Abstract
Leishmaniasis is caused by infection with the protozoan parasite, Leishmania, that parasitizes human cells, and the cellular immune response is essential for controlling infection. In order to measure the host T cell response to Leishmania infection, we have measured the expansion, activation state and functional potential of specific T cells as identified by their T cell receptor Vβ region expression. In a group of cutaneous leishmaniasis (CL) patients, we evaluated these characteristics in nine different T cell subpopulations as identified by their Vβ region expression, before and after specific Leishmania antigen stimulation. Our results show: (1) an increase in CD4+ T cells expressing Vβ 5·2 and Vβ 24 in CL compared to controls; (2) a Leishmania antigen-induced increase in CD4+ T cells expressing Vβ 5·2, 11, 12 and 17; (3) a profile of previous activation of CD4+ Vβ 5·2-, 11- and 24-positive T cells, with higher expression of CD45RO, HLA-DR, interferon-γ, tumour necrosis factor-α and interleukin-10 compared to other Vβ-expressing subpopulations; (4) a positive correlation between higher frequencies of CD4+Vβ5·2+ T cells and larger lesions; and (5) biased homing of CD4+ T cells expressing Vβ 5·2 to the lesion site. Given that CL disease involves a level of pathology (ulcerated lesions) and is often followed by long-lived protection and cure, the identification of specific subpopulations active in this form of disease could allow for the discovery of immunodominant Leishmania antigens important for triggering efficient host responses against the parasite, or identify cell populations most involved in pathology.
Keywords: CD4 T cells, cytokines, human leishmaniasis, T cell receptor, tegumentary lesions
Introduction
The establishment of an effective and regulated immune response directed against Leishmania is critical for resolution of infection and limitation of pathology. Leishmaniasis is considered as an emergent and re-emergent disease and encompasses visceral and tegumentary forms, including cutaneous and mucocutaneous forms [1–3]. Infection with the protozoa parasite Leishmania braziliensis can cause several clinical forms of disease, and in Brazil it is responsible for at least two major clinical forms: cutaneous (CL) and mucosal (ML) leishmaniasis [1,2]. Human tegumentary leishmaniasis is usually limited to the skin and lymphatic system, but it may recur in the mucous membranes of the mouth, nose or pharynx in ML [4,5].
In experimental CL, development of protective immunity is dependent upon the generation of specific cytokine-producing T cells with a regulated T helper type 1 (Th1)-like profile [6,7]. In the majority of CL patients, effective cell-mediated immunity, as evidenced by a positive delayed-type hypersensitivity (DTH) reaction [8,9], as well as production of interferon (IFN)-γ and tumour necrosis factor (TNF)-α by peripheral T cells and cutaneous lesion cells found in inflammatory infiltrates, show the same profile seen in experimental models [10–13]. IFN-γ is an important cytokine that activates infected macrophages to eliminate parasites and improve antigen processing and presentation, as well as aiding in creating an effective microenvironment for generation of Th1 T cells. At the same time, the lack of proper regulation of this response may lead to the formation of exacerbated lesions, as seen in mucosal disease [12–14].
Recently, we demonstrated that Leishmania-specific T cells from CL patients displayed a regulated inflammatory T cell response as measured by correlation between the frequency of proinflammatory (IFN-γ and TNF-α) and anti-inflammatory (IL-10) cytokine-producing cells [10,13]. Interestingly, our group also observed positive correlations between immunological and clinical measurements in CL patients. This work demonstrated a positive correlation between the Montenegro skin test (MST) size and the frequency of recent activated CD4+ T cells analysed ex vivo. Moreover, the larger the lesions, the higher the frequencies of inflammatory cytokine (IFN-γ or TNF-α)-producing Leishmania-specific lymphocytes [15].
Given that specific T cell responses against Leishmania antigens play a critical role in the formation of protective and pathogenic immune responses in human leishmaniasis, it is clear that the elucidation of which T cell subpopulations are involved in the response will aid in the identification of possible dominant antigens used by the human immune response. Thus, we designed the present study to identify T cells involved in possible dominant responses with the hope of one day using them as tools for identifying dominant antigens and understanding more clearly the progression of human disease and the formation of protective responses in human CL.
The α/β T cell repertoire is made up of T cells expressing diverse T cell receptors (TCR) composed of disulphide-bound α and β TCR chains. These TCR recognize antigens as peptides bound to major histocompatibility complex (MHC) molecules [16] that, together with co-stimulatory molecules, develop an effective immune response [17]. The α and β chains are the most common among peripheral T cells and are composed of subregions V and J, or V, D and J, respectively, which combine to provide the TCR's fine specificity. Antigen recognition diversity is generated in part by the use of specific V region gene segments encoding for each polypeptide chain of the TCR [18,19].
Furthermore, study of the T cell receptor (TCR) repertoire can contribute to understanding disease pathogenesis and, for this reason, has been an important focus of research in several diseases [20–22]. Studies of the TCR Vβ repertoire have also described the role played by microbial toxins or superantigens in activating the human immune system [23,24]. Superantigen stimulation of the immune system or stimulation by dominant antigens leads to proliferation of specific T cell populations followed by clonal deletion [25].
In human leishmaniasis, the adaptive immune response is predominantly T cell-mediated. It has been demonstrated that the predominant T cells in CL lesions bear the αβ TCR [26,27]. Studies using polymerase chain reaction (PCR) in CL patient lesions caused by L. braziliensis have demonstrated that the TCR Vβ repertoire presented expansions of Vβ families 3, 6·6/6·7 and 7 in 50% of the patients studied; however, as CD4+ and CD8+ T cells were not separated, interpretation of what proportion of these dominant responses are due to CD4+ or CD8+ or both is impossible [28]. Another study has shown an expansion of CD4+ and CD8+ T cells expressing Vβ 12 after stimulation with soluble Leishmania antigen (SLA) of L. amazonensis among CL patients infected with L. braziliensis, and thus points to this population as a dominant responding population in this disease [29]. Specific subpopulations of T cells can be identified using monoclonal antibodies directed against the TCR β chain region and thus, using flow cytometry, we are able to examine the relative frequency, activation state and functional activity of these populations either ex vivo or after specific antigenic stimulation in vitro. Eventually, through the identification of specific T cell populations involved in the response, we can use this information to identify antigens involved in the response against Leishmania. Thus, to understand more clearly the role that subpopulations of CD4+ T cells expressing distinct Vβ usage may have in the human immune response against L. braziliensis, we analysed the TCR Vβ repertoire as well as activation state, memory markers and the cytokine profile of these cells, focusing on populations that may be involved actively in the formation of protective or pathogenic immune responses. We also performed correlations between the frequency of proinflammatory and anti-inflammatory cytokines, as well clinical indicators related to human CL.
Materials and methods
Ethics statement
These studies were approved by the National Ethical Clearance Committee of Brazil (CONEP), as well as by the UFMG and UFBA local Institutional Review Boards, all of which adhere to the principles laid out in the Declaration of Helsinki. All participants in this study provided informed written consent.
Patients and controls
The peripheral blood mononuclear cells (PBMC) analysed were obtained from 12 infected individuals from the village of Corte de Pedra, in the state of Bahia, Brazil, an area endemic for leishmaniasis caused by L. braziliensis infection. The data presented are from a group of 12 individuals, ranging between 14 and 50 years of age (mean 25·08 ± 11·15). Cutaneous lesions (n = 3) were collected at the Corte de Pedra health-care facility. Diagnosis of leishmaniasis was based on clinical findings, a positive skin test for Leishmania antigens [30–32] and/or positive parasitological examination. All presented with one or more ulcerated lesions between 8 days and 4 months of duration. None of the individuals had been treated previously for leishmaniasis and reported no previous infections with Leishmania. The blood was drawn immediately before treatment was initiated. All individuals participated in the study through informed consent, and received treatment whether or not they chose to participate in the study. PBMC were also obtained from a group of six healthy donors from Bahia, Brazil, with ages ranging between 23 and 33 years (mean 27·6 ± 3·97).
Skin biopsy procedure
Skin fragment specimens were taken from the borders of active lesions, using a 4-mm-diameter punch, after application of a local anaesthetic. Lesions were maintained in a 30% sucrose solution for 30 min at 4°C and then transferred to octreotide (OCT) Tissue Tek (Sakura Seiki Co. Ltd, SSC and SCL, Tokyo, Japan) freezing medium and placed immediately in dry ice. The material was stored at −70°C until analysis, as described in Faria et al. [12].
L. braziliensis antigen
The SLA of L. braziliensis was provided by the Leishmaniasis Laboratory (ICB/UFMG/Brazil; Dr W. Mayrink) and is a freeze/thawed antigen preparation. Briefly, L. braziliensis promastigotes (MHOM/BR/75M2903) were washed and adjusted to 108 promastigotes/ml in phosphate-buffered saline (PBS) (Sigma-Aldrich, St Louis, MO, USA) followed by repeated freeze/thaw cycles and a final ultrasonication. After centrifugation the supernatant was harvested and the protein concentration was measured using the Lowry method. All antigens were titrated using PBMC from patients infected with L. braziliensis.
Preparation of PBMC
PBMC from cutaneous leishmaniasis patients and non-infected individuals were obtained by separating blood cells in a Ficoll gradient (Sigma-Aldrich), as described by Gazzinelli et al. [33]. Cells were washed three times in media and counted. PBMC from cutaneous leishmaniasis patients and non-infected individuals were used for in vitro culture TCR-usage analysis. Cultures were set up using a concentration of 2·5 × 105 cells in 96-well plates in the presence or absence of SLA (10 µg/ml final concentration) and were incubated for approximately 20 h. During the last 4 h of culture, Brefeldin-A (Sigma-Aldrich) (1 µg/ml), which impairs protein secretion by the Golgi complex, was added to the cultures. After the incubation period, cultures were harvested and submitted to flow cytometric analysis to evaluate T cell repertoire, surface markers and cytokine profile.
Monoclonal antibodies (mAbs)
The antibodies used for staining were immunoglobulin fluorescein isothiocyanate (FITC) and phycoerythrin (PE) controls (PharMingen, San Diego, CA, USA), anti-Vβ2-biot, anti-Vβ3-biot, anti-Vβ5·1-biot, anti-Vβ5·2-biot, anti-Vβ11-biot, anti-Vβ17-biot, anti-Vβ 24-biot (Immunotech, Burlingame, CA, USA) anti-Vβ8-FITC, anti-Vβ 12-FITC (Immunotech), SA-FITC (PharMingen), anti-CD69 PE (Ebioscience, San Diego, CA, USA), anti-HLA-DR-PE, anti-CD45RO-PE (PharMingen) and anti-CD4-PE-Cy5 (Ebioscience). The anti-cytokines antibodies used were PE-labelled anti-IFN-γ, anti-TNF-α (PharMingen) and anti-IL-10 (Caltag, Carlsbad, CA, USA).
Cell surface and single-cell cytoplasmic cytokine staining
PBMC were analysed for their repertoire, surface markers and intracellular cytokine expression pattern. Briefly, 2·5 × 105 PBMC were cultured in 96-well plates in 200 µl cultures for 20 h with either media alone or SLA (at 10 µg/ml final concentration). Brefeldin-A (1 µg/ml) was added during the last 4 h of culture to impair protein secretion, allowing for cytokine intracellular staining, as performed previously by us [11]. The cells were then stained for T cell receptor Vβ repertoire and surface markers, and fixed using 4% formaldehyde (Sigma-Aldrich). Cells were then permeabilized with a solution of saponin (Sigma-Aldrich) and stained, for 30 min at 4°C, using anti-cytokine monoclonal antibodies directly conjugated with PE. PE-labelled immunoglobulin control antibodies and a control of unstimulated PBMC were included in all experiments. Preparations were washed and fixed as described in the previous section and analysed using fluorescence activated cell sorter analysis (FACS), selecting the total lymphocyte population (Fig. 1). In all cases both cytokine and surface marker staining were associated with T cell receptor Vβ repertoire staining for studying the expression of cytokines and surface markers together and the phenotype of the cells that produced them. At least 40 000 gated events were acquired for later analysis.
Fig. 1.

Representative flow cytometry graphs of CD4+ T cells expressing distinct Vβ region from cutaneous leishmaniasis patients. Peripheral blood mononuclear cells (PBMC) from cutaneous leishmaniasis patients were stained after culture and analysed using flow cytometry as described in Materials and methods. Flow cytometry dot-plots and histograms demonstrate the data analysed in CD4+ T lymphocytes expressing different Vβ regions.
Flow cytometry data analysis
CD4+ T lymphocytes were analysed for their T cell receptor Vβ repertoire, intracellular cytokine and surface marker expression patterns and frequencies using the CellQuest program (Becton & Dickinson, San José, CA, USA) and FlowJo program (Tree Star, Ashland, OR, USA). Limits for the quadrant markers were always set based on negative populations and isotype controls. Three different fluorochromes were associated for each analysis, for example, anti-Vβ-biot-SA-FITC, anti-X-PE, with X representing a surface marker or a cytokine and anti-CD4-PE-Cy5 (Fig. 1). In this manner, for example, the region upper right of the dot-plot was selected, where the cells were double-positive for Vβ (FITC) and CD4 (PE-Cy5) (Fig. 1) and then histograms were generated for evaluation of frequency of cells producing the given surface markers or cytokines (Fig. 1).
Histological and immunofluorescence staining
Individual 4–5-µm cryosections were prepared as described by Faria et al. [12]. Briefly, cryosections were placed in silane-precoated slides and fixed for 10 min with acetone (Merck, Damstadt, Hessen, Germany). Slides were incubated with PBS for 30 min and subjected to either haematoxylin and eosin staining or immunofluorescence staining using specific monoclonal antibodies. Standard haematoxylin and eosin (Merck) staining was performed to ensure tissue integrity, as well as for evaluation of the intensity of the inflammatory infiltrate. Immunofluorescence reactions involved incubation with labelled monoclonal antibodies directed to surface receptors Vβ 2 FITC and CD4 (PE-Cy5) or Vβ 5·2 FITC and CD4 PE-Cy5. Sections were incubated with antibody mixtures overnight at 4°C. After staining, preparations were washed extensively with phosphate-buffered saline, counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), and mounted using Antifade mounting medium (Molecular Probes, Eugene, OR, USA). Slides were kept at 4°C, protected from light, until acquisition in a laser scanning confocal microscope (Zeiss, Jena, Turingia, Germany). Isotype controls (Caltag) were analysed separately to confirm the lack of non-specific staining.
Haematoxylin and eosin-stained sections were analysed using light microscopy (Axiovert, Zeiss-Jena, Turingia, Germany). We analysed 16 fields/sample using a power magnification of 400×. Confocal analysis were performed using a Meta-510 Zeiss laser scanning confocal system running LSMix software (Zeiss-Jena) coupled to a Zeiss microscope (Axiovert 100) with an oil immersion Plan-Apochromat objective (63×, 1·2 numerical aperture) and Bio-Rad MRC 1024 laser scanning confocal system running LaserSharp 3·0 software (Bio-Rad, Hercules, CA, USA) coupled to a Zeiss microscope (Axiovert 100) with a water immersion objective (40×, 1·2 numerical aperture). A water-cooled argon ultraviolet (UV) laser (488 nm) or a krypton/argon laser was used to excite the preparation (through its 363-nm; 488-nm or 633-nm line), and light emitted was selected with band-pass filters (505/35 for FITC or LP700 for PE-Cy5). For DAPI visualization a mercury lamp was used to excite the preparation (through its 20/80 nm line), and light emitted was selected with band-pass filters (363/90 for DAPI). For each section, the inflammatory infiltrate present in the connective tissue adjacent to the epithelia was located and an area presenting with a uniform infiltrate was selected for analysis. Within this inflammatory area, a minimum of six images (fields) were collected. Image analysis and processing were performed with LSMix (Zeiss) or LaserSharp, Confocal Assistant, Adobe Photoshop (Adobe Systems Incorporated, San Jose, CA, USA) and Image Tool software (UTHSCSA, San Antonio, TX, USA). Analyses were performed by counting the total number of cells in six to nine fields acquired and calculating the average cell number per field for each patient. This procedure was performed for each parameter analysed, allowing determination of the total number of inflammatory cells (total number of DAPI+ cells within the inflammatory infiltrate), the number of FITC (TCR Vβ regions) or PE-Cy5 (CD4+) single-positive cells, and the number of double-positive cells. The counts were performed blindly for each parameter for each patient. The results are representative of two experiments per patient.
Statistical analysis
Statistical analysis was performed as indicated in each figure legend. For comparison of means between control versus CL, individual Student's t-tests were used for each given Vβ-expressing population. For comparison of specific Vβ-expressing CD4+ T cell populations between media alone and SLA, paired Student's t-tests were used. For comparison of the percentage of cells within each Vβ population expressing a given marker (CD45RO, cytokines, etc.), the data were treated with the Tukey–Kramer analysis of variance (anova) test within the jmp statistical package (SAS Institute Inc., Cary, NC, USA).
All correlation analyses were performed using Spearman's correlation coefficient contained within the jmp statistical package (SAS Institute Inc.) and reported with its associated r2 and P-value.
Results
Clinical characteristics of CL patients
The clinical characteristics of the 12 patients with CL used in this study are shown in Table 1. All patients were from an endemic area near Salvador, Brazil (see Materials and methods) and participated in the study after informed consent through the donation of peripheral blood. Regardless of participation in the study, all patients received medical care. The patient ages ranged between 14 and 50 years (mean 25·08 ± 11·15) and time of lesion, as reported by the patient, ranged from 8 to 120 days at time the blood was taken and measurements were made. The total area measured of ulcers varied from 12 to 272 mm2 (mean 151·44 ± 103·87). All patients presented with positive Leishmania skin tests (MST), while measurements existed for 11 patients, ranging from 72 to 910 mm2 (mean 329·72 mm2 ± 229·66).
Table 1.
Clinical profile of patients with cutaneous leishmaniasis.
| Patient identification | Sex | Age (years) | Total lesion area (mm2)† | Montenegro skin test (MST) (mm2)‡ | Time of lesion (days)§ |
|---|---|---|---|---|---|
| S1 | Male | 18 | 48 | n.d. | 60 |
| S2 | Male | 16 | 255 | 255 | 120 |
| S3 | Male | 14 | n.d. | 420 | 60 |
| S4 | Male | 21 | 272 | 240 | 60 |
| S5 | Female | 50 | n.d. | 72 | 15 |
| S6 | Male | 28 | 16 | 225 | 8 |
| S7 | Male | 29 | 220 | 210 | 60 |
| S8 | Male | 29 | 90 | 910 | 120 |
| S9 | Male | 18 | 12 | 270 | 35 |
| S10 | Male | 18 | n.d. | 442 | 35 |
| S11 | Male | 22 | 270 | 121 | 35 |
| S12 | Male | 21 | 180 | 462 | 120 |
‡Area of two diameters for each lesion or MST.
Area of two diameters for each lesion or MST.
Time of lesion as reported by the patients; n.d.: not determined.
Cutaneous leishmaniasis patients displayed a slight increase in the frequency of T cells expressing TCR Vβ 5·2 and 24 compared to non-infected controls
We performed a comparative analysis of the frequency of T cells expressing given Vβ regions 2, 5·1, 5·2, 8, 11, 12, 17 and 24 from CL and from non-infected individuals. The mean frequency of cells expressing Vβ 5·2 and 24 was increased slightly in the actively infected CL group compared to the non-infected control group (P = 0·006 and P = 0·02, respectively) (Fig. 2).
Fig. 2.
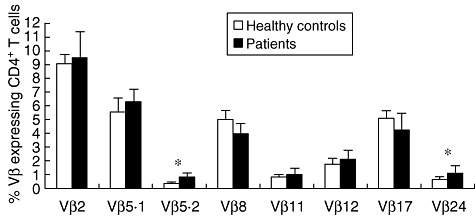
Differential frequencies of subpopulations of specific Vβ+ CD4+ T cells. Comparison between CD4+ T cells expressing distinct Vβs from healthy controls (white bars) and patients (black bars) with cutaneous leishmaniasis (CL) are shown. Peripheral blood mononuclear cells (PBMC) from control individuals and CL patients were stained for T cell receptor (TCR) Vβ region and CD4 expression followed by flow cytometry analysis. The bars represent the mean ± standard error for each group. Means were compared using Student's t-test with a P-value of < 0·05 considered significant; *significant differences between the means of control and CL individuals.
In vitro stimulation of PBMC from CL patients with soluble Leishmania antigen induces an increase in the frequency of CD4+ T cells expressing Vβs 5·2, 11, 12 and 17
In order to detect a specific T cell expansion involved in the anti-Leishmania T cell response in infected CL patients, we compared the Vβ repertoire in vitro of CD4+ T cells before and after stimulation with SLA. We analysed the frequency of CD4+ T cells expressing Vβ 2, 3, 5·1, 5·2, 8, 11, 12, 17 or 24. Paired analysis of Vβ expression before and after SLA stimulation revealed the specific expansion of CD4+ T cells expressing Vβ 5·2, 11, 12 and 17 among the patient group (Fig. 3) using a significance of P < 0·05. In all four cases, > 80% of the individuals displayed an antigen-induced expansion of the specific Vβs, while the other Vβ-expressing T cells expand only in some patients in response to in vitro stimulation (Fig. 3).
Fig. 3.
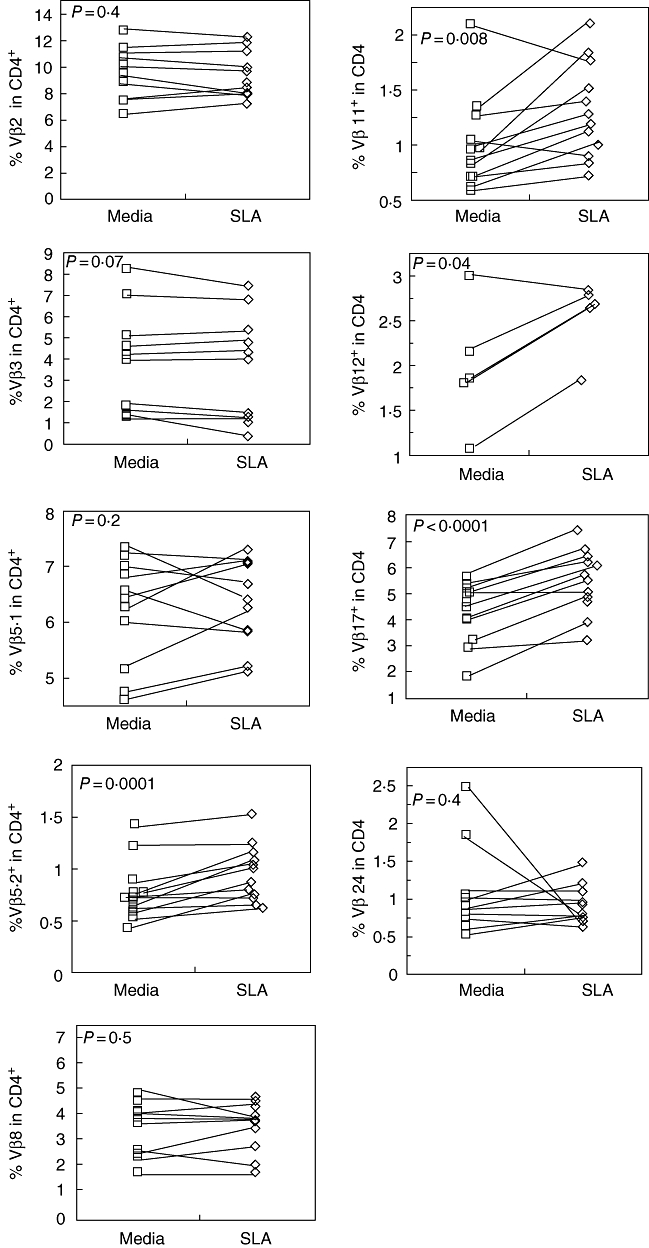
Frequency of CD4+ T cells expressing specific Vβ regions from cutaneous leishmaniasis patients after antigen-specific stimulation. Peripheral blood mononuclear cells (PBMC) from cutaneous leishmaniasis patients were maintained in culture without stimulus, as well as with soluble Leishmania antigen [soluble Leishmania antigen (SLA)-10µg/ml]. After 20 h of culture, PBMC were stained for Vβ regions and CD4 expression as described in Materials and methods. SLA induced a significantly higher expression of Vβ 5·2, 11, 12 and 17 among CD4+ T cells from patients with cutaneous leishmaniasis. All groups have n = 12 except for Vβ 12, which had n = 6. Significance was set at P < 0·05.
Vβ 5·2, 11 and 24 CD4+ T cells display a higher frequency of ‘experienced’ T cells than other Vβ-expressing T cells in CL patients
To determine the activation state and previous antigenic experience of CD4+ T cells expressing distinct Vβ from CL patients, we evaluated activation and memory molecule expression (HLA-DR and CD45RO, respectively) within each Vβ subpopulation. The proportion of specific Vβ-expressing CD4+ T cells expressing HLA-DR or CD45RO was compared among the various Vβ-expressing T cell populations without in vitro stimulation as a measure of in vivo experience in actively infected patients. CD4+ T cell subpopulations defined by Vβ 5·2, 11 and 24 expressed a higher percentage of CD45RO+ T cells compared to all the other Vβ-expressing populations studied (Fig. 4a). Interestingly, the same three subpopulations defined by T cells expressing Vβ 5·2, 11 and 24 had a significantly higher expression of HLA-DR compared to CD4+ T cells expressing Vβ 2 and Vβ 5·1. All other CD4+ T cell populations displayed frequencies statistically equivalent to one another (Fig. 4b). Thus, two indicators of previous in vivo antigenic stimulation (CD45RO, a memory/experienced T cell marker, and HLA-DR, a late activation marker) are increased in CD4+ T cell subpopulations expressing TCR Vβ regions 5·2, 11 and 24, compared to other subpopulations among actively infected leishmaniasis patients.
Fig. 4.

CD45RO and Human Leucocyte Antigen-DR (HLA-DR) expressing CD4+ T cells bearing different Vβ regions of T cell receptor (TCR). Peripheral blood mononuclear cells (PBMC) from cutaneous leishmaniasis patients were stained for Vβ region, HLA-DR, CD45RO and CD4 expression and analysed as described in Materials and methods. The graphs represent the frequency of CD45RO (a) and HLA-DR (b) within CD4+ T cells expressing different Vβ regions without stimulus. CD4+ T cells expressing Vβ 5·2, 11 and 24 had a higher CD45RO and HLA-DR expression when compared with other Vβ-specific CD4+ T cell subpopulations. The data represent the mean ± standard error for each Vβ region without in vitro stimulation. All groups have n = 12 except for Vβ 12, which had n = 6. *P ≤ 0·05 for the indicated Vβ subpopulations compared to the other populations using analysis of variance Tukey–Kramer test.
The same Vβ CD4+ T cell subpopulations (Vβ 5·2, 11 and 24) that display a bias ‘experienced’ phenotype also display an increased frequency of pro- and anti-inflammatory cytokine expression following antigenic stimulation in vitro compared to other Vβ subpopulations
Effective CD4+ T cell responses and subsequent cytokine production are critical for the cure, and possibly the exacerbation, of human leishmaniasis. We have shown previously that CD4+ Th1 T cells are associated with human CL [11], and that these cells are also accompanied by the production of IL-10 [10]. In addition to co-regulation of the frequency of IFN-γ- and TNF-α-producing T cells, we also identified co-regulation of IL-10-producing T cells [11]. Interestingly, higher frequencies of IFN-γ-producing T cells were also associated with lesion size [15]. Thus, in attempts to identify possible specific T cell subpopulations that could be involved in these responses, we measured the frequency of individual Vβ-expressing CD4+ T cell subpopulations producing inflammatory (TNF-α and IFN-γ) and anti-inflammatory (IL-10) cytokines.
The analysis of antigen-specific TNF-α-producing CD4+ T cells within each Vβ-expressing subpopulation revealed a diverse array of frequencies as expected, given the complex nature of Leishmania and the hundreds, if not thousands, of possible antigenic peptides contained within Leishmania antigenic preparations. However, of all the Vβ subpopulations analysed, three (Vβ 5·2, 11 and 24) displayed a significantly higher frequency of TNF-α-producing cells compared to all but one of the other Vβ (that defined by Vβ 12) subpopulations (Fig. 5a).
Fig. 5.

Antigen-specific stimulation increases expression of tumour necrosis factor (TNF)-α, interferon (IFN)-γ and interleukin (IL)-10 cytokines in CD4+ T cells bearing distinct Vβ regions of T cell receptor (TCR). Peripheral blood mononuclear cells (PBMC) from cutaneous leishmaniasis patients were maintained in culture without stimulus, as well as with soluble Leishmania antigen (SLA-10µg/ml). After approximately 20 h of culture, PBMC were stained for Vβ region, TNF-α, IFN-γ, IL-10 and CD4 expression. Data were collected using flow cytometry and analysed using FlowJo software. (a) TNF-α-producing CD4+ T cells expressing different Vβ regions; (b) the graphs represent the frequency of IFN-γ within CD4+ T cells expressing different Vβ regions and (c) frequency of IL-10 within CD4+ T cells expressing different Vβ regions. The data represent the mean ± standard error for each Vβ region with SLA (white bars). All groups have n = 12 except for Vβ 12, which had n = 6. *P ≤ 0·05 for the indicated Vβ subpopulations compared to at least four of the other Vβ populations using analysis of variance Tukey–Kramer test.
The same general profile was seen for the frequency of cells expressing IFN-γ, with T cells expressing Vβ 5·2, 11 and 24 also having a higher commitment to IFN-γ production compared to at least four other Vβ families (Fig. 5b). In order were Vβ 5·2 (greater than Vβ 2, 5·1, 8 and 17), followed by Vβ 11 and 24 (greater than Vβ 2, 5·1, 8 and 17).
Finally, given that our earlier published studies have shown a consistent co-production of IL-10 together with IFN-γ and TNF-α[1], we analysed the frequency of IL-10-producing cells among the same Vβ subpopulations (Fig. 5c). Again, the same Vβ-expressing CD4+ T cells (Vβ 5·2, 11 and 24) displayed a higher frequency of antigen-induced IL-10-producing T cells than at least four of the other Vβ-expressing T cells. In order were Vβ 5·2 (greater than Vβ 2, 5·1, 8 and 17), followed by Vβ 11 and 24 (greater than Vβ 2, 3, 5·1, 8, 12 and 17). In all cases we reported only Vβ subpopulations that displayed a significantly higher percentage of cells through analysis of all pairs using the Tukey–Kramer anova test.
Thus, these results suggest a disproportionate role for a group of CD4+ T cells expressing Vβ 5·2, 11 and 24 that are highly committed to the response against Leishmania, and express cytokine and activation profiles consistent with a regulated, yet activated T cell response.
A positive correlation between IFN-γ- and TNF-α- producing CD4+ T cells was identified among restricted Vβ subpopulations
To investigate the possibility that specific subpopulations of CD4+ T cells defined by Vβ expression were involved in the formation of the co-regulated cytokine response among T cells producing IFN-γ and TNF-α, as we demonstrated for the total CD4+ T cell population in an earlier publication [10], we performed a correlation analysis between the frequency of specific CD4+ Vβ-expressing T cells producing IFN-γ or TNF-α with one another following SLA stimulation.
Of the three Vβ subpopulations that showed a higher SLA-specific production of IFN-γ and TNF-α compared to the other Vβ subpopulations, both CD4+ T cells expressing Vβ 5·2 and 11, but not Vβ 24, showed a positive correlation between the frequency of T cells expressing TNF-α and IFN-γ (Fig. 6a and b). Of all the subpopulations analysed, in addition to these two subpopulations, only T cells expressing Vβ regions 8 and 17 also had this correlation (Fig. 6c and d). Interestingly, Vβ 17-expressing cells, despite showing an expansion following SLA stimulation in CL patients, did not display higher frequency of activated or cytokine-producing cells compared to the other subpopulations.
Fig. 6.

Interferon (IFN)-γ and tumour necrosis factor (TNF)-α expression is correlated positively with CD4+ T cells expressing different Vβ regions after stimulation with soluble Leishmania antigen (SLA). Correlation between the frequencies of IFN-γ and TNF-α in CD4+ T cells expressing Vβ region of T cell receptor (TCR) analysed in vitro of cutaneous leishmaniasis patients (n = 12). (a) Demonstrates the positive correlation between expression levels of IFN-γ and TNF-α in CD4+ T cells expressing Vβ 5·2. (b) Positive correlation between expression levels of IFN-γ and TNF-α in CD4+T cells expressing Vβ 11. (c) Positive correlation between expression levels of IFN-γ and TNF-α in CD4+ T cells expressing Vβ 8 or (d) positive correlation between expression levels of IFN-γ and TNF-α in CD4+ T cells expressing Vβ 17. Correlation analyses were performed using Spearman's correlation coefficient and results were considered significant with P < 0·05; n = 12 in all cases.
Proinflammatory IFN-γ and TNF-α are correlated positively with anti-inflammatory IL-10 cytokines among CD4+ T cells expressing Vβ 5·2 or 24 regions
In CL patients, cytokine-producing cells can be related to cure or exacerbation of disease and may be associated with a regulated or unregulated cellular immune response. In our earlier study we demonstrated co-regulation of inflammatory with anti-inflammatory CD4+ T cells in CL disease [10]. In order to understand more clearly the possible role of the specific Vβ CD4+ T cell subpopulations in CL disease, correlation analyses were performed between the frequency of proinflammatory (IFN-γ and TNF-α) and anti-inflammatory (IL-10) cytokine-producing cells for each of the specific Vβ CD4+ T cell subpopulations following stimulation with SLA.
Among the three Vβ families that demonstrated higher frequencies of TNF-α-, IFN-γ- and IL-10-producing cells, two of them, Vβ 5·2 and 24, demonstrated strong positive correlations between the frequency of cells producing IL-10 and TNF-α or IFN-γ (Vβ 5·2) (Fig. 7). In addition, the Vβ 8 subpopulation (P = 0·02, data not shown) demonstrated a positive correlation.
Fig. 7.

Proinflammatory cytokines and interleukin (IL)-10-producing antigen-specific cells are correlated with CD4+ T cells expressing different Vβ regions. Correlation between the frequencies of interferon (IFN)-γ or tumour necrosis factor (TNF)-α and IL-10 in CD4+ T cells expressing Vβ region of T cell receptor (TCR) analysed in vitro of cutaneous leishmaniasis patients (n = 12). (a) Demonstrates the positive correlation between expression levels of IFN-γ and IL-10 in CD4+ T cells expressing Vβ 5·2 after soluble Leishmania antigen (SLA) stimulation; (b) positive correlation between TNF-α and IL-10 expressing antigen-specific CD4+ T cells bearing Vβ 5·2; (c) positive correlation between TNF-α- and IL-10-expressing CD4+ T cells bearing Vβ 24, after SLA stimulation. Correlation analyses were performed using Spearman's correlation coefficient and results were considered significant with P < 0·05; n = 12 in all cases.
CD4+ T cells expressing Vβ 5·2 are correlated with larger lesion sizes
Our earlier data demonstrated a direct correlation between the frequency of both activated T cells and IFN-γ-producing lymphocytes and the size of ulcerated cutaneous lesions in CL disease [15]. Thus, it was of great interest to verify if any of the specific CD4+ Vβ subpopulations also correlated with lesion size as a method of identifying possible T cell subpopulations involved with the local immune response and possible tissue damage. Interestingly, correlation analyses revealed a positive correlation between higher frequencies of Vβ 5·2 CD4+ T cells and larger lesion areas (Fig. 8).
Fig. 8.

Vβ 5·2-expressing CD4+ T cells are correlated with clinical characteristics. Correlation between lesion area (mm2) and the frequency of CD4+ T cells expressing the T cell receptor (TCR) Vβ 5·2 region after soluble Leishmania antigen (SLA) stimulation was performed. The correlation analysis was performed using Spearman's correlation coefficient and results were considered significant with P < 0·05. A total of nine patients were included in this analysis for which lesion area measurements were available.
Thus, three Vβ subpopulations (Vβ 5·2, 11 and 24) were identified as having a significant and consistent bias towards involvement with the anti-Leishmania response as measured by a variety of indicators, such as overall frequency, portion of cells committed to an ‘experienced’ phenotype and cytokine production. One of these, Vβ 5·2, also showed a positive correlation with lesion size.
CD4+ Vβ 5·2-expressing T cells accumulate in the lesion compared to their frequency in the blood
Given that there is intense trafficking of lymphocytes from the local draining lymph nodes through the blood and to lesions, we have seen that the blood often reflects what is happening at lesion sites in CL and mucosal disease when considering the overall immunoregulatory profile [10,12,13,34]. However, specific T cell subpopulations could be expected to accumulate in lesions if they express receptors specific for a prevalent antigen. This preferential accumulation would be identified by a higher percentage of cells expressing a given TCR Vβ segment in the inflammatory infiltrate compared to the percentage of these same TCR Vβ-expressing cells in the blood.
Given the positive correlation of CD4+ Vβ 5·2-expressing T cells with lesion size and their greater frequency of activation and cytokine production as measured by all criteria examined in this study, we analysed the percentage of these cells among CD4+ T cells in the inflammatory infiltrate of lesions from a group of CL patients. In addition, we determined the percentage of CD4+ Vβ 2-expressing T cells among CD4+ T cells as a control population that did not show an increase in activation or cytokine production compared to the other populations. Strikingly, CD4+ Vβ5·2 + T cells account for 29·3 ± 5% of the CD4+ T cells on average (n = 3), while CD4+ Vβ2 + T cells account for 21·3 ± 7% on average (Fig. 9). Thus, CD4+ Vβ5·2 + T cells showed an approximately 15-fold increase, on average, in the lesions compared to their frequency in blood, while CD4+ Vβ2 + T cells did not show a significant increase.
Fig. 9.

Analyses of CD4+ T cells expressing Vβ region in cutaneous leishmaniasis (CL) lesions. Representative images from confocal microscopy analyses for determination of frequency of CD4+ cells expressing Vβ 5·2 (a) and Vβ 2 (b) in CL lesions. Tissue sections were stained with fluorescein isothiocyanate (FITC)-labelled anti-Vβ 2 or Vβ 5·2 and anti-CD4 phycoerythrin (PE) cyanin 5 (Cy5)-labelled monoclonal antibodies and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), as described in Material and methods. The five optical sections for each patient were obtained simultaneously with lines 363, 488 and 633 of the agon/krypton laser and the proper filters. Overlays for Vβ 2 or Vβ 5·2 (green), CD4 (red) and DAPI (blue) in CL lesions are shown. Cells that are double-positive for CD4 and Vβ 2 or Vβ 5·2 appear in yellow. Three CL lesions were analysed.
Discussion
The human immune system joins a variety of factors to combat infection, while maintaining a well-balanced state within the host. Upon infection, the necessity to combat the pathogen, while maintaining this balanced state, is key for the health of the host. Understanding the events that lead to effective cellular immune responses in humans infected with intracellular pathogens such as Leishmania is key to the development of effective vaccines, immunotherapeutic approaches and specific diagnostics. To elucidate fully the role of T cells in the establishment and maintenance of effective immune responses to pathogens it is critical to study the dynamics of specific T cell subpopulations in individuals infected with pathogens.
One powerful way to monitor the T cell response is by studying individual T cell subpopulations based on their T cell receptor expression. Due to the availability of a panel of anti-Vβ TCR monoclonal antibodies, together with multi-parameter flow cytometry, we are able to follow the progression of T cell responses in infected patients with the hope of identifying specific T cell subpopulations that are most involved in the establishment of a protective or pathogenic immune response. We are able to determine the involvement of these subpopulations by studying not only the frequency of these specific subpopulations, but also the functional status via cytokine production and activation state by looking at memory and activation markers.
Through studies of the T cell repertoire, one can detect dominant T cell responses directed against specific MHC-peptide or major histocompatibility complex (MHC)-superantigen complexes [19,28]. Thus, by using flow cytometry to measure subpopulations of T cells based on their Vβ TCR chain from actively infected individuals, we aimed to determine the role of specific subpopulations in human CL.
Previous work studying the T cell repertoire in human and experimental infectious diseases has been carried out with the goal of identifying specific cellular subpopulations associated with disease development. Regarding experimental models in leishmaniasis, it has been demonstrated that IL-4-producing CD4+ T cells, which are responsible by directing the immune response towards Th2 cells, and therefore leading to pathology, preferentially express Vα8Vβ4 TCR [35,36].
Human leishmaniasis studies have demonstrated that cure of CL caused by L. braziliensis is associated with a higher percentage of T cells and higher IFN-γ production [14,37,38]. In CL caused by L. braziliensis, T cells present a strong Th1 profile with high levels of IFN-γ and TNF-α production [11,12,39], and CD4+ T cells are the major source of IFN-γ in peripheral blood [11] as well as in lesions of CL patients [12,34].
Comparative analyses of repertoire between non-infected individuals and CL patients were performed in the present study. The frequency of CD4+ T cells presenting specific Vβ subregions presented great heterogeneity in both groups, as expected, based on previous TCR repertoire studies in humans [21,40]. The majority of Vβ subpopulations were present in equivalent frequencies in non-infected controls and in L. braziliensis-infected individuals with CL disease. However, CD4+ T cells expressing Vβ5·2 and 24 from CL patients were present at increased frequencies compared to control donors in the absence of in vitro stimulation (Fig. 2). This may indicate that these subpopulations are involved in the response against Leishmania and play an important role in human CL. In acute pathogen-induced diseases, T cells involved in a response can have two distinct overall outcomes with regard to their frequency, depending on the nature of the antigenic stimulus and the disease at hand. T cells involved directly in the response and recognizing a specific antigenic peptide or superantigen can be measured either in an expansion phase or during a deletion phase. Both phases can be a reflection of antigenic stimulation, with one leading to an expansion of a specific T cell subpopulation and the other leading to deletion due to chronic re-stimulation and subsequent death of T cells [21,40]. While these results highlighted a group of T cells related to active disease, the determination of their antigen-specific response is also critical for determining their possible role in the response against Leishmania. Thus, we also performed comparative studies of cells before and after antigenic stimulation (Fig. 3). In this study we observed that after stimulus with the SLA, CD4+ T cells expressing regions Vβ 5·2, 11, 12 and 17 undergo statistically significant expansion, which suggests that they are involved in the response against Leishmania. Together with the results comparing non-infected to infected individuals, and the antigen-specific response, we identified several candidate subpopulations as being involved in the response against Leishmania in CL disease. One population in particular displayed an increased frequency when comparing both infected and non-infected individuals, as well as after antigenic stimulation, which was the CD4+ T cells expressing Vβ 5·2. Interestingly, studies of the repertoire in human Chagas disease demonstrated that PBMC from chronic cardiac patients displayed an expansion of the CD4+ T cells expressing Vβ5, which suggests that this subpopulation may play an important role in Chagas disease after contact with parasite antigens [20]. While these two parasites are distinct pathogens with differing life cycles and clinical manifestations, it is interesting that the same subpopulation of T cells expressing the Vβ5 region have arisen as T cells that are associated with the presence of the two diseases.
The antigen-specific responses among individuals infected with L. braziliensis also revealed a significant expansion of T cells expressing Vβ12 (Fig. 3). Interestingly, this was the same subpopulation identified by Clarencio et al. in CL caused by L. braziliensis and stimulated by SLA of L. amazonensis[29]. This finding may suggest an existence of common dominant response between different species of Leishmania leading to the expansion of a similar subpopulation of T cells.
Frequency differences are only one possible measure of the involvement of a specific subpopulation of T cells in an active immune response. It is possible that slight changes or no global change in the frequency of T cell subpopulations will be noted due to a balance between expansion and death of responding T cells. However, by determining the portion of a given subpopulation committed to an activated phenotype, memory phenotype or producing specific cytokines, we can determine their relative involvement and possible functional role in a protective or pathogenic immune response. Thus, we performed comparative analyses between the different Vβ subpopulations of the proportion of cells expressing either a marker of late T cell activation, the class II molecule, HLA-DR, or a marker associated with many memory T cells, CD45RO. These markers were measured without in vitro antigenic restimulation with the goal of determining their involvement in the host actively infected with Leishmania. Strikingly, CD4+ T cells expressing Vβ regions 5·2, 11 and 24 displayed a significantly higher portion of cells expressing CD45RO and HLA-DR (Fig. 4). Thus, these subpopulations demonstrated a phenotype consistent with greater involvement in an ongoing immune response than the other T cell subpopulations. Importantly, two of these subpopulations (Vβ5·2 and Vβ11 CD4+ T cells) also displayed an expansion after antigen specific stimulation in vitro (Fig. 3).
In order to understand more clearly the functional potential of specific subpopulations of CD4+ T cells based on Vβ expression, we went on to measure their relative commitment to production of antigen-specific proinflammatory (IFN-γ and TNF-α) and anti-inflammatory (IL-10) cytokines. Strikingly, the same three Vβ-expressing subpopulations arose as having a disproportionately high percentage of the SLA-stimulated T cells committed to cytokine production compared to the majority of the other Vβ-expressing T cell populations (Fig. 5). Thus, CD4+ T cell subpopulations defined by Vβ 5·2, 11 and 24 in CL patients displayed higher production of IFN-γ, TNF-α and IL-10 compared to several other subpopulations of T cells in CL patients.
An important aspect of human leishmaniasis and other infectious diseases is the balance of inflammatory and down-regulatory cytokines. In human CL there is a controlled immune response against Leishmania that often leads to protective immunity and is marked by the co-regulation of not only TNF-α- and IFN-γ-producing T cells, but also of inflammatory (TNF-α and IFN-γ) and anti-inflammatory (IL-10) cytokine-producing T cells [10,13,15].
Interestingly, the same Vβ subpopulations that demonstrated a higher proportion of cells committed to previously activated or memory T cells, as well as higher frequencies of cytokine-producing cells, were among those that showed the co-regulation of IFN-γ-, TNF-α-producing T cell subpopulations. The only other T cell subpopulations that demonstrated this co-regulation of frequencies were those represented by Vβ8 and 17 subpopulations (Fig. 6). In addition to the co-regulation of inflammatory cytokines, the only Vβ subpopulations that showed co-regulation of inflammatory and anti-inflammatory cytokine, IL-10, were those identified by Vβ 5·2 and 24, which also showed involvement in the response as determined by a number of other indicators (Figs 3–7). These findings agree with earlier findings by our group demonstrating co-regulation of these same cytokine-producing cells at the level of total CD4+ T cells stimulated with SLA from CL patients [10]. This result suggests that these CD4+ T cell subpopulations expressing specific Vβs are involved significantly in the response during active infection with L. braziliensis in patients with CL disease. Thus, the T cell subpopulations identified in this study based on their Vβ expression are consistent with the overall profile seen in the CD4+ T cell population, and have functional significance for control and possibly pathology of human CL disease. While the co-regulation of TNF-α and IFN-γ with IL-10 was seen in only one of the Vβ T cell subpopulations, it is one of the populations that were demonstrated consistently to be involved in all aspects of the response from an increased frequency to higher proportions in memory and cytokine production. When performing analysis of associations between the frequency of CD4+ T cell subpopulations with lesion size using measurements from both non-stimulated and antigen stimulated cultures, only the subpopulation expressing Vβ 5·2 displayed a positive correlation between higher frequencies of T cells and larger lesion area. This is striking, given that none of the other eight Vβ subpopulations demonstrated this significant correlation for both non-stimulated and antigen-stimulated measurements. Importantly, CD4+ Vβ 5·2-expressing T cells are greatly over-represented at the lesion site compared to the blood, further suggesting a key role in the response during CL (Fig. 9).
In summary, in this study we have demonstrated the existence of distinct CD4+ T lymphocyte subpopulations defined by their TCR Vβ regions that are involved consistently in several aspects of the immune response in individuals infected with L. braziliensis and with active CL disease. Given that the great majority of individuals with CL disease typically resolve infection and go on to establish long-lived protective immunity, we hypothesize that these cells are involved in the formation of a protective immune response that could also be associated with pathology in the case of the ulcerated lesion. Moreover, we continue to add to the evidence that modulatory cytokines, such as IL-10, are co-regulated with macrophage-activating cytokines such as IFN-γ and TNF-α. Further studies are under way to directly measure these T cell subpopulations at the lesion site and in other clinical forms of leishmaniasis. Moreover, the use of this information in attempts to define the antigens responsible for the preferential use of the subpopulations defined here could aid in the selection of immunodominant antigens used by the human immune response against Leishmania.
Acknowledgments
We thank the funding agencies: NIH-TMRC, NIH-R03AI066253-02, FAPEMIG-Infra, CNPq-INCT-DT and CNPq for fellowships.
Disclosure
None.
References
- 1.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–77. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–423. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Exp Rev Anti Infect Ther. 2010;8:419–33. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho EM, Barral A, Costa JM, Bittencourt A, Marsden P. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 1994;56:315–25. doi: 10.1016/0001-706x(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 5.Barral A, Barral-Netto M, Almeida R, et al. Lymphadenopathy associated with Leishmania braziliensis cutaneous infection. Am J Trop. 1992;47:587–92. doi: 10.4269/ajtmh.1992.47.587. [DOI] [PubMed] [Google Scholar]
- 6.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 7.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 8.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 9.Mendonca SC, De Luca PM, Mayrink W, et al. Characterization of human T lymphocyte-mediated immune responses induced by a vaccine against American tegumentary leishmaniasis. Am J Trop Med Hyg. 1995;53:195–201. doi: 10.4269/ajtmh.1995.53.195. [DOI] [PubMed] [Google Scholar]
- 10.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Gollob KJ. Antigen-specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation. Clin Exp Immunol. 2004;136:341–8. doi: 10.1111/j.1365-2249.2004.02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottrel RL, Dutra WO, Martins FA, et al. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun. 2001;69:3232–9. doi: 10.1128/IAI.69.5.3232-3239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faria DR, Gollob KJ, Barbosa J, Jr, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–9. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaze ST, Dutra WO, Lessa M, et al. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol. 2006;63:70–8. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]
- 14.Gomes-Silva A, de Cassia BR, Dos Santos NR, et al. Can interferon-gamma and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol. 2007;149:440–4. doi: 10.1111/j.1365-2249.2007.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101:226–30. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Meuer SC, Cooper DA, Hodgdon JC, et al. Identification of the receptor for antigen and major histocompatibility complex on human inducer T lymphocytes. Science. 1983;222:1239–42. doi: 10.1126/science.6606228. [DOI] [PubMed] [Google Scholar]
- 17.Lennon GP, Sillibourne JE, Furrie E, Doherty MJ, Kay RA. Antigen triggering selectively increases TCRBV gene transcription. J Immunol. 2000;165:2020–7. doi: 10.4049/jimmunol.165.4.2020. [DOI] [PubMed] [Google Scholar]
- 18.Brenner MB, Trowbridge IS, Strominger JL. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985;40:183–90. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- 19.Herman A, Kappler JW, Marrack P, Pullen AM. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–72. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 20.Costa RP, Gollob KJ, Fonseca LL, et al. T-cell repertoire analysis in acute and chronic human Chagas' disease: differential frequencies of Vbeta5 expressing T cells. Scand J Immunol. 2000;51:511–19. doi: 10.1046/j.1365-3083.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot AP, Kotzin BL, Comment CE, Newman LS. Expansions of T-cell subsets expressing particular T-cell receptor variable regions in chronic beryllium disease. Am J Respir Cell Mol Biol. 1998;18:581–9. doi: 10.1165/ajrcmb.18.4.2981. [DOI] [PubMed] [Google Scholar]
- 22.Giacoia-Gripp CB, Neves I, Jr, Galhardo MC, Morgado MG. Flow cytometry evaluation of the T-cell receptor Vbeta repertoire among HIV-1 infected individuals before and after antiretroviral therapy. J Clin Immunol. 2005;25:116–26. doi: 10.1007/s10875-005-2817-z. [DOI] [PubMed] [Google Scholar]
- 23.Mancia L, Wahlstrom J, Schiller B, et al. Characterization of the T-cell receptor V-beta repertoire in Kawasaki disease. Scand J Immunol. 1998;48:443–9. doi: 10.1046/j.1365-3083.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- 24.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 25.Gollob KJ, Palmer E. Divergent viral superantigens delete V beta 5+ T lymphocytes. Proc Natl Acad Sci USA. 1992;89:5138–41. doi: 10.1073/pnas.89.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modlin RL, Tapia FJ, Bloom BR, et al. In situ characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Exp Immunol. 1985;60:241–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Pirmez C, Cooper C, Paes-Oliveira M, Schubach A, Torigian VK, Modlin RL. Immunologic responsiveness in American cutaneous leishmaniasis lesions. J Immunol. 1990;145:3100–4. [PubMed] [Google Scholar]
- 28.Uyemura K, Pirmez C, Sieling PA, Kiene K, Paes-Oliveira M, Modlin RL. CD4+ type 1 and CD8+ type 2 T cell subsets in human leishmaniasis have distinct T cell receptor repertoires. J Immunol. 1993;151:7095–104. [PubMed] [Google Scholar]
- 29.Clarencio J, de Oliveira CI, Bomfim G, et al. Characterization of the T-cell receptor Vbeta repertoire in the human immune response against Leishmania parasites. Infect Immun. 2006;74:4757–65. doi: 10.1128/IAI.00265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayrink W, Schettini AP, Williams P, et al. Histological observations on Montenegro's reaction in man. Rev Inst Med Trop Sao Paulo. 1989;31:256–61. doi: 10.1590/s0036-46651989000400008. [DOI] [PubMed] [Google Scholar]
- 31.Follador I, Araujo C, Bacellar O, et al. Epidemiologic and immunologic findings for the subclinical form of Leishmania braziliensis infection. Clin Infect Dis. 2002;34:E54–E58. doi: 10.1086/340261. [DOI] [PubMed] [Google Scholar]
- 32.Jose FF, da Silva IM, Araujo MI, Almeida RP, Bacellar O, Carvalho EM. [Evaluation of the sensitization power of Montenegro skin test] Rev Soc Bras Med Trop. 2001;34:537–42. doi: 10.1590/s0037-86822001000600007. [DOI] [PubMed] [Google Scholar]
- 33.Gazzinelli G, Katz N, Rocha RS, Colley DG. Immune responses during human Schistosomiasis mansoni. X. Production and standardization of an antigen-induced mitogenic activity by peripheral blood mononuclear cells from treated, but not active cases of schistosomiasis. J Immunol. 1983;130:2891–5. [PubMed] [Google Scholar]
- 34.Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–40. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiner SL, Wang ZE, Hatam F, Scott P, Locksley RM. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993;259:1457–60. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- 36.Rogers KA, DeKrey GK, Mbow ML, Gillespie RD, Brodskyn CI, Titus RG. Type 1 and type 2 responses to Leishmania major. FEMS Microbiol Lett. 2002;209:1–7. doi: 10.1111/j.1574-6968.2002.tb11101.x. [DOI] [PubMed] [Google Scholar]
- 37.Da-Cruz AM, Conceicao-Silva F, Bertho AL, Coutinho SG. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect Immun. 1994;62:2614–18. doi: 10.1128/iai.62.6.2614-2618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coutinho SG, Oliveira MP, Da-Cruz AM, et al. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–55. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- 39.Castes M, Trujillo D, Rojas ME, et al. Serum levels of tumor necrosis factor in patients with American cutaneous leishmaniasis. Biol Res. 1993;26:233–8. [PubMed] [Google Scholar]
- 40.Menezes CA, Rocha MO, Souza PE, Chaves AC, Gollob KJ, Dutra WO. Phenotypic and functional characteristics of CD28+ and CD28- cells from chagasic patients: distinct repertoire and cytokine expression. Clin Exp Immunol. 2004;137:129–38. doi: 10.1111/j.1365-2249.2004.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


