Abstract
Sweet’s syndrome or acute febrile neutrophilic dermatosis is characterised by the abrupt onset of painful erythematous plaques or nodules, pyrexia (>38°F) and histopathologic evidence of a dense neutrophilic infiltrate without vasculitis. It has been reported in association with many diseases, however, its association with Hashimoto’s thyroiditis is rare. A 47-year-old Filipino woman with a 30-year history of an asymptomatic anterior neck mass developed painful, erythematous annular plaques on her arms with associated fever. Skin biopsy confirmed the diagnosis of Sweet’s syndrome. The anterior neck mass was confirmed to be Hashimoto’s thyroiditis. This is a rare association with only two reported cases in the literature. There are no published cases in the Philippines on Sweet’s syndrome and Hashimoto’s thyroiditis to date.
Background
The association of Hashimoto’s thyroiditis and Sweet’s syndrome is rare.
Sweet’s syndrome responded to colchicine treatment and cutaneous lesions disappeared after total thyroidectomy.
Autoimmune condition like Hashimoto’s thyroiditis should be ruled out in patients with Sweet’s syndrome who are negative for cancer and parainflammatory diseases.
Case presentation
A 47-year-old Filipino woman presented with a 1-week history of tender, erythematous plaques on her forearms accompanied by a 4-day history of low-grade fever (38.5°C). The plaques were annular, well-defined, slightly oedematous and with a partially mammillated surface. There was no ulceration, scaling or pruritus (figure 1).
Figure 1.
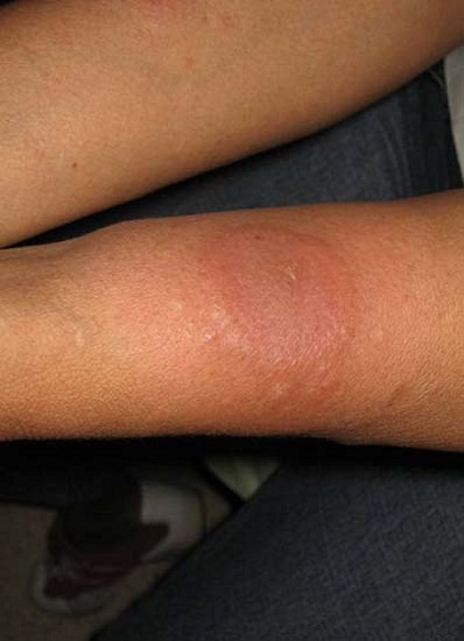
Tender, annular erythematous plaques on ventral forearm.
The patient had a 30-year history of a gradually enlarging anterior neck mass with no associated symptoms. One year prior to consult at the Section of Dermatology, Philippine General Hospital, she noted a sudden increase in the size of the neck mass with accompanying dysphagia. She was seen at the institution’s Department of Otorhinolaryngology where fine needle aspiration biopsy was performed which showed a follicular tumour. She was advised thyroidectomy and endocrinology consult.
Medical history revealed pulmonary tuberculosis that was treated 13 years ago and a previous oophorectomy done as a complication of an ectopic pregnancy. The patient denied any recent infections or intake of medications. Family medical history was non-contributory.
Physical examination was unremarkable aside from the enlarged, firm, multinodular anterior neck mass that moved with deglutition (figure 2). There were no clinical signs of hypo- or hyperthyroidism or other extracutaneous manifestations. Impression at this time was Sweet’s syndrome with follicular thyroid tumour; rule out thyroid carcinoma.
Figure 2.
Firm, multinodular anterior neck mass.
Investigations
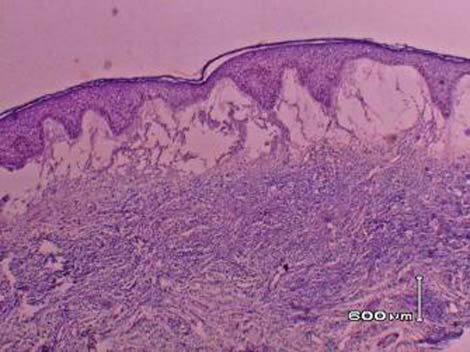
A skin biopsy showed marked dermal papillary oedema and a dense infiltrate of neutrophils and neutrophilic nuclear dusts throughout the upper dermis (figure 3). Microscopic changes of vasculitis were not evident in the sections examined.
Figure 3.
Skin biopsy showing prominent oedema and a dense neutrophilic infiltrate.
Laboratory workup done showed elevated erythrocyte sedimentation rate, and a normal complete blood count except for the presence of a mild normochromic anaemia. Electrolytes, urinalysis, renal function tests, ECG and chest radiograph were all normal. Free thyroxine (3.9 pmol/l NV 11–24 pmol/l) was decreased and thyroid stimulating hormone (9.5 mIU/l N.V. 0.3–3.8 mlU/l) was increased indicating primary hypothyroidism.
CT of the chest was normal except for the finding of a non-specific pulmonary fibrosis which was most probably secondary to the previous tuberculous infection. Carcinoembryonic antigen level was normal. Transvaginal ultrasound showed a normal uterus, a normal right ovary and a surgically absent left ovary.
Treatment
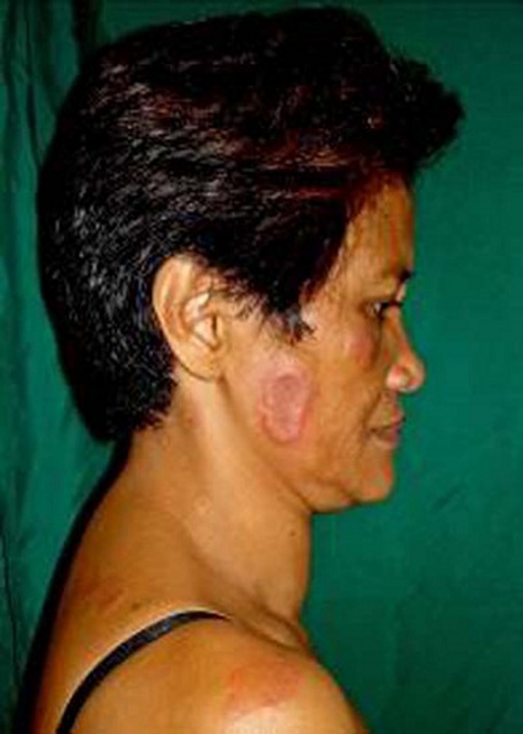
The patient was treated with colchicine 0.5 mcg/tab three times a day. Response to therapy was excellent, with resolution of the painful plaques within a week of treatment (figure 4). Levothyroxine 25 µg once daily was given to control the patient’s hypothyroidism. There were recurrences of the tender plaques occurring on her face, neck, upper back and arms (figure 5). Each episode would resolve rapidly after treatment with colchicine. Repeat skin biopsy showed findings consistent with Sweet’s syndrome.
Figure 4.
Resolution of plaques after colchicine treatment.
Figure 5.
Recurrence on the face and upper back prior to thyroidectomy.
Total thyroidectomy was performed. Final pathologic diagnosis of the thyroid mass showed Hashimoto’s thyroiditis. The discrepancy between the patient’s cytology results and final histopathology is most probably due to inadequate sampling during the fine needle aspiration biopsy.
Outcome and follow-up
There was no recurrence of skin lesions after total thyroidectomy. The patient is currently maintained on levothyroxine 150 µg once daily.
Discussion
Sweet’s syndrome is characterised by the abrupt onset of tender, erythematous plaques on the face, neck, upper trunk and extremities with associated fever (>38°F) and malaise. Typical inflammatory papules tend to coalesce into irregular plaques, usually forming an annular pattern.1 It was first described by Dr Robert Sweet in 1964. Although initially considered a rare entity, more than 500 cases have been documented in the literature.2
Histologically, it is characterised by a moderate to marked neutrophilic infiltrate in the upper and lower dermis and marked papillary dermal oedema.3 There may be leukocytoclasia but vasculitis is not usually seen.
Classic Sweet’s syndrome usually occurs in middle aged women after a non-specific infection of the respiratory or gastrointestinal tract. The dermatosis may also be associated with parainflammatory processes such as infections, autoimmune disorders and vaccination. Pregnancy, and intake of certain drugs most commonly occurring with administration of granulocyte-colony stimulating factor are also included.4 As a cutaneous paraneoplastic syndrome, it has been associated with haematologic malignancies, most commonly acute myelogenous leukaemia and to a lesser extent solid tumours.5
The exact pathogenesis of Sweet’s syndrome remains to be definitely determined. It has been suggested that it results from a hypersensitivity reaction to a bacterial, viral or tumour antigen. Cytokines, either directly or indirectly, may also have an aetiologic role in the development of Sweet’s syndrome.4 Further evidence for the role of cytokines comes from the association of Sweet’s syndrome occurring in patients with thyroid disease.
On the other hand, Hashimoto’s thyroiditis is a chronic inflammatory autoimmune disease of the thyroid gland. It is sometimes used to refer to goitrous thyroiditis but may be considered in a broad sense, a synonym of chronic thyroiditis or autoimmune thyroiditis, including atrophic and non-goitrous thyroiditis.6 Clinically, it may present as a continuum. In the early stages, patients are euthyroid with absence of goitre or presence of a very small goitre with the only evidence of autoimmune thyroiditis as a positive reaction for antithyroid antibodies. As the disease progresses, patients commonly develop a firm, diffuse goitre of small to moderate size. Thyroid function test vary from euthyroidism to thyrotoxicosis. The more advanced disease present as large, firm goitre or the classical goitrous Hashimoto’s disease. It may then eventually lead to atrophy due to cytotoxic autoimmune reaction. The first variety of chronic thyroiditis, struma lymphomatosa was described by Hakaru Hashimoto in 1912.
Hashimoto’s thyroiditis is characterised clinically by hypothyroidism and diffuse goitre. Patients may also present with an enlarged, rubbery, non-tender and nodular thyroid gland.7 Histologically, follicular structure is replaced by lymphocytic and plasma cell infiltrates with the formation of lymphoid germinal clusters. Thyroid follicles may remain isolated or in small clusters; are small or atrophic and are empty or contain sparse colloid. In our case, initial histologic diagnosis was a follicular tumour. Upon histopathology review after thyroid surgery, few atypical thyrocytes were seen in clusters. Final histopathology of the excised thyroid gland showed Hashimoto’s thyroiditis in all lobes.
In the US, around 1.5 million adults and 200 000 children are afflicted with this condition,8 approximately 1 in 182 or 0.55%. It is five times more common in women than men and in patients with chromosomal abnormalities such as trisomy 21 and Turner’s syndrome.7 The association of Sweet’s syndrome with Hasimoto’s thyroiditis is uncommon. Only two cases are reported in the literature. In 1993, the first case of Sweet’s syndrome associated with aortitis and Hashimoto’s thyroiditis in a 39-year-old woman was reported by Nakayama.9 Medeiros reported a second case of Sweet’s syndrome and Hashimoto’s thyroiditis in a 65-year-old female in September 2008.10 Our patient is the third reported case of such an association.
The possible link between Sweet’s syndrome and thyroid disease is supported by the proposed role of immune-mediated T cytokine expression in both diseases. Magri11 reported the case of a 50-year-old female who had a relapse of Sweet’s syndrome associated with changes in thyroid autoimmunity. She was documented with antithyroid peroxidase (TPO) and antithyroglobulin positivity and a free T4 within the low normal range during the relapse. The authors stated that the cytokine cascade in Sweet’s syndrome can stimulate human leukocyte antigen class II expression on immune thyroid epithelial cells and may have a cytotoxic effect on thyroid cells. Kalmus reported a 63-year-old woman who developed Sweet’s syndrome 1 week after the onset of subacute thyroiditis.12
Thorough assessment of patients with new-onset Sweet’s syndrome is imperative, and majority of authors recommend that initial assessment include a complete history and physical examination, complete blood cell count, serum chemical studies, urinalysis and chest roentgenography.4 13 In addition to this, it is suggested that serological evaluation of thyroid function should be done in the evaluation of patients because of the increasing evidence of an association between Sweet’s syndrome and thyroid diseases.
Systemic corticosteroids are the therapeutic gold standard for Sweet’s syndrome, but colchicine and potassium iodide are also considered as first-line therapeutic alternatives.4 Other treatment options include dapsone, clofazimine, indomethacin and cyclosporine. In our patient, colchicine was given as first line of treatment rather than systemic corticosteroids in order to decrease the risk of perioperative complications prior to the scheduled for thyroidectomy. The response to colchicine was rapid in both primary and recurrent lesions with resolution after a few days of treatment.
Patients with Hashimoto’s thyroiditis and euthyroidism develop hypothyroidism at a rate of approximately 5% per year.5 Euthyroid patients treated with levothyroxine (1.0 to 2.0 µg/kg/day) showed decrease in anti-TPO antibodies and thyroid B lymphocytes. This suggests that prophylactic levothyroxine therapy might be useful to stop the progression of disease.11 However, long term data about the benefit of this treatment is still lacking. When hypothyroidism develops, levothyroxine replacement should be given. Painful subacute exacerbation of goitrous Hashimoto’s thyroiditis may be treated with corticosteroid therapy and recurrent episodes of which would benefit from surgical removal of the thyroid gland or administration of I131 therapy.
Learning points.
-
▶
Sweet’s syndrome or acute febrile neutrophilic dermatosis is characterised by the abrupt onset of painful erythematous plaques or nodules, pyrexia (>38°F) and histopathologic evidence of a dense neutrophilic infiltrate without vasculitis.
-
▶
It is associated with malignancy, parainflammatory disorders and autoimmune processes.
-
▶
It may be associated with Hasimoto’s thyroiditis though, rarely.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Callen JP. Neutrophilic dermatoses. Dermatol Clin 2002;20:409–19 [DOI] [PubMed] [Google Scholar]
- 2.von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol 1994;31:535–56 [DOI] [PubMed] [Google Scholar]
- 3.Lee YS, Kuo TT. Sweet’s syndrome: clinicopathologic study of eleven cases. Int J Dermatol 1994;33:425–32 [DOI] [PubMed] [Google Scholar]
- 4.Cohen PR: Sweet’s syndrome Orphanet Encyclopedia. http://www.orpha.net/data/patho/GB/uk-Sweet.pdf (accessed November 2007). [Google Scholar]
- 5.Cohen PR, Holder WR, Tucker SB, et al. Sweet syndrome in patients with solid tumors. Cancer 1993;72:2723–31 [DOI] [PubMed] [Google Scholar]
- 6.DeGroot LJ, Jameson JL. Endocrinology. Fifth edition Philadelphia, PA: Elsevier Saunders; 2006:2055–65 [Google Scholar]
- 7.Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis, and dermatologic manifestations. J Am Acad Dermatol 2003;48:641–59; quiz 660–2 [DOI] [PubMed] [Google Scholar]
- 8.Rose NR, Mackay IR. The Autoimmune Disease. Third Edition San Diego, CA: Academic Press; 1998 [Google Scholar]
- 9.Nakayama H, Shimao S, Hamamoto T, et al. Neutrophilic dermatosis of the face associated with aortitis syndrome and Hashimoto’s thyroiditis. Acta Derm Venereol 1993;73:380–1 [DOI] [PubMed] [Google Scholar]
- 10.Medeiros S, Santos R, Carneiro V, et al. Sweet syndrome associated with Hashimoto thyroiditis. Dermatol Online J 2008;14:10. [PubMed] [Google Scholar]
- 11.Magri F, Gabellieri E, Sorrentino AR, et al. Sweet’s syndrome and thyroid diseases: is there a link? Endocrine Abstracts 2006;11:64 [Google Scholar]
- 12.Kalmus Y, Kovatz S, Shilo L, et al. Sweet’s syndrome and subacute thyroiditis. Postgrad Med J 2000;76:229–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc 1995;70:234–40 [DOI] [PubMed] [Google Scholar]