 1 mm MgCl2, 1 mm DTT). Fractions (2.5 ml) were collected and analyzed by immunoblotting with anti-Cdc27 and anti-Tsg24 (BIME) antibodies. Fractions containing peak amounts of both the Cdc27 and BIME proteins were collected, desalted on PD10 columns (Pharmacia), and concentrated to 500 μl by use of Centriprep-10 concentrators (Amicon). The concentrated fractions were aliquoted and stored at −70°C.
Preparation of recombinant His6–Cdc27 in bacteria
To prepare a full-length recombinant human Cdc27 with a six-histidine tag at its amino-terminal end (His6–Cdc27) in bacteria, a PCR product containing the entire cDNA of Cdc27 was first prepared with pSTU16 as the template (Tugendreich et al. 1993). In this construct, the initiating codon (ATG) was converted into an NdeI restriction site with the primers 5′-TGAGTCCACATATGACGGTGCTGCAGGAACCC-3′ and 5′-CGGAATTCCCAGAAGTTAAAATTCATCACTTTCAGCTGC-3′. After digestion of the PCR product with NdeI and EcoRI, it was then subcloned into the bacterial expression vector pET9-His6 (Shou and Dunphy 1996) that had been digested with NdeI and EcoRI. The His6–Cdc27 protein was expressed in BL21(DE3)pLysS cells and purified by nickel agarose chromatography.
Miscellaneous
Preparation of recombinant His6–p9 in Sf9 insect cells, preparation of antibodies against a carboxy-terminal peptide of p9, and depletion of p9 from M-phase and interphase egg extracts with these anti-p9 antibodies bound to protein A–Sepharose beads (Sigma) were performed as reported previously (Patra and Dunphy 1996). Antibodies against the human cyclin B1 were obtained from Upstate Biotechnology (Lake Placid, NY). Antibodies against Xenopus Cdc25, Wee1, and Myt1 were described previously (Kumagai and Dunphy 1992; Mueller et al. 1995a,b). Antibodies against human Cdc27 were generously supplied by Dr. P. Hieter (University of British Columbia, Vancouver, B.C., Canada), and antibodies against the mouse Tsg24 (BIME; Starborg et al. 1994) were kindly provided by Dr. C. Hoog (Karolinska Institutet, Stockholm, Sweden). Immunoblotting with various antibodies was done as described (Coleman et al. 1993) by use of 125I-labeled protein A (ICN, Cleveland, OH) or 125I-labeled sheep anti-mouse IgG antibodies (Amersham, Arlington Heights, IL). Detection of p9 in Figure 4B and the Cdc27 and BIME proteins in Figure 5B was performed by chemiluminescence (ECL, Amersham) with a goat anti-rabbit-IgG–HRP conjugate (Bio-Rad). Coimmunoprecipitation of Cdc27 with p9 from egg extracts was performed with 100 μg/ml of anti-p9 antibodies as described (Mueller et al. 1995a; Patra and Dunphy 1996). Coimmunoprecipitation of p9 with Cdc27 from egg extracts was performed by use of a rabbit polyclonal antiserum (10 μl of serum per 100 μl of egg extract) directed against the human Cdc27 protein (King et al. 1995; Tugendreich et al. 1995). Purification of active Plx1 from Sf9 cells treated with okadaic acid was performed as described previously (Kumagai and Dunphy 1996). Protein concentrations were determined by use of a Bio-Rad protein assay kit with bovine serum albumin or lysozyme as the standard.
Acknowledgments
We thank our colleagues for comments on the manuscript. We are grateful to Philip Hieter for the human Cdc27 cDNA clone and for antibodies against the human Cdc27 protein, and to Christer Hoog for antibodies against the mouse Tsg24 protein (BIME). D.P. wishes to thank Sophie Wang for Xenopus Plx1 and Mary Kennedy and her laboratory members for the use of the FPLC (Pharmacia) system. D.P. has been supported by fellowships from the Leukemia Society of America and the American Cancer Society (California Division, Inc.). This work was supported by the Howard Hughes Medical Institute, in which W.G.D. is an investigator.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL dunphy@cco.caltech.edu; FAX (626) 449-0679.
References
- Amon A, Irniger S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. doi: 10.1016/0092-8674(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Arvai AS, Bourne Y, Hickey MJ, Tainer JA. Crystal structure of the human cell cycle protein CksHs1: Single domain fold with similarity to kinase N-lobe domain. J Mol Biol. 1995;249:835–842. doi: 10.1006/jmbi.1995.0341. [DOI] [PubMed] [Google Scholar]
- Basi G, Draetta G. p13suc1 of Schizosaccharomyces pombe regulates two distinct forms of the mitotic cdc2 kinase. Mol Cell Biol. 1995;15:2028–2036. doi: 10.1128/mcb.15.4.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y, Watson MH, Hickey MJ, Holmes W, Rocque W, Reed SI, Tainer JA. Crystal structure and mutational analysis of the human Cdk2 kinase complex with cell cycle-regulatory protein CksHs1. Cell. 1996;84:863–874. doi: 10.1016/s0092-8674(00)81065-x. [DOI] [PubMed] [Google Scholar]
- Brandeis M, Hunt T. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 1996;15:5280–5289. [PMC free article] [PubMed] [Google Scholar]
- Brizuela L, Draetta G, Beach D. p13suc1 acts in the fission yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J. 1987;6:3507–3514. doi: 10.1002/j.1460-2075.1987.tb02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O, Peters J-M, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes & Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Tang Z, Dunphy WG. Negative regulation of the Wee1 protein kinase by direct action of the Nim1/Cdr1 mitotic inducer. Cell. 1993;72:919–929. doi: 10.1016/0092-8674(93)90580-j. [DOI] [PubMed] [Google Scholar]
- Dawson IA, Roth S, Artavanis-Tsakonas S. The Drosophila cell cycle gene fizzy is required for normal degradation of cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J Cell Biol. 1995;129:725–737. doi: 10.1083/jcb.129.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P, Nigg EA. The polo-like kinase Plx1 is required for M-phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The Cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Brizuela L, Beach D, Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988;54:423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Félix MA, Cohen P, Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: Evidence from the effects of okadaic acid. EMBO J. 1990a;9:675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix MA, Labbé JC, Dorée M, Hunt T, Karsenti E. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature. 1990b;346:379–382. doi: 10.1038/346379a0. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- Gautier J, Norbury C, Lohka M, Nurse P, Maller J. Purified maturation promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+ Cell. 1988;54:433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990;60:487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. Cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Hadwiger JA, Wittenberg C, Mendenhall MD, Reed SI. The Saccharomyces cerevisiae CKS1 gene, a homolog of the Schizosaccharomyces pombe suc1+ gene, encodes a subunit of the Cdc28 protein kinase complex. Mol Cell Biol. 1989;9:2034–2041. doi: 10.1128/mcb.9.5.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayles J, Beach D, Durkacz B, Nurse P. The fission yeast cell cycle control gene cdc2: Isolation of a sequence suc1 that suppresses cdc2 mutant function. Mol Gen Genet. 1986a;202:291–293. doi: 10.1007/BF00331653. [DOI] [PubMed] [Google Scholar]
- Hayles J, Aves S, Nurse P. suc1 is an essential gene involved in both the cell cycle and growth in fission yeast. EMBO J. 1986b;5:3373–3379. doi: 10.1002/j.1460-2075.1986.tb04653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ganoth D, Sudakin V, Dahan A, Cohen LH, Luca FC, Ruderman JV, Eytan E. Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2. J Biol Chem. 1994;269:4940–4946. [PubMed] [Google Scholar]
- Hindley J, Phear G, Stein M, Beach D. Suc1+ encodes a predicted 13-kilodalton protein that is essential for cell viability and is directly involved in the division cycle of Schizosaccharomyces pombe. Mol Cell Biol. 1987;7:504–511. doi: 10.1128/mcb.7.1.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA. Eliminating all obstacles: Regulated proteolysis in the eukaryotic cell cycle. Cell. 1997;91:149–151. doi: 10.1016/s0092-8674(00)80396-7. [DOI] [PubMed] [Google Scholar]
- Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81:269–277. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus Cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessus C, Rime H, Haccard O, Van Lint J, Goris J, Merlevede W, Ozon R. Tyrosine phosphorylation of p34cdc2 and p42 during meiotic maturation of Xenopus oocyte: Antagonistic action of okadaic acid and 6-DMAP. Development. 1991;111:813–820. doi: 10.1242/dev.111.3.813. [DOI] [PubMed] [Google Scholar]
- Juang Y-L, Huang J, Peters J-M, McLaughlin ME, Tai C-Y, Pellman D. APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle. Science. 1997;275:1311–1314. doi: 10.1126/science.275.5304.1311. [DOI] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- King RW, Peters J-M, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters J-M, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- Kotani S, Tugendreich S, Fujii M, Jorgensen P-M, Watanabe N, Hoog C, Hieter P, Todokoro K. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell. 1998;1:371–380. doi: 10.1016/s1097-2765(00)80037-4. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Fesquet D, Johnson AL, Johnston LH. Budding yeast RSI1/APC2, a novel gene necessary for initiation of anaphase, encodes an APC subunit. EMBO J. 1998;17:498–506. doi: 10.1093/emboj/17.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J, Ashorn CL. At least two kinases phosphorylate the MPM-2 epitope during Xenopus oocyte maturation. J Cell Biol. 1993;123:859–868. doi: 10.1083/jcb.123.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- ————— Control of the Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol Biol Cell. 1995;6:199–213. doi: 10.1091/mbc.6.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Kusubata M, Tokui T, Matsuoka Y, Okumura E, Tachibana K, Hisanaga S, Kishimoto T, Yasuda H, Kamijo M, Ohba Y, Tsujimura K, Yatani R, Inagaki M. p13suc1 suppresses the catalytic function of p34cdc2 for intermediate filament proteins, in vitro. J Biol Chem. 1992;267:20937–20942. [PubMed] [Google Scholar]
- Lahav-Baratz S, Sudakin V, Ruderman J, Hershko A. Reversible phosphorylation controls the activity of cyclosome-associated cyclin-ubiquitin ligase. Proc Natl Acad Sci. 1995;92:9303–9307. doi: 10.1073/pnas.92.20.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JR, Michaud WA, Sikorski RS, Hieter PA. Cdc16p, Cdc23p and Cdc27p form a complex essential for mitosis. EMBO J. 1994;13:4321–4328. doi: 10.1002/j.1460-2075.1994.tb06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Stanton JJ, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates cdc2 on threonine 14 and localizes to the endoplasmic reticulum and golgi complex. Mol Cell Biol. 1997;17:571–583. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca FC, Ruderman JV. Control of programmed cyclin destruction in a cell-free system. J Cell Biol. 1989;109:1895–1909. doi: 10.1083/jcb.109.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca FC, Shibuya EK, Dohrmann CE, Ruderman JV. Both cyclin A Δ60 and B Δ97 are stable and arrest cells in M-phase, but only cyclin B Δ97 turns on cyclin destruction. EMBO J. 1991;10:4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CH, Russell P. Human Wee1 kinase inhibits cell-division by phosphorylating p34cdc2 exclusively on tyr15. EMBO J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski KL, Dunphy WG, Russell P, Gould SJ, Newport JW. Cloning and characterization of Xenopus Cdc2, a component of MPF. Cold Spring Harbor Symp Quant Biol. 1991;56:377–384. doi: 10.1101/sqb.1991.056.01.045. [DOI] [PubMed] [Google Scholar]
- Moreno S, Hayles J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases-engines, clocks and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995a;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: A membrane-associated inhibitory kinase that phosphorylates cdc2 on both threonine-14 and tyrosine-15. Science. 1995b;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Murray A. Cyclin ubiquitination: The destructive end of mitosis. Cell. 1995;81:149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell-cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin-B complex by the human Wee1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- Patra D, Dunphy WG. Xe-p9, a Xenopus Suc1/Cks homolog, has multiple essential roles in cell cycle control. Genes & Dev. 1996;10:1503–1515. doi: 10.1101/gad.10.12.1503. [DOI] [PubMed] [Google Scholar]
- Peters J-M, King RW, Hoog C, Kirschner MW. Identification of BIME as a subunit of the anaphase-promoting complex. Science. 1996;274:1199–1201. doi: 10.1126/science.274.5290.1199. [DOI] [PubMed] [Google Scholar]
- Picard A, Labbé JC, Barakat H, Cavadore JC, Dorée M. Okadaic acid mimics a nuclear component required for cyclin B-Cdc2 kinase microinjection to drive starfish oocytes into M-phase. J Cell Biol. 1991;115:337–344. doi: 10.1083/jcb.115.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HE, Stueland CS, Thomas J, Russell P, Reed SI. Human cDNAs encoding homologs of the small p34cdc28/cdc2-associated protein of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Genes & Dev. 1990;4:1332–1344. doi: 10.1101/gad.4.8.1332. [DOI] [PubMed] [Google Scholar]
- Schwab M, Lutum AS, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The polo-like kinase Cdc5p and the WD-repeat protein Cdc20/Fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou W, Dunphy WG. Cell cycle control by Xenopus p28Kix1, a developmentally regulated inhibitor of cyclin-dependent kinases. Mol Biol Cell. 1996;7:457–469. doi: 10.1091/mbc.7.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Jacobs H, Stratmann R, Lehner CF. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J. 1995;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starborg M, Brundell E, Gell K, Hoog C. A novel murine gene encoding a 216-kDa protein is related to a mitotic checkpoint regulator previously identified in Aspergillus nidulans. J Biol Chem. 1994;269:24133–24137. [PubMed] [Google Scholar]
- Solomon MJ, Lee T, Kirschner MW. Role of phosphorylation in p34cdc2 activation: Identification of an activating kinase. Mol Biol Cell. 1992;3:13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6:185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Shteinberg M, Ganoth D, Hershko J, Hershko A. Binding of activated cyclosome to p13suc1. J Biol Chem. 1997;272:18051–18059. doi: 10.1074/jbc.272.29.18051. [DOI] [PubMed] [Google Scholar]
- Tang Y, Reed SI. The Cdk-associated protein Cks1 functions both in G1 and G2 in Saccharomyces cerevisiae. Genes & Dev. 1993;7:822–832. doi: 10.1101/gad.7.5.822. [DOI] [PubMed] [Google Scholar]
- Tugendreich S, Boguski MS, Seldin MS, Hieter P. Linking yeast genetics to mammalian genomes: Identification and mapping of the human homolog of Cdc27 via the expressed sequence tag (EST) data base. Proc Natl Acad Sci. 1993;90:10031–10035. doi: 10.1073/pnas.90.21.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Visintin R, Prinz S, Amon A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. Regulation of the human Wee1hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Kumada K, Yanagida M. Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J Cell Sci. 1997;110:1793–1804. doi: 10.1242/jcs.110.15.1793. [DOI] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Hunt T. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J. 1996;15:5268–5279. [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, Nakaseko Y, Samejima I, Kumada K, Yamada H, Michaelson D, Yanagida M. 20S cyclosome complex formation and proteolytic activity inhibited by the cAMP/PKA pathway. Nature. 1996;384:276–279. doi: 10.1038/384276a0. [DOI] [PubMed] [Google Scholar]
- Yu H, Peters J-M, King RW, Page AM, Hieter P, Kirschner MW. Identification of a cullin homology region in a subunit of the anaphase-promoting complex. Science. 1998;279:1219–1222. doi: 10.1126/science.279.5354.1219. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Shin TH, Galova M, Obermaier B, Nasmyth K. Identification of subunits of the anaphase-promoting complex of Saccharomyces cerevisiae. Science. 1996;274:1201–1204. doi: 10.1126/science.274.5290.1201. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Shevchenko A, Andrews PD, Ciosk R, Galova M, Stark MJR, Mann M, Nasmyth K. Mass spectrometric analysis of the anaphase-promoting complex from yeast: Identification of a subunit related to cullins. Science. 1998;279:1216–1219. doi: 10.1126/science.279.5354.1216. [DOI] [PubMed] [Google Scholar]