Abstract
Ultraviolet B (UVB) damage is recognized as the most important etiological factor in the development of skin cancer. Human papillomaviruses (HPV) have also been implicated in the disease, although the mechanism of action of these viruses remains unknown. We present evidence here that Bak protein is involved in signaling apoptosis in the skin in response to UVB damage, and that cutaneous HPV E6 proteins target and abrogate Bak function by promoting its proteolytic degradation both in vitro and in regenerated epithelium. Additionally, HPV positive skin cancers had undetectable levels of Bak in contrast to HPV negative cancers, which expressed Bak. This study supports a link between the virus and UVB in the induction of HPV-associated skin cancer and reveals a survival mechanism of virally infected cells.
Keywords: HPV, skin cancer, apoptosis, UV, proteolysis
Apoptosis, or programmed cell death, triggers a series of events leading to the efficient elimination of a cell. In actively proliferating tissues, such as the epidermis of the skin, apoptosis-like phenomena are often found, as seen in the regression of hair follicles (Sieberg et al. 1995; Lindner et al. 1997) and in terminal differentiation (McCall and Cohen 1991; Haake and Polakowska 1993; Polakowska et al. 1994). The formation of “sunburn cells”, frequently observed in epidermis treated with UVB, have the apoptotic characteristic of condensed nuclei (Young 1987; Schwarz et al. 1995), the response to UVB radiation being in part dependent upon the expression of p53 (Ziegler et al. 1994). This p53-driven response, often termed cellular proofreading, eliminates rather than repairs, severely damaged cells, however p53-independent pathways have also been described (Allday et al. 1995; Gniadecki et al. 1997).
Solar UVB radiation represents one of the major environmental impacts for humans (Miralles et al. 1998) resulting in about 40,000 new cases of nonmelanoma skin cancer (NMSC) arising annually in the UK and 1,000,000 in the USA. In particular, it is the UVB portion (280–320 nm) of sunlight which stimulates the induction of somatic mutations through the formation of pyrimidine dimers and photoproducts (Herzinger et al. 1995). It has been suggested that failure to repair this DNA damage or to remove severely damaged cells by apoptosis may lead to the replication of deleterious mutations and ultimately to carcinogenesis (for review, see Griffiths et al. 1998). There may also be a role for other factors including immune response, genetic disposition, and infection by viruses such as HPV (for review, see Proby et al. 1996). Populations at most risk of developing HPV-associated NMSC are individuals with the rare inherited disease, Epidermodysplasia verruciformis (EV), and immunosuppressed patients, in particular renal transplant recipients (RTRs) who have a well-documented 50- to 100-fold increased risk of cutaneous squamous cell carcinoma (SCC). In both EV and immunocompromised patients, warts and SCCs contain a diverse spectrum of HPV types, the virus being present in ∼80% of lesions from immunocompromised patients and ∼30% of those from immunocompetent patients (Storey et al. 1998; Harwood et al. 2000, and references therein). The co-localization of warts and cancers at sun-exposed sites suggests a possible interaction between HPV and UVB irradiation (for review, see Proby et al. 1996).
The pro-apoptotic effector Bak is expressed in human epidermal keratinocytes (Mitra et al. 1997; Tomkova et al. 1997) and is a target of the E6 protein of anogenital HPVs (Thomas and Banks 1998). Here we demonstrate that the impact of cutaneous HPV E6 proteins resulting in Bak dysfunction has important physiological implications with regard to skin cancer development.
Results
Accumulation of Bak following UVB irradiation is abrogated by HPV E6 proteins
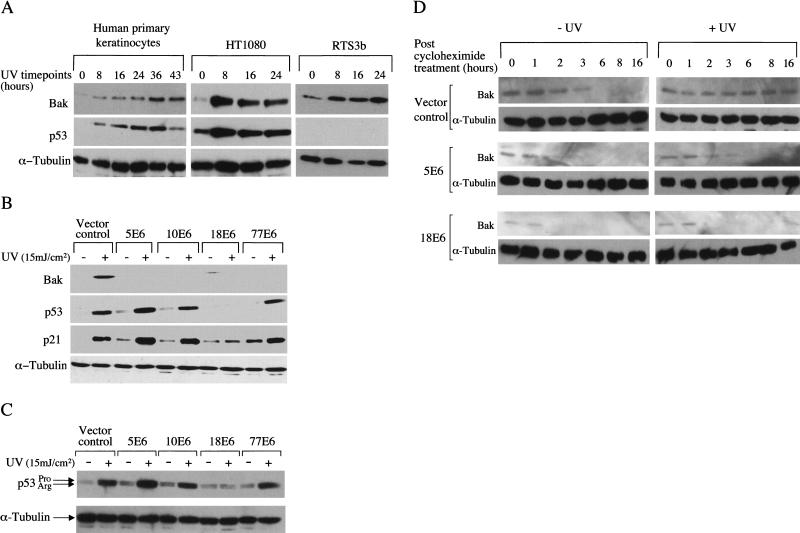
To investigate whether UVB damage might be a physiologically relevant inducer of Bak in the skin, primary normal human keratinocytes (the natural target of cutaneous HPVs), HT1080 cells which express wild-type p53 and human p53-null keratinocytes (RTS3b, Rapp et al. 1997) were treated with UVB and harvested at 0, 8, 16, 24, 36, and 43 h postirradiation. Equal amounts of protein extract were fractionated by SDS-PAGE and analyzed by Western blotting using antibodies specific for Bak and p53. The results demonstrate that UVB was a potent inducer of Bak in each cell line tested, including RTS3b cells, indicating that this induction can occur independently of p53 (Fig. 1A).
Figure 1.
Induction of Bak by UVB and abrogation of the Bak pathway by E6 proteins. (A) Human primary keratinocytes, HT1080 cells and human p53-null keratinocytes (RTS3b line) were treated with UVB (15 mJ/cm2) and the cells harvested at 0–43 h (only human primary keratinocytes survived after 24 h). Equal protein samples were resolved by SDS-PAGE, with Bak, p53 and α-tubulin levels detected by Western blotting using monoclonal antibodies. (B) HT1080 E6 polyclonal cell lines were treated with UVB (15 mJ/cm2) and the cells harvested 24 h later. Proteins were detected as in A. The membrane was exposed to X-ray film for 1 min prior to development. (C) Polymorphic forms of p53 from B were resolved on a 10% PAG and detected as previously. (D) HT1080 E6 cells were treated with 60 μg/mL cycloheximide 5 min post UV treatment (15 mJ/cm2). Protein extracts were prepared at the indicated time points following cycloheximide treatment and Bak levels determined by Western blotting. The membrane was exposed to X-ray film for 20 min prior to development to detect low levels of Bak.
We then asked whether the E6 proteins from a spectrum of HPV types could abrogate the increase in Bak protein levels following UVB treatment. We chose to investigate E6 proteins derived from diverse HPV groups, including HPV 5 (found in cutaneous carcinomas in EV), HPV 10 (found in benign warts and SCCs of RTRs), and HPV 77 (a novel type identified in SCCs and warts from RTRs), and compared their activity with that of the anogenital type HPV 18. UVB irradiation of HT1080 cell lines expressing the different E6 proteins (Jackson and Storey 2000) increased Bak protein levels in the HT1080 vector control cells as before but not in cells expressing the E6 proteins (Fig. 1B). In the HPV18 E6 cells, p53 protein failed to accumulate following UVB exposure as its degradation is efficiently promoted by anogenital HPV types (Scheffner et al. 1990; Werness et al. 1990), thereby blocking the induction of p21. In contrast, the HPV 5, 10, and 77 E6-expressing cells, in which the cutaneous E6 proteins are unable to degrade p53, have increased levels of both p53 and p21 protein following UVB damage. This suggests that the activity of the cutaneous E6 proteins, unlike those of other mucosal HPVs, is specifically directed toward Bak rather than p53. Furthermore, analysis of two other pro-apoptotic members of the Bcl-2 family, Bax and Bik, were not induced by UVB and were not targeted for degradation by E6 (S. Jackson and A. Storey, unpubl.). A common polymorphism at codon 72 of p53 results in either an arginine or proline at that position, the arginine being more susceptible to proteolytic degradation promoted by anogenital HPV E6 proteins (Storey et al. 1998). Because HT1080 cells express both p53 isoforms, which are easily identified by their different mobilities on gels, we tested whether the cutaneous E6 proteins were also able to preferentially target the arginine form. Resolution of the p53 isoforms on a lower percentage polyacrylamide gel showed that neither was targeted for degradation by the cutaneous HPV E6 proteins (Fig. 1C).
Analysis of Bak mRNA by Northern blotting showed that it was expressed at low levels in all the cell types used above and did not increase following treatment with UVB (data not shown) implying that the increase in Bak protein detected above was due to stabilization of the protein. To confirm this, the half-life of Bak was examined in UVB-irradiated HT1080 cells (Fig. 1D). Analysis of Bak from UVB-irradiated cells incubated with cycloheximide showed that the Bak half-life was significantly extended. In agreement with our previous observations, this increase in Bak half-life was not seen in cells expressing either the HPV5 or HPV18 E6 protein. In HPV5 E6-expressing cells the Bak half-life following UVB treatment was reduced to the level seen in the untreated vector control cells, whereas it was reduced even further by HPV18 E6 either with or without UVB irradiation.
E6 promotes proteolytic degradation of Bak
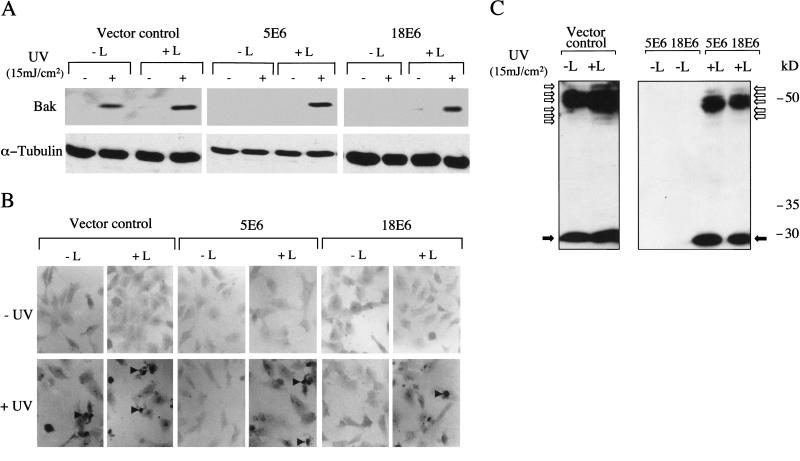
To investigate whether HPV5 E6 and HPV18 E6 promoted proteolytic degradation of Bak, cell lines were incubated with lactacystin, a specific inhibitor of proteasome activity (Fenteany et al. 1994, 1995; Dick et al. 1996; Craiu et al. 1997). The results in Figure 2A demonstrate that, although Bak protein levels increased in the vector control cells following UVB damage, no increase was seen in the HPV5 and HPV18 E6 cells treated with UVB alone. Only when lactacystin was added to UVB-treated E6-expressing cells prior to harvesting was Bak then detected at levels comparable to the control cells, suggesting that, at least in these cells, additional DNA damage was required to initiate Bak accumulation. This restoration of Bak levels in the E6 expressing cells was also accompanied by an increase in the number of apoptotic cells as determined by TUNEL staining (Fig. 2B). Further analysis of proteins extracted from lactacystin-treated UVB-irradiated cells in the presence of iodoacetimide revealed the presence of many higher molecular weight, modified Bak species (Fig. 2C).
Figure 2.
Ubiquitin-dependent proteolytic degradation of Bak and induction of apoptosis following UV treatment. (A) Western blot analysis of endogenous Bak levels in HT1080 vector control cells, 5E6 cells and 18E6 cells grown in the presence (+L) and absence (−L) of the proteasome inhibitor lactacystin (5 μM) with (+) and without (−) UVB (15 mJ/cm2) treatment. Lactacystin was added 2 h prior to the 24-h cell harvesting timepoint. (B) Parallel cultures of cells seeded onto glass coverslips were treated with UV and lactacystin as in A. Cells were fixed in paraformaldehyde and apoptotic cells detected by TUNEL (Magnification, 200×). (C) HT1080 cells were exposed to UVB (15 mJ/cm2) and were grown in the presence (+L) and absence (−L) of lactacystin for 2 h prior to cell harvesting, as in A. Cells were lysed 24 h post UVB in SDS sample buffer diluted in RIPA buffer containing iodoacetamide (10 mM), ubiquitinated Bak was detected by Western blotting.
Bak is induced in normal skin by UVB radiation
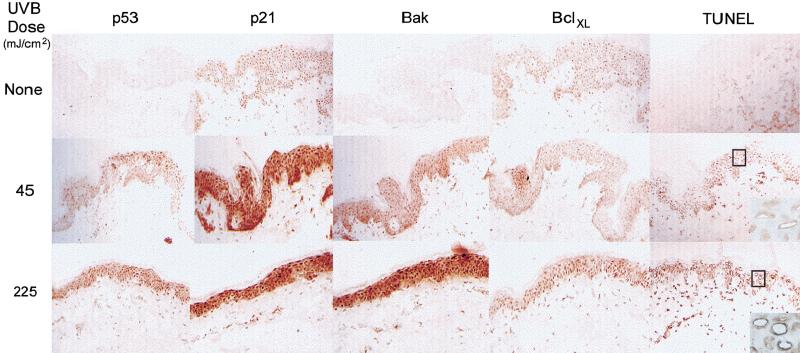
We extended our observations in monolayer cell cultures to normal skin specimens treated with UVB. Skin was obtained from an abdominal biopsy and subsequently maintained in organ culture, UVB-irradiated (45 and 225 mJ/cm2) and processed for immunohistochemistry. We observed strong p21 staining throughout the epidermis in accordance with its role in terminal differentiation (Ponten et al. 1995; Missero et al. 1996). The induction of both p53 and Bak proteins following UVB damage was not restricted to a particular stage of keratinocyte differentiation but instead occurred throughout the epidermal layers in the skin, including the basal cell compartment where putative stem cells reside (Fig. 3). Spontaneously apoptosing cells in non-UVB-treated skin were rarely detected; however, upon UVB exposure, apoptotic cells were readily detected throughout the epidermis using TUNEL staining, with the staining concentrated in a rim of condensed chromatin.
Figure 3.
Bak induction in normal skin by UVB. Skin specimens were irradiated with 45 or 225 mJ/cm2 UVB and snap frozen 24 h posttreatment for immunohistochemical analysis. Apoptotic cells were detected using TUNEL (original magnification, 100×, insert, 400×).
HPVs inhibit Bak accumulation and apoptosis in regenerated human skin
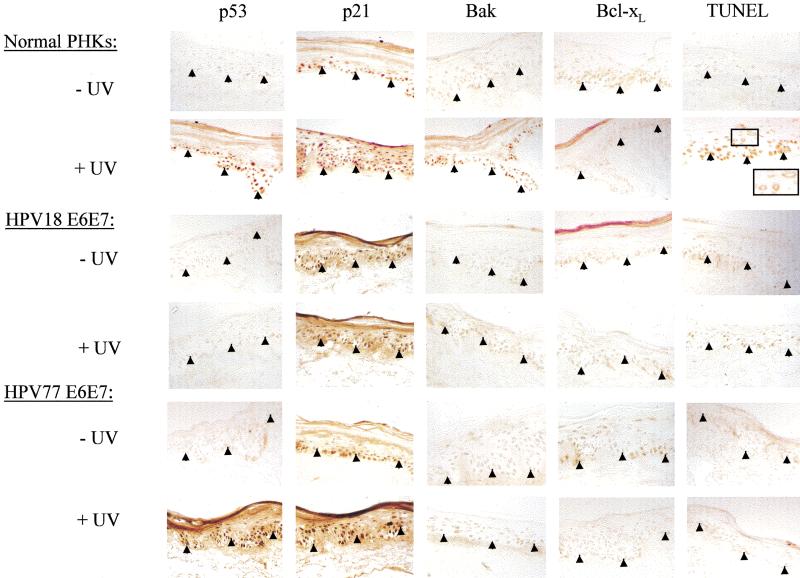
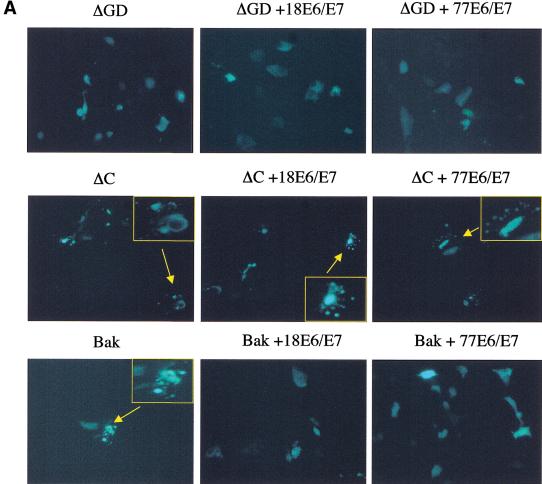
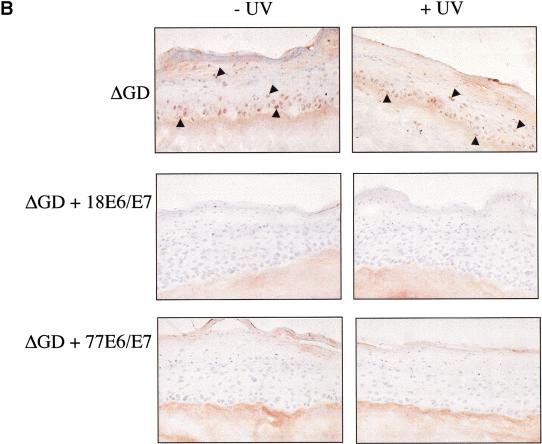
The finding that UV up-regulated Bak expression in skin, coupled with the promotion of Bak proteolysis by E6 proteins following UV treatment, suggests that E6 expression is likely to have significant effects in modulating the cellular responses of skin exposed to UV. To explore this further, we used a more physiologically appropriate organotypic cell culture system, which permits the regeneration of stratified differentiated epithelium in vitro and can be used to mimic viral infection. Primary human keratinocytes were transfected with the E6 and E7 genes of either HPV77, or for comparative purposes, HPV18. For cultures where HPV genes were transfected, the E7 gene, in addition to E6, is required to obtain optimal epithelial regeneration. Reconstituted epidermal sheets were then irradiated with UV doses, which caused apoptosis in the explanted skin and were processed 24 h later. As expected, control cells not transfected with HPV genes showed strong induction of both p53 and Bak in all cell layers following UV treatment, with many apoptotic cells being detected throughout the epidermis as assessed by TUNEL staining (Fig. 4). In contrast, keratinocytes transfected with HPV E6 and E7 genes of either HPV77 or HPV18 showed no increase in Bak levels following irradiation. In agreement with our findings in monolayer cultures, p53 levels increased in both control and HPV77 transfected cells but not in cells transfected with HPV18 genes. Nevertheless, apoptotic cells were not detected in any cell layers of the epithelia regenerated from keratinocytes transfected with either HPV77 or HPV18, despite the increased p53 levels in the HPV77 transfected cells (Fig. 4). To gain further insights into the mechanism and causal effects of Bak degradation by E6 in our regenerated skin model, we used HA-tagged Bak mutants that were either unable to be proteolytically degraded (ΔC) or signal apoptosis (ΔGD) (Chittenden et al. 1995), to investigate whether Bak degradation was required to regenerate an epithelium in vitro. Transfection of primary keratinocytes with plasmids expressing either ΔC or Bak resulted in apoptotic death. In contrast to the wild-type protein, the activity of the ΔC mutant was not inhibited by cotransfection with either HPV18 or HPV77 E6 (Fig. 5A). Only the ΔGD- or ΔGD/E6-transfected cells survived transfection and these were then seeded onto dermis. Both formed a differentiated epithelium with evidence of cornification that was histologically comparable to normal primary keratinocytes. The tagged protein is readily detectable in the ΔGD-transfected cells using an anti-HA monoclonal antibody, with no increase in expression following UV irradiation (Fig. 5B). By comparison, the ΔGD protein was not detected in the E6 transfected cells even following UV treatment.
Figure 4.
Effects of irradiation on HPV18 and 77 E6/E7 transfected keratinocytes grown in organotypic culture. Endogenous p53, p21, Bak and Bcl-xL protein levels were detected by immunohistochemistry in HPV 18 and 77 E6/E7 cultures 24 h post UV treatment (45 mJcm-2) and compared to control PHK raft cultures (original magnification, 100×).
Figure 5.
Inhibition of Bak apoptotic activity but not degradation is required to regenerate epidermis in vitro. (A) Normal keratinocytes were transfected with either Bak or the ΔGD or ΔC mutants either alone or together with HPVE6/E7. EGFP was used as a marker of transfection. 16 h post-transfection the cells were monitored for morphological signs of apoptosis (membrane blebbing, nuclear condensation and fragmentation) (Magnification, 200×; inserts, 400×). (B) ΔGD and ΔGD/E6/E7 cells were seeded onto de-epidermalized dermis and allowed to reform an epithelium. The cells were irradiated with 45 mJcm−2 UVB, harvested 8 h posttreatment and processed for immunohistochemistry. Sections were counterstained with haematoxylin. Positively stained cells are indicated by arrowheads. (Magnification, 200×)
Bak expression in nonmelanoma skin cancers
The elimination of Bak in the epidermis by different E6 proteins invokes a mechanism by which these diverse viral types could play a role in skin cancer development. To test this idea we initiated a pilot study to investigate whether HPV-positive skin lesions had detectable levels of Bak protein. After HPV typing, SCC biopsies that were either HPV-positive or -negative were selected for analysis. Immunohistochemistry showed that the Bak protein was not present in HPV-positive lesions but in contrast was detected to varying degrees in all five HPV-negative biopsies tested (Table 1). The p53 positive staining in the HPV-negative tumor suggests that this may be reflective of p53 gene mutations as these are frequently found in cutaneous lesions (Ziegler et al. 1994; Jonason et al. 1996; Ren et al. 1996). In contrast, p21 protein levels are high in all the biopsies processed, even in the absence of detectable p53. Interestingly, protein levels of Bcl-xL, the anti-apoptotic partner of Bak, were negligible in all of the biopsies tested. In summary, Bak protein was detected in 5/5 HPV-negative SCC lesions, whereas in marked contrast 5/5 HPV-positive SCC biopsies had negligible levels of Bak protein (P = 0.004). These results suggest that the HPV-containing tumors may have a higher proliferative potential. Staining for the proliferation marker Ki67 in conjunction with TUNEL staining showed that, although both the HPV-positive and -negative tumors showed similar levels of Ki67 expression, the HPV-negative tumors contained many more apoptotic cells compared to the HPV-positive tumors.
Table 1.
TUNEL and endogenous p53, p21, Bak, Bcl-xL, Ki67 levels detected by immunostaining in HPV positive/negative skin lesions
| Biopsy code
|
Lesion
|
HPV type(s)
|
p53
|
p21
|
Bak
|
Bcl-xL
|
Ki67
|
TUNEL
|
|---|---|---|---|---|---|---|---|---|
| HPV positive lesions | ||||||||
| 7F | SCC | 37 | − | +++ | − | − | +++ | + |
| 7L | SCC | 10, 23 | ++ | − | − | − | + | +/− |
| 7J | Bowen's/Dysplastic | RTR × 32 | ++ | − | − | − | ++ | + |
| 7B | Keratosis | 77, vs 92–1, 14 | − | − | − | − | + | − |
| 9A | SCC | pso × 1 | +/− (focal) | +++ | − | − | ++ | + |
| HPV negative lesions | ||||||||
| 9E | SCC | − | + (focal) | − | +/− | − | ++ | ++ |
| 9K | SCC | − | − | + | ++ | − | − | ++ |
| 9H | SCC | − | − | ++ | ++ | − | + | +++ |
| 7H | SCC/Bowen's | − | +/− | ++ | +/− | − | ++ | +++ |
| 7V | SCC | − | +++ | +++ | +++ | − | ++ | ++ |
SCC, Squamous cell carcinoma. Fisher's exact test of Bak staining between HPV positive and negative lesions: P = 0.004.
Discussion
Our previous studies have shown that both p53-dependent and p53-independent apoptotic pathways are inhibited by cutaneous E6 proteins (Jackson and Storey 2000). Such an overall effect may influence lesion development by altering the balance between proliferation and apoptosis, a key factor in determining the net growth of a tumor (Arends et al. 1994). We demonstrated that both normal human keratinocytes and HT1080 cells treated with UVB had dramatically increased levels of the Bak protein brought about by an increase in half-life, pointing to a role for Bak in promoting apoptosis in UVB-damaged skin. In contrast, HT1080 cell lines expressing the anogenital and cutaneous HPV E6 proteins showed no such increase in Bak levels following UVB damage. Bak is the first identified target of cutaneous E6 proteins. The mechanism of degradation, shared with anogenital HPVs, indicates that the cutaneous E6 proteins are able to discriminate between p53 and Bak as targets. This is critical to HPV77 since it utilizes transcriptionally active p53 to increase viral gene transcription (Purdie et al. 1999). Analysis of clonal p53 mutant cell patches in human skin demonstrated that they had little or no precancerous potential (Jonason et al. 1996; Ren et al. 1996). Our results suggest that the elimination of Bak protein by E6 leads to a decrease in apoptosis in UV-irradiated cells, which could in turn promote tumor formation. This posed the question as to what was the mechanism for the abrogation of Bak by the E6 proteins following UVB damage. We conclude from experiments using the proteasome inhibitor lactacystin, that the E6 proteins promoted proteolytic degradation of Bak.
To place the UVB induction of Bak in a physiologically meaningful context, we investigated Bak induction in UVB-treated skin, as Bak does not appear to be induced by γ-radiation (Burger et al. 1998). UVB induced Bak and p53 throughout the epidermal layer. These findings, together with our cellular experiments, suggested that HPV E6 proteins may have the potential to inhibit Bak-induced apoptosis in skin following UVB damage, resulting in the accumulation of deleterious mutational changes, which further increase the genetic instability of HPV-containing lesions. To test this hypothesis, we expressed E6 and E7 genes in an organotypic model of skin. Irradiation of epidermis regenerated from HPV-transfected keratinocytes showed that the cells failed to accumulate Bak and did not undergo apoptosis. This underscores the importance of the elimination of cells damaged by UV and how such cells can persist in HPV infected lesional tissue. Our results demonstrate that the apoptotic function of Bak must be tightly regulated in tissue development. Although low levels of Bak are required to regenerate the epidermis in vitro, the fact that the ΔGD mutant, which cannot signal apoptosis, does not interfere with keratinocyte terminal differentiation indicates that Bak degradation per se is not required to obtain regenerated skin.
The perturbation of apoptotic responses by HPVs has important clinical implications for NMSC development. Immunostaining of biopsies demonstrated that the majority of HPV-negative SCCs were positive for the Bak staining. In contrast, all the HPV-positive SCCs had negligible levels of the Bak protein. Although this initial pilot study is small and would need to be extended, it nevertheless indicates that the detection of Bak in the HPV-negative tumors may reflect that some measure of response to endogenous growth control still exists to promote active cell death in these cancers. This is in agreement with reports where the detection of Bak protein positively correlates with the onset of apoptosis in human colon carcinomas (Partik et al. 1998). The failure to detect endogenous Bak protein in the HPV-positive tumors, together with a marked decrease in the number of apoptotic cells compared to the HPV negative tumors and the continued expression of proliferation markers, suggests that the balance between apoptosis and proliferation is altered in HPV-containing lesions. The fact that HPV-associated NMSC occurs predominantly at sun-exposed body sites indicates that it is the abrogation by E6 of UV-induced, rather than spontaneous apoptosis that is important in tumor formation and progression of HPV-containing lesions.
In summary, we have shown that Bak functions in a pathway that removes precancerous cells from the epidermis resulting from UVB damage. Evidence that HPV-positive NMSC lesions have undetectable levels of Bak protein, together with results suggesting that anogenital and cutaneous HPVs may possess the ability to use a common antiapoptotic mechanism, raise the exciting possibility that the abrogation of Bak by the HPV E6 proteins is a common means of promoting the survival of virally infected cells. This may provide a useful target for intervention against skin lesions harboring a wide variety of HPV types.
Materials and methods
Cells and transfections
HT1080 polyclonal cell lines expressing HPV 5, 10, 18, and 77 E6 were made as described previously (Jackson and Storey 2000). Plasmids encoding HA-tagged Bak, the ΔGD and ΔC mutants (0.5 μg) (Chittenden et al. 1995) and the E6 and E7 genes (3 μg) of the different HPV types were transfected into primary cultures of human epidermal keratinocytes using TransFast (Promega). Human primary keratinocytes and p53-null keratinocytes (RTS3b, Rapp et al. 1997) were grown in DMEM/Ham's F12 (3 : 1) supplemented with 10% FCS and growth factors. Tissue specimens used for immunohistochemistry were frozen in Cryobed mountant and stored at −70°C. Keratinocytes were transfected using Transfast (Promega) with an efficiency of ∼90% as judged by the fluorescence resulting from cotransfection of an EGFP plasmid. Organotypic cultures were essentially prepared on de-epidermalized dermis as described by Prunerias et al. (1983). Briefly, the reticular dermal surface was repopulated with human fibroblasts and keratinocytes were seeded on the papillary dermal surface and allowed to form a confluent monolayer. The culture was then raised to the air–liquid interface for a further 14 d before harvesting. Where required, cells were irradiated with UVB using a UVP CL-1000 ultraviolet cross-linker with F8T5 bulbs giving a spectral peak at 312 nm.
Antibodies
The monoclonal antibodies used were Bak (Ab-2, Calbiochem), p53 (D01, ICRF), p21 (Santa Cruz), Ki67 (Dako), α-Tubulin (Calbiochem), and anti-HA (12CA5, ICRF). The Bcl-x rabbit polyclonal antibody was obtained from Calbiochem.
Western blot analysis
Cells were incubated with lysis buffer (50 mM HEPES at pH 7.4, 250mM NaCl, 0.1% Nonidet-P40 and 1× cocktail protease inhibitors [Boehringer Mannheim]) at 4°C for 20 min prior to harvesting. The supernatants were resolved by SDS-PAGE and transferred onto PVDF membrane according to standard procedures. The membrane was probed with specific antibodies and developed using the Amersham ECL +Plus kit according to the manufacturers instructions.
For Western blot analysis of ubiquitinated Bak, the cells were lysed in SDS sample buffer diluted in RIPA buffer (1 : 3) containing iodoacetamide (10 mM) as described previously (Rodriguez et al. 1999).
Half-life measurements
HT1080 E6 cells were UV irradiated (15mJ/cm2) 5 min prior to the addition of 60μg/mL cycloheximide and the cells incubated for the indicated time periods. Protein extracts were prepared in lysis buffer and Bak levels determined by Western blotting.
Immunohistochemistry
Biopsies were cut into 5 μm sections, placed onto APES (3-aminopropyltriethoxy-silane/acetone) coated slides and fixed in methanol/acetone (1 : 1) for 10 min at room temperature. Endogenous peroxidase was removed by treatment with 1% hydrogen peroxide/PBS for 20 min at room temperature. The sections were incubated overnight with primary antibody at 4°C and the secondary and tertiary antibodies were supplied in the ABC PK-6200 Universal kit (Vector Laboratories) and were used in accordance with the manufacturer's instructions. The slides were developed in DAB (6 mg of DAB-tetrahydrochloride, 10 μL of 30% hydrogen peroxide, 100 μL of 0.1 M nickel chloride, 10 mL PBS). TUNEL staining of tissue sections was carried out according to manufacturer's instructions (Promega, DeadEnd colorimetric apoptosis detection system), using 5 μg/mL Proteinase K incubation for 5 min. HPV typing of biopsies was performed as described previously (Storey et al. 1998).
Acknowledgments
We thank R. Cerio and A.G. Quinn for advice on pathology of the tissue biopsies, E. Rugg for critical reading of this manuscript, and R. Hay for helpful advice. We are grateful to T. Chittenden for the gift of the plasmids encoding Bak and Bak mutants. This work was supported in part by a studentship to S.J. from the Biotechnology and Biological Sciences Research Council (BBSRC) and Roche Products Ltd., Welwyn Garden City, U.K. L.B. acknowledges the support of the Associazione Italiana per la Ricerca sul Cancro.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL a.storey@icrf.icnet.uk; FAX 44-207-882-7171.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.182100.
References
- Allday M, Inman G, Crawford D, Farrell P. DNA damage in human B cells can induce apoptosis, proceeding from G1/S when p53 is transactivation competent and G2/M when it is transactivation defective. EMBO J. 1995;14:4994–5005. doi: 10.1002/j.1460-2075.1995.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends M, McGregor A, Wyllie A. Apoptosis is inversely related to necrosis and determines net growth in tumours bearing constitutively expressed myc, ras, and HPV oncogenes. Am J Path. 1994;144:1045–1057. [PMC free article] [PubMed] [Google Scholar]
- Burger H, Hooter K, Boersma AWM, Kortland CJ, Van Den Berg AP, Stoter G. Expression of p53, p21Waf/Cip, Bcl-2, Bax, Bcl-X and Bak in radiation-induced apoptosis in testicular germ cell tumour lines. Int J Radiation Oncology Biol Phys. 1998;41:415–424. doi: 10.1016/s0360-3016(98)00065-0. [DOI] [PubMed] [Google Scholar]
- Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan D, Chinnadurai G, Lutz RJ. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craiu A, Gaczynska M, Akopian T, Gramm CF, Fenteany G, Goldberg AL, Rock KL. Lactacystin and clasto-lactacystin β-lactone modify multiple proteosome β-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- Dick LR, Cruikshank AA, Grenier L, Melandri FD, Nunes SL, Stein RL. Mechanistic studies on the inactivation of the proteosome by lactacystin. J Biol Chem. 1996;271:7273–7276. doi: 10.1074/jbc.271.13.7273. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Standaert RF, Riechard GA, Corey EJ, Schreiber SL. A β-lactone related to lactacystin induces neurite outgrowth in neuroblastoma cell line and inhibits cell cycle progression in an osteosarcoma cell line. Proc Natl Acad Sci. 1994;91:3358–3362. doi: 10.1073/pnas.91.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteosome activities and subunit-specific amino terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- Gniadecki R, Hansen M, Wulf HC. Two pathways for the induction of apoptosis by ultraviolet radiation in cultured human keratinocytes. Soc Invest Dermatol. 1997;109:163–169. doi: 10.1111/1523-1747.ep12319216. [DOI] [PubMed] [Google Scholar]
- Griffiths H, Mistry P, Herbert K, Lunec J. Molecular and cellular effects of ultraviolet light-induced genotoxicity. Crit Rev Clin Lab Sci. 1998;35:189–237. doi: 10.1080/10408369891234192. [DOI] [PubMed] [Google Scholar]
- Haake A, Polakowska R. Cell death by apoptosis in epidermal biology. J Invest Dermatol. 1993;101:107–112. doi: 10.1111/1523-1747.ep12363594. [DOI] [PubMed] [Google Scholar]
- Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, Proby CM. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289–297. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Herzinger T, Funk JO, Hillmer K, Eick D, Wolf DA, Kind P. Ultraviolet B irradiation-induced G2 cell cycle arrest in human keratinocytes by inhibitory phosphorylation of the cdc cell cycle kinase. Oncogene. 1995;11:2151–2156. [PubMed] [Google Scholar]
- Jackson S, Storey A. E6 proteins from diverse cutaneous HPV types inhibit apoptosis in response to UV damage. Oncogene. 2000;19:592–598. doi: 10.1038/sj.onc.1203339. [DOI] [PubMed] [Google Scholar]
- Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, Leffell DJ, Tarone RE, Brash DE. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner G, Botchkarev V, Botchkareva N, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression (catagen) Am J Pathol. 1997;151:1601–1607. [PMC free article] [PubMed] [Google Scholar]
- McCall C, Cohen J. Programmed cell death in terminally differentiating keratinocytes, role of endogenous endonuclease. J Inv Dermatol. 1991;97:111–114. doi: 10.1111/1523-1747.ep12478519. [DOI] [PubMed] [Google Scholar]
- Miralles F, Parra M, Caelles C, Nagamine Y, Felez J, Munoz-Canoves P. UV irradiation induces the murine urokinase-type plasminogen activator gene via the c-jun N-terminal kinase signaling pathway, requirement of an AP1 enhancer element. Mol Cell Biol. 1998;18:4537–4547. doi: 10.1128/mcb.18.8.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C, Di Cunto F, Kiykawa H, Koff A, Dotto G. The absence of p21Cip1/Waf1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes & Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- Mitra R S, Wrone-Smith T, Simonian P, Foreman KE, Nunez G, Nickoloff BJ. Apoptosis in keratinocytes is not dependent on induction of differentiation. Lab Invest. 1997;76:99–107. [PubMed] [Google Scholar]
- Partik G, Kahl-Rainer P, Sedivy R, Ellinger A, Bursch W, Marian B. Apoptosis in human colorectal tumours, ultrastructure and qualitative studies on tissue localisation and association with Bak expression. Virchows Arch. 1998;432:415–426. doi: 10.1007/s004280050185. [DOI] [PubMed] [Google Scholar]
- Polakowska R, Piacentini M, Bartlett R, Goldsmith L, Haake A. Apoptosis in human skin development, morphogenesis, periderm and stem cells. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- Ponten F, Berne B, Ren ZP, Nister M, Ponten J. Ultraviolet light induces expression of p53 and p21 in human skin: effect of sunscreen and constitutive p21 expression in skin appendages. Soc Invest Dermatol. 1995;105:402–406. doi: 10.1111/1523-1747.ep12321071. [DOI] [PubMed] [Google Scholar]
- Proby C, Storey A, McGregor J, Leigh I. Does human papillomavirus infection play a role in non-melanoma skin cancer? Papillomavirus Rep. 1996;7:53–60. [Google Scholar]
- Prunerias M, Regnier M, Woodley D. Methods of cultivation of keratinocytes at an air liquid interface. J Invest Derm. 1983;81:28–33. doi: 10.1111/1523-1747.ep12540324. [DOI] [PubMed] [Google Scholar]
- Purdie KJ, Pennington J, Proby CM, Khalaf S, de Villiers E-M, Leigh IM, Storey A. The promoter of a novel human papillomavirus (HPV 77) associated with skin cancer displays UV responsiveness, which is mediated through a consensus p53 binding sequence. EMBO J. 1999;18:5359–5369. doi: 10.1093/emboj/18.19.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Pawellek A, Kraetzer F, Schaefer M, May C, Purdie K, Grassman K, Iftner T. Cell-type-specific separate regulation of the E6 and E7 promoters of human papillomavirus type 6a by the viral transcription factor E2. J Virol. 1997;71:6956–6966. doi: 10.1128/jvi.71.9.6956-6966.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZP, Hedrum A, Ponten F, Nister M, Ahmadian A, Lundeberg J, Uhlen M, Ponten J. Human epidermal cancer and accompanying precursors have identical p53 mutations different from p53 mutations in adjacent areas of clonally expanded non-neoplastic keratinocytes. Oncogene. 1996;12:765–773. [PubMed] [Google Scholar]
- Rodriguez MS, Desterro JMP, Lain S, Midgley CA, Lane DP, Hay RT. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 1999;18:6455–6461. doi: 10.1093/emboj/18.22.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by HPV types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Bhardwaj R, Argane Y, Mahnke K, Reimann H, Metze D, Lugar T, Schwarz T. Ultraviolet B-induced apoptosis of keratinocytes: Evidence for partial involvement of tumour necrosis factor-alpha in the formation of sunburn cells. J Invest Dermatol. 1995;104:922–927. doi: 10.1111/1523-1747.ep12606202. [DOI] [PubMed] [Google Scholar]
- Sieberg M, Marthinuss J, Stenn K. Changes in expression of apoptosis-associated genes in skin mark early catagen. J Invest Dermatol. 1995;104:78–82. doi: 10.1111/1523-1747.ep12613555. [DOI] [PubMed] [Google Scholar]
- Storey A, Thomas M, Kalita A, Harwood C, Gardiol D, Mantovani F, Breuer J, Leigh I, Matlashewski G, Banks L. Role of p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393:229–234. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene. 1998;17:2943–2954. doi: 10.1038/sj.onc.1202223. [DOI] [PubMed] [Google Scholar]
- Tomkova H, Fujimoto W, Arata J. Expression of Bcl-2 antagonist Bak in inflammatory and neoplastic skin diseases. Br J Dermatol. 1997;137:703–708. [PubMed] [Google Scholar]
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Young A. The sunburn cell. Photodermatol. 1987;4:127–134. [PubMed] [Google Scholar]
- Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]