In the recent collection of articles in Perspectives on: Ion selectivity, several authors summarized the current state of knowledge on ion channel selectivity, predominated by experimental and theoretical approaches to understanding K+/Na+ discrimination of passive ion channels. Considerable insight has been gained by complementary analyses of crystal structures as well as by molecular simulations of models ranging from fully atomistic scenarios to simple “toys” that represent essential, localizable features of the overall phenomenon. On the theoretical side, much emphasis has been put on the thermodynamic aspect of selectivity, namely modeling and understanding the free energy change associated with K+/Na+ exchange in the selectivity filter of K+ channels. Numerous studies have convincingly shown (e.g., Dixit and Asthagiri, 2011; Roux et al., 2011; Varma et al., 2011) that thermodynamic selectivity results from a complex interplay between the chemical nature and number of coordinating ligands and the restricted inherent local atomic flexibility. However, both experimental (kinetic and structural analyses) and computational studies (multi-ion free energy surfaces) are directed toward understanding the ionic interplay and competition in the selectivity filter of K+ channels that give rise to complex kinetic features that are equally important signatures of ion selectivity (Alam and Jiang, 2011; Nimigean and Allen, 2011). As emphasized in the Perspective’s editorial (Andersen, 2011), further progress relies on converging thermodynamic (single ion) and kinetic (multi-ion) approaches into a unifying picture. The success of the latter depends strongly on the adequate treatment of physiological reference states. These are (in contrast to infinite dilution states, often implicitly assumed in free energy simulations) characterized by finite ion concentrations as needed for kinetic modeling, allowing for direct ion competition as a result of partitioning.
Theoretical progress can be made by methodologies that are capable of predicting ion concentration profiles. Here, we apply the 3-D reference interaction site model (RISM) integral equation theory (Beglov and Roux, 1997; Kovalenko and Hirata, 1998), adapted to the special case of a charged solute immersed in a salt solution (Kloss and Kast, 2008), to a truncated fragment comprising the selectivity filter of the KcsA channel in the conducting conformation (Zhou et al., 2001). The 3-D RISM approach is an approximate equilibrium solvation theory that retains the molecular detail of the solvent and that is therefore in principle capable of ion size discrimination on the basis of molecular force fields. Although it has well-known shortcomings in its established form, such as the neglect of intramolecular solute flexibility, it is nevertheless highly valuable for addressing problems that are difficult to study by molecular simulation, such as the partitioning and ion competition problem. We can turn the disadvantages into advantages by studying a sequence of progressively simpler, toy-like models that ultimately reduce the protein environment to the essential, minimal features that still exhibit one specific property, in our case the selectivity as measured by ratios of partition constants. By examining the solvent response of a simplified filter to pure monocationic K+ and Na+ solutions on one hand in comparison with the effect of a bicationic K+/Na+ mixture on the other hand, we are entitled to compare the relative trends of the reduction sequence, although the absolute numbers are of course only qualitatively meaningful. In particular, the 3-D RISM approach provides hints and answers to the following questions: (1) How different is the thermodynamic selectivity in comparison with a measure based on concentration ratios? (2) How important is the chemical composition of the residues for this measure? (3) Is the presence of more than one filter binding site relevant for the partitioning selectivity?
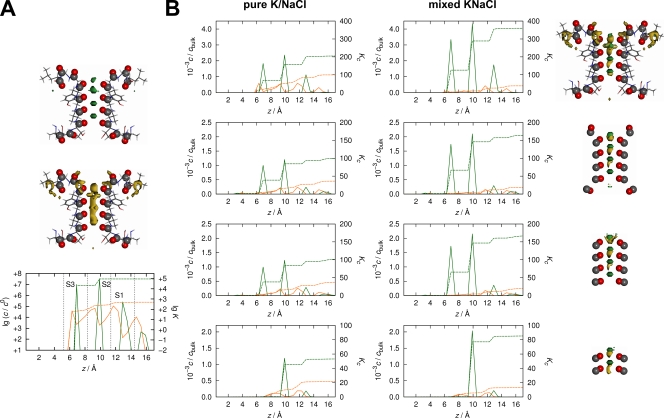
We apply two variants of the 3-D RISM theory, the finite concentration solute–solvent (uv) equations in conjunction with the hypernetted chain (HNC) closure and the infinite dilution HNC solute–solute (uu) equation between a molecule and a single ion site partner (Kloss and Kast, 2008). The resulting pair distribution functions are then used for computing uu thermodynamic binding constants (K) and uv mass action partition constants (Kc) by integration of axial concentration profiles within the pore (Kloss, 2007). We proceed by applying both theories to a truncated tetrameric selectivity filter fragment in the conducting conformation (Protein Data Bank accession no. 1K4C) comprising residues 73–81, followed by a sequence of uv calculations for drastically reduced filter models composed of mere carbonyl groups enclosing the binding sites S0–S4, S1–S3, and S2 only. The results are summarized in Fig. 1.
Figure 1.
K+ and Na+ concentration profiles and isocontour surfaces of ion distributions. K+, green; Na+, yellow. (A) top, K+; middle, Na+; bottom, superposition of ion concentration profiles. (A and B) Results at uu infinite dilution conditions (A) and at uv finite bulk concentration (B) are depicted. (B) Profiles and their axial integral functions are shown for pure 0.1 M KCl (water density of 0.03324 Å−3) and 0.1 M NaCl (0.03327 Å−3) solutions (left) and mixed 0.05:0.05 M K/NaCl solution (middle). (right) Depictions of the corresponding structures and isocontour surfaces in mixed solution. Calculations were performed at 298.15 K on 64 × 64 × 64 grids with 0.6-Å spacing according to protocols that were described previously (Tayefeh et al., 2007). Integrals were computed over the volume generated by HOLE software (Smart et al., 1996) of 91.8 Å3. The uu results in A and the uv data in the top row of B have been computed for the truncated high K+ structure (ATTVGYGDL), which is followed in B from the second row to the bottom by results for carbonyl groups enclosing binding sites S0–S4 (TTVGYG), for carbonyl groups enclosing binding sites S1–S3 (TVGY), and for carbonyl groups enclosing binding site S2 only (VG). The largest values of the integral functions reflect the equilibrium binding constants K (A) and partitioning constants Kc (B), the ratios of which can be assigned to the equilibrium constant (A) and mass action constants (B) for the reaction Na+–Chaq + K+aq→K+–Chaq + Na+aq. The infinite dilution result (A) is K = 186.4. The finite concentration results (B) are Kc = 2.1 (pure) and 9.5 (mixed) for the atomically detailed model (top), Kc = 2.8 (pure) and 8.8 (mixed) for the S0–S4 carbonyl group model (second row), Kc = 2.7 (pure) and 8.6 (mixed) for the S1–S3 model (third row), and Kc = 2.5 (pure) and 6.5 (mixed) for the S2 model (bottom row). Isocontour values were chosen as (uu) gK+ = gNa+ = 800 (A) and (uv) gK+ = gNa+ = 80 (B). Throughout, only two opposing subunits are shown. Structural images were created with MOLCAD software (MOLCAD GmbH).
Examining the concentration profiles, we find close correspondence with other studies. The peaks indicating a large probability of finding an ion at the respective position along the filter axis show the typical eightfold K+ coordination (Zhou et al., 2001; Alam and Jiang, 2011) as well as preferential location of Na+ between K+ binding sites in the carbonyl planes (Alam and Jiang, 2011; Nimigean and Allen, 2011). The ratio of K+ and Na+ binding constants from uu calculations translates into a free energy difference for the Na+→K+ exchange reaction of −3.1 kcal mol−1 (K = 186.4), which is fairly in line with simulation data (Roux et al., 2011).
More interesting are the uv results in pure monocationic 0.1 M KCl and NaCl solution in comparison with 0.05:0.05 M mixed K/NaCl. We find basically identical coordination geometries as before and irrespective of the level of reduction, whereas the selectivity measured by the Kc ratios in mixed solution differ drastically from the infinite dilution reference state, maximally reaching 9.5. This value compares well with early single-channel data and flux measurements on KcsA (Cuello et al., 1998; Meuser et al., 1999). Although this ratio stays basically the same throughout the multisite models (though absolute numbers change), it drops to some extent if only S2 is left. In contrast, the Kc ratios in monocationic solutions remain unaffected for all stages of reduction.
In summary, the relative trends observed suggest the following answers to the questions above: (1) Taking finite concentrations into account has massive impact on selectivity in comparison with infinite dilution. (2) The chemical composition of the environment plays a minor role in the partitioning selectivity as long as channels with similar average filter geometry and flexibility are compared. (3) Ionic competition emerging from in-plane Na+ displacement by adjacent in-cage K+ coordination is an important ingredient for understanding selectivity. This means in particular that only a multi (at least two)-site filter motif is fully capable of exhibiting K+/Na+ selectivity, which is in line with structural observations (Alam and Jiang, 2011). This emphasizes the importance of kinetics and the impact of competition effects for understanding the selectivity phenomenon fully.
Acknowledgments
Olaf S. Andersen served as editor.
References
- Alam A., Jiang Y. 2011. Structural studies of ion selectivity in tetrameric cation channels. J. Gen. Physiol. 137:397–403 10.1085/jgp.201010546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O.S. 2011. Perspectives on: Ion selectivity. J. Gen. Physiol. 137:393–395 10.1085/jgp.201110651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglov D., Roux B. 1997. An integral equation to describe the solvation of polar molecules in liquid water. J. Phys. Chem. B. 101:7821–7826 10.1021/jp971083h [DOI] [Google Scholar]
- Cuello L.G., Romero J.G., Cortes D.M., Perozo E. 1998. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry. 37:3229–3236 10.1021/bi972997x [DOI] [PubMed] [Google Scholar]
- Dixit P.D., Asthagiri D. 2011. Thermodynamics of ion selectivity in the KcsA K+ channel. J. Gen. Physiol. 137:427–433 10.1085/jgp.201010533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss T. 2007. Quantitative Berechnung thermodynamischer Observablen mittels dreidimensionaler Integralgleichungstheorien. PhD thesis. Technische Universität Darmstadt, Darmstadt, Germany: 108 pp [Google Scholar]
- Kloss T., Kast S.M. 2008. Treatment of charged solutes in three-dimensional integral equation theory. J. Chem. Phys. 128:134505 10.1063/1.2841967 [DOI] [PubMed] [Google Scholar]
- Kovalenko A., Hirata F. 1998. Three-dimensional density profiles of water in contact with a solute of arbitrary shape: a RISM approach. Chem. Phys. Lett. 290:237–244 10.1016/S0009-2614(98)00471-0 [DOI] [Google Scholar]
- Meuser D., Splitt H., Wagner R., Schrempf H. 1999. Exploring the open pore of the potassium channel from Streptomyces lividans. FEBS Lett. 462:447–452 10.1016/S0014-5793(99)01579-3 [DOI] [PubMed] [Google Scholar]
- Nimigean C.M., Allen T.W. 2011. Origins of ion selectivity in potassium channels from the perspective of channel block. J. Gen. Physiol. 137:405–413 10.1085/jgp.201010551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Bernèche S., Egwolf B., Lev B., Noskov S.Y., Rowley C.N., Yu H. 2011. Ion selectivity in channels and transporters. J. Gen. Physiol. 137:415–426 10.1085/jgp.201010577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart O.S., Neduvelil J.G., Wang X., Wallace B.A., Sansom M.S.P. 1996. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14:354–360: 376 10.1016/S0263-7855(97)00009-X [DOI] [PubMed] [Google Scholar]
- Tayefeh S., Kloss T., Thiel G., Hertel B., Moroni A., Kast S.M. 2007. Molecular dynamics simulation of the cytosolic mouth in Kcv-type potassium channels. Biochemistry. 46:4826–4839 10.1021/bi602468r [DOI] [PubMed] [Google Scholar]
- Varma S., Rogers D.M., Pratt L.R., Rempe S.B. 2011. Perspectives on: Ion selectivity. Design principles for K+ selectivity in membrane transport. J. Gen. Physiol. 137:479–488 10.1085/jgp.201010579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.F., Morais-Cabral J.H., Kaufman A., MacKinnon R. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 414:43–48 10.1038/35102009 [DOI] [PubMed] [Google Scholar]