Abstract
The authors describe a case of episodic acute pneumonitis occurring after repeated doses of bortezomib, a drug being used increasingly to treat multiple myeloma. The delay in presentation each time with fever, dyspnoea, hypoxia and interstitial radiological changes contributed to under-recognition of this complication, which has so far been described in only a handful of patients, but the pattern seen may offer clues to pathogenesis and potential treatments. This case highlights the need for both physicians and patients consenting to bortezomib treatment to be more aware of this potentially fatal adverse effect.
Background
Bortezomib (velcade) is a proteasome inhibitor used increasingly since 2006 for the treatment of multiple myeloma in patients who have received at least one prior therapy1 and now also as first line treatment in patients with renal failure at presentation. Common adverse effects of bortezomib are fatigue, gastrointestinal symptoms, thrombocytopenia and neuropathy.2
There are only seven cases described in the literature of acute pneumonitis following this treatment.3–6 This is the first case in which multiple discrete episodes of pneumonitis were seen, each occurring with a delay after administration of the bortezomib dose. This delay, along with the common constellation of symptoms, contributed to mistaken diagnoses of pulmonary oedema or infection, which suggests that bortezomib-induced pneumonitis may be under-reported.
In previous cases, this complication has been treated with systemic steroids with variable degrees of success. To increase chemotherapeutic efficacy, the latest bortezomib protocols followed here advocate concurrent steroid administration,2 yet pneumonitis occurred despite this. There is, therefore, a need to find other treatment options for this complication, and we highlight that co-trimoxazole (trimethoprim/sulphamethoxazole) may have had a protective effect when co-administered with one of the doses in this case.
Case presentation
A Caucasian male smoker in his mid-50s presented with renal failure due to a relapse of multiple myeloma, 3 years after first line therapy. He was retreated with bortezomib and dexamethasone according to the Clinical Response and Efficacy Study of bortezomib in the Treatment of refractory myeloma trial protocol,2 receiving five doses (1.25 courses) of 2.1 mg intravenous bortezomib. Initial pulsed steroids (dexamethasone 40 mg daily) were reduced to 20 mg daily for 2 days in association with bortezomib doses four and five due to suspected infection.
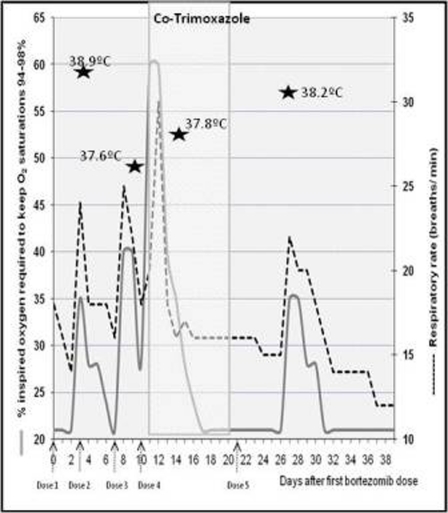
He developed four episodes of hypoxia, fever and radiographic interstitial changes, occurring with a delay of 3–6 days after all but the fourth bortezomib dose. Each episode lasted between 3 and 6 days (figure 1).
Figure 1.
Relationship of respiratory signs to the timing of bortezomib dose administration. Fevers of above 37.5°C are shown on the days on which they occurred.
In each episode he had cough and dyspnoea, with wheeze and bi-basal crackles on auscultation. Oxygen saturation on air dropped to 83–89% from his baseline of 95%, thus increasing his inspired oxygen requirements.
Investigations
Blood tests showed normal white cell and platelet counts; C-reactive protein remained at a baseline of 50–60 mg/l.
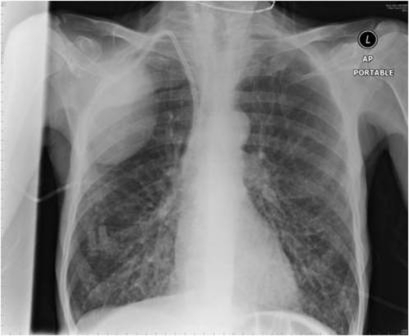
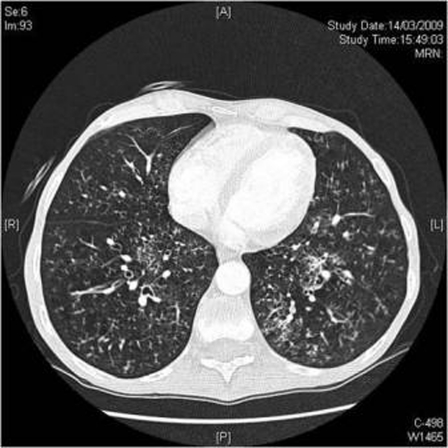
Serial chest radiographs showed episodic development and resolution of bi-basal reticular shadowing and pleural effusions (figure 2). Four days after the third bortezomib dose, CT pulmonary angiogram showed extensive nodular interstitial shadowing in both lungs (figure 3).
Figure 2.
Plain chest radiograph showing bi-basal reticular shadows. A haematoma sustained during dialysis line insertion is also seen.
Figure 3.
CT from day 11 showing bilateral interstitial nodules and ‘tree-in-bud’ peribronchial thickening, after resolution of a pleural effusion on the right.
Differential diagnosis
This common pattern of presentation is seen in cardiogenic pulmonary oedema and bacterial pneumonia, with or without bronchospasm.
Treatment
The patient did not respond to empirical piperacillin/tazobactam, bronchodilators or diuretics. Co-trimoxazole (960 mg twice daily intravenously) was initiated following the third and most severe episode for suspected Pneumocystis jiroveci pneumonia, although PCR assays for pneumocystis infection were subsequently negative. The co-trimoxazole course coincided with the fourth dose of bortezomib, the only dose not followed by a delayed acute pneumonitis.
Outcome and follow-up
The patient’s myeloma markers indicated a good response to bortezomib, but the drug was discontinued after the fifth dose. He recovered to his baseline respiratory function with a normal chest radiograph, but unfortunately 28 days later, developed clostridium difficile sepsis and died.
Discussion
The clinical and radiological features of pneumonitis occurring here after bortezomib treatment are similar to those in the seven previously described cases.3–6 However, this is the first case of repeated discrete episodes of pneumonitis, which we have demonstrated by tracking the patient’s respiratory rate, oxygen requirements and temperature in relation to his bortezomib doses. We highlight that it is easy to miss this diagnosis as the symptoms are both easily mistaken for pulmonary oedema or infection, and only occur several days after the drug is given.
This delay in manifestation of symptoms may also help to identify the mechanism by which the lung injury occurs. Previously described cases have shown a similar delay in development of pulmonary complications occurring between 3 to 8 days after bortezomib administration.3 During phase two trials of this drug, thrombocytopenia occurred in dose-related cycles in which platelet counts started to fall 4–8 days after administration, reaching a nadir around day 112.
Bortezomib given intravenously disappears rapidly from the vascular compartment and has a biological half-life of approximately 24 h. Our patient developed his most severe symptoms after his third dose, which may be a reflection of accumulated drug. There was a drug-free period between the fourth and fifth doses which may account for the milder pneumonitis which occurred finally.
The cyclical pattern of nadir and recovery suggests a reversible element to this pneumonitis. In six of the seven previously described cases,3–6 high dose steroids (1000 mg methylprednisolone) given at the time of respiratory decompensation had some benefit, although most did require invasive ventilation. The case we describe here differs by the use of steroids given concomitantly with the bortezomib dose. Although our patient had steroids earlier and in lower doses, he still developed acute pneumonitis, albeit to a milder degree.
It is difficult to comment on the significance of the lower steroid doses given with the final two bortezomib infusions, as it is confounded by the drug-free period between cycles and the possible cumulative effects of treatments. However it is interesting that the only bortezomib dose not to be followed by respiratory decompensation was that given during the course of co-trimoxazole. Small studies have suggested that co-trimoxazole may have a role in treating interstitial lung disease,7 and trials are ongoing to clarify this.
In conclusion, bortezomib-induced lung injury may be under-recognised due to the delay in onset of symptoms after the drug is given and all patients, including those given early steroids, should be monitored carefully for this possible severe adverse event.
Learning points.
-
▶
Bortezomib-induced pneumonitis may be more common than we realise.
-
▶
Further steroids may be required to treat pneumonitis, even if already given concurrently with the bortezomib dose.
-
▶
Co-trimoxazole may have a role in treating this interstitial pneumonitis.
Footnotes
Competing interests None.
Patient consent Not obtained.
References
- 1.Kane RC, Farrell AT, Sridhara R, et al. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res 2006;12:2955–60 [DOI] [PubMed] [Google Scholar]
- 2.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 2004;127:165–72 [DOI] [PubMed] [Google Scholar]
- 3.Miyakoshi S, Kami M, Yuji K, et al. Severe pulmonary complications in Japanese patients after bortezomib treatment for refractory multiple myeloma. Blood 2006;107:3492–4 [DOI] [PubMed] [Google Scholar]
- 4.Kang W, Kim JS, Cho SH, et al. Nonspecific interstitial pneumonitis after bortezomib and thalidomide treatment in a multiple myeloma patient. Yonsei Med J 2010;51:448–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zappasodi P, Dore R, Castagnola C, et al. Rapid response to high-dose steroids of severe bortezomib-related pulmonary complication in multiple myeloma. J Clin Oncol 2007;25:3380–1 [DOI] [PubMed] [Google Scholar]
- 6.Boyer JE, Batra RB, Ascensao JL, et al. Severe pulmonary complication after bortezomib treatment for multiple myeloma. Blood 2006;108:1113. [DOI] [PubMed] [Google Scholar]
- 7.Varney VA, Parnell HM, Salisbury DT, et al. A double blind randomised placebo controlled pilot study of oral co-trimoxazole in advanced fibrotic lung disease. Pulm Pharmacol Ther 2008;21:178–87 [DOI] [PubMed] [Google Scholar]