Abstract
Little is known about the ability of hepatitis C virus (HCV) to alter early innate immune responses in infected patients. Previous studies have shown that natural killer (NK) cells are functionally impaired after interaction of recombinant HCV glycoprotein E2 with the co-stimulatory CD81 molecule in vitro; however, the functional consequences of a prolonged contact of NK cells with HCV particles have remained unclear. We have examined the phenotypes of purified, interleukin-2-activated NK cells from healthy donors and HCV genotype 1b patients after culture for 5 days with HCV pseudoparticles (HCVpp) and serum samples containing HCV genotype 1b. NK cells from healthy donors and chronic HCV patients were found to up-regulate receptors associated with activation (NKp46, NKp44, NKp30, NKG2D), while NK receptors from the killer cell immunoglobulin-like receptor family (KIR/CD158), predominantly having an inhibitory function, were significantly down-modulated after culture in the presence of HCV particles compared with control cultures of NK cells. HCV-infected sera and HCVpp elicited significantly higher secretion of the NK effector lymphokines interferon-γ and tumour necrosis factor-α. Furthermore, HCV stimulated the cytotoxic potential of NK cells from normal donors and patients. The enhanced activation of NK cells after prolonged culture with HCVpp or HCV-containing sera for 5 days suggests that these innate effector cells may play an important role in viral control during early phases of HCV infection.
Keywords: hepatitis C virus, natural killer cells, NK cell receptors, viral hepatitis
Introduction
Natural killer (NK) cells play an important role in innate immune responses against hepatitis C virus (HCV) [1–4]. NK cells can inhibit HCV expression through interferon (IFN)-γ[5] and directly lyse hepatoma cells harbouring HCV replicons [6]. The presence of particular killer immunoglobulin-like receptors (KIR) with inhibitory function and human leucocyte antigen-C (HLA-C) alleles is associated with a more efficient clearance of low-dose acute HCV infections [7]. Increased expression of the activating receptors, NKp46 and NKp30, has been reported for NK cells from chronically infected HCV patients [8], whereas other authors described reduced levels of natural cytotoxicity receptors in viraemic patients [9]. NK cells from HCV-infected patients were reported to express increased amounts of the inhibitory receptors CD94/NKG2A and to produce the inhibitory cytokines interleukin (IL)-10 and transforming growth factor (TGF)-β[9–11]. Some groups [9,12,13], but not others [14–16], described a depressed natural cytotoxicity in NK cells from HCV patients. Furthermore, generally reduced NK cell frequencies and significant alterations in the subsets of CD56+/CD16+ NK cells in chronic HCV-infected individuals have been reported [9,11,15,17–20]. An activated NK phenotype was found in blood samples from patients with acute HCV infections [21].
Cross-linking of the tetraspanin CD81 on NK cells by an immobilized, recombinant form of the HCV envelope protein E2 has been shown to inhibit NK cytotoxicity as well as CD16- or IL-2-induced IFN-γ secretion by NK cells [22–24]. The findings suggested a direct impairment of NK cell functions through direct contact with HCV virions or HCV-infected cells. This view has been challenged recently by a study using infectious HCV particles produced in a cell culture system for co-cultivation with NK cells from healthy donors. Exposure to high concentrations of HCV particles had a neutral effect as it did not alter CD16-stimulated NK activation and IFN-γ production [25]. In this study, we asked whether HCV pseudoparticles (HCVpp) or HCV-containing sera would enhance or block activation of NK cells under stimulatory culture conditions including allogeneic feeder cells. Unexpectedly, we found that HCVpp as well as infected sera containing high titres of HCV consistently induced an activation-associated surface marker phenotype in NK cells purified from the blood of healthy donors and chronic HCV patients, and stimulated effector lymphokine secretion as well as cytolytic activity.
Materials and methods
HCV serum samples
Ten ml of venous blood were withdrawn from untreated individuals infected with HCV genotype 1b. Informed consent was obtained from each patient included in the study. The whole blood sample was left to coagulate and then centrifuged at 2100 g for 10 min. Serum was collected and stored in small aliquots at −20°C. For co-cultivation with NK cells, we used serum samples from four chronic patients with HCV1b RNA titres ranging between 33·9 and 41·5 × 106 RNA copies/ml.
Production of HCV pseudotype particles
To generate HCV pseudotype particles (HCVpp), HEK293T cells were transfected with expression vectors encoding the viral E1/E2 glycoproteins [phCMVDCE1-E2(Con1)], human immunodeficiency virus (HIV)-Gag-Pol (pCMV-DR8·74), and the packaging-competent green fluorescent protein (GFP)-containing retroviral transfer vector pHR-CMV-Emd, which were kindly provided by Dr R. Bartenschlager, Department of Molecular Virology, University of Heidelberg [26,27]. For an expression control, we used the plasmid pcz vesicular stomatitis virus (VSV)-G [26] instead of phCMVDCE1-E2(Con1). For transfection a calcium phosphate transfection kit was used (Clontech, Heidelberg, Germany), according to the manufacturer's instructions, using 8 µg of each plasmid. Supernatants containing HCVpp were harvested 40–48 h after transfection, clarified by low-speed centrifugation for 15 min, filtered through membranes with 0·45 µm pores and concentrated using Amicon Ultra-15 molecular filters with an exclusion size of 30 kDa (Millipore, Bedford, MA, USA). We usually concentrated the particles 20-fold and stored them at −80°C.
HCVpp infection assay
Huh-7 human hepatocellular carcinoma cells were seeded the day before infection at 1 × 105 Huh-7 cells per well in a 12-well tissue culture plate. One h before infection, cells were washed three times with warm phosphate-buffered saline (PBS) and incubated with plain Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Karlsruhe, Germany). Then dilutions of viral supernatants containing the HCVpp were added to the cells and incubated for 3 h. The supernatants were removed and the cells incubated in DMEM supplemented with 10% (v/v) fetal calf serum (FCS) (Biochrom, Berlin, Germany) and 2 mm l-glutamine (Invitrogen) for 72 h at 37°C. The infectious titres, expressed as transducing units (TU)/ml, were determined as the percentage of GFP-positive cells measured by fluorescence activated cell sorter (FACS) analysis using the formula [(number of Huh-7 target/volume of HCVpp) × (percentage of GFP-positive cells/100)]. Infected Huh-7 cells were trypsinized, suspended in PBS with 0·5% bovine serum albumin (Sigma-Aldrich) and analysed for GFP fluorescence by cytofluorometry.
NK cell isolation and culture
Peripheral blood mononuclear cells (PBMC) were prepared from anti-coagulated blood from two chronic HCV patients (genotype 1b, untreated, viral load 12 and 18 × 106 RNA copies/ml, respectively) and peripheral blood from voluntary, uninfected blood donors. Blood was diluted 1:1 with Dulbecco's PBS (Invitrogen) and PBMC prepared using lymphocyte separation medium LSM 1077 (PAA, Pasching, Austria). After washing the harvested leucocyte-rich interphase in PBS, up to 250 × 106 PBMC were used for the purification of NK cells by using the ‘Dynabeads Untouched Human NK cells’ kit (Invitrogen Dynal, Oslo, Norway) following the manufacturer's instructions. Purified NK cells were resuspended in complete NK medium at 1 × 106/ml [Iscove's modified Dulbecco's medium (IMDM)] cell culture medium supplemented with 10% heat-inactivated human antibody serum, 100 U of penicillin/ml, 100 µg of streptomycin/ml, 1% sodium pyruvate and 1% non-essential amino acids (all from Invitrogen). To provide for a basic activation of NK cells, we added recombinant human IL-2 (100 U/ml) (Proleukin, Novartis, Basle, Switzerland), phytohaemagglutinin-P (PHA-P) (1 µg/ml) (Sigma-Aldrich, Taufkirchen, Germany) and allogeneic PBMC as feeder cells at 1 × 106/ml (γ-irradiated with 50 Gy). Cells were finally plated at 1 × 105 per well in 96-well round-bottomed plates in 100 µl complete NK medium. Irradiated, allogeneic feeder cells decayed completely during the first 2–3 days of culture.
Stimulation of NK cells
For the culture of NK cells with HCV particles and medium controls, we added either 100 µl of complete IMDM medium, 100 µl of complete Dulbecco's modified Eagle's medium (DMEM) conditioned for 48 h by untransfected HEK293T cells, 100 µl of human serum from healthy donors, HCVpp (23·000 TU/ml) contained in 100 µl complete DMEM or 100 µl HCV1b-containing sera (∼38 × 106 RNA copies/ml), respectively, to 96-well plates containing NK cells. NK cells were harvested 5 days later and washed extensively before analysis by flow cytometry or use in the cytotoxicity assay. In some experiments, 1 µl/well goat anti-HCV polyclonal antibodies (Antigenix America, Huntington Station, NY, USA) were included during the culture period. In other experiments, the monoclonal antibody I·3·3·22 (abcam, Cambridge, UK) against CD81/TAPA1 was added at 1 µg/well at the beginning of the culture period.
Flow cytometry analysis of NK cells
To 2–3 × 105 NK cells used per sample, human immunoglobulin (Ig)G (Venimmun N, CSL Behring, Marburg, Germany) was added at 2·5 mg/ml in FACS buffer (Dulbecco's PBS/1% FCS) in order to block Fc receptors. Then the samples were incubated in 100 µl FACS buffer supplemented with 2 µl AlexaFluor 488-conjugated anti-CD56 (B159; BD Pharmingen, Heidelberg, Germany), 1 µl AlexaFluor 647-conjugated mouse anti-human CD16 (3G8; BioLegend, San Diego, CA, USA), 0·5 µl phycoerythrin-cyanin-5·5 (PE-Cy5·5)-conjugated mouse anti-human CD3 (UCHT1; Southern Biotech, Birmingham, AL, USA) and one of the following PE-conjugated monoclonal antibodies (mAb) recognizing human NK cell receptors: NKp46/CD335 (9E2; Miltenyi Biotec, Bergisch-Gladbach, Germany, 5 µl), NKp44/CD336 (2·29; Miltenyi Biotec, 5 µl), NKp30/CD337 (AF29-4D12; Miltenyi Biotec, 5 µl), anti-NKG2D/CD314 (1D11; BioLegend, 5 µl), anti-CD158/KIR (180704; R&D Systems, Wiesbaden, Germany, 1·5 µl), anti-CD94 (DX22; BioLegend, 2 µl), anti-AIRM1/p75/Siglec-7 (eBioQA79; eBioscience, San Diego, CA, USA 5 µl) and anti-CD81/TAPA1 (JS-81; BD Pharmingen, 5 µl), respectively, for 45 min on ice. After two washes with FACS buffer, cells were resuspended in FACS buffer with 1·3 µg/ml propidium iodide (PI) (Sigma-Aldrich). Control samples stained with anti-CD16 and anti-NKp46 alone showed 85–95% purity of NK cells after co-cultivation for 5 days with irradiated PBMC. For instrument settings and compensation, samples stained with individual fluorescent probes were used. Flow cytometry analysis was performed with a FACSCalibur cytometer and data were analysed with CellQuest Pro software from Becton Dickinson (Heidelberg, Germany). Frequencies of NK cells, double-stained for an NK cell marker and either CD56 or CD16, from different treatment modalities were compared by Student's t-test.
Cytokine production by NK cells
Cell-free culture supernatants were harvested after 5 days of NK cell culture. Appropriate dilutions assayed for IFN-γ and TNF-α contents using human IFN-γ and TNF-α enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go kits (eBioscience). The actual cytokine concentrations in pg/ml were determined by using standard reagents as provided by the manufacturer.
Cytotoxicity assay
NK cells cultured in the presence of HCVpp or HCV1b, or IMDM medium for control, were used as effector cells. The human pancreatic carcinoma line BxPC3 and the human T×B hybrid cell line T2 were used as targets. The target cells were labelled with specific antibodies, 5 µl HEA125-fluorescein isothiocyanate (FITC) and 10 µl Muromonab-CD3 (OKT3)-FITC (kind gifts from Dr G. Moldenhauer, DKFZ, Heidelberg) for BxPC3 and T2, respectively, for 30 min on ice in the dark followed by two washes in FACS buffer; 2 × 105 target cells and 106 or 2 × 106 effectors cells were resuspended in complete RPMI-1640 medium. Cells were pelleted at 100 g for 1 min, incubated at 37°C in a CO2 incubator for 4 h and then washed twice with FACS buffer. The cell mixture was resuspended in 200 µl FACS buffer with 1·3 µg/ml PI to label dead cells. For instrument settings and compensations, individual samples of FITC-labelled target cells and NK cells were used. An analysis gate was set on the target cells and the percentage of FITC+/PI+ cells was determined using CellQuest Pro software.
Results
Infectivity of HCVpp
HEK293T cells were transfected with expression vectors encoding the viral E1 and E2 glycoproteins, HIV retroviral core proteins and packaging-competent GFP-containing retroviral transfer vectors. HEK293T cells showed ∼40% GFP-positive cells 48 h after transfection indicating HCVpp production (Fig. 1a). Huh-7 human hepatocellular carcinoma cells were infected with HEK293T supernatants containing HCVpp or with VSV-G pseudotype particles as positive control, or left untransfected. Flow cytometry analysis showed 14·5–22·3% GFP-positive cells after infection with HCVpp-containing supernatant (Fig. 1b), demonstrating that the HCVpp preparation was indeed infective.
Fig. 1.
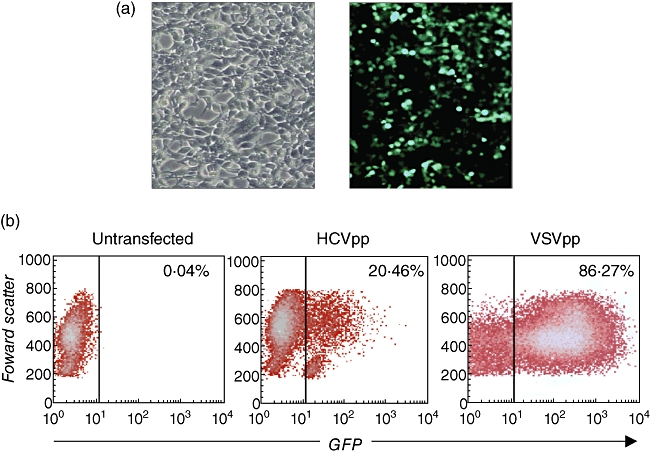
Production and infectivity of hepatitis C virus pseudoparticles (HCVpp). (a) HEK293T cells were transfected transiently with expression vectors encoding the HCV E1 and E2 glycoproteins, retroviral core proteins and a packaging-competent green fluorescent protein (GFP)-containing retroviral transfer vector to produce HCVpp. Left: phase contrast microscopical picture of HEK293T cells 48 h after transfection. Right: fluorescence picture of HEK293T cells 48 h after transfection showing ∼40% GFP-positive cells confirming HCVpp production. (b) Infectivity of HCVpp produced by HEK293T cells. Huh-7 human hepatocellular carcinoma cells were infected with 500 µl HEK293T supernatant containing HCVpp, or vesicular stomatitis virus (VSV)-Gpp as a positive control, or left untransfected; 72 h after infection Huh-7 cells were analysed by flow cytometry for GFP expression.
Enhanced expression of activating NK receptors after in vitro exposure to HCV
NK cells were isolated from healthy donors and chronic HCV patients followed by culture for 5 days in the presence of IL-2, PHA-P and irradiated allogeneic PBMC to provide for a basic stimulation. To these cultures, HCVpp-containing HEK293T supernatant, control HEK293T supernatant, HCV1b-containing serum samples, normal human serum or IMDM medium were added, respectively. NK cells were analysed by multi-colour flow cytometry using AlexaFluor 488- and AlexaFluor 647-labelled mAbs against the NK cell markers CD56 and CD16, together with various PE-conjugated mAbs recognizing NK receptors. Using a PE-Cy5-labelled anti-CD3 mAb, few remaining CD3+ T cells were gated out together with PI-labelled dead cells. The purity of NK cells as judged by stainings with NKp46, CD56 and CD16 ranged between 85–95% after the stimulation period (data not shown).
As representative examples, we show stainings obtained with NK cells from normal donor 1 for the activating receptor NKp44, the inhibitory receptor CD94 (KLRD1) and the HCV-E2 receptor CD81 (Fig. 2). The cells were co-stained for the NK cell marker CD16 (FcγRIIIb). Upon culture with HCVpp and HCV1b serum, the frequencies of NKp44+/CD16+ double-positive NK cell subsets were increased compared with control-cultured NK cells, whereas the sizes of CD94+/CD16+ subsets were clearly reduced. The expression rates of CD81 were not influenced by HCVpp or HCV1b serum.
Fig. 2.
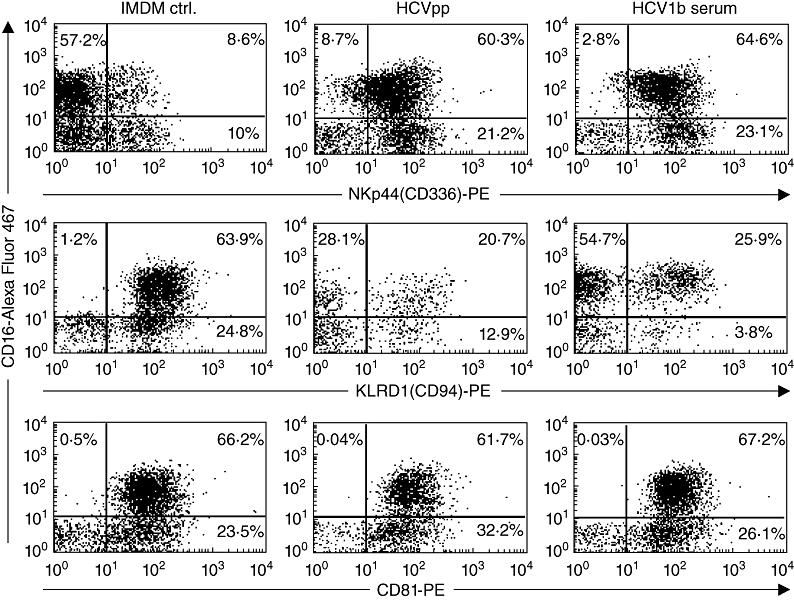
Flow cytometry analysis of primary natural killer (NK) cells cultured in the presence of hepatitis C virus (HCV). NK cells from normal donor 1 were cultured for 5 days in Iscove's modified Dulbecco's medium/interleukin-2 (IMDM/IL-2) medium alone, in IMDM supplemented with HCV pseudoparticles (HCVpp)-containing supernatant, or in IMDM supplemented with HCV1b-containing human sera. Cells were stained with AlexaFluor 647-conjugated anti-CD16 and either anti-NKp44-phycoerythrin (PE), anti-CD94-PE or anti-CD81-PE monoclonal antibodies, respectively.
The results from flow cytometry analyses of NK cells derived from five normal donors and three chronic HCV patients are summarized in Fig. 3. We studied the expression levels of a panel of surface receptors with activating and inhibitory functions [28–30]. The activating NK receptors studied were NKp46 (NCR1/CD335), NKp44 (NCR2/CD336), NKp30 (NCR3/CD337) and NKG2D (KLRK1/CD314). Using a pan-KIR/CD158 mAb, we analysed expression of the mostly inhibitory killer cell immunoglobulin-like receptors (KIR), of CD94 (KLRD1), which is part of the NKG2A/CD94 inhibitory receptor, of the inhibitory receptor AIRM1 (CD328/p75/Siglec-7) and the HCV-E2 receptor CD81 (TAPA1). In NK cell samples from normal donors and patients treated with HCVpp or HCV1b serum, a relative increase in the frequencies of CD56bright and CD16+ NK cells co-expressing NKp46, NKp44, NKp30 or NKG2D was observed consistently compared with control-treated NK cells (Fig. 3a). By contrast, culture of NK cells from all donors with HCVpp or HCV1b serum resulted in significantly reduced frequencies of CD56+ and CD16+ NK cells expressing CD158 compared with NK cells cultured without HCV (Fig. 3b). We also noted a clear trend of NK cells to down-modulate the inhibitory receptors CD94 and AIRM1 upon culture with HCVpp and HCV1b serum, as indicated by reduced mean values of respective NK cell frequencies averaged over all donors (Fig. 3b). Due to the up-regulation of CD94 or AIRM1 in a minority of samples, however, these reduced expression levels did not reach statistical significance. Expression of the tetraspanin CD81 in NK cell subsets was, on average, found to be slightly increased after culture in the presence of HCVpp or HCV1b serum.
Fig. 3.
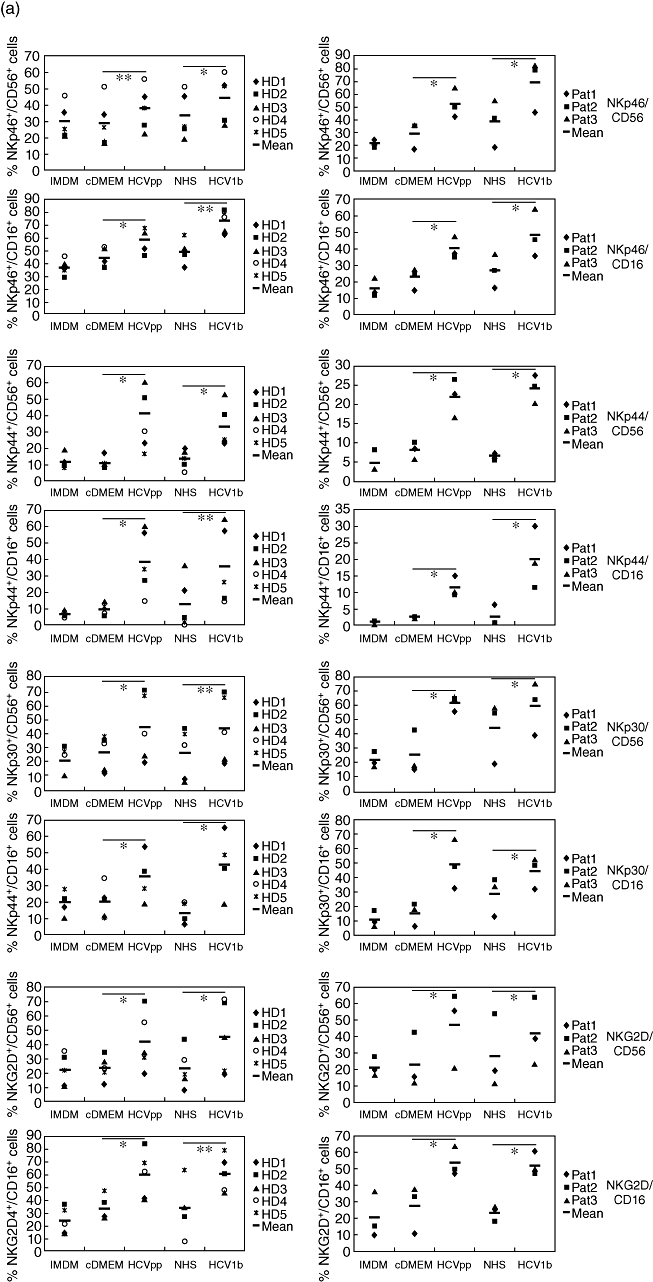
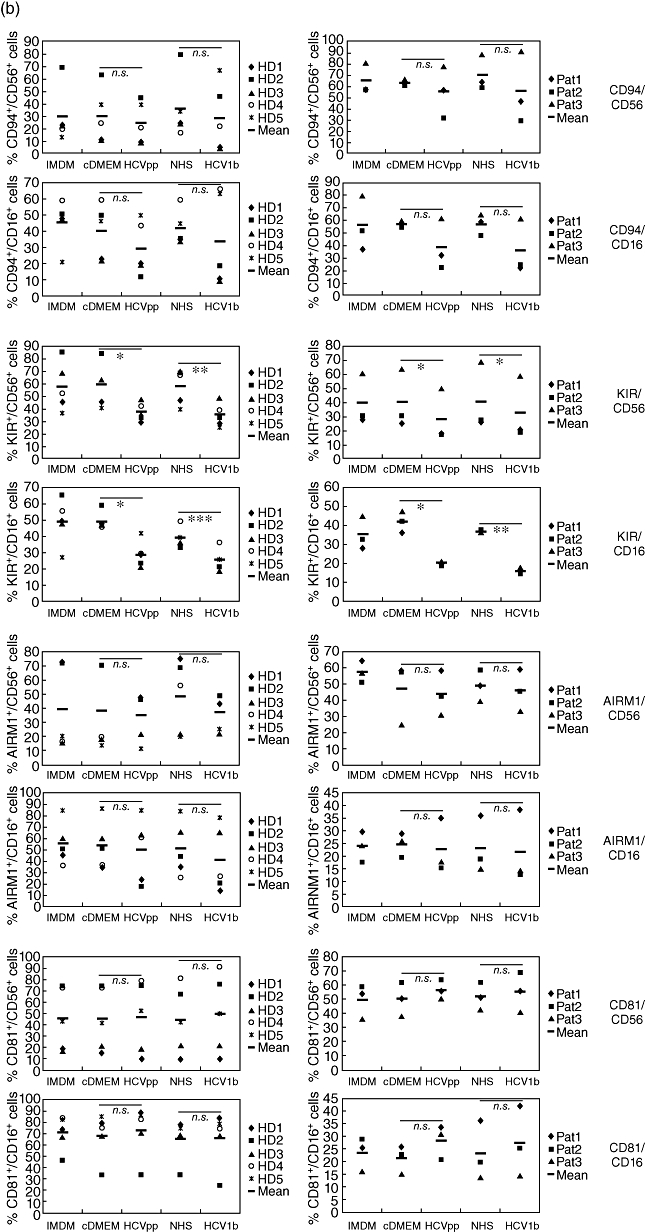
Increased expression of activating receptors and decreased expression of inhibitory receptors on natural killer (NK) cells after exposure to hepatitis C virus (HCV). NK cells from five healthy donors (HD, panels on left side) and three chronic HCV patients (Pat., panels on right side) were cultured for 5 days in either NK cell medium [Iscove's modified Dulbecco's medium (IMDM)] with interleukin-2/phytohaemagglutinin-P (IL-2/PHA-P)/feeders alone, or supplemented (1:1) with Dulbecco's modified Eagle's medium (DMEM) conditioned by untransfected HEK293T cells (cDMEM), HCV pseudoparticles (HCVpp)-containing supernatant from transfected HEK293T cells, normal human serum (NHS) or human serum containing HCV1b, as indicated. Cells were analysed by multi-colour flow cytometry. (a) Frequencies of cells stained for either CD56 or CD16, and for one of the activating receptors NKG2D, NKp46, NKp44 or NKp30 are indicated. (b) Frequencies of cells stained for CD56 or CD16, and for one of the inhibitory receptors CD94/KLRD1 or AIRM1/p75, the KIR/CD158 receptor family, or for the cellular HCV-E2 ligand CD81/TAPA1 are indicated. The mean values of NK cell frequencies are shown. The differences between the cDMEM- and HCVpp-, as well as between the NHS- and HCV1b serum-treated groups, were evaluated by a paired two-tailed Student's t-test; n.s.: not significant; *P < 0·05; **P < 0·01; ***P < 0·001.
Because HCVpp and HCV1b are supposed to interact with NK cells through the E2 receptor CD81, we asked whether anti-CD81 antibodies would counteract the up-regulation of NK activating receptors cultured in the presence of HCVpp or HCV1b serum. Compared with cultures to which no antibody was added, to a large extent the addition of an anti-CD81 antibody blocked the HCV-induced upregulation of NKp46, NKp44, NKp30 and NKG2D (Fig. 4).
Fig. 4.
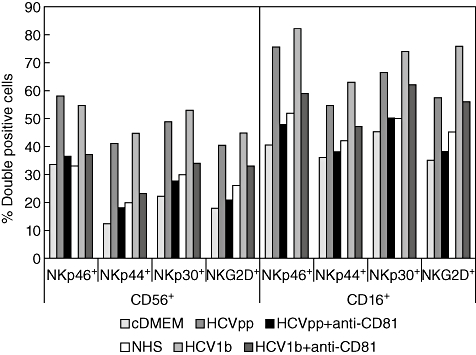
Anti-CD81 antibodies counteract the induction of activating natural killer (NK) receptors by hepatitis C virus (HCV). Purified NK cells were cultured as described in the legend to Fig. 3. The anti-CD81 monoclonal antibody I·3·3·22 was added to culture wells at 1 µg/well as indicated. Frequencies of NK cells labelled for either CD56 or CD16, and one of the activating receptors, NKp46, NKp44, NKp30 or NKG2D, are shown.
CD56+/CD16+ NK subsets are not altered by HCV particles
Because chronic HCV infection was found to be associated with alterations in the CD56+ and CD16+ NK cell subsets [9,11,15,17–20], we studied NK phenotypes after 5 days of stimulation with IL-2, PHA-P and allogeneic feeders. A larger fraction of NK cells from normal donors and HCV patients displayed the CD56dim/CD16++ phenotype of NK cells (Table 1), which has been reported to possess a high cytolytic capacity [31]. The frequencies of cytokine-secreting CD56bright/CD16- NK cells, that are prone to cytokine secretion [31], varied considerably among the NK cell donors. This NK subset was considerably larger in healthy donors 2 and 5 and patient 1 than in the other donors. These variations probably represented interindividual differences. Except for slightly increased CD56dim/CD16++ frequencies in NK cells from patients 1 and 3 after co-cultivation with HCV1b serum, however, we did not note an influence of the used in vitro culture modalities on the sizes of CD56+/CD16+ NK cell subsets (Table 1).
Table 1.
Changes in CD56+/CD16+ natural killer (NK) cell subsets after exposure to hepatitis C virus (HCV) particles.
| IMDM | Cond. DMEM | HCVpp | Normal human serum | HCV1b serum | |||||
|---|---|---|---|---|---|---|---|---|---|
| CD56dim/CD16++ | CD56bright/CD16- | CD56dim/CD16++ | CD56bright/CD16- | CD56dim/CD16++ | CD56bright/CD16- | CD56dim/CD16++ | CD56bright/CD16- | CD56dim/CD16++ | CD56bright/CD16- |
| Healthy donor 1 | |||||||||
| 54·7 | 8·5 | 53·3 | 13·3 | 51·4 | 13·6 | 54·2 | 13·6 | 55·8 | 13·4 |
| Healthy donor 2 | |||||||||
| 35·5 | 23·1 | 37·0 | 21·0 | 31·0 | 26·0 | 27·3 | 24·0 | 35·2 | 25·7 |
| Healthy donor 3 | |||||||||
| 66·8 | 14·9 | 59·2 | 15·7 | 66·5 | 13·0 | 68·4 | 12·8 | 64·0 | 13·5 |
| Healthy donor 4 | |||||||||
| 83·7 | 5·0 | 82·7 | 6·3 | 75·7 | 9·3 | 83·7 | 5·6 | 83·7 | 3·8 |
| Healthy donor 5 | |||||||||
| 55·3 | 36·7 | 58·2 | 33·2 | 62·4 | 31·7 | 56·8 | 40·2 | 56·6 | 40·6 |
| Patient 1 | |||||||||
| 43·0 | 34·2 | 40·5 | 41·1 | 54·2 | 31·8 | 47·5 | 39·2 | 59·3 | 26·0 |
| Patient 2 | |||||||||
| 43·9 | 16·1 | 48·8 | 21·0 | 45·7 | 17·8 | 50·4 | 19·0 | 47·5 | 15·8 |
| Patient 3 | |||||||||
| 63·6 | 15·5 | 65·7 | 15·2 | 64·9 | 16·5 | 69·2 | 10·1 | 84·2 | 11·6 |
Natural killer (NK) cells from five healthy donors and three chronic hepatitis C virus (HCV) patients were cultured for 5 days under the conditions indicated. Cells were analysed by multi-colour flow cytometry. Frequencies of CD56dim/CD16++ and CD56bright/CD16- are shown. DMEM: Dulbecco's modified Eagle's medium; IMDM: Iscove's modified Dulbecco's medium; HCVpp: HCV pseudoparticles.
Enhanced cytokine secretion and NK cytotoxicity after in vitro exposure to HCV
Because the cytofluorometric analyses indicated an enhanced activation of NK cells by HCV rather than an inhibited phenotype, we studied functional aspects of NK cell activation. In culture supernatants from NK cells derived from healthy donors and in HCV patients we detected significantly larger amounts of the effector cytokine IFN-γ into the culture medium when cultured for 5 days in the presence of HCVpp or HCV1b (Fig. 5a). Similarly, we observed a strongly enhanced production of TNF-α by NK cells cultured with HCV serum, and to a lesser extent also with HCVpp (Fig. 5b). In the experiments with NK cells from normal donors we added a polyclonal anti-HCV antibody to the wells containing HCV1b sera. Inclusion of anti-HCV partially blocked the enhanced production of IFN-γ and TNF-α, suggesting that an interaction of HCV envelope glycoproteins with NK surface receptors was responsible for this effect (Fig. 5a,b).
Fig. 5.
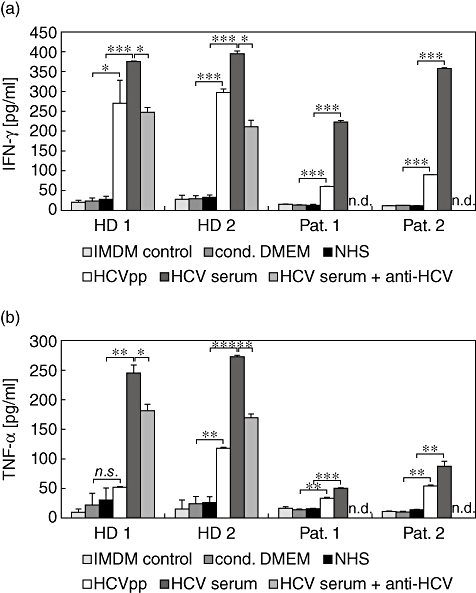
Augmented cytokine secretion by natural killer (NK) cells after culture in the presence of hepatitis C virus (HCV). Secretion of interferon (IFN)-γ (a) and tumour necrosis factor (TNF)-α (b) by NK cells from healthy donors (HD) and chronic HCV patients (Pat.) cultured for 5 days in either Iscove's modified Dulbecco's medium (IMDM) with interleukin-2/phytohaemagglutinin-P (IL-2/PHA-P)/feeders alone, or IMDM supplemented (1:1) with conditioned Dulbecco's modified Eagle's medium (DMEM), normal human serum, HCV pseudoparticles (HCVpp) or HCV1b-infected human serum, as indicated. Anti-HCV serum was added to wells as indicated. Values shown are means ± standard error of the mean from triplicate samples; statistical significances were determined by Student's t-test. *P < 0·05; **P < 0·01; ***P < 0·001.
Next, we assessed the cytolytic capacity of NK cells from the healthy donor 1 and HCV patient 1. We used the human major histocompatibility complex (MHC) class I-deficient human lymphoblastoid line T2 and BxPC3 carcinoma cells as target cells in a cytofluorimetric cytotoxicity assay. In most cases, culture of NK cells in the presence of HCVpp or HCV1b serum increased the cytolytic capacity significantly against either target cell line when compared with the lytic capacity of control-treated NK cells (Fig. 6). In conclusion, stimulation of NK cells from healthy donors and HCV patients in the presence of HCVpp or HCV1b-containing sera resulted in a higher degree of activation in terms of cell surface receptors, lymphokine secretion and cytotoxicity.
Fig. 6.
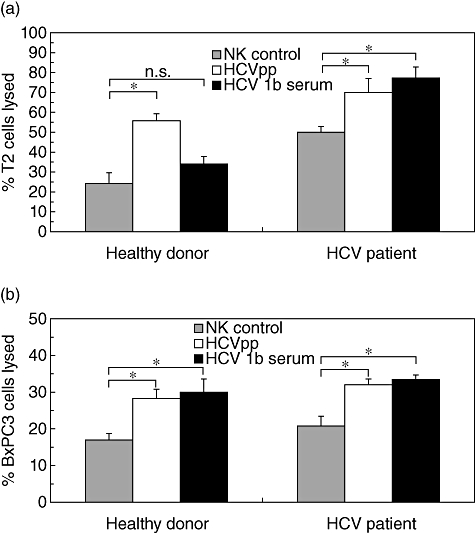
Augmented cytotoxicity by natural killer (NK) cells after culture in the presence of hepatitis C virus (HCV). Cytotoxicity assay using NK cells from healthy donor 1 and chronic HCV patient 1 cultured in Iscove's modified Dulbecco's medium/interleukin-2/phytohaemagglutinin-P (IMDM/IL-2/PHA-P) medium for control, or in IMDM supplemented with HCV pseudoparticles (HCVpp)-containing supernatant or with HCV-infected serum. T2 cells (a) and BxPC3 cells (b) were labelled with Muromonab-CD3-fluorescein isothiocyanate (OKT3-FITC) and HEA125-FITC, respectively, and used as targets for NK cells at a effector to target ratio of 10:1. Lysis is presented as percentage of dead cells (propidium iodide-positive cells) from all FITC-labelled target cells. The percentage of propidium-iodide-positive target cells in the absence of effector cells has been subtracted. Values shown are means ± standard error of the mean from duplicate samples; statistical significances were determined by Student's t-test. *P < 0·05.
Discussion
Our results indicate that a prolonged culture of NK cells from healthy donors, as well as chronic HCV patients in the presence of HCVpp and HCV1b-containing serum, not only induced the expression of activation-associated NK cells receptors, but also led to a diminished expression of inhibition-associated NK cell receptors. The culture with HCV particles also stimulated significantly the secretion of the NK effector lymphokines IFN-γ and TNF-α. Furthermore, the cytotoxic capacity of NK cells cultured in the presence of HCV was found to be enhanced. These results are at variance with a recent study by the Reherman group, showing that infectious HCV particles did not influence NK cell activation or effector function, whereas cross-linking of CD81 with plate-bound anti-CD81 antibodies had an inhibitory effect [25]. The latter study utilized HCV genotypes 2a and 1 particles produced in the Huh7·5 tissue culture system while we used HCV1b-containing patient sera and HCVpp produced by HEK293T cells. We cannot exclude that HCV particles produced by Huh7·5 cells have a lesser stimulatory effect; however, we consider the prolonged culture period of 120 h employed in this investigation, compared with 18 h used by Yoon et al., to be responsible for this discrepancy. Furthermore, we included irradiated allogeneic PBMC as feeder cells to provide for a more physiological situation, because in vivo NK cells represent only 5–15% of blood lymphocytes. Although the feeder cells decayed rapidly in the culture, they might have provided for potent co-stimulatory signals elicited by HCV-mediated activation of T cells [22–24].
To our knowledge, this is the first report to analyse the expression of a larger panel of activating and inhibitory NK cell receptors on the cell surface of in vitro HCV-exposed NK cells together with the common NK markers, CD56 and CD16. Previously, the expression levels of the natural cytotoxicity receptors NKp46, NKp44 and NKp30 as well as NKG2D were analysed in fresh blood samples from larger panels of chronic HCV patients. In one study, the expression of NKp30 and NKp46 was found to be augmented in NK cells from HCV-infected donors while NKG2D was unaltered [8]. In another study, however, NKp46 and NKp30 were reduced significantly in HCV RNA+ but not in RNA- blood samples, compared with healthy donors [9]. These reduced expression levels were maintained after 2 weeks of culture in the presence of IL-2 [9].
In this study we consistently observed an increased expression of NKp46, NKp44, NKp30 and NKG2D in CD56bright and CD16+ NK cell subsets from healthy donors and patients after culture with HCVpp or HCV1b sera. This effect occurred regardless of the varying expression levels of activating receptors in control-treated NK cell samples from the five healthy donors and the three patients. This finding is consistent with a generally higher activation state of acutely HCV-exposed NK cells [21], which is also reflected by the augmented cytotoxicity and significantly increased lymphokine secretion observed. Thus, infected sera and HCVpp seemed to have a co-stimulatory effect on naive NK cells concerning the acquisition of activating receptors. We speculate that this situation may be different in chronic HCV patients, where inhibitory effects exerted by liver-derived dendritic cells or liver sinusoidal endothelial cells may become dominant [2,9–13]. Interestingly, a suppressed NK phenotype, which we, however, did not verify for the patients' NK cells used here, appears to be reversible ex vivo, as in our hands patients' NK cells were not refractory to the stimulation protocols in vitro. The removal of a suppressive environment may be sufficient to allow for a proper activation in vitro.
In previous studies, an increased expression of the inhibitory NK receptor NKG2A/CD94 in NK cells purified from the blood of persistently HCV-infected patients was noted [9–11]. Rather, we detected a reduced frequency of CD94+ NK cells after culture with HCV1b or HCVpp, except for NK cells from one healthy donor and one patient showing increased or unaltered CD94+ expression after culture with HCV1b or HCVpp. Thus, upon HCV exposure, CD94 expression levels may exhibit larger interindividual variations. Stainings with an anti-CD158 antibody, which covers various types of the killer cell immunoglobulin-like (KIR) receptors that, in quantitative terms mainly have an NK-inhibitory function [28–30], were consistently lower in HCV-treated CD56+ and CD16+ NK subsets from healthy donors and patients. Moreover, in the majority of samples the inhibitory AIRM1 receptor was also down-modulated after exposure to HCV. Taken together, this relative loss of inhibitory surface receptors is also consistent with the increased NK cytototoxicity observed. With regard to the modulation of inhibitory NK receptors, no general differences between NK cells from healthy donors and chronic HCV patient were obvious.
Short-term culture in the presence of plate-bound recombinant HCV E2 glycoprotein or anti-CD81 antibodies was shown previously to inhibit cytokine secretion and cytotoxicity of NK cells [22–25]. In line with Yoon and colleagues [25], we could not confirm an inhibitory effect of infectious HCV particles harbouring E2. Using an anti-CD81 antibody added to the cultures of NK cells with either HCV1b or HCVpp, we were able to partially block the enhanced expression of activating receptors. This result suggests that, also in the NK cell stimulations performed here, CD81 was involved in the interaction of HCV with NK cells. In contrast to the inhibitory effect on primary NK cells, CD81 ligation by immobilized recombinant E2 protein or anti-CD81 was reported to provide a co-stimulatory signal for T cells [22–24]. Thus, tetraspanin engagement can have opposite outcomes, dependent on the cellular context, which was attributed to enhanced or reduced phosphorylation of CD3ζ and extracellular-regulated kinase (Erk) signal-transducing proteins in T cells and NK cells, respectively [24]. We speculate that a prolonged culture of NK cell in the presence of HCV might have induced a T cell-like behaviour allowing for NK cell co-activation by HCV E2. Alternatively, it is possible that irradiated T cells that were initially present as feeder cells in the culture-bound HCV were activated and contributed, in part, to an enhanced production of TNF-α and IFN-γ.
HCV envelope proteins E2 or E1 were likely to be involved in the reported NK cell activation reported in this study, as they represent the common denominator of HCVpp and HCV-infected sera. The partial blockade of the HCV-induced IFN-γ and TNF-α secretion by NK cells by inclusion of a polyclonal anti-HCV anti-serum supports this contention; however, HCV-derived proteins appear to be incompletely masked due to limiting amounts of the anti-serum. Similarly, the enhanced expression of activating receptors by NK cells could be blocked by the anti-HCV anti-serum in some experiments (data not shown).
Previous studies have reported decreased frequencies of CD16+/CD56dim cytolytic NK cells in the peripheral blood of patients persistently infected with HCV, while CD56bright cytokine-secreting NK cells were expanded [11,15,17,18,20]. Despite decreased frequencies of CD56dim NK cells, the overall levels of natural cytotoxicity, however, were not always impaired [14,15]. While we did not analyse the phenotype of NK cells from chronic HCV patients in comparison to healthy donors directly ex vivo, we did find a large interindividual heterogeneity, including an increased CD56bright subset in chronic HCV patient 1. After culture with HCV1b serum the CD56bright subset from this patient was diminished at the expense of CD56dim/CD16++ NK cells, which would be consistent with their enhanced cytolytic capacity (see Fig. 6). NK cells from patient 3, which were not tested for cytotoxicity, also showed an increased frequency of CD56dim/CD16++ NK cells after HCV1b exposure in vitro.
In summary, in this study, HCVpp or HCV1b sera imparted a phenotypic and functional activation when purified NK cells were cultured in vitro for several days in the presence of low-dose IL-2, PHA-P and allogeneic feeder cells. This is of relevance for the acute phase of HCV infection and implies that NK cells could essentially be involved in the early clearance of this virus until inhibitory effects may become dominant during the chronic stage of the infection.
Acknowledgments
This research was supported, in part, by the Tumorzentrum Heidelberg/Mannheim (grant D.100 27 965). We thank Professor Dr Rolf Bartenschlager, University of Heidelberg and his group for their kind gift of HCV expression plasmids, Dr Franziska Voigt for her help with the establishment of HCVpp production, Silvia Dykstra for collecting the patients' sera and Dr Gerhard Moldenhauer, DKFZ Heidelberg, for his kind gift of antibodies.
Disclosure
None.
References
- 1.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 2.Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies. Liver Transpl. 2006;12:363–72. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 3.Szabo G, Chang S, Dolganiuc A. Altered innate immunity in chronic hepatitis C infection – cause or effect? Hepatology. 2007;46:1279–90. doi: 10.1002/hep.21938. [DOI] [PubMed] [Google Scholar]
- 4.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–54. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Zhang T, Ho C, et al. Natural killer cells inhibit hepatitis C virus expression. J Leukoc Biol. 2004;76:1171–9. doi: 10.1189/jlb.0604372. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J, Bost A, Glass JI, et al. Cytokine-activated natural killer cells exert direct killing of hepatoma cells harboring hepatitis C virus replicons. J Interferon Cytokine Res. 2006;26:854–65. doi: 10.1089/jir.2006.26.854. [DOI] [PubMed] [Google Scholar]
- 7.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 8.De Maria A, Fogli M, Mazza S, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–55. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 9.Nattermann J, Feldmann G, Ahlenstiel G, et al. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–77. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jinushi M, Takehara T, Tatsumi T, et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–81. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 11.Golden-Mason L, Madrigal-Estebas L, McGrath E, et al. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121–8. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- 12.Corado J, Toro F, Rivera H, et al. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;109:451–7. doi: 10.1046/j.1365-2249.1997.4581355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pár G, Rukavina D, Podack ER, et al. Decrease in CD3-negative-CD8dim+ and Vd2/Vγ9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J Hepatol. 2002;37:514–22. doi: 10.1016/s0168-8278(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 14.Düesberg U, Schneiders AM, Flieger D, et al. Natural cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) is not impaired in patients suffering from chronic hepatitis C. J Hepatol. 2001;35:650–7. doi: 10.1016/s0168-8278(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 15.Morishima C, Paschal DM, Wang CC, et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–80. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 16.Oliviero B, Varchetta S, Paudice E, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–60. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Meier UC, Owen RE, Taylor E, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–74. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin AW, Gonzalez SA, Cunningham-Rundles S, et al. CD56+dim and CD56+bright cell activation and apoptosis in hepatitis C virus infection. Clin Exp Immunol. 2004;137:408–16. doi: 10.1111/j.1365-2249.2004.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okumura A, Ishikawa T, Maeno T, et al. Changes in natural killer T cells subsets during therapy in type C hepatitis and hepatocellular carcinoma. Hepatol Res. 2005;32:213–17. doi: 10.1016/j.hepres.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Zarife MA, Reis EA, Carmo TM, et al. Increased frequency of CD56Bright NK-cells, CD3-CD16+CD56- NK-cells and activated CD4+ T-cells or B-cells in parallel with CD4+CD25High T-cells control potentially viremia in blood donors with HCV. J Med Virol. 2009;81:49–59. doi: 10.1002/jmv.21340. [DOI] [PubMed] [Google Scholar]
- 21.Amadei B, Urbani S, Cazaly A, et al. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–45. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng CT, Klimpel GR. Binding of hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–9. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crotta S, Stilla A, Wack A, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–42. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotta S, Ronconi V, Ulivieri C, et al. Cytoskeleton rearrangement induced by tetraspanin engagement modulates the activation of T and NK cells. Eur J Immunol. 2006;36:919–29. doi: 10.1002/eji.200535527. [DOI] [PubMed] [Google Scholar]
- 25.Yoon JC, Shiina M, Ahlenstiel G, et al. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koutsoudakis G, Kaul A, Steinmann E, et al. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J Virol. 2006;80:5308–20. doi: 10.1128/JVI.02460-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietschmann T, Zayas M, Meuleman P, et al. Production of infectious genotype 1b virus particles in cell culture and impairment by replication enhancing mutations. PLoS Path. 2009;5:e1000475. doi: 10.1371/journal.ppat.1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McQueen KL, Parham P. Variable receptors controlling activation and inhibition of NK cells. Curr Opin Immunol. 2002;14:615–21. doi: 10.1016/s0952-7915(02)00380-1. [DOI] [PubMed] [Google Scholar]
- 29.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 30.Bryceson YT, March ME, Ljunggren HG, et al. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer cells subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]


