Abstract
We investigated whether the multisubunit holoenzyme complex of RNA polymerase II (Pol II) and mediator is universally required for transcription in budding yeast. ΔCTD Pol II lacking the carboxy-terminal domain of the large subunit cannot assemble with mediator but can still transcribe the CUP1 gene. CUP1 transcripts made by ΔCTD Pol II initiated correctly and some extended past the normal poly(A) site yielding a novel dicistronic mRNA. Most CUP1 transcripts made by ΔCTD Pol II were degraded but could be stabilized by deletion of the XRN1 gene. Unlike other genes, transcription of CUP1 and HSP82 also persisted after inactivation of the CTD kinase Kin28 or the mediator subunit Srb4. The upstream-activating sequence (UAS) of the CUP1 promoter was sufficient to drive Cu2+ inducible transcription without Srb4 and heat shock inducible transcription without the CTD. We conclude that the Pol II holoenzyme is not essential for all UAS-dependent activated transcription in yeast.
Keywords: RNA Pol II holoenzyme, CTD, transcriptional activation
RNA polymerase II (Pol II) holoenzymes containing core polymerase and many accessory proteins have been identified in budding yeast (Thompson et al. 1993; Kim et al. 1994; Koleske and Young 1994) and in mammals (Ossipow et al. 1995; Maldonado et al. 1996; Pan et al. 1997). In yeast up to 50% of Pol II is bound to a complex of ∼20 proteins called mediator (Kim et al. 1994; Myers et al. 1998). Addition of mediator to core Pol II in vitro elevates basal transcription and permits stimulation of transcription in response to activators (Kim et al. 1994; Koleske and Young 1994). In addition to the mediator, holoenzyme has also been isolated in association with other proteins including TFIIB, TFIIF, TFIIH, and Swi/Snf (Koleske and Young 1994; Wilson et al. 1996).
Several lines of evidence suggest that the carboxy-terminal domain (CTD) of the Pol II large subunit is essential for the physical and functional integrity of the holoenzyme. Purified mediator binds to the CTD (Myers et al. 1998) and the Pol II-mediator complex is disrupted by a monoclonal antibody against the CTD (Kim et al. 1994). Furthermore, addition of mediator to Pol II lacking the CTD did not stimulate either basal or activated transcription (Myers et al. 1998). The CTD is composed of a repeated heptad sequence whose consensus (YSPTSPS) is absolutely conserved between yeast and mammals. The CTD undergoes a cycle of hyperphosphorylation and dephosphorylation that accompanies the transcription cycle (Dahmus 1996) and may be linked to recycling of the mediator (Svejstrup et al. 1997). Mediator also strongly stimulates CTD phosphorylation by the Kin28 kinase subunit of TFIIH (Kim et al. 1994). It has been suggested that phosphorylation of the CTD counteracts a negative effector of transcription in crude extracts (Li and Kornberg 1994) and that it enhances transcriptional elongation (O’Brien et al. 1994; Lee and Greenleaf 1997). Truncation of the yeast CTD from 26 to between 13 and 11 repeats causes cold and temperature sensitivity for growth and further deletion to 10 or fewer repeats is lethal (Nonet et al. 1987b; West and Corden 1995). Nine SRB (suppressor of RNA pol B) genes were identified, which as mutants suppressed the cold-sensitive phenotype associated with CTD truncation (Nonet and Young 1989; Hengartner et al. 1995). Both dominant and recessive srb mutants were obtained, implying that both gain and loss of Srb function can suppress the effects of CTD truncation. The three essential SRB genes, SRB-4, SRB-6, and SRB-7, all encode subunits of the mediator (Thompson et al. 1993; Kim et al. 1994; Myers et al. 1998).
Holoenzyme but not core Pol II is able to respond to activators in a defined transcription system in vitro (Kim et al. 1994; Koleske and Young 1994). Moreover, artificial tethering of holoenzyme subunits to promoters in vivo can activate transcription (Farrell et al. 1996) and CTD truncation mutants have a reduced response to some activators (Allison and Ingles 1989; Scafe et al. 1990; Liao et al. 1991; Gerber et al. 1995). On the basis of these observations it has been suggested that activators work by making protein–protein contacts that recruit holoenzyme to the promoter (Ptashne and Gann 1997). It is not known, however, whether all activators work by this mechanism.
The role of the holoenzyme in vivo has been addressed genetically in yeast. Mutations that inactivate Srb4, Srb6, and Kin28 abolish synthesis of stable mRNAs from most genes (Cismowski et al. 1995; Thompson and Young 1995; Valay et al. 1995). On the other hand, the principal effect of mutations affecting the nonessential holoenzyme components Srb8-11, Rox3, Sin4, and Gal11 is to derepress a particular subset of genes (Carlson 1997). One limitation of these genetic studies is that it is not known how extensively the various mutations disrupt holoenzyme structure in vivo.
We have studied the role of holoenzyme in mRNA synthesis by asking what happens when the CTD is deleted completely. Deletion of the CTD is expected to prevent interaction of core Pol II with mediator and therefore, to preclude assembly of holoenzyme. Pol II without the CTD is capable of transcription in vitro from certain promoters in partially purified systems (Zehring and Greenleaf 1990; Kang and Dahmus 1993; Li and Kornberg 1994), but its function in vivo has not been investigated. We examined transcription in a yeast strain where the only functional Pol II at the restrictive temperature lacks the CTD. Run-on transcription in permeabilized cells (Elion and Warner 1986) was used to measure the average density of polymerases engaged on a gene at a particular instant. Surprisingly, we found that the Pol II minus the CTD was able to transcribe the CUP1 gene at a high level. The holoenzyme subunits Srb4 and Kin28 were also found not to be essential for CUP1 transcription. These results show that in some circumstances, activated Pol II transcription in vivo can occur independently of the “core + mediator” form of holoenzyme.
Results
A strategy to study transcription in vivo by CTD-deleted Pol II
To determine whether Pol II could function in vivo without the CTD, we introduced a plasmid pFL38–rpb1ΔCTD, which expresses the large subunit with a deletion of the CTD, into a yeast strain (Y260) with a temperature-sensitive mutation in the RPB1 gene (rpb1-1; Nonet et al. 1987a). When this strain is shifted to 37°C, the full-length large subunit is inactivated within 5–15 min (Nonet et al. 1987a) and the only potentially functional Pol II is the one that has incorporated the CTD-deleted large subunit. Because there is only one copy of the large subunit in each Pol II complex (Kolodziej et al. 1990), interallelic complementation is highly unlikely. More than 99.9% of cells in each culture were temperature sensitive, eliminating the possibility of contamination by recombinants between the rpb1-1 and rpb1ΔCTD alleles. As controls, the Y260 strain was also transformed with vector pFL38 (Bonneaud et al. 1991) or pFL38–RBP1, which expresses the wild-type Rpb1 protein and complements the temperature-sensitive phenotype. The pFL38–rpb1ΔCTD plasmid slowed growth of the cells significantly. Doubling times for strains harboring pFL38, pFL38–RPB1, and pFL38–rpb1ΔCTD at 30°C were 6, 4, and 9.5 hr, respectively. Therefore, expression of the CTD-deleted large subunit has a dominant negative effect on growth.
A new CUP1 mRNA transcribed by ΔCTD Pol II
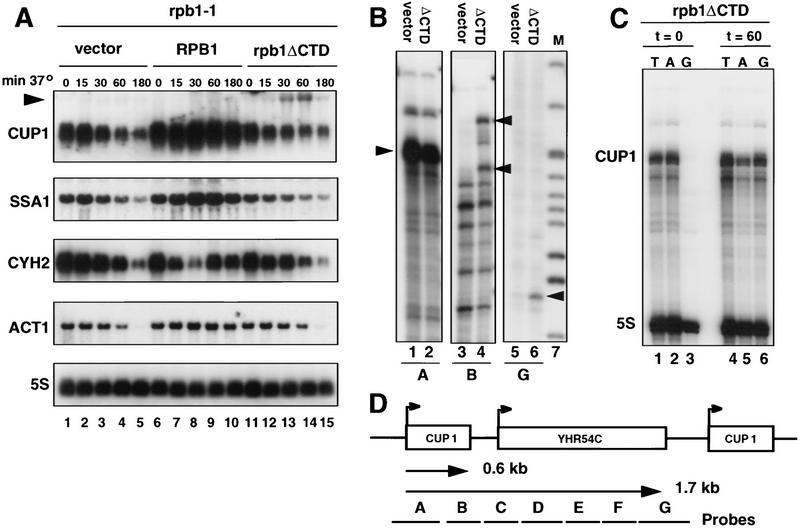
Expression of several genes was examined in these derivatives of Y260 by Northern blot of RNA isolated at intervals after shifting the cells to the restrictive temperature. The steady-state mRNA levels for CUP1, CYH2, SSA1, and ACT1 decayed in pFL38 vector-transformed cells at 37°C (Fig. 1A, lanes 1–5) as expected when Pol II is inactivated. In contrast, Y260 containing the pFL38–RPB1 plasmid at 37°C behaved like wild type. The cells maintained high ACT1 mRNA, induced CUP1 (Silar et al. 1991) and SSA1 mRNA, and transiently repressed CYH2 mRNA in response to heat shock (Fig. 1A, lanes 6–10). In rpb1ΔCTD-expressing cells, ACT1, CYH2, SSA1, and CUP1 mRNAs decayed with similar kinetics to the pFL38 vector control (Fig. 1A, lanes 11–15). Therefore, Pol II without a CTD is unable to sustain wild-type levels of these mRNAs. We noticed, however, that although the major 0.6-kb CUP1 mRNA decayed in cells with pFL38–rpb1ΔCTD at 37°C, a new 1.7-kb CUP1 transcript was induced transiently after 30–60 min at the restrictive temperature (see arrow Fig. 1A, lanes 13,14). Synthesis of the 1.7-kb CUP1 transcript was observed in two different rpb1-1 strains harboring the pFL38–rpb1ΔCTD plasmid and therefore, is not peculiar to a particular genetic background (data not shown). It is highly unlikely that this transcript is made by a polymerase other than ΔCTD Pol II because it is expressed at a significant level only in rpb1-1 strains containing the pFL38–rpb1ΔCTD plasmid and not in cells with the vector or pFL38–RPB1 (Fig. 1A). The induction of a new CUP1 transcript at 37°C, which is specific to strains with pFL38-rpb1ΔCTD, suggests that Pol II without the CTD has some transcriptional activity in vivo.
Figure 1.
Synthesis of a novel CUP1 transcript by ΔCTD Pol II. (A) Northern blots of mRNA from rpb1-1ts strains at intervals after shifting to the restrictive temperature of 37°C. Strain Y260 was transformed with pFL38 vector (lanes 1–5), pFL38–RPB1 (lanes 6–10), or pFL38–rpb1ΔCTD (lanes 11–15). Note the appearance of a new 1.7-kb CUP1 transcript specific to the rpb1ΔCTD strain. (B) 5′ and 3′ end mapping of CUP1 transcripts. RNase protection analysis with antisense probes A (lanes 1,2), B (lanes 3,4), and G (lanes 5,6; see D) of RNA from Y260 containing pFL38 grown at 30°C (vector; lanes 1,3,5) or pFL38–rpb1ΔCTD grown for 1 hr at 37°C (ΔCTD; lanes 2,4,6). Note that CUP1 RNAs have identical 5′ ends in the two samples (arrowhead; lanes 1,2), but the ΔCTD Pol II-expressing cells have transcripts that extend past the normal poly(A) site (arrowheads; lane 4). Multiple bands in lane 3 are attributable in part to artifactual cleavage at AU rich regions in probe B. The 3′ ends of the readthrough RNAs map to the end of the YHR54C ORF (arrowhead; lane 6). Markers (M, lane 7) are MspI cut pBR322 from 427–123 bp. (C) 5′ end mapping of hybrid selected transcripts from the CUP1 locus in pFL38–rpb1ΔCTD cells grown at 30° (t = 0, lanes 1–3) and for 60 min at 37°C (t = 60; lanes 4–6). Total RNA (T; lanes 1,4) and RNA hybrid selected with a 5S rDNA and either CUP1 A or G (see D) were analyzed by RNase protection with probes for 5S rRNA and the CUP1 5′ end (probe A). Note that only at 37°C (lane 6) did transcripts homologous to region G appear and their 5′ ends were identical to those of normal CUP1 transcripts made at 30°C (lanes 1,2). (D) Map of the CUP1 repeat unit with 0.6- and 1.7-kb transcripts and fragments A–G indicated.
The 1.7-kb CUP1 transcript made by ΔCTD Pol II was characterized in detail. The CUP1 locus is a 2.0-kb tandem repeat that is reiterated up to 15 times (Karin et al. 1984). The repeat contains two ORFs, CUP1 and YHR54C, which are 460 bp apart and transcribed in the same direction (Fig. 1D). The CUP1 gene, which encodes a metallothionein homolog, has a high basal level of expression and is inducible by heat shock and copper ions (Thiele and Hamer 1986; Silar et al. 1991; Yang et al. 1991). Like other genes transcribed by Pol II, basal and induced CUP1 transcription were inhibited by a mutation of TATA-binding protein (TBP), which is defective for interaction with TFIIA (Stargell and Struhl 1995). YHR54C gene is an ORF of unknown function that is normally expressed at a lower level than CUP1 (Karin et al. 1984). To map the ends of the 1.7-kb CUP1 transcripts produced by ΔCTD Pol II, we conducted RNase protection experiments with a series of seven antisense probes (A–G), which covers completely the CUP1 repeat. RNA samples used in Figure 1A from Y260 cells containing either pFL38 vector at 30°C (lane 1) or pFL38–rpb1ΔCTD after 1 hr at 37°C (lane 14) were analyzed. CUP1 5′ ends mapped by probe A did not differ between the control and ΔCTD Pol II-expressing cells (arrowhead, Fig. 1B, lanes 1,2). In contrast, mapping with probe B showed that a significant fraction of transcripts read through the CUP1 poly(A) site in rpb1ΔCTD-expressing cells (arrowheads, Fig. 1B, lane 4) but not in the control. Antisense probes C–F were fully protected by transcripts from rpb1ΔCTD-expressing cells, but no protection was observed in the control (data not shown). The 3′ end of the ΔCTD Pol II transcripts, which traverse the entire CUP1 repeat unit, was mapped to a site ∼150 bp downstream of the YHR54C termination codon (probe G; see arrowhead Fig. 1B, lane 6). Therefore, these RNase protection results are entirely consistent with the 1.7-kb estimated length of the novel transcript detected by Northern blot in Figure 1A (lanes 13,14).
To map the start site of the 3′ extended CUP1 transcripts unambiguously, first they were purified by hybrid selection. RNA was isolated from cells with pFL38–rpb1ΔCTD at 30°C (t = 0) or after 1 hr at 37°C (t = 60) and hybridized to single-stranded M13 clones of fragments A or G (Fig. 1D). As a control for recovery, 5S rRNA was also hybrid selected. Selected RNA and total RNA (T) were analyzed by RNase protection with the CUP1 5′ probe A and with a 5S probe (Fig. 1C). This experiment provides independent confirmation that the long CUP1 transcripts, which hybridize to fragment G, are specific to rpb1ΔCTD-expressing cells at the restrictive temperature (Fig. 1C, cf. lane 3 with lane 6) and that their 5′ ends map to the correct start sites in the CUP1 promoter. In summary, the 1.7-kb transcript is a dicistronic mRNA that starts at the CUP1 promoter and continues to the 3′ end of the downstream gene (YHR54C) where it is cleaved and polyadenylated (data not shown). The specific induction of this new transcript in rpb1ΔCTD-expressing cells at the restrictive temperature suggests that Pol II without a CTD recognizes the CUP1 promoter and correctly initiates transcription in vivo. The precise coincidence of the start sites for the ΔCTD-specific 1.7-kb RNA and the normal 0.6-kb RNA makes it very unlikely that the former is made by nonspecific Pol II initiation or by another RNA polymerase.
Run-on analysis of CTD-independent CUP1 transcription
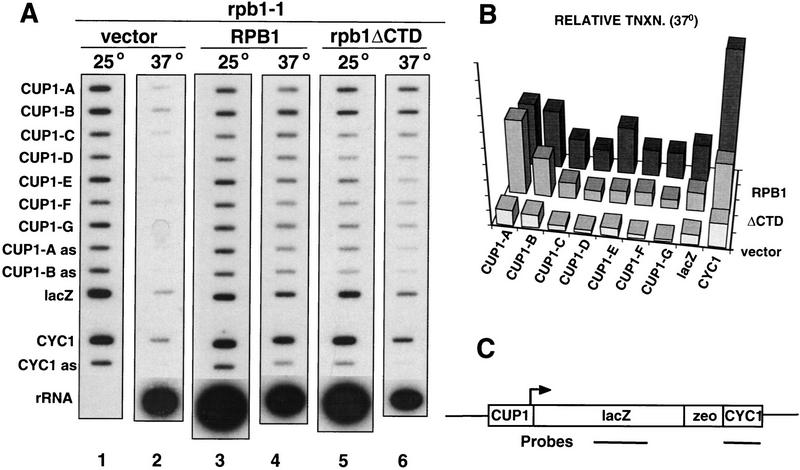
Steady-state RNA measurements are not reliable indicators of transcription rate because they are influenced by mRNA stability. Furthermore, after shifting cells to the restrictive temperature, new synthesis may be obscured by persistence of mRNAs synthesized before the shift. To obtain instantaneous measurements of polymerase density over CUP1, run-on assays (Elion and Warner 1986) were conducted in cells permeabilized with Sarkosyl, which prevents initiation of transcription. In these experiments, 32P-UTP-labeled nascent transcripts from equal numbers of cells were hybridized to slot-blotted strand-specific M13 probes. When the control rpb1-1 strain DBY120 with pFL38 vector was shifted to 37°C for 1 hr, polymerase density over the CUP1 repeat unit declined sharply, although a residual background of transcription was detected (Fig. 2A, lane 2). In contrast, cells with the wild-type RPB1 plasmid maintained a high polymerase density over CUP1 at 37°C (Fig. 2A, lane 4). Remarkably, polymerase density was approximately equally high over the 5′ end of the CUP1 gene in cells containing pFL38–rpb1ΔCTD after 1 hr at 37°C (Fig. 2A, cf. lane 2 with lane 6, and 2B). Similar results were obtained after 3 hr at 37°C (data not shown). ΔCTD Pol II is almost certainly responsible for this run-on transcription at the restrictive temperature because it is dependent on the rpb1ΔCTD gene. These experiments do not address what fraction of CUP1 transcription is carried out by ΔCTD Pol II at the permissive temperature where presumably it competes with wild-type Pol II. The ratio of 5′-to-3′ polymerase densities on the CUP1 gene at the restrictive temperature was always higher for ΔCTD Pol II than for wild-type Pol II (Fig. 2A,B; cf. CUP1-A and CUP1-B in lanes 4 and 6). This observation is consistent with CTD-mediated enhancement of transcriptional elongation (Akhtar et al. 1996; Lee and Greenleaf 1997). The distribution of polymerases on the CUP1 gene suggests that transcripts made by wild-type and ΔCTD Pol II terminate normally in the region between probes B and C (Fig. 2A, lane 6, and 2B). Apparently the dicistronic readthrough RNA is a relatively small fraction of total transcripts made by the truncated Pol II. In some experiments, significant run-on signals for ENO2, ACT1, and CYH2 were also detected in cells expressing ΔCTD Pol II at 37°C. Unlike CUP1, however, the signals relative to background in the pFL38 vector control varied considerably between different experiments. Therefore, we chose to concentrate on CTD-independent transcription of CUP1.
Figure 2.
Run-on analysis of CUP1 transcription by ΔCTD Pol II. (A) DBY120 (rpb1-1) was transformed with pRS314 CUP1–lacZ–CYC1 and either pFL38 vector (lanes 1,2), pFL38–RPB1 (lanes 3,4), or pFL38–rpb1ΔCTD (lanes 5,6). Cells were harvested at 25°C or after 60 min at 37°C. [32P]UTP-labeled run-on transcripts were hybridized to single-stranded M13 clones of fragments A–G from the CUP1/YHR54C repeat unit (Fig. 1D). lacZ and CYC1 clones are shown in C. (as) Antisense controls. The U content of transcripts hybridizing to each probe are CUP1A 56, CUP1-B 105, CUP1-C 117, CUP1-D 118, CUP1-E 70, CUP1-F 79, CUP1-G 138, lacZ 166, and CYC1 99. The rRNA probe was omitted from lane 1. Note the transcription by ΔCTD Pol II at 37°C (lane 6) relative to the vector control (lane 2). Autoradiography was for 3.5 days. (B) Quantitation of run-on signals at 37°C. PhosphorImager signals were normalized for cell number and corrected for U content. Note that Pol I transcription of 35S rRNA fell by four- to eightfold in all heat-shocked cells. The apparent build up of polymerases over the downstream CYC1 sequence relative to lacZ could indicate polymerase stalling at the 3′ end of this gene. (C) Map of the CUP1–lacZ–CYC1 fusion gene with lacZ and CYC1 M13 probes indicated.
The CUP1 promoter is sufficient for CTD-independent transcription
Because the normal CUP1 start sites are used by ΔCTD Pol II (Fig. 1C, lane 6), we investigated whether the mutant polymerase could also support transcription of a heterologous gene under the control of the CUP1 promoter. The CUP1 promoter was inserted upstream of a lacZ–zeor fusion gene with the CYC1 3′ processing site in the plasmid pRS314 CUP1–lacZ–CYC1 (Fig. 2C). This promoter fragment (−394 to +37 relative to the major start site) includes the CUP1 upstream-activating sequence (UAS) with binding sites for Ace1 and HSF transcription factors (Silar et al. 1991). Polymerase density over the lacZ reporter gene was consistently higher in the pFL38–rpb1ΔCTD-containing strain than in the vector control at 37°C (Fig. 2A, cf. lane 2 with lane 6). No run-on signal was observed for the lacZ or CYC1 probes in strains without the reporter plasmid (Fig. 5A, lane 4). In summary, these results show that the CUP1 promoter is sufficient to support CTD-independent transcription of a lacZ reporter gene.
Figure 5.
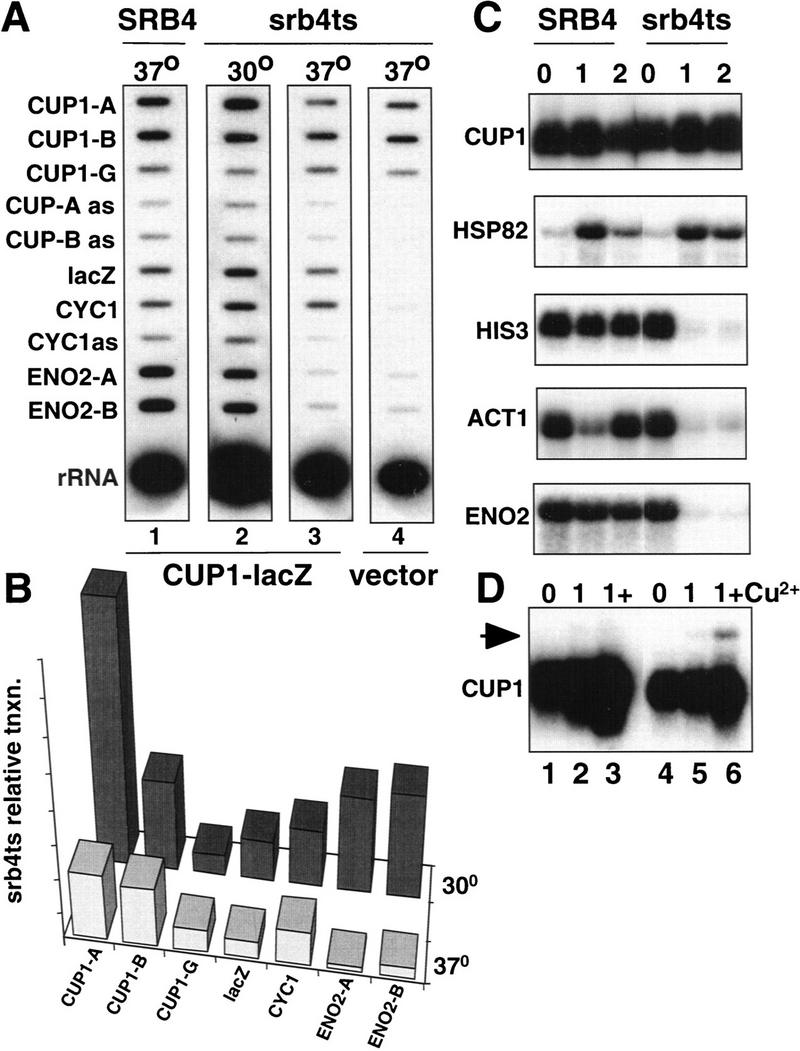
Srb4-independent transcription of CUP1 and HSP82. (A) Run-on transcription of strains Z579 (SRB4, lane 1) and Z628 (srb4ts, lanes 2–4). In lanes 1–3 the strains were transformed with the pRS316 CUP1–lacZ–CYC1 reporter plasmid and in lane 4 with pRS316 vector. U contents of the hybridizing transcripts are CUP1A 56, CUP1-B 105, CUP1-G 138, lacZ 166, CYC1 99, ENO2-A 106, and ENO2-B 114. Note the continued transcription of CUP1 and the CUP1–lacZ–CYC1 reporter gene in srb4ts cells at the restrictive temperature (lanes 3,4). Autoradiography was for 2 days. (B) Quantitation of run-on signals as in Fig. 2B. (C) Northern blots of mRNAs in Z579 (SRB4) and Z628 (srb4ts) strains at 30°C and after 1 and 2 hr at 37°C. Note the induction of HSP82 mRNA after heat shock in the srb4ts strain. (D) Northern blot analysis of CUP1 mRNA from Z579 (SRB4) and Z628 (srb4ts) at 30°C and after 1 hr at 37°C plus and minus Cu2+, which was added 30 min after shifting to 37°C (lanes 3,6). Note the induction of the 1.7-kb CUP1 mRNA (arrow) with Cu2+ at the restrictive temperature in the srb4ts strain (lane 6).
CUP1 transcripts made by ΔCTD Pol II are unstable
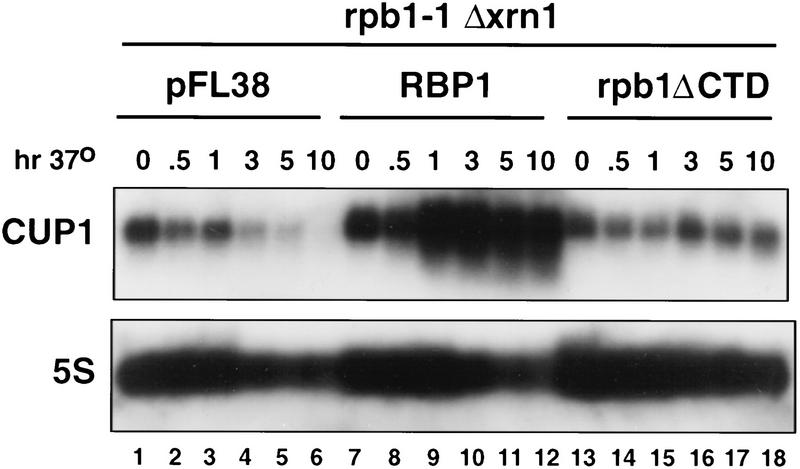
The run-on experiments showed high transcriptional activity of ΔCTD Pol II on the CUP1 gene at 37°C (Fig. 2A, lane 6) yet the steady-state level of the major 0.6-kb mRNA species decayed at this temperature (Fig. 1A, lanes 11–15). The failure to accumulate 0.6-kb transcripts could be attributable to the specific degradation of transcripts made by ΔCTD Pol II. This hypothesis was tested by analyzing CUP1 transcripts made by ΔCTD Pol II in a rpb1-1, Δxrn1 double mutant. Deletion of XRN1, which encodes a 5′-to-3′ RNA exonuclease, stabilizes uncapped Pol II transcripts that would normally be rapidly degraded (Hsu and Stevens 1993). RNA was isolated from the double mutant strain (DBY121) containing either pFL38, pFL38–RPB1, or pFL38–rpb1ΔCTD at intervals after shifting to 37°C and analyzed by Northern blot (Fig. 3). After 10 hr at 37°C, the 0.6-kb CUP1 mRNA had decayed completely in the pFL38 vector control (Fig. 3, lanes 1–6), but it remained at a constant level in cells expressing rpb1ΔCTD (Fig. 3, lanes 13–18). Although transcription by ΔCTD Pol II maintained a steady level of CUP1 mRNA for a long period in the Δxrn1 background, it did not support the approximately threefold induction in response to heat shock that occurred in the RPB1 strain (Fig. 3, lanes 7–12). Failure to induce the RNA could be attributable to inefficient transcriptional elongation (see Fig. 2B, cf. CUP1-A and CUP1-B) by ΔCTD Pol II or to degradation of the transcripts by an XRN1-independent pathway. The shortening of CUP1 RNAs with time at 37°C (Fig. 3, lanes 7–12 and 13–18) is presumably attributable to 3′-5′ degradation (Hsu and Stevens 1993). In contrast to the 0.6-kb transcript, the 1.7-kb dicistronic CUP1 RNA was not greatly stabilized by XRN1 deletion (data not shown). In summary, the experiments in Figures 1–3 show that at the restrictive temperature, the 0.6-kb CUP1 transcript is synthesized by ΔCTD Pol II, but does not accumulate unless it is stabilized by inactivating Xrn1. These results provide an explanation for the high transcription rate measured by run-on experiments without a corresponding high level of CUP1 mRNA. We conclude that a major effect of CTD deletion on CUP1 expression is to cause the transcripts to be unstable.
Figure 3.
Inactivation of XRN1 stabilizes CUP1 transcripts made by ΔCTD Pol II. Northern blot of 0.6 kb CUP1 mRNA and 5S rRNA loading control in strain DBY121 (rpb1-1, Δxrn1) containing pFL38 vector (lanes 1–6), pFL38-RPB1 (lanes 7–12), or pFL38–rpb1ΔCTD (lanes 13–18). RNA was isolated at intervals after shifting to 37°C as indicated. Note the persistance of the 0.6-kb CUP1 transcript in the rpb1ΔCTD strain.
CUP1 transcription independent of Kin28
As CUP1 transcription does not absolutely require the CTD, we hypothesized that the CTD kinase Kin28 may also be dispensable. To test this idea, we used a kin28ts mutant that at 37°C causes almost immediate decay of many mRNAs (Cismowski et al. 1995; Valay et al. 1995). Run-on analysis showed that transcription of CUP1 relative to rRNA was maintained at close to the wild-type level in kin28ts cells after 1 hr at 37°C (Fig. 4A, cf. lane 2 with lane 4). In contrast to CUP1, the run-on signal for ENO2 was almost completely abolished at 37°C. ACT1 and CYH2 run-on signals were also significantly inhibited by Kin28 inactivation but to a lesser extent than ENO2 (Fig. 4A,B). At 37°C, the ratios of 5′ CUP1 run-on transcription (corrected for U content) to 5′ ENO2, CYH2, and ACT1 were 6.6, 1.8, and 6.0, respectively, in the KIN28 strain, compared to 146, 25, and 16 in the kin28ts strain.
Figure 4.
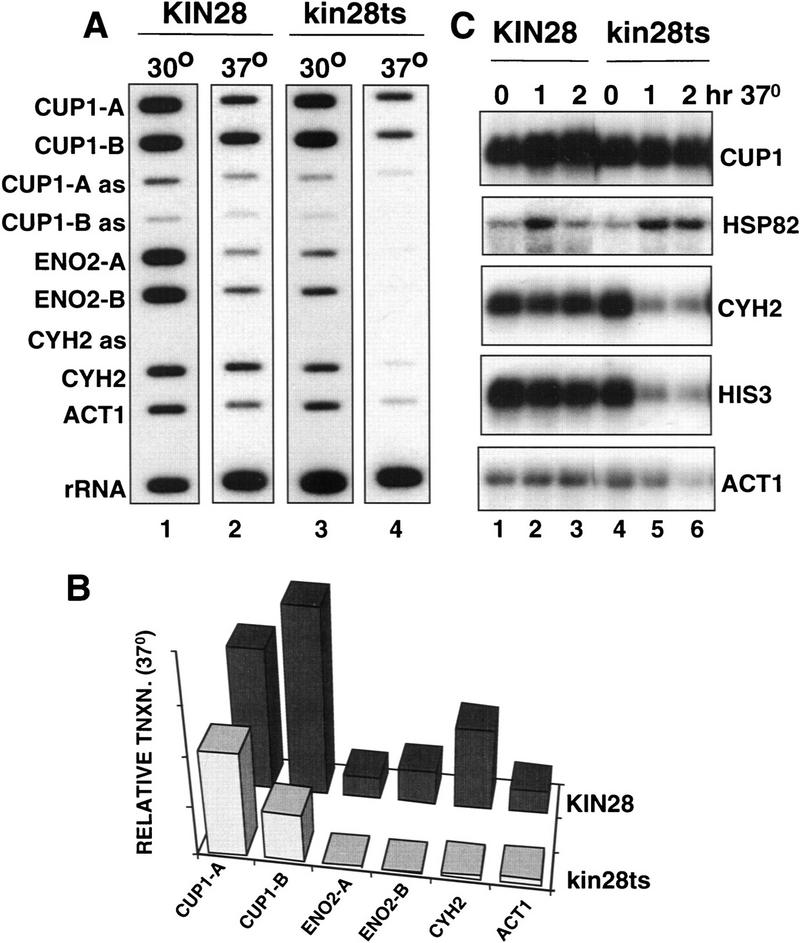
Kin28-independent transcription of CUP1 and HSP82. (A) Run-on analysis in isogenic KIN28 (GFY262, lanes 1,2) and kin28ts3 (lanes 3,4) strains at 30°C and after 1 hr at 37°C. U contents of the transcripts hybridizing to the M13 probes are CUP1A 56, CUP1-B 105, ENO2-A 106, ENO2-B 114, CYH2 88, ACT1 153. Autoradiography of lanes 2 and 4 was for approximately twice as long as for lanes 1 and 3. (B) Quantitation of run-on signals at 37°C. PhosphorImager signals were normalized as in Fig. 2B. (C) Northern blots of mRNA from KIN28 (GFY262, lanes 1–3) and kin28ts3 strains (lanes 4–6) at 30°C and after 1 and 2 hr at 37°C. Note the induction of HSP82 mRNA after heat shock in the kin28ts3 cells.
Steady-state mRNA levels in kin28ts cells were measured by Northern blot of samples isolated at intervals after shifting to 37°C (Fig. 4C). As expected, high levels of CUP1 mRNA were sustained for at least 2 hr at the restrictive temperature, whereas CYH2, HIS3, and ACT1 mRNAs rapidly decayed. Interestingly, HSP82 mRNA was actually induced by heat shock in the kin28ts mutant to the same degree as it was in the wild type (Fig. 4C). For reasons we do not understand, the peak HSP82 mRNA level was also maintained longer in the kin28ts mutant. These observations suggest that like CUP1, HSP82 can also be transcribed independently of the Kin28 kinase. This conclusion is subject to the reservation that it has not been confirmed by run-on analysis as signals from the HSP82 gene were very low.
These results show that transcription of different genes depends on the Kin28 kinase to different extents. After inactivation of Kin28, CUP1 and probably also HSP82 were transcribed at close to normal levels; ACT1 and CYH2 were transcribed at a much reduced level and transcription of ENO2 was not detectable.
SRB4-independent transcription of CUP1
We reasoned that transcription from a promoter that does not require the CTD may also not require the mediator. To test this idea, we asked whether Srb4, an essential subunit of mediator, is required for transcription of CUP1 using a temperature-sensitive mutant that inactivates the protein within 15 min at 37°C (Thompson and Young 1995). Transcription of CUP1 was investigated by the run-on assay in srb4ts cells at 30°C and after 1 hr at 37°C. Remarkably, polymerase densities for the chromosomal CUP1 gene and the pRS316 CUP1–lacZ–CYC1 fusion gene were only slightly reduced when Srb4 was inactivated (Fig. 5A, cf. lane 1 with lane 3). In a specificity control, no lacZ or CYC1 run-on signal was observed in cells with the pRS316 vector plasmid (lane 4). In contrast to CUP1, polymerase densities over ENO2 (Fig. 5A, cf. lane 2 with lane 3) and ACT1 (P. Atadja and D. Bentley, unpubl.) were dramatically reduced at 37°C in srb4ts cells. The ratio of 5′ CUP1 run-on signal to 5′ ENO2 in srb4ts cells was 2.7 at the permissive temperature compared to 14.1 at the restrictive temperature.
Consistent with the run-on results, Northern blot of steady-state mRNA from srb4ts cells showed that CUP1 transcripts remained high for at least 2 hr at the restrictive temperature, whereas HIS3, ACT1, and ENO2 transcripts decayed rapidly (Fig. 5C, lanes 4–6). In contrast, HSP82 mRNA was induced by heat shock equally in the srb4ts and isogenic SRB4 strains (Fig. 5C, cf. lane 2 with lane 5). HSP82 mRNA also remained at a high level for a longer time in the srb4ts mutant than in the wild type.
We tested whether Cu2+ induction of CUP1 still operated in srb4ts cells at the restrictive temperature. The addition of Cu2+ 30 min after shifting the cells to 37°C caused a modest induction of the 0.6-kb CUP1 transcript (Fig. 5D, lanes 4–6) relative to that which occurs in the wild-type SRB4 strain (Fig. 5D, lanes 1–3). Interestingly, the 1.7-kb dicistronic CUP1 transcript was specifically induced by Cu2+ at 37°C in the srb4ts mutant and not in the wild type (see arrow, Fig. 5D). In summary, the inducibility of HSP82 and CUP1 in srb4ts cells at the restrictive temperature suggests that some activation of transcription in response to stress stimuli can occur in the absence of this essential holoenzyme subunit.
Activation by the CUP1 UAS independent of Srb4
To identify the sequence elements required for Srb4-independent transcription of CUP1, we analyzed several 5′ deletions of the CUP1 promoter in the plasmid pRS316 CUP1–lacZ–CYC1. Deletion to positions −227, −169, −144, −129, and −87 relative to the ATG eliminated almost completely expression of lacZ mRNA at both the permissive and restrictive temperatures (data not shown). The −227 deletion removes two Ace1-binding sites and an HSF-binding site, but leaves the basal promoter region including the TATA box at −141 intact. Therefore, Srb4-independent transcription requires the CUP1 UAS and is not a property of the basal promoter alone. Our deletion analysis, however, does not identify individual binding sites that may be necessary for Srb4-independent transcription.
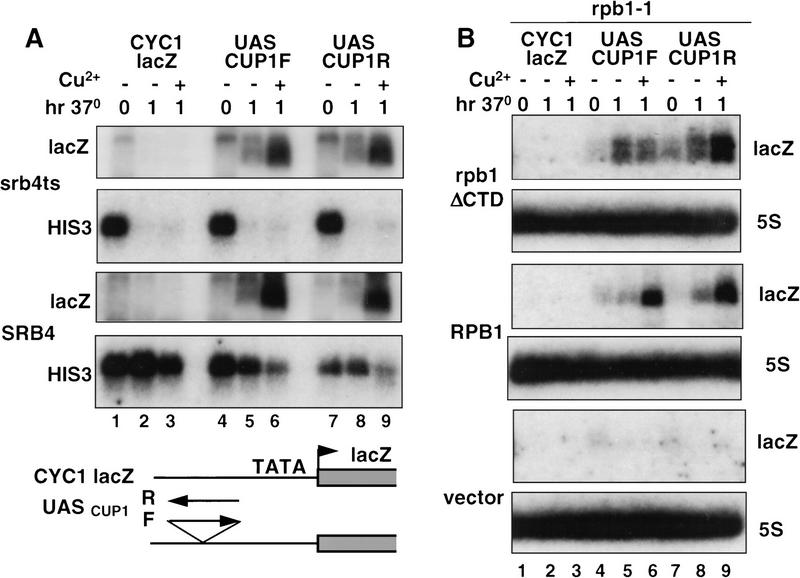
To test whether the CUP1 UAS is sufficient for Srb4-independent transcription, it was inserted as a 107-bp fragment in the forward and reverse orientations (designated F and R) upstream of the basal CYC1 promoter–lacZ fusion gene on multicopy plasmids. The plasmids were transformed into the isogenic SRB4 and srb4ts strains and lacZ transcripts were analyzed by Northern blot. After shifting the cells to 37°C for 1 hr, there was a clear induction of lacZ mRNA in the srb4ts mutant, whereas the HIS3 control mRNA decayed rapidly (Fig. 6A, top, lanes 4,5 and 7,8). Little or no lacZ mRNA was detected from the CYC1–lacZ parent plasmid showing that expression is dependent on the CUP1 UAS (Fig. 6A, lanes 1–3). When Cu2+ was added 30 min after shifting to 37°C, a significant further induction of lacZ mRNA was observed 30 min later (Fig. 6A, top, lanes 5,6 and 8,9). The magnitude of lacZ mRNA induction in response to heat shock and Cu2+ was almost identical in the srb4ts and SRB4 wild-type strains (Fig. 6A, cf. top and bottom lacZ lanes). These results show that CUP1 UAS-dependent inducible transcription of lacZ on a multicopy plasmid is almost completely independent of Srb4.
Figure 6.
The CUP UAS supports activated transcription independent of Srb4 and the CTD. (A) Northern blots of lacZ and HIS3 control mRNA from Z628 (srb4ts, top) and Z579 (SRB4, bottom) strains transformed with Gal5–CYC1–lacZ (lanes 1–3) and derivatives pJM1505 (lanes 4–6) and pJM1506 (lanes 7–9) with the CUP1 UAS in forward (F) and reverse (R) orientations (see diagram). RNA was isolated at 30°C (t = 0) and after 1 hr at 37°C with or without the addition of Cu2+ (0.1 mm), which was added after 30 min at 37°C (lanes 3,6,9). Note the induction of lacZ mRNA with heat shock and Cu2+ at the restrictive temperature in the srb4ts strain. (B) Northern blot analysis of lacZ and 5S rRNA control in DBY120 (rpb1-1) transformed with pFL38–rpb1ΔCTD, pFL38–RPB1, or pFL38 vector as indicated. Each strain was also transformed with Gal5–CYC1–lacZ–TRP (lanes 1–3) or derivatives with the CUP1 UAS in forward (F) and reverse (R) orientations (pJM1503, lanes 4–6; pJM1504, lanes 7–9). RNA was isolated from cultures treated as in A.
ΔCTD Pol II responds to the CUP1 UAS
We also tested whether the CUP1 UAS could function in the absence of the CTD. The UASCUP1–CYC1–lacZ plasmids were transformed into the rpb1-1 strain DBY120 with pFL38, pFL38–RPB1, or pFL38–rpb1ΔCTD. lacZ mRNA in cells grown at 30°C and 37°C was detected by Northern blot (Fig. 6B). lacZ transcripts were induced approximately two- to fourfold in the pFL38–rpb1ΔCTD-containing strain after shifting to 37°C (Fig. 6B, lanes 4,5 and 7,8). These transcripts were dependent on expression of ΔCTD Pol II and were not present in the pFL38 vector control (Fig. 6B, bottom). Furthermore, this transcription by ΔCTD Pol II was dependent on the CUP1 UAS, as very few transcripts of the CYC1–lacZ parent plasmid were detected (Fig. 6B, lane 1–3). At least as much lacZ mRNA was induced in response to a 1-hr heat shock at 37°C in ΔCTD Pol II-expressing cells as in the wild type (Fig. 6B, lanes 5,8, cf. top and middle). Interestingly, lacZ transcripts made by ΔCTD Pol II migrated more diffusely than those made by wild-type Pol II. This altered mobility may reflect a difference in RNA processing or degradation. CUP1 UAS-driven lacZ mRNA expression was not induced as much by Cu2+ in ΔCTD Pol II-expressing cells as in the wild type; however, clear activation was observed for the construct with the UAS in the reverse orientation (Fig. 6B, lanes 8,9). The induction of lacZ reporter gene mRNA in the absence of Srb4 or the CTD (Fig. 6) was always greater than that observed for CUP1 mRNA (Figs. 1A and 5D), possibly because of the higher stability of lacZ transcripts (Durrin et al. 1992). In summary, these results show that ΔCTD Pol II can carry out activated transcription dependent on the CUP1 UAS.
Discussion
Pol II transcription in vivo without the CTD
The question of whether holoenzyme is universally required for Pol II transcription in vivo has not been previously explored in detail. We investigated transcription in budding yeast by Pol II that lacks the CTD and, therefore, is unable to assemble with mediator into a conventional holoenzyme complex. These experiments used a yeast strain that expresses the temperature-sensitive rpb1-1 allele for the full-length Pol II large subunit (Nonet et al. 1987a) and the CTD-deleted rpb1ΔCTD allele. At the restrictive temperature, the only active Pol II molecules are those that lack the CTD. Under these conditions, ΔCTD Pol II was engaged on the CUP1 gene at significantly higher density than in the control strain without a Pol II expression plasmid (Fig. 2). It is conceivable that in the absence of the CTD, mediator makes alternative contacts with Pol II; however, this possibility is not consistent with the fact that CTD deletion prevents mediator from working in an in vitro transcription system (Myers et al. 1998). Therefore, these observations strongly suggest that holoenzyme is not essential for Pol II to transcribe all protein-encoding genes in yeast. It remains to be determined whether other genes in addition to CUP1 can also be transcribed in vivo independently of the CTD.
The CTD has been implicated previously in the stimulation of transcriptional elongation by activators such as Drosophila HSF (O’Brien et al. 1994). We observed consistently that heat shock elevated the ratio of 3′-to-5′ polymerase densities on CUP1 (cf. CUP1-B to CUP1-A run-on signals in Fig. 2A, lanes 3,4 and Fig. 5A, lanes 1,2). This apparent increase in the processivity of CUP1 transcription was prevented by CTD deletion (Fig. 2A,B) and Kin28 inactivation (Fig. 4A) but not by inactivation of Srb4 (Fig. 5A, cf. lane 2 with lanes 3,4). These results are consistent with the idea that the CTD and Kin28 enhance elongation in yeast (Akhtar et al. 1996). It is possible that yeast HSF, like its mammalian and Drosophila counterparts, is responsible for this enhancement of transcriptional processivity.
We do not know in what form ΔCTD Pol II transcribes CUP1 in vivo. It is possible that a holoenzyme complex independent of mediator with a distinct set of accessory proteins is involved. Such an alternative holoenzyme was described recently by Shi et al. (1997). It is also possible that CTD-independent transcription is carried out by free core Pol II. This possibility is consistent with the fact that 50%–95% of Pol II in yeast is estimated to exist in free form (Kim et al. 1994; Koleske and Young 1994). The CTD is not essential for formation of preinitiation complexes containing mammalian Pol II, TFIIB, TFIID, and TFIIE/TFIIF on promoter DNA in vitro (Buratowski and Sharp 1990). Furthermore, Pol II without a CTD can initiate correctly at some promoters and can respond to certain activators in vitro (Zehring and Greenleaf 1990; Buermeyer et al. 1992; Kang and Dahmus 1993; Li and Kornberg 1994).
Abnormal transcripts made by ΔCTD Pol II
Paradoxically, transcription of CUP1 by ΔCTD Pol II occurs without accumulation of stable mRNA (Fig. 1A, lanes 11–15). We surmise that most of the transcripts made by CTD-deleted Pol II are unstable. This conclusion is supported by the observation that decay of CUP1 transcripts in rpb1ΔCTD cells at 37°C is blocked by deletion of the XRN1 gene, which encodes a 5′–3′ exonuclease involved in mRNA turnover (Hsu and Stevens 1993; Fig. 3). Unlike CUP1 mRNA, lacZ transcripts made by ΔCTD Pol II do accumulate (Fig. 6B), perhaps because they are exceptionally stable. One reason why most transcripts made by ΔCTD Pol II are unstable could be failure to receive a 5′ cap. In mammalian cells CTD truncation disrupts capping (McCracken et al. 1997a) and the yeast capping enzymes Ceg1 and Abd1 both bind directly to the phosphorylated CTD (Cho et al. 1997; McCracken et al. 1997a). Contrary to this expectation, most of the 1.7-kb CUP1 RNA made by ΔCTD Pol II was capped (perhaps post-transcriptionally) and was not stabilized by XRN1 deletion (S. McCracken, N. Fong, J.B. McNeil, unpubl.). Other possible reasons for the instability of transcripts made by ΔCTD Pol II include failure to engage appropriate RNA-binding proteins, inappropriate localization in the cell, or failure of other RNA processing steps.
In this regard, it is of interest that 3′ processing of CUP1 transcripts is less efficient when the gene is transcribed by CTD-deleted Pol II relative to wild-type causing the appearance of the dicistronic CUP1–YHR54C transcript (Fig. 1). Failure to cleave at the poly(A) site is characteristic of transcripts made by CTD-truncated Pol II in mammalian cells (McCracken et al. 1997b). These observations suggest that some degree of CTD-dependent coupling between 3′ processing and transcription occurs at CUP1; however, this phenomenon is not as general in yeast as it is in mammalian cells. Transcripts made by ΔCTD Pol II are cleaved efficiently at the CYC1 poly(A) site (data not shown) and at the putative poly(A) site of the YHR54C gene (Fig. 1B, probe G). Furthermore, Pol I transcripts of the HIS4 gene are polyadenylated in yeast (Lo et al. 1998). Interestingly, a dicistronic mRNA was also made when the HIS4 gene was transcribed by Pol I (Lo et al. 1998). CTD-dependent 3′ processing may be restricted to a particular subset of poly(A) sites in yeast. Unusually long CUP1 transcripts have been observed previously in RNA processing and transport mutants (Forrester et al. 1992; Chanfreau et al. 1996). The 1.7-kb CUP1 RNA was also induced by Cu2+ specifically in the srb4ts mutant at 37°C (Fig. 5D). Defective 3′ processing at the CUP1 poly(A) site may, therefore, be a general characteristic of transcription by abnormal Pol II complexes.
Diverse modes of transcriptional activation
Pol II that lacks all of the CTD recognizes the CUP1 promoter and initiates transcription at the correct start sites in vivo (Fig. 1C). Consistent with the lack of a CTD requirement, transcription of CUP1 was also observed after inactivation of the CTD-associated mediator subunit Srb4 and the CTD kinase Kin28 (Figs. 4 and 5). Transcription of CUP1 and SSA4 after inactivation of either Kin28 or Srb4 also has been reported by Lee and Lis (1998). The HSP82 gene was also expressed independently of Srb4 and Kin28 (Figs. 4 and 5). Dissection of the CUP1 promoter showed that it is the UAS and not the basal promoter elements that permit CTD-independent transcription (data not shown; Fig. 6B). In this respect the CUP1 UAS contrasts sharply with the GAL1 and INO1 UAS elements whose activity is severely inhibited by partial truncation of the CTD (Allison and Ingles 1989; Scafe et al. 1990). The CUP1 UAS fused to the CYC1 basal promoter could also stimulate a normal amount of transcription independent of Srb4 (Fig. 6A). The CUP1 gene differs from CYH2, ACT1, and ENO2, which have far more stringent requirements for Kin28 and Srb4 (Figs. 4 and 5; data not shown). Therefore, CUP1 and also HSP82 seem to be exceptional genes that can be transcribed independently of many holoenzyme components. The holoenzyme-independent activity of the CUP1 promoter is apparently not related to activation of the same promoter by histone depletion, which is independent of the UAS (Durrin et al. 1992).
These observations reinforce the principal that there are different requirements for components of the basal transcriptional machinery at different promoters in vivo. It was shown previously that TBP-associated factors (TAFs) are only essential for the function of a small subset of promoters in yeast (Moqtaderi et al. 1996; Walker et al. 1996) and that this requirement is dictated by basal promoter elements (Shen and Green 1997).
The relaxed holoenzyme requirement for activation by the CUP1 UAS is probably a consequence of the mechanism of activation by Ace1 or HSF, which bind to this element. HSF also stimulates transcription of the HSP82 gene which, like CUP1, is heat inducible in the absence of Srb4 and Kin28 (Figs. 4 and 5). These observations suggest that under some circumstances HSF can contribute to activation of transcription in the absence of intact holoenzyme. This ability may have evolved to allow transcription under stressful conditions where holoenzyme is disrupted or limiting. There is much support for the model that transcriptional activation works by recruitment of holoenzyme to promoters through protein–protein contacts with sequence-specific transcription factors (Ptashne and Gann 1997). The existence of a holoenzyme-independent mode of activation by the CUP1 UAS shows that this model does not apply in all cases and that diverse mechanisms of activation may operate at different promoters.
Materials and methods
Yeast strains and growth conditions
Yeast cultures (Table 1) were grown in synthetic complete (SC) medium plus 2% glucose without tryptophan or uracil as necessary, to maintain plasmid DNAs. Copper induction was by addition of copper sulfate to 0.1 mm, 30 min after shifting to the restrictive temperature. The TRP1 and XRN1 genes in Y260 were disrupted by one-step integration of homologous fragments containing URA3 flanked by hisG repeats. Ura− derivatives were then selected on FOA containing plates.
Table 1.
Yeast strains and growth conditions
| Strain |
Genotype
|
Source
|
|---|---|---|
| Y260 | MATa, ura3-52 rpb1-1 | Nonet et al. (1987a) |
| DBY120 | MATa ura3-52 rpb1-1 trp1::hisG | isogenic with Y260 (this study) |
| DBY121 | MATa, ura3-52 rpb1-1 trp1::hisG Δxrn1::hisG | isogenic with Y260 (this study) |
| Z579 | MATa his3Δ200 leu2-3,112 ura3-52 srb4Δ2::HIS3 [pRY2844 (LEU2 CEN SRB4)] | Thompson and Young (1995) |
| Z628 | MATa his3Δ200 leu2-3,112 ura3-52 srb4Δ2::HIS3 [pRY2882 (LEU2 CEN srb4-138)] | Thompson and Young (1995) |
| GF262 | MATα leu2 trp1 ura3 his3 | Valay et al. (1993) |
| kin28ts3 | MATα leu2 trp1 ura3 his3 kin28ts3 | Valay et al. (1993) |
Northern blotting and RNase protection
For Northern blots total RNA prepared by the hot phenol method (20 μg) was electrophoresed on 1.2% formaldehyde or on 1% 0.5× TBE nondenaturing agarose gels and transferred to Genescreen (New England Nuclear). Antisense riboprobes for CUP1, SSA1, CYH2, ACT1, ENO2, HIS3, HSP82, and lacZ mRNA and 5S rRNA were made from pWF1, pBS–SSA1, pBS–CYH2, pVZ–ACT1, pBS–ENO2, pVZ–HIS3, pBS–HSP82, pVZ–lacZ, and pBS–5S, respectively. For RNase protection, 20 μg of RNA was hybridized overnight to antisense probes in 0.4 m NaCl, 0.5 mm EDTA, 20 mm PIPES (pH 6.4), 80% formamide at 50°C. RNase digestion was in 0.3 m NaCl, 10 mm Tris (pH 7.5), 5 mm EDTA, 5 μg/ml RNase T1, 1.0 μg/ml RNase A for 30 min at 37°C.
Hybrid selection
Two micrograms of EcoRI-digested, heat-denatured pBS-5S, and 1 μg of single-stranded M13 mp19 CUP1-A or CUP1-G were mixed and slot-blotted onto Genescreen (Dupont). One hundred micrograms of total yeast RNA was incubated overnight with the filters at 65°C in 0.4 ml of 0.4 m NaCl, 0.5 mm EDTA, 20 mm PIPES (pH 6.4). The filters were washed two times at 60°C in 1× SSC and 1% SDS and then heated at 100°C for 10 min in H2O. Ten micrograms of total Escherichia coli RNA was added and the eluted RNA was precipitated with ethanol. CUP1-A and 5S antisense probes were used for RNase protection of selected RNA.
Transcriptional run-on analysis and M13 probes
Run-on reactions were performed as described previously in Sarkosyl-permeabilized cells (Elion and Warner 1986; Akhtar et al. 1996). All run-on probes were single-stranded M13s. The data were quantified by PhosphorImager (Molecular Dynamics). All values were normalized for U content and cell number and are expressed in arbitrary units.
M13 CUP1-A–G contain fragments of the CUP1 repeat generated by PCR. The sequences in each clone numbered according to Karin et al. (1984) are as follows: A 1454–1714; B 1710–20; C 25–392; D 362–675; E 658–888; F 886–1117; G 1076–1456. The PCR primers were A, 5′-GCCGGGATCCGTGCAATATCATATAGAAGTCATC and 5′-CGGCGGATCCCAGAGCAGCATGACTTCTTGG; B, 5′-GCCGGGATCCGGGAAATGAAACGAATAGTC and 5′-CGGCGGATCCAGCAGCGGGTACCATGAAT; C, 5′-CGGCGGATCCTATCTCCGATACCTGCCTC and 5′-CGGCGGATCCTTTGCACATCTTTCAGAG; D, 5′-CGGCGGATCCGACCTCGAACTCTGAAAGATGTG and 5′-CGGCGGATCCAAAATCTTGTCATGAATC; E, 5′-CGGCGGATCCGATTCATGACAAGATTTTGG and 5′-GCCGGGATCCGAGAACATTTTTGTTCTTCG CC; F, 5′-CGGCGGATCCTCTTGAAATTCCCCGTTAGTG and 5′-GCCGGGATCCGAACATATGCACGTATAGCGCCC; G, 5′-CGGCGGATCCCATTACCGACATTTGGG and 5′-CGGCGGATCCACTAACAAGAAGATATTATAATGC.
CYC1 sense and antisense probes were derived from a 321-bp PCR fragment comprising bases +290 to +610 relative to the ATG in the HindIII–SalI site of M13mp18 and M13mp19, respectively. The PCR primers used were 5′-GCGGAAGCTTGAAACGACTTAATTACCTACTTG and 5′-CGCCCTCGAGCTTGCAAATTAAAGCCTTCGAGCG.
CYH2 sense and antisense probes contain the 378-bp PCR fragment derived from the cDNA in pAS2 (Clontech), comprising −23 to the EcoRI site at +355 relative to the ATG, inserted into the EcoRI–SalI sites of M13mp18 and M13mp19, respectively. The forward PCR primer was 5′-GGCGGTCGACAACAATCATCCAACTAATC.
ENO2-A is a PCR fragment comprising bases −32 to +385 relative to the ATG in M13mp19. The PCR primers were 5′-ATGGATCCAAGCAACTAATACTATAAC and 5′-ATCTCGAGGGACGTTCTTTTCAGCAGC.
ENO2-B is a PCR fragment comprising bases +888 to +1302 relative to the ATG cloned into M13mp19. The PCR primers were 5′-ATGGATCCATTTGCTGAAGATGAC and 5′-ATCTCGAGCACCGTGGTGGAGTTTTCAC.
ACT1 is a 382-bp PCR fragment comprising bases +1407 to + 1787 relative to the ATG cloned in M13mp18. The PCR primers were 5′-CCGGATCCATCTATCGTTCACCACAAG and 5′-CCGAATTCTCCCAAGA AGGGCAATTGCA.
The lacZ (L2) and 35S rRNA M13 probes have been described (Akhtar et al. 1996).
Plasmids
pFL38 is a CEN-based URA3 plasmid (Bonneaud et al. 1991).
pFL38–RPB1 was made by inserting a 6042 bp EcoRI–PstI fragment of RPB1 into pFL38 after destroying the HindIII site in the polylinker.
pFL38–rpb1ΔCTD was made by replacing the 885-bp BsiWI–HindIII fragment in pFL38–RPB1, which contains codons 1510 through the carboxyl terminus, and 230 bp of 3′ flanking sequence with a BsiWI–HindIII cut PCR fragment encoding residues 1510 to 1533. Codon 1533 is at position 4 of the first heptad repeat. Amino acids SFKW were inserted as a result of a PCR error after codon 1533 and before the termination codon.
pRS314 CUP1–lacZ–CYC1 and pRS316 CUP1–lacZ–CYC1 contain the CUP1 promoter (−394 to +37 relative to the major transcriptional start site), lacZ (codons 10–1024) and zeor coding regions, and the CYC1 3′ processing signal (bases +336 to +610 relative to the CYC1 ATG) cloned between the NotI and KpnI sites of pRS314 (CEN, TRP1) and pRS316 (CEN, URA3) (Sikorski and Hieter 1989), respectively. The lacZ–zeor–CYC1 cassette was derived from pUT357 (Cayla, Toulouse, France).
Gal5–CYC1–lacZ (2 mμm, URA3) was described (Akhtar et al. 1996).
Gal5–CYC1–lacZ–TRP1 was derived from Gal5–CYC1–lacZ as follows. The URA3 marker was excised partially by StuI digestion and replaced with a blunt-ended BglII TRP1 fragment derived from pFL35 (Bonneaud et al. 1991).
pJM1503 and pJM1504 are derivatives of Gal5–CYC1–lacZ–TRP1 with insertion of a 107-bp PCR fragment that includes the CUP1 UAS (bases 1255–1362 according to Karin et al. 1984) inserted into the XhoI site between the Gal4-binding sites and the CYC1 promoter in the forward and reverse orientations, respectively. The PCR primers were 5′-GGCGGTCGACATTTTTGCTGTCAGTCAC and 5′-GGCGGTCGACGTCTTTTTTGCTGGAACGGTTC.
pJM1505 and pJM1506 were made by insertion of the CUP1 UAS fragment into Gal5–CYC1–lacZ (URA3) in forward and reverse orientations, respectively.
pBS-5S contains a 245-bp fragment of yeast 5S rRNA (with 40 bases of 5′ and 84 bases of 3′ flank) (Challice and Segall 1989) in pBSKS−.
pBS–HSP82 is a 515-bp PCR fragment (−313 to +202 relative to the HSP82 ATG) cloned into the BamHI–XhoI site of pBSKS−. The primers used were 5′-CGGCGGATCCTCATACGCTTGTCACATATTGTTC and 5′-GCCGCTCGAGTGATTCTAATAAAGAGATCTGG.
pBS–SSA1 is a 395-bp PCR fragment (−191 to +204 relative to the SSA1 ATG) cloned into the BamHI–XhoI site of pBSKS−. The primers used were 5′-CGGCGGATCCTGATCGTTTCGAGGACTTCAAGG and 5′-GCCGCTCGAGCGTCGAAAACGGTATTCGAAGG.
pBS–CYH2 contains the same 378-bp CYH2 cDNA fragment used for the M13 clones (see above) inserted into the EcoRI–SalI site of pBSKS−.
pBS–ENO2 contains the same PCR fragment as M13 ENO2-A (see above) in the BamHI–SalI site of pBSKS−.
pBS–CUP1-A, B, and G (see Fig. 1D) contain PCR fragments described above for the respective M13s cloned into pBSKS−.
pVZ–ACT1, pVZ–lacZ, pVZ–HIS3 (Akhtar et al. 1996), and the CUP1 clone pWF1 (Forrester et al. 1992) have been described.
Acknowledgments
We thank P. Atadja for his help in starting this project. We thank J. Segall, S. Dalton, M. Wickens, D. Jansma, J. Friesen, R. Young, and G. Faye for gifts of plasmids and yeast strains. We thank S. McCracken, J. Greenblatt, and L. Harrington for comments on the manuscript, S. McCracken and N. Fong for technical help, and M. Luchico and E. Schaeffer for expert secretarial and computing assistance. We are grateful to J. Lis for communicating data before publication. This work was supported in part by a grant from the Medical Research Council of Canada to D.B.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL bentley_d@defiance.uchsc.edu; FAX (303) 315-8215.
References
- Akhtar A, Faye G, Bentley D. Distinct activated and non-activated RNA polymerase II complexes in yeast. EMBO J. 1996;15:4654–4664. [PMC free article] [PubMed] [Google Scholar]
- Allison LA, Ingles CJ. Mutations in RNA polymerase II enhance or suppress mutations in GAL4. Proc Natl Acad Sci. 1989;86:2794–2798. doi: 10.1073/pnas.86.8.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneaud N, Ozier K-O, Li GY, Labouesse M, Minvielle S-L, Lacroute F. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast. 1991;7:609–615. doi: 10.1002/yea.320070609. [DOI] [PubMed] [Google Scholar]
- Buermeyer AB, Thompson NE, Strasheim LA, Burgess RR, Farnham PJ. The HIP1 initiator element plays a role in determining the in vitro requirement of the dihydrofolate reductase gene promoter for the C-terminal domain of RNA polymerase II. Mol Cell Biol. 1992;12:2250–2259. doi: 10.1128/mcb.12.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S, Sharp PA. Transcription initiation complexes and upstream activation with RNA polymerase II lacking the C-terminal domain of the largest subunit. Mol Cell Biol. 1990;10:5562–5564. doi: 10.1128/mcb.10.10.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M. Genetics of transcriptional regulation in yeast: Connections to the RNA polymerase II CTD. Annu Rev Cell Dev Biol. 1997;13:1–23. doi: 10.1146/annurev.cellbio.13.1.1. [DOI] [PubMed] [Google Scholar]
- Challice JM, Segall J. Transcription of the 5 S rRNA gene of Saccharomyces cerevisiae requires a promoter element at +1 and a 14-base pair internal control region. J Biol Chem. 1989;264:20060–20067. [PubMed] [Google Scholar]
- Chanfreau G, Noble SM, Guthrie C. Essential yeast protein with unexpected similarity to subunits of mammalian cleavage and polyadenylation specificity factor (CPSF) Science. 1996;274:1511–1514. doi: 10.1126/science.274.5292.1511. [DOI] [PubMed] [Google Scholar]
- Cho E-J, Takagi T, Moore C, Buratowski S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes & Dev. 1997;11:3319–3326. doi: 10.1101/gad.11.24.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismowski M, Laff G, Solomon M, Reed S. KIN28 encodes a C-terminal domain kinase that controls mRNA transcription in Saccharomyces cerevisiae but lacks cyclin-dependent kinase-activating kinase (CAK) activity. Mol Cell Biol. 1995;15:2983–2992. doi: 10.1128/mcb.15.6.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmus ME. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J Biol Chem. 1996;271:19009–19012. doi: 10.1074/jbc.271.32.19009. [DOI] [PubMed] [Google Scholar]
- Durrin LK, Mann RK, Grunstein M. Nucleosome loss activates cup1 and his3 promoters to fully induced levels in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:1621–1629. doi: 10.1128/mcb.12.4.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion EA, Warner JR. An RNA polymerase I enhancer in Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:2089–2097. doi: 10.1128/mcb.6.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell S, Simkovich N, Wu Y, Barberis A, Ptashne M. Gene activation by recruitment of the RNA polymerase II holoenzyme. Genes & Dev. 1996;10:2359–2367. doi: 10.1101/gad.10.18.2359. [DOI] [PubMed] [Google Scholar]
- Forrester W, Stutz F, Rosbash M, Wickens M. Defects in mRNA 3′-end formation, transcription initiation, and mRNA transport associated with the yeast mutation prp20: Possible coupling of mRNA processing and chromatin structure. Genes & Dev. 1992;6:1914–1926. doi: 10.1101/gad.6.10.1914. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hagmann M, Seipel K, Georgiev O, West MA, Litingtung Y, Schaffner W, Corden JL. RNA polymerase II C-terminal domain required for enhancer-driven transcription. Nature. 1995;374:660–662. doi: 10.1038/374660a0. [DOI] [PubMed] [Google Scholar]
- Hengartner CJ, Thompson CM, Zhang J, Chao DM, Liao SM, Koleske AJ, Okamura S, Young RA. Association of an activator with an RNA polymerase II holoenzyme. Genes & Dev. 1995;9:897–910. doi: 10.1101/gad.9.8.897. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Stevens A. Yeast cells lacking 5′ → 3′ exoribonuclease 1 contain mRNA species that are poly(A) deficient and partially lack the 5′ cap structure. Mol Cell Biol. 1993;13:4826–4835. doi: 10.1128/mcb.13.8.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang ME, Dahmus ME. RNA polymerase IIA and polymerase IIO have distinct roles during transcription from the TATA-less murine dihydrofolate reductase promoter. J Biol Chem. 1993;268:25033–25040. [PubMed] [Google Scholar]
- Karin M, Najarian R, Haslinger A, Valenzuela P, Welch J, Fogel S. Primary structure and transcription of an amplified genetic locus: The CUP1 locus of yeast. Proc Natl Acad Sci. 1984;81:337–341. doi: 10.1073/pnas.81.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Bjorklund S, Li Y, Sayre MH, Kornberg RD. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Koleske AJ, Young RA. An RNA polymerase II holoenzyme responsive to activators. Nature. 1994;368:466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, Woychik N, Liao SM, Young RA. RNA polymerase II subunit composition, stoichiometry, and phosphorylation. Mol Cell Biol. 1990;10:1915–1920. doi: 10.1128/mcb.10.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-K, Lis J. Transcriptional activation independent of TFIIH kinase and the RNA polymerase II mediator in vivo. Nature. 1998;393:389–392. doi: 10.1038/30770. [DOI] [PubMed] [Google Scholar]
- Lee JM, Greenleaf AL. Modulation of RNA polymerase II elongation efficiency by C-terminal heptapeptide repeat domain kinase I. J Biol Chem. 1997;272:10990–10993. doi: 10.1074/jbc.272.17.10990. [DOI] [PubMed] [Google Scholar]
- Li Y, Kornberg RD. Interplay of positive and negative effectors in function of the C-terminal repeat domain of RNA polymerase II. Proc Natl Acad Sci. 1994;91:2362–2366. doi: 10.1073/pnas.91.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SM, Taylor IC, Kingston RE, Young RA. RNA polymerase II carboxy-terminal domain contributes to the response to multiple acidic activators in vitro. Genes & Dev. 1991;5:2431–2440. doi: 10.1101/gad.5.12b.2431. [DOI] [PubMed] [Google Scholar]
- Lo HJ, Huang HK, Donahue TF. RNA polymerase I-promoted HIS4 expression yields uncapped, polyadenylated mRNA that is unstable and inefficiently translated in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:665–675. doi: 10.1128/mcb.18.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado E, Shiekhattar R, Sheldon M, Cho H, Drapkin R, Rickert P, Lees E, Anderson C, Linn S, Reinberg D. A human RNA polymerase II associated complex with SRB and DNA-repair proteins. Nature. 1996;381:86–89. doi: 10.1038/381086a0. [DOI] [PubMed] [Google Scholar]
- McCracken S, Fong N, Rosonina E, Yankulov K, Brothers G, Siderovski D, Hessel A, Foster S, Shuman S, Bentley D Amgen EST Program. 5′-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes & Dev. 1997a;11:3306–3318. doi: 10.1101/gad.11.24.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken S, Fong N, Yankulov K, Ballantyne S, Pan GH, Greenblatt J, Patterson SD, Wickens M, Bentley DL. The C-terminal domain of RNA polymerase II couples messenger RNA processing to transcription. Nature. 1997b;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- Moqtaderi Z, Bai Y, Poon D, Weil PA, Struhl K. Tbp-associated factors are not generally required for transcriptional activation in yeast. Nature. 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- Myers L, Gustafson C, Bushnell D, Lui M, Erdument-Bromage H, Tempst P, Kornberg R. The Med proteins of yeast and their function through the RNA polymerase II carboxy-terminal domain. Genes & Dev. 1998;12:45–54. doi: 10.1101/gad.12.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Young RA. Intragenic and extragenic suppressors of mutations in the heptapeptide repeat domain of Saccharomyces cerevisiae RNA polymerase II. Genetics. 1989;123:715–724. doi: 10.1093/genetics/123.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M, Scafe C, Sexton J, Young R. Eukaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol Cell Biol. 1987a;7:1602–1611. doi: 10.1128/mcb.7.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M, Sweetser D, Young RA. Functional redundancy and structural polymorphism in the large subunit of RNA polymerase II. Cell. 1987b;50:909–915. doi: 10.1016/0092-8674(87)90517-4. [DOI] [PubMed] [Google Scholar]
- O’Brien T, Hardin S, Greenleaf A, Lis JT. Phosphorylation of RNA polymerase II C-terminal domain and transcriptional elongation. Nature. 1994;370:75–77. doi: 10.1038/370075a0. [DOI] [PubMed] [Google Scholar]
- Ossipow V, Tassan JP, Nigg EA, Schibler U. A mammalian RNA polymerase II holoenzyme containing all components required for promoter-specific transcription initiation. Cell. 1995;83:137–146. doi: 10.1016/0092-8674(95)90242-2. [DOI] [PubMed] [Google Scholar]
- Pan G, Aso T, Greenblatt J. Interaction of elongation factors TFIIS and elongin A with a human RNA polymerase II holoenzyme capable of promoter-specific initiation and responsive to transcriptional activators. J Biol Chem. 1997;272:24563–24571. doi: 10.1074/jbc.272.39.24563. [DOI] [PubMed] [Google Scholar]
- Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- Scafe C, Chao D, Lopes J, Hirsch JP, Henry S, Young RA. RNA polymerase II C-terminal repeat influences response to transcriptional enhancer signals. Nature. 1990;347:491–494. doi: 10.1038/347491a0. [DOI] [PubMed] [Google Scholar]
- Shen WC, Green MR. Yeast TAF(II)145 functions as a core promoter selectivity factor, not a general coactivator. Cell. 1997;90:615–624. doi: 10.1016/s0092-8674(00)80523-1. [DOI] [PubMed] [Google Scholar]
- Shi XM, Chang MP, Wolf AJ, Chang CH, Frazerabel AA, Wade PA, Burton ZF, Jaehning JA. Cdc73p and paf1p are found in a novel RNA polymerase II-containing complex distinct from the Srbp-containing holoenzyme. Mol Cell Biol. 1997;17:1160–1169. doi: 10.1128/mcb.17.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silar P, Butler G, Thiele DJ. Heat shock transcription factor activates transcription of the yeast metallothionein gene. Mol Cell Biol. 1991;11:1232–1238. doi: 10.1128/mcb.11.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stargell LA, Struhl K. The TBP–TFIIA interaction in the response to acidic activators in vivo. Science. 1995;269:75–78. doi: 10.1126/science.7604282. [DOI] [PubMed] [Google Scholar]
- Svejstrup JQ, Li Y, Fellows J, Gnatt A, Bjorklund S, Kornberg RD. Evidence for a mediator cycle at the initiation of transcription. Proc Natl Acad Sci. 1997;94:6075–6078. doi: 10.1073/pnas.94.12.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele DJ, Hamer DH. Tandemly duplicated upstream control sequences mediate copper-induced transcription of the Saccharomyces cerevisiae copper-metallothionein gene. Mol Cell Biol. 1986;6:1158–1163. doi: 10.1128/mcb.6.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CM, Young RA. General requirement for RNA polymerase II holoenzymes in vivo. Proc Natl Acad Sci. 1995;92:4587–4590. doi: 10.1073/pnas.92.10.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CM, Koleske AJ, Chao DM, Young RA. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell. 1993;73:1361–1375. doi: 10.1016/0092-8674(93)90362-t. [DOI] [PubMed] [Google Scholar]
- Valay JG, Simon M, Faye G. The kin28 protein kinase is associated with a cyclin in Saccharomyces cerevisiae. J Mol Biol. 1993;234:307–310. doi: 10.1006/jmbi.1993.1587. [DOI] [PubMed] [Google Scholar]
- Valay J-G, Simon M, Dubois M-F, Bensaude O, Facca C, Faye G. The KIN28 gene is required for RNA polymerase II mediated transcription and phosphorylation of the Rpb1p CTD. J Mol Biol. 1995;249:535–544. doi: 10.1006/jmbi.1995.0316. [DOI] [PubMed] [Google Scholar]
- Walker SS, Reese JC, Apone LM, Green MR. Transcription activation in cells lacking TAF(II)s. Nature. 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- West M, Corden J. Construction and analysis of yeast RNA polymerase II CTD deletion and substitution mutations. Genetics. 1995;140:1223–1233. doi: 10.1093/genetics/140.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chao DM, Imbalzano AN, Schnitzler GR, Kingston RE, Young RA. RNA polymerase II holoenzyme contains Swi/Snf regulators involved in chromatin remodeling. Cell. 1996;84:235–244. doi: 10.1016/s0092-8674(00)80978-2. [DOI] [PubMed] [Google Scholar]
- Yang WM, Gahl W, Hamer D. Role of heat shock transcription factor in yeast metallothionein gene expression. Mol Cell Biol. 1991;11:3676–3681. doi: 10.1128/mcb.11.7.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehring WA, Greenleaf AL. The carboxyl-terminal repeat domain of RNA polymerase II is not required for transcription factor Sp1 to function in vitro. J Biol Chem. 1990;265:8351–8353. [PubMed] [Google Scholar]