Abstract
Background
The use of highly active antiretroviral therapy (HAART) has been associated with multiple metabolic complications whose pathogenesis is poorly understood at the present time.
Methods
We performed a cross-sectional analysis of whole-body, lumbar spine (L1 – L4) and proximal femur bone mineral density in 112 male subjects (HIV-infected patients on HAART that included a protease inhibitor, HIV-infected patients not receiving a protease inhibitor and healthy seronegative adults) using dual energy x-ray absorptiometry.
Results
Men receiving protease inhibitors had a higher incidence of osteopenia and osteoporosis according to World Health Organization definitions: relative risk = 2.19 (95% confidence interval 1.13–4.23) (P = 0.02). Subjects receiving protease inhibitors had greater central : appendicular adipose tissue ratios than the other two groups (P < 0.0001). There was no relationship between the central : appendicular fat ratio and the lumbar spine or proximal femur bone mineral density t- or z- scores, suggesting that osteoporosis and body fat redistribution are independent side effects of HAART.
Conclusions
Osteopenia and osteoporosis are unique metabolic complications associated with protease inhibitor-containing potent antiretroviral regimens, that appear to be independent of adipose tissue maldistribution.
Keywords: bone mineral metabolism, bone densitometry, osteoporosis, adiposity, aspartyl protease inhibitors, HIV infection
Introduction
Dramatic advances in the treatment of HIV infection have occurred over the past few years. Availability of HIV protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors allowed the use of highly active combinations of antiretroviral drugs (HAART) that can inhibit HIV replication. The use of HAART has dramatically reduced the morbidity and mortality rates from HIV infection [1,2]. This progress is not without problems. All components of HAART regimens have major acute and long term toxicities that are only partially understood [3–11]. Among these toxicities are metabolic problems including diabetes mellitus, hyperlipidemia, lipodystrophy, lipoatrophy and dyslipidemia. The mechanism(s) by which antiretroviral therapy might cause these abnormalities is unknown.
There have been anecdotal reports of bone disorders such as avascular necrosis of the hip and compression fracture of the lumbar spine in HIV-infected patients receiving HAART [12–17]. These are recognized complications of severe osteoporosis, but avascular necrosis might be attributed to hyperlipidemia. We have used dual energy X-ray absorptiometry (DEXA) to evaluate bone mineral density (BMD) and body adiposity in patients with HIV. We have observed a lower lumbar and thoracic spine BMD in up to 30% of the HIV-infected individuals treated with PIs. This implies that osteopenia and osteoporosis are additional metabolic complications associated with HAART.
Methods
Patients
A cross-sectional analysis was performed on 122 subjects (112 men and 10 women). Three different subject groups were enrolled: (a) HIV-infected patients receiving PIs (n = 64); (b) HIV-infected patients not receiving PIs (n = 36); and (c) healthy adults (HIV negative controls) (n = 22). We focused our analysis on the 112 males. This study represents the findings of consecutive DEXA scans carried out on our HIV-infected patient population over a period of time of more than 2 years. When an individual had several scans we selected the first scan available if it was done before starting therapy. If no scan was available before starting therapy then, the last scan on PI treatment was used. The population of controls was actively enrolled later on. All studies were performed after an overnight fast. All subjects gave written informed consent.
Dual energy X-ray absorptiometry
An Hologic QDR-2000 enhanced-array whole-body DEXA scanner (Hologic, Waltham, Massachusetts, USA), Hologic enhanced-array whole body software (version 5.71A) and regional array software (version 4.74A:1) were used to determine BMD of the whole-body, the lumbar spine (L1–L4), and proximal femur. The soft tissue bar was used to quantitate whole-body adipose tissue mass, regional adipose tissue mass (arms, legs, trunk) and fat-free mass. The central : appendicular adipose tissue ratio was calculated as the amount of adipose tissue in the trunk region/amount of adipose tissue in both arms and legs. Each scan was acquired and processed by an experienced radiology technologist. A subset of patients also underwent localized bone densitometry of the lumbar spine and the hip during the same examination (see below).
Statistical methods
To evaluate whether the lumbar spine BMD derived from the whole body DEXA scan was similar to the lumbar spine BMD derived from a DEXA scan localized to the L1–L4 region, we performed both determinations in a subset of 41 patients and calculated the Pearson’s correlation coefficient between these measurements.
Then, using the manufacturers normative population data established by gender, race and age, we calculated the normalized t- and z- scores for the lumbar spine (L1–L4) in all subjects, according to the following formulas: t-score = (measured BMD − population mean BMD at age 30 years)/standard deviation (BMD at age 30 years); z-score = (measured BMD − population mean BMD for same age subject)/standard deviation (BMD at same age).
Using World Health Organization (WHO) definitions of osteopenia and osteoporosis we classified the patients into the following categories: normal = t-score > −1, osteopenic = t-score from −1 to −2.5, and osteoporotic = t-scores < −2.5. In addition, any subject with a BMD z-score < −2 (less than 2 SD of what is considered normal for his or her age and race) was classified as osteoporotic. Non-parametric statistical tests (Kruskal–Wallis) were used to compare t- and z-scores for spine and hip BMD between groups. Normally distributed variables [age, body mass index (BMI), adiposity] were compared using analysis of variance.
Results
We enrolled 122 subjects into the study (112 men and 10 women). The demographic and anthropometric characteristics are summarized in Table 1. The BMI (kg/m2) were well balanced between groups. Controls were slightly younger than the HIV-infected patients. To adjust for the age difference, the t- and z-scores for the regional BMDs were used to compare the three groups. We focused our analysis on the 112 men (60 on PIs, 35 never-treated or on regimens that did not include a PI and 17 non-HIV-infected controls) because the group of women was too small to make meaningful gender comparisons. The median exposure to PIs in the group of subjects receiving them was 104 weeks (range, 16–363) and double PI regimens were counted as the sum of the exposures to each individual PI. Forty percent of the patients had received two or more PIs at the time the DEXA scan was done. The treatment with PIs represented a total of 6730 weeks of PI exposure (27% nelfinavir, 27% indinavir, 24% ritonavir and 22% saquinavir, with the last two generally being used in combination). Amprenavir was only used for 10 weeks (0.14% of the total PI exposure).
Table 1.
Characteristics of the 112 men included in this study.
| HIV+ PI+ n = 60 |
HIV + PI− n = 35 |
Controls n = 17 |
P-values | |
|---|---|---|---|---|
| Age (years)a | 41 ± 8 | 37 ± 7 | 33 ± 9 | 0.001 |
| Body mass index (kg/m2)a | 24 ± 4 | 22 ± 6 | 23 ± 4 | 0.706 |
| Median lumbar spine BMD | 0.9860 | 1.0690 | 1.0660 | 0.002 |
| Median t-score | −1.005 | −0.382 | −0.227 | 0.02 |
| Median z-score | −0.923 | −0.382 | 0.145 | 0.04 |
| % of subjects with osteopenia or osteoporosis according to WHO | 50 | 23 | 29 | 0.02 |
| % with severe osteoporosis (z-score < −2) | 21 | 11 | 6 | 0.19 |
Values are mean + SD. HIV+ PI+, HIV-positive patients taking protease inhibitors; HIV+PI− HIV-positive patients not taking protease inhibitors; control, healthy, uninfected subjects; BMD, bone mineral density; WHO, World Health Organization.
Correlative measures of lumbar spine BMD derived from the whole-body and localized lumbar spine scans were carried out in 41 subjects. We observed a strong correlation between lumbar spine BMD using regional and whole-body scans (r = 0.97, P < 0.0001). This indicated that the lumbar spine BMD determined using a whole-body scan was representative of lumbar spine (L1–L4) BMD determined by a scan localized to this region. Therefore, lumbar spine BMD determined using whole-body DEXA scanning was used to estimate the lumbar spine BMD t- and z-scores for all the subjects.
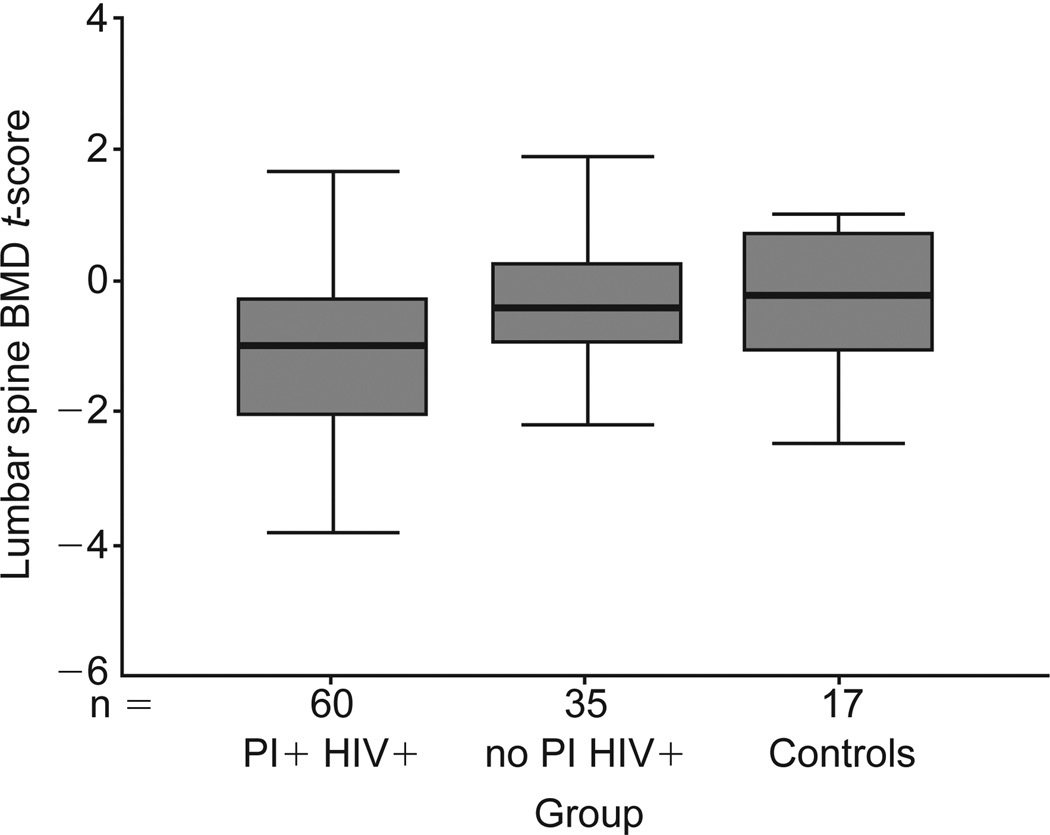
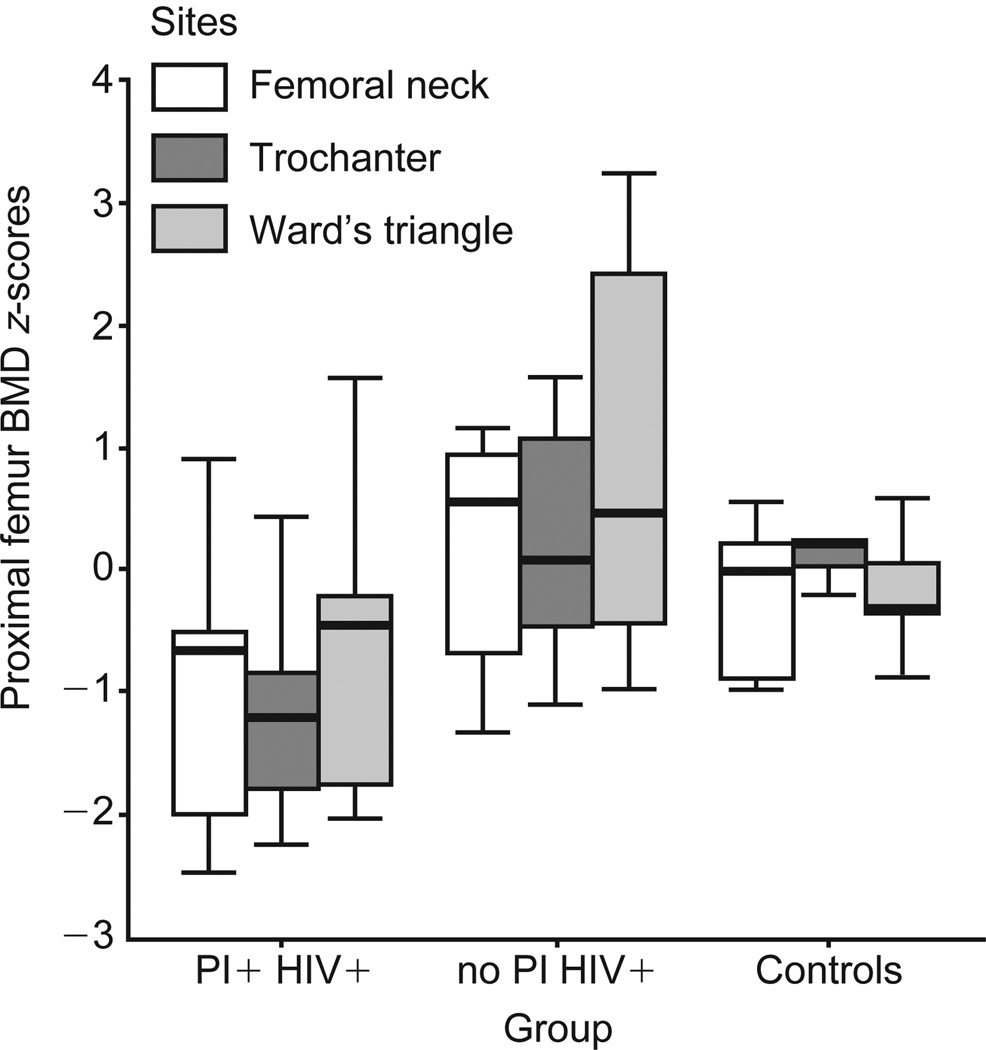
Men receiving PIs had lower median t-scores for the lumbar spine BMD than the other two groups (Table 1 and Fig. 1; P = 0.02). Median z-scores for the lumbar spine BMD were also lower in PI recipients (P = 0.04). Median z-scores for BMD in the trochanter, femoral neck, and Ward’s triangle regions of the proximal femur were significantly lower in PI-treated subjects than in HIV-infected subjects who were not receiving PI’s and in controls (Fig. 2; P = 0.01, 0.08 and 0.09 respectively).
Fig. 1.
Lumbar spine bone mineral density (BMD) t-scores for the 112 men included in this study. The box represents the inter-quartile range, the thick line the median and the thin lines the range of the t-scores. The median lumbar spine BMD (P = 0.002) and the t-score for the HIV-positive patients on protease inhibitors (HIV+ PI+) group (P = 0.02) were lower than the other two groups. Controls, healthy, uninfected subjects; no PI HIV+, HIV-positive patients not taking protease inhibitors.
Fig. 2.
HIV-positive patients taking protease inhibitors (HIV+ PI+) had significantly lower bone mineral density (BMD) z-scores in the neck (P = 0.08), trochanteric (P = 0.01) and Ward’s triangle (P = 0.09) regions of the proximal femur than the other two groups (Kruskal–Wallis test). Controls, healthy, uninfected subjects; no PI HIV+, HIV-positive patients not taking protease inhibitors.
Using lumbar spine BMD t-scores, 50% of the subjects on PIs were classified as osteopenic or osteoporotic according to the WHO classification (Table 1; P = 0.02). The relative risk for osteoporosis in these subjects was 2.19 (95% confidence interval, 1.13–4.23) when compared with HIV-infected subjects not receiving PI’s. Twenty-one percent of the patients receiving PIs had a lumbar spine BMD more than 2 SD’s lower than the mean value expected for their age, sex and race and met our definition of osteoporosis (Table 1). Only 6% of the controls and 11% of the HIV-infected patients not taking PIs were classified as osteoporotic using these same criteria.
Men receiving PIs had a greater central : appendicular adipose tissue ratio than the other two groups (Fig. 3; P < 0.0001), consistent with previous observations by others [8,18]. Whether this was a result of HAART or existed before HAART was initiated, cannot be determined in this cross-sectional study. There was no correlation between the central : appendicular fat ratio and the lumbar or proximal femur BMD t- or z-scores (Fig. 3). This suggests that osteoporosis and body fat redistribution are independent side effects of potent antiretroviral regimens (r2 = 0.0012, P = NS), which might be mediated through different mechanisms.
Fig. 3.
Patients on protease inhibitors had greater central : appendicular adipose tissue ratios than the other two groups (P 0.0001). Controls, healthy, uninfected subjects; PI+ HIV+, HIV-positive patients taking protease inhibitors; no PI HIV+, HIV-positive patients not taking protease inhibitors.
Patients with more prolonged use of PIs tended to have lower t-scores in the lumbar spine (Pearson correlation coefficient = −0.19, P = 0.14), but this correlation did not reach statistical significance.
Discussion
Gradual demineralization of bone is a normal feature of aging. Men and women naturally begin to lose bone around 35 years of age, at a rate of 0.5–1% per year. Women lose bone at an accelerated rate after the menopause. Bone densitometry reports refer to z- or t-score. The z-score (which uses age-matched controls) compares the patient with a population adjusted for age, race and sex; the t-score (using young normal controls) compares the patient with a sex-adjusted population at peak bone mass [19].
Bone densitometry is a widely accepted tool to assess bone mineralization. For an individual patient osteopenia (t-score between −1 and −2.5) carries a two-fold increase in risk for fracture compared with normal BMD, and osteoporosis (t-score < −2.5) (without fracture) carries a four- to five-fold increase in fracture risk. Severe osteoporosis (t-score < −2.5 plus the presence of a fracture) increases the risk for further fractures by 20 times [20–22]. Osteoporosis is a multifactorial disease. Recognized risk factors for the development of osteoporosis include postmenopausal state, ovarian-deficient status, primary hyperparathyroidism, hypogonadism, cancer chemotherapy and systemic steroid administration. None of these risk factors were present in the subjects studied.
Prior to the availability of PIs, low BMD was rarely observed in HIV-infected individuals [23]. However, serum markers of bone turnover (osteocalcin) were decreased and resorption (C-teleopeptide) increased in HIV-infected subjects with advanced clinical disease and high serum tumor necrosis factor-α (TNF-α) levels [24]. Treatment with HAART (16 subjects, 2 years) reduced serum TNF-α levels and increased serum osteocalcin to levels in excess of normal controls, whereas serum C-teleopeptide remained in the high-normal range [24]. Elevated serum osteocalcin indicates that bone cell turnover was accelerated after HAART was initiated, but it is not direct proof that bone density was altered. Collectively, these findings suggest that HAART may increase bone cell turnover, but in approximately 30% of those treated, BMD (especially in the hip and spine) will decline.
Our findings indicate that HIV-infected individuals receiving PI-based HAART are more likely to have significant bone demineralization. Therefore, HAART might be associated with an increasing prevalence of osteopenia and osteoporosis, which may increase the risk for fracture in people living with HIV/AIDS. The lack of a clear association with the total exposure to PIs has to be interpreted with caution because the numbers are small, and we do not know the baseline BMD t-scores of these patients before starting therapy. Patients that started therapy with a high t-score (if this problem is causally related to therapy) might take longer to reach a value in the osteopenic/osteoporotic range, suggesting, inaccurately, that there is no relationship between length of treatment time on drug and BMD. A prospective study is necessary to evaluate this question. As osteoporosis is slow to progress, several years of HAART may accelerate the typical loss of bone mineral that occurs with advancing age. Prospective sequential studies comparing antiretroviral regimens with and without HIV-PIs are needed to definitely establish the role of the individual components of the antiretroviral regimens in the pathogenesis of this apparent complication of therapy.
The cross-sectional nature of the study design diminishes our ability to attribute cause but it does suggest that an association exists, that requires further investigation. The situation is similar to other well-known metabolic complications associated with antiretroviral therapy, all of which have only been reported in cross-sectional studies. Their etiology and the role of each individual medication in the antiretroviral regimen are still unclear.
This association between HAART and osteopenia requires prompt examination. The increasing number of case reports of avascular necrosis, osteonecrosis and hip fracture among patients receiving antiretroviral therapy suggest that this is a real phenomenon and not a spurious association. Given the number of HIV-infected people who are currently being treated with these medications, and the adverse health consequences associated with osteoporosis, the combination could exacerbate the high morbidity and mortality rates associated with HIV disease.
Acknowledgments
Sponsorship: This study was supported by National Institutes of Health grants DK49393, DK54163, AI25903, K23AI01612, and the Campbell Foundation.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Update: trends in AIDS incidence – United States, 1996. MMWR. 1997;46:861–867. [PubMed] [Google Scholar]
- 3.Visnegarwala F, Krause KL, Musher DM. Severe diabetes associated with protease inhibitor therapy. Ann Intern Med. 1997;127:947. doi: 10.7326/0003-4819-127-10-199711150-00016. [DOI] [PubMed] [Google Scholar]
- 4.Eastone JA, Decker CF. New-onset diabetes mellitus associated with use of protease inhibitor. Ann Intern Med. 1997;127:948. doi: 10.7326/0003-4819-127-10-199711150-00017. [DOI] [PubMed] [Google Scholar]
- 5.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. “Buffalo hump” in men with HIV-1 infection. Lancet. 1998;351:867–870. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 7.Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 9.Behrens G, Dejam A, Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–F70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 10.Henry K, Melroe H, Huebsch J, et al. Severe premature coronary artery disease with protease inhibitors. Lancet. 1998;351:1328. doi: 10.1016/S0140-6736(05)79053-X. [DOI] [PubMed] [Google Scholar]
- 11.Yarasheski KE, Tebas P, Sigmund C, et al. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr. 1999;21:209–216. doi: 10.1097/00126334-199907010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer D, Behrens G, Schmidt RE, Stoll M. Osteonecrosis of the femoral head in patients receiving HIV protease inhibitors. AIDS. 1999;13:1147–1148. doi: 10.1097/00002030-199906180-00025. [DOI] [PubMed] [Google Scholar]
- 13.Manzaneque Gonzalez L, Mayoral Martin L, Jimenez Ocana C, Corzo Delgado JE, Sanchez-Matas Rodriguez P, Grilo Reina A. Bilateral avascular necrosis of the femur head in an HIV-positive male. Anales de Medicina Interna. 1994;11:601–603. [PubMed] [Google Scholar]
- 14.Gibert C, Koller E, Mann M, Bacsanyi J, Malozowski S. Avascular necrosis in AIDS patients receiving megestrol acetate. Fifth Conference on Retroviruses and Opportunistic Infections; 1998; Chicago. [abstract 478] [Google Scholar]
- 15.De Truchis P, Saillour M, Pigne E, et al. Avascular necrosis of bone in HIV-infected patients with antiphospholipid antibodies. XI International Conference on AIDS; 1996; Vancouver. [abstract MoB1298] [Google Scholar]
- 16.Stovall D, Jr, Young TR. Avascular necrosis of the medial femoral condyle in HIV-infected patients. Am J Orthop. 1995;24:71–73. [PubMed] [Google Scholar]
- 17.Gerster JC, Camus JP, Chave JP, Koeger AC, Rappoport G. Multiple site avascular necrosis in HIV infected patients. J Rheumatol. 1991;18:300–302. [PubMed] [Google Scholar]
- 18.Grinspoon S, Corcoran C, Miller K, et al. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting [published erratum appears in J Clin Endocrinol Metab 1997 82:3360] J Clin Endocrinol Metab. 1997;82:1332–1337. doi: 10.1210/jcem.82.5.3907. [DOI] [PubMed] [Google Scholar]
- 19.Kanis JA, Delmas P, Burckhardt P, Cooper C, Torgerson D. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int. 1997;7:390–406. doi: 10.1007/BF01623782. [DOI] [PubMed] [Google Scholar]
- 20.Nevitt MC, Johnell O, Black DM, Ensrud K, Genant HK, Cummings SR. Bone mineral density predicts non-spine fractures in very elderly women. The Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4:325–331. doi: 10.1007/BF01622192. [DOI] [PubMed] [Google Scholar]
- 21.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [see comments]. [DOI] [PubMed] [Google Scholar]
- 22.Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919–923. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 23.Paton NIJ, Macallan DC, Griffin GE, Pazianas M. Bone mineral density in patients with human immunodeficiency virus infection. Calcif Tissue Int. 1997;61:30–32. doi: 10.1007/s002239900288. [DOI] [PubMed] [Google Scholar]
- 24.Aukrust P, Haug CJ, Ueland T, et al. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: Indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab. 1999;84:145–150. doi: 10.1210/jcem.84.1.5417. [DOI] [PubMed] [Google Scholar]