NIK expression in thymic dendritic cells is required for the development of effector T cells and their ability to promote EAE.
Abstract
The canonical NF-κB pathway is a driving force for virtually all aspects of inflammation. Conversely, the role of the noncanonical NF-κB pathway and its central mediator NF-κB–inducing kinase (NIK) remains poorly defined. NIK has been proposed to be involved in the formation of TH17 cells, and its absence in TH cells renders them incapable of inducing autoimmune responses, suggesting a T cell–intrinsic role for NIK. Upon systematic analysis of NIK function in cell-mediated immunity, we found that NIK signaling is dispensable within CD4+ T cells but played a pivotal role in dendritic cells (DCs). We discovered that NIK signaling is required in DCs to deliver co-stimulatory signals to CD4+ T cells and that DC-restricted expression of NIK is sufficient to restore TH1 and TH17 responses as well as cell-mediated immunity in NIK−/− mice. When CD4+ T cells developed in the absence of NIK-sufficient DCs, they were rendered anergic. Reintroduction of NIK into DCs allowed developing NIK−/− CD4+ T cells to become functional effector populations and restored the development of autoimmune disease. Therefore, our data suggest that a population of thymic DCs requires NIK to shape the formation of most αβ CD4+ T effector lineages during early development.
Noncanonical NF-κB signaling is a prerequisite for the anlage of secondary lymphoid tissues (SLTs). Mice carrying lesions in elements of this pathway are often alymphoplastic (absence of lymph nodes) and lack the specific lymphoid organization in spleen and thymus (Weih and Caamaño, 2003). The notion that cell-mediated immunity commences exclusively in SLTs provides a tangible explanation for the immunodeficiency of alymphoplastic mice. Because of their inability to generate germinal centers, alymphoplastic mice such as lymphotoxin-β receptor–deficient (LTβR−/−), LTα−/−, NIKaly/aly, and NIK−/− animals are all defective in immunoglobulin class-switch (Miyawaki et al., 1994; Banks et al., 1995; Shinkura et al., 1996; Fütterer et al., 1998). However, T cell responses and cell-mediated immunity are severely reduced in NIKaly/aly mice when compared with other alymphoplastic mice (Greter et al., 2009).
NF-κB–inducing kinase (NIK) is a key mediator of the noncanonical NF-κB pathway (Sun and Ley, 2008). It transduces signals from distinct members of the TNFR family and induces via phosphorylation of IκB-specific kinase α (IKK-α) the cleavage of p100-RelB to p52-RelB, which then translocates as heterodimer into the nucleus (Senftleben et al., 2001; Xiao et al., 2004). The activity of NIK is tightly regulated on several levels, generally using the TNFR-associated factors 2/3 (TRAF2/3), cytosolic inhibitor of apoptosis 1 (cIAP1), and cIAP2 (Varfolomeev et al., 2007; Vince et al., 2007), which prevent basal activation of this pathway. The signal-induced activation of the noncanonical pathway results in the degradation of TRAF2 and TRAF3 and thus in the stabilization of NIK protein (Liao et al., 2004). NIKaly/aly mice contain a point mutation that is located in the C-terminal region of NIK and is responsible for the physical interaction with the upstream TRAFs and IKK-α (Shinkura et al., 1999). Thus, the levels of nuclear p52 in several tissues and cell types of NIKaly/aly mice are virtually ablated (Xiao et al., 2001b).
There is evidence that noncanonical NF-κB signaling within hematopoietic cells is involved in several human diseases such as lymphoid cancers, including EBV-positive Hodgkin’s lymphoma and HTLV-1–transformed T cell lymphoma (Xiao et al., 2001a; Atkinson et al., 2003; Eliopoulos et al., 2003). Also, mutations in NIK have been correlated with the development of multiple myeloma (Annunziata et al., 2007). Thus, NIK poses an attractive pharmacological target for the treatment of a variety of diseases (Staudt, 2010), and it is thus important that its role and function within the immune system are resolved.
For many years, it has been believed that the noncanonical NF-κB pathway is preferably activated by ligands either important for the lymphoid organogenesis (through LTβR) or in B cell responses (through CD40 and BAFF-R; Youssef and Steinman, 2006). However, it has become increasingly evident that the noncanonical NF-κB pathway can be triggered by many different ligands such as RANK, LIGHT, TWEAK, CD70, and CD28 (Darnay et al., 1999; Yin et al., 2001; Ramakrishnan et al., 2004; Sánchez-Valdepeñas et al., 2006; Nadiminty et al., 2007; Bhattacharyya et al., 2010; Maruyama et al., 2010; Sanz et al., 2010). Furthermore, it was reported that NIK can also signal into the classical NF-κB pathway (Ramakrishnan et al., 2004; Zarnegar et al., 2008; Staudt, 2010; Sasaki et al., 2011). The vast variety of triggers suggests that noncanonical NF-κB signaling is not exclusively active in the development of SLTs but also plays a role in B and T cell responses as well as in the function of APCs. NIK-deficient T cells have been shown to be defective in secretion of IL-2 and GM-CSF (Sánchez-Valdepeñas et al., 2006). They are further limited in their proliferative capacity as well as TH17 differentiation and fail to become pathogenic in experimental autoimmune encephalomyelitis (EAE), graft versus host disease, and in models of transplantation (Yamada et al., 2000; Matsumoto et al., 2002; Ishimaru et al., 2006; Sánchez-Valdepeñas et al., 2006, 2010; Greter et al., 2009; Jin et al., 2009).
Apart from the involvement in T cell function, alternative NF-κB signaling has been controversially discussed in the induction of central tolerance by regulating the development and function of Aire+ medullary thymic epithelial cells (mTECs; Chin et al., 2003; Kajiura et al., 2004; Venanzi et al., 2007; Akiyama et al., 2008). The impaired mTEC function in NIKaly/aly or RelB-deficient mice provides a potential explanation for the reported autoimmune phenotypes. However, the reason for disturbed cell-mediated immunity in NIKaly/aly animals remains poorly understood.
Even though the immunodeficiency in NIKaly/aly mice was thought to be caused by their alymphoplasia, we have previously reported that the defect in cell-mediated immunity in NIKaly/aly mice is not connected to their lack of SLTs but that NIK activity is critical for cellular immune function (Greter et al., 2009). Cell-mediated immunity could be induced in the total absence of SLTs when splenectomized NIKaly/aly mice were reconstituted with a WT hematopoietic system. However, cell-mediated immunity could not be induced in mice featuring normal SLTs but carrying the NIKaly/aly lesion only in hematopoietic cells (Greter et al., 2009). Although a critical function for NIK has been suggested specifically in T cells by several studies (Yamada et al., 2000; Matsumoto et al., 2002; Ishimaru et al., 2006; Sánchez-Valdepeñas et al., 2006; Jin et al., 2009), the data presented in this study suggest that the loss of T cell function in NIKaly/aly mutants was not T cell intrinsic, but rather resulted from a defect in hematopoietic accessory leukocytes, namely DCs.
We show in this study that NIK-lesioned T cells were indeed capable of secreting effector cytokines and acquiring full effector functions, depending on the thymic environment in which they developed. Instead of a T cell–intrinsic lesion, loss of NIK in thymic DCs imprinted a long-lasting halt in T cell effector function. However, if NIK-deficient T cells matured within a thymic environment that hosts NIK, T cells gained proper effector functions regardless of their NIK deficiency. Thus, we propose that thymic DCs, which so far have only been implemented in negative selection (Gallegos and Bevan, 2004) and the induction of natural regulatory T cells (nTreg cells; Proietto et al., 2009), are capable of imprinting subsequent T cell effector function onto developing αβ thymocytes. This ability of thymic DCs is strictly dependent on NIK.
RESULTS
Loss of NIK function results in reduced T cell proliferation, differentiation, and production of effector cytokines
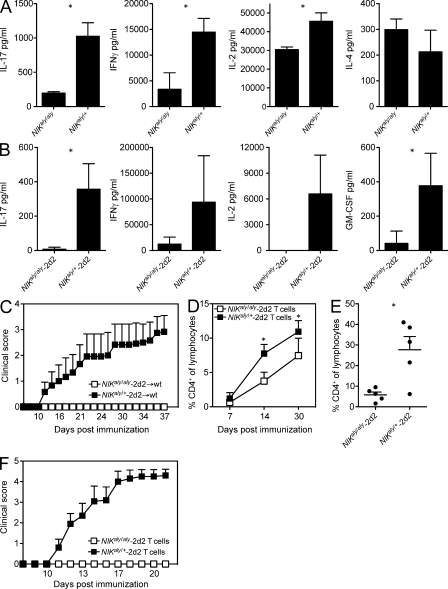
We have recently reported that NIKaly/aly mice are resistant to the induction of EAE because of the loss of function of NIK within the hematopoietic compartment and more specifically because of a defect in T cell priming, independent of the lack of SLTs (Greter et al., 2009). Furthermore, NIKaly/aly T cells have been reported to be defective in proliferation and secretion of IL-17, IL-2, and GM-CSF (Matsumoto et al., 2002; Sánchez-Valdepeñas et al., 2006; Jin et al., 2009). In line with these previous studies, we observed that in vitro, polyclonally activated NIKaly/aly CD4+ T cells produced lower amounts of effector cytokines (IL-2, IFN-γ, and IL-17) than heterozygous NIKaly/+ controls, whereas the production of IL-4 was unaffected (Fig. 1 A). This suggests a T cell intrinsic impairment of polarization conferred by the ablation of NIK signaling. To further investigate the requirement of NIK for antigen-specific T cell activation, we crossed NIKaly/aly mice with TCR transgenic 2d2 mice, in which the TCR recognizes the immunodominant epitope of the myelin oligodendrocyte glycoprotein (MOG35–55). As expected, NIKaly/aly-2d2 T cells also failed to secrete effector cytokines upon encountering their cognate antigen (Fig. 1 B). To exclude the possibility that the observed defects were caused by the developmental malformations of SLTs in NIKaly/aly mice, we generated BM chimeric mice (BMCs) in which WT mice were reconstituted with hematopoietic stem cells from either NIKaly/aly-2d2 or NIKaly/+-2d2 mice. We found that even if most of the CD4+ T cells carry the cognate antigen-specific TCR, NIKaly/aly-2d2 → WT BMCs retained their EAE resistance upon MOG35–55/CFA immunization (Fig. 1 C), emphasizing the critical role of NIK signaling for the development of T cell–mediated autoimmune responses.
Figure 1.
NIKaly/aly mice are resistant to EAE because of the lack of NIK signaling in immune cells. (A) CD4+ T cells of NIKaly/aly or NIKaly/+ mice were stimulated in vitro with plate-bound α-CD3/α-CD28 for 48 h. Cytokine secretion was analyzed by ELISA. (B) Splenocytes from NIKaly/aly-2d2 or NIKaly/+-2d2 mice were stimulated in vitro with MOG35–55 and α-CD28 for 48 h. Cytokine secretion was analyzed by ELISA. (C) NIKaly/aly-2d2 → WT or NIKaly/+-2d2 → WT BMCs were immunized with MOG35–55/CFA and monitored daily for clinical signs of EAE (n = 6). (D–F) CD4+ T cells from NIKaly/aly-2d2 → WT or NIKaly/+-2d2 → WT BMCs were transferred into Rag1−/− mice. Homeostatic expansion was observed by analyzing the percentage of CD4+ T cells within total lymphocytes in the peripheral blood (D). 30 d after adoptive CD4+ T cell transfer, Rag1−/− mice were immunized with MOG35–55/CFA and observed for antigen-driven expansion by FACS analysis of blood at day 7 after immunization (E) and clinical signs of EAE (n = 6; F). Each graph shows one representative of three independent experiments. (A, B, D, and E) Error bars indicate SD. *, P ≤ 0.05. (C and F) Error bars indicate SEM.
Because T cells from NIKaly/aly-2d2 → WT BMCs failed to acquire pathogenic properties, we addressed their behavior in antigen-independent homeostatic expansion in lymphopenic Rag1−/− mice. After adoptive transfer of CD4+ T cells from NIKaly/aly-2d2 → WT BMCs into Rag1−/− mice, we observed a reduction in homeostatic expansion when compared with CD4+ T cells from NIKaly/+-2d2 → WT BMCs (Fig. 1 D). Upon immunization of those mice with MOG35–55/CFA, NIKaly/aly-2d2 T cells further failed to respond to their cognate antigen, whereas control NIKaly/+-2d2 T cells strongly expanded (Fig. 1 E). In addition, Rag1−/− mice reconstituted with T cells from NIKaly/aly-2d2 → WT BMCs remained completely resistant to EAE, suggesting that NIKaly/aly-2d2 T cells cannot be primed by NIK-sufficient accessory cells (Fig. 1 F). Collectively, the data support the notion that NIK deficiency indeed leads to a T cell–intrinsic lesion.
Loss of NIK results in a primary APC defect
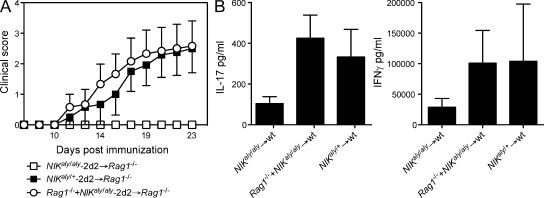
Our data and a previous study (Jin et al., 2009) support a T cell–intrinsic role of NIK in cell-mediated immunity. However, we further aimed to identify the role of NIK in the accessory cell compartment. BMCs were generated by transferring a 4:1 mixture of Rag1−/− and NIKaly/aly-2d2 BM into Rag1−/− mice. In those mice, NIKaly/aly-2d2 T cell progenitors are developing within an NIK-sufficient accessory cell environment. Surprisingly, Rag1−/− + NIKaly/aly-2d2 → Rag1−/− BMCs developed EAE comparable with NIKaly/+-2d2 → Rag1−/− BMCs (Fig. 2 A). This finding demonstrates that NIK plays a vital role in hematopoietic accessory cells rather than in T cells to develop autoimmune inflammation. Complementing this result, polyclonally in vitro activated CD4+ T cells from Rag1−/− + NIKaly/aly → WT BMCs were rescued in their ability to secrete IL-17 and IFN-γ (Fig. 2 B), suggesting that the production of effector cytokines by T cells requires intact NIK signaling in the accessory cell compartment but not the T cell compartment.
Figure 2.
Loss of NIK signaling in accessory cells determines the fate of T cells early in development. (A) Lethally irradiated Rag1−/− mice were reconstituted with BM of NIKaly/aly-2d2, NIKaly/+-2d2, or a 4:1 mixture of Rag1−/− and NIKaly/aly-2d2 mice. 6 wk after reconstitution, BMCs were immunized with MOG35–55/CFA and observed for clinical signs of EAE (n = 6). Error bars indicate SEM. (B) Lethally irradiated WT mice were reconstituted with BM of NIKaly/aly, NIKaly/+, or a 4:1 mixture of Rag1−/− and NIKaly/aly mice. 6 wk after reconstitution, CD4+ T cells were isolated and stimulated with plate-bound α-CD3/α-CD28. Supernatants were analyzed by ELISA. Each graph shows one representative of three independent experiments. Error bars indicate SD.
DC maturation and co-stimulation are dependent on NIK signaling
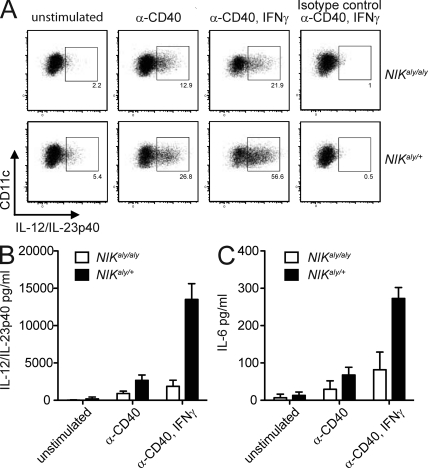
The most prominent accessory cells involved in the induction of cell-mediated immunity and antigen presentation are DCs. It has been reported that in vitro NIKaly/aly DCs have defects in the expression of CD80, CD86, and MHCII as well as in antigen presentation and their ability to drive T cell expansion (Garceau et al., 2000; Tamura et al., 2006; Lind et al., 2008). Also the ability of T cells to secrete effector cytokines is largely dependent on the capacity of APCs to provide T cell instructive cytokines. In particular, the cytokines IL-12, IL-23, and IL-6 have a major impact on the polarization of effector T cells. We investigated the ability of splenic NIKaly/aly DCs to secrete these factors after stimulation with anti-CD40, thereby mimicking T cell–APC interactions. Interestingly, activated NIKaly/aly DCs secreted significantly lower levels of the proinflammatory cytokine subunit IL-12/IL-23p40 (Fig. 3, A and B; and Fig. S1, A and B). We could further observe a strong reduction of the transcripts for IL-12p35 and IL-23p19 (Fig. S1 C) and protein levels of IL-6 (Fig. 3 C). These data suggest that NIK signaling in DCs upon DC–T cell interaction via CD40 is critical for the capacity of DCs to secrete T cell-instructive cytokines.
Figure 3.
NIKaly/aly DCs are restrained in secretion of proinflammatory cytokines. (A–C) Splenic NIKaly/aly or control DCs were stimulated in vitro with α-CD40 and IFN-γ for 24 h. GolgiPlug was added for the last 4 h of culture. Intracellular anti–IL-12/IL-23p40 FACS staining was performed together with cell surface staining for CD11c (A). Supernatants were analyzed by ELISA for IL-12/IL-23p40 (B) and IL-6 (C). Each graph shows one representative of three independent experiments. (B and C) Error bars indicate SD.
NIKaly/aly DCs are restrained in T cell priming and fail to induce EAE
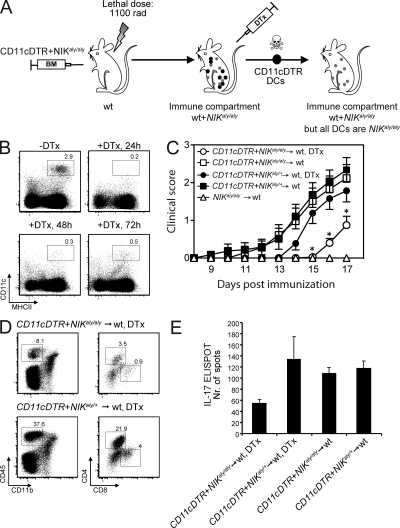
To verify the reduced priming capacity of NIKaly/aly DCs in vivo, we established a model based on diphtheria toxin (DTx)–mediated cell ablation. We created mice with an immune compartment containing both NIK-deficient and -sufficient T cells, whereas the vast majority of DCs carry the mutated NIKaly/aly protein. To do so, a 1:1 mixture of CD11cDTR and NIKaly/aly BM was transferred into irradiated WT recipients (Fig. 4 A). Upon injection of DTx, DCs of CD11cDTR origin (NIK+/+) were depleted, whereas mutant NIKaly/aly DCs were retained. The efficiency of DC ablation in CD11cDTR mice was >90% (Fig. 4 B). We observed a significant delay in disease onset (Fig. 4 C) with decreased central nervous system (CNS) infiltration of T cells (Fig. 4 D), and particularly IL-17–producing T cells (Fig. 4 E), in DTx-treated CD11cDTR + NIKaly/aly → WT BMCs compared with DTx-treated control CD11cDTR + NIKaly/+ → WT BMCs. This finding strongly supports our previous in vitro (Fig. 3) and in vivo (Fig. 2 A) data and demonstrates that NIK signaling in DCs is critical for their ability to instruct T cell polarization and effector function.
Figure 4.
Absence of NIK signaling in DCs significantly delays EAE. (A) Lethally irradiated WT mice were reconstituted with a 1:1 mixture of CD11cDTR and NIKaly/aly or CD11cDTR and NIKaly/+ BM. Upon i.p. injection of DTx, CD11cDTR (NIK+/+) DCs are depleted, whereas NIKaly/aly or NIKaly/+ DCs remain. All other immune cells are still present as NIK+/+. (B) Efficiency of DC depletion in DTx-treated CD11cDTR mice was analyzed by flow cytometry. (C) CD11cDTR + NIKaly/aly → WT and CD11cDTR + NIKaly/+ → WT BMCs were immunized with MOG35–55/CFA and treated with DTx every second day. Mice were observed for clinical signs of EAE. Three individual experiments were pooled (n ≥ 15/group). Error bars indicate SEM. *, P ≤ 0.05. (D) FACS analysis of CNS-infiltrating lymphocytes in MOG35–55/CFA-immunized DTx-treated CD11cDTR + NIKaly/aly → WT and CD11cDTR + NIKaly/+ → WT BMCs at peak disease (17 d after immunization). Several brains and spinal cords of each experimental group were pooled. (E) Splenocytes were isolated from MOG35–55/CFA-immunized DTx-treated/untreated CD11cDTR + NIKaly/aly → WT and CD11cDTR + NIKaly/+ → WT BMCs and rechallenged in vitro with 50 µg/ml MOG35–55 peptide, followed by IL-17 ELISPOT. Triplicates of pooled splenocytes of one experiment are shown (n ≥ 5/group). Error bars indicate SD.
Restoration of NIK signaling in DCs is sufficient to generate pathogenic T cells
To ascertain a primary role of NIK signaling in the DC compartment, we generated mice in which NIK expression is restricted to DCs. R26StopFLNIKWT mice express NIKWT preceded by a loxP-flanked neoR-Stop cassette and followed by an Frt-flanked IRES-eGFP within the ubiquitously active ROSA26 locus (Sasaki et al., 2008). Upon crossing to CD11c-cre mice, the neoR-Stop is excised, and NIKWT will be expressed in CD11c+ cells, which are mainly DCs (these mice are hereafter called DCNIK). Upon further breeding those animals onto the NIK−/− background, we generated mice that express NIKWT in DCs, whereas other cells and tissues lack the ability to express NIK. To verify the cell type–specific targeting of the NIKWT transgene, we analyzed GFP expression in different immune compartments, which confirmed the transgene expression in DCs (Fig. S2 A).
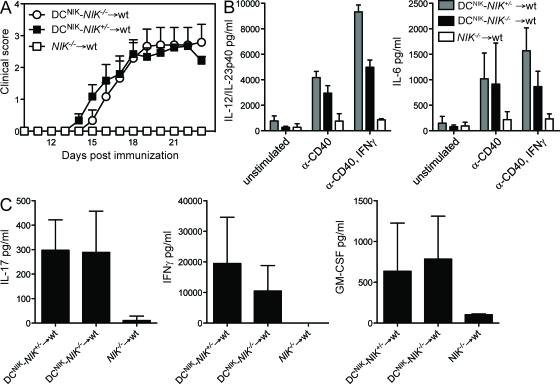
To manipulate the expression of NIK only within the hematopoietic compartment and to provide a normal lymphoid organ structure, we again generated BMCs by transferring BM of either DCNIK-NIK−/− or control DCNIK-NIK+/− and NIK−/− mice into lethally irradiated WT mice. 6 wk after reconstitution, these BMCs were immunized with MOG35–55/CFA and observed for the development of clinical disease. Strikingly, DCNIK-NIK−/− → WT BMCs were fully susceptible to EAE, even though most T cells are NIK−/− (Fig. 5 A). Furthermore, the secretion of proinflammatory cytokines IL-12/IL-23p40 and IL-6 in DCs of DCNIK-NIK−/− → WT BMCs was largely restored (Fig. 5 B). Also, the restoration of NIKWT in DCs rescued the secretion of T effector cytokines IL-17, IFN-γ, and GM-CSF (Fig. 5 C) after antigen restimulation. These findings demonstrate that NIK signaling in DCs is sufficient to generate autoaggressive CD4+ T cells, even if these T cells themselves do not express functional NIK.
Figure 5.
Expression of NIK in DCs is sufficient to restore EAE susceptibility in NIK−/− mice. (A) DCNIK-NIK−/− → WT, DCNIK-NIK+/− → WT, and NIK−/− → WT BMCs were immunized with MOG35–55/CFA and observed for clinical signs of EAE. Shown is one representative of three independent experiments (n = 6). Error bars indicate SEM. (B) Splenic DCs were isolated from DCNIK-NIK−/− → WT and DCNIK-NIK+/− → WT BMCs and stimulated in vitro with α-CD40 and IFN-γ for 24 h. IL-12/IL-23p40 and IL-6 secretion was measured by ELISA. (C) Splenocytes of MOG35–55/CFA-immunized DCNIK-NIK−/− → WT and DCNIK-NIK+/− → WT BMCs were isolated at the peak of disease and restimulated in vitro with MOG35–55 for 48 h. Supernatants were analyzed by ELISA. (B and C) Each graph shows one representative of three independent experiments. Error bars indicate SD.
In addition to verifying the transgene expression in DCs, we thoroughly analyzed other leukocyte populations for inadvertent ectopic expression. Macrophages were found to be negative, and although microglia can express transient levels of CD11c upon activation, they can be excluded as effectors in this model because NIK−/− → WT BMCs have NIK-sufficient microglia but remained EAE resistant (Fig. 5 A). In DCNIK-NIK−/− → WT BMCs, we detected a minor percentage of GFP+CD4+ and CD8+ T cells (Fig. S2 A). To ensure that this population of NIK-expressing T cells does not contribute to the EAE susceptibility of DCNIK-NIK−/− → WT BMCs, we further bred TNIK-NIK−/− mice by crossing R26StopFLNIKWT mice to CD4-cre and NIK−/− mice. TNIK-NIK−/− → WT BMCs were, similar to NIK−/− → WT BMCs, completely EAE resistant (Fig. S2 B). Thus, the reintroduction of NIKWT into the DC pool fully restored EAE susceptibility.
Loss of NIK signaling critically impairs thymic DC function
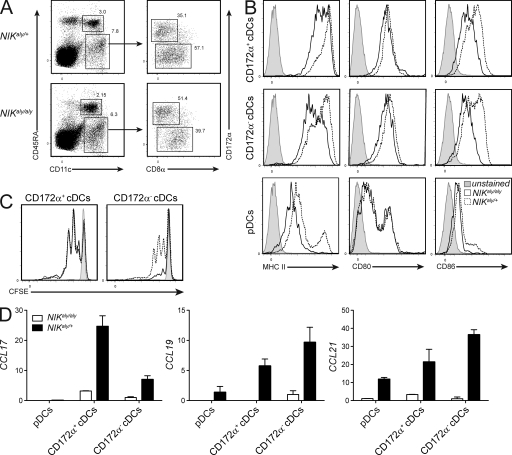
Our data thus far clearly showed that NIK signaling in DCs is critical for T effector function. However, if the restoration of NIK signaling in DCs alone reinstates T effector function, the fact that adoptively transferred mature NIKaly/aly T cells into NIK-sufficient recipients fail to acquire effector function represents a contradiction (Fig. 1). Thus, we hypothesized that NIK signaling in thymic DCs is required to enable developing thymocytes to exit the thymus as fully functional T cells. Therefore, the thymic DC compartment of NIKaly/aly and NIKaly/+ mice was analyzed in detail. All three thymic DC subsets (Wu and Shortman, 2005; Proietto et al., 2008a; Li et al., 2009), namely migratory and resident conventional DCs (cDCs; CD11c+CD172α+ and CD172α−, respectively) and plasmacytoid DCs (pDCs; CD11cintCD45RA+), were found in comparable numbers in NIKaly/aly and NIKaly/+ mice (Fig. 6 A). However, both cDCs and pDCs in NIKaly/aly mice expressed reduced levels of CD80 and CD86 and even more drastically MHCII (Fig. 6 B), with the strongest reduction in the resident DC subset. This indicates some degree of functional impairment of APC properties, which we further assessed by co-culturing NIKaly/aly and NIKaly/+ thymic DCs with 2d2 CD4+ single-positive (SP) thymocytes in the presence of MOG35–55. NIKaly/aly thymic resident DCs elicited reduced proliferation in 2d2 CD4+ SP thymocytes (Fig. 6 C).
Figure 6.
NIKaly/aly thymic DCs show reduced APC capacity. (A) Flow cytometric analysis of thymic DC subsets from NIKaly/aly and control animals. pDCs are CD11cintCD45RA+, thymic resident DCs are CD11c+CD172a−, and thymic migratory DCs are CD11c+CD172a+. Numbers in the plots indicate the percentage of the respective DC subset of the total thymic DC fraction. (B) Expression analysis of MHCII, CD80, and CD86 on thymic DC subsets. Dotted lines represent heterozygous control DCs, solid lines NIKaly/aly DCs, and gray histograms unstained controls. (C) Proliferation of CFSE-labeled 2d2 CD4+ SP thymocytes after 3 d of co-culture with thymic migratory (left) and resident (right) DCs in the presence of 10 µg/ml MOG35–55. Dotted lines represent CFSE profile after stimulation with heterozygous control DCs, solid lines show NIKaly/aly DCs, and gray histograms show unstimulated cells. (D) RNA of FACS-sorted thymic DC subsets from NIKaly/aly and NIKaly/+ mice was transcribed into cDNA and analyzed by qRT-PCR for expression of different chemokines. Shown is the fold change in expression level compared with CD172α− NIKaly/aly cDCs, which was set to 1. Data in all panels are representative of three independent experiments. Error bars indicate SD.
To further investigate the phenotypic properties of NIKaly/aly thymic DCs, we performed quantitative RT-PCR (qRT-PCR) analysis for various chemokine ligands and receptors involved in thymic DC function (Proietto et al., 2008a). NIKaly/aly thymic DCs revealed a strong reduction in the expression of CCL17, CCL19, and CCL21 (Fig. 6 D), which are crucial for the migration of developing thymocytes (Ueno et al., 2004; Proietto et al., 2008a). Furthermore, the analysis of chemokine receptors revealed an overall reduction in the levels of CCR2, CCR5, CCR6, and CCR7 but increased expression of CCR9 and TLR9 (Fig. S3). Collectively, we found that NIKaly/aly thymic DCs are phenotypically and functionally distinct from those in NIKaly/+ mice, suggesting a causative link between the altered function of thymic DCs and the subsequent loss of T effector function.
Restoration of NIK in DCs rescues Foxp3, RORγt, and Tbet expression in developing thymocytes
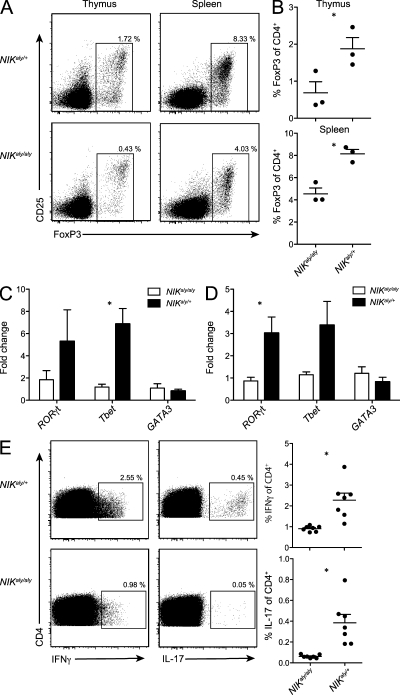
Thymic DCs have primarily been implicated in mediating negative selection (Gallegos and Bevan, 2004). However, there is increasing evidence that thymocyte development not only selects the T cell receptor repertoire but also imprints effector function onto thymic emigrants. In particular, NIK has been suggested to be involved in the expansion of CD25+CD4+ T cells (Lu et al., 2005; Tamura et al., 2006). We found a 50% reduction in FoxP3+ Treg cells in both developing and mature T cells (Fig. 7, A and B). Given the reduced effector cytokine expression of NIKaly/aly T cells, we speculated whether the observed decrease of natural occurring nTreg cells in NIKaly/aly thymi is the result of altered licensing of T cells during their development. Recently, thymic T cell lineage commitment has been expanded to other lineages including TH17 cells (Marks et al., 2009). Therefore, we analyzed the gene expression of lineage-specific transcription factors in CD4+ SP cells of NIKaly/aly thymi (Fig. 7 C) and spleens (Fig. 7 D) and found that in addition to Foxp3, the expression of RORγt and Tbet was decreased, whereas GATA3 was not affected. We further found that the percentage of IL-17– and IFN-γ–producing CD4+ SP thymocytes was strongly reduced in naive NIKaly/aly mice (Fig. 7 E). Interestingly, a very recent study described that RelB, which is one of the target molecules of NIK, is essential for LTβR-dependent thymic commitment of γδ T cells toward IL-17 production (Powolny-Budnicka et al., 2011). In line with this, our observations suggest that thymic commitment toward the TH1 or TH17 lineage might also occur for αβ T cells and that NIK signaling in thymic DCs is crucial for this process.
Figure 7.
NIKaly/aly mice show strongly reduced percentage of thymic committed nTreg cells and TH17 and TH1 cells. (A and B) NIKaly/aly and NIKaly/+ thymocytes and splenocytes were stained intracellularly for FoxP3 expression. Plots are gated on CD4+ CD8− cells (A). Shown are means of three mice each (B). (C and D) RNA of FACS-sorted SP CD4+ thymic (C) and splenic (D) T cells of NIKaly/aly and NIKaly/+ mice was transcribed into cDNA and analyzed by qRT-PCR for expression of RORγt, Tbet, and GATA3. Shown is fold change of expression levels compared with one of three NIKaly/aly samples, which was set to 1. Data are representative of three independent experiments (n = 3). (E) NIKaly/aly and NIKaly/+ CD4+ SP thymocytes of naive mice were stimulated in vitro with PMA and ionomycin and stained intracellularly for the secretion of IL-17 and IFN-γ. Data are pooled from two independent experiments (n = 7). (B–E) Error bars indicate SD. *, P ≤ 0.05.
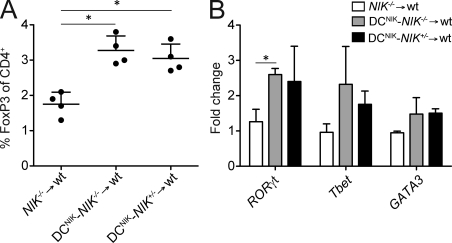
To verify that DC-restricted expression of NIKWT is capable of restoring early lineage commitment, we again generated DCNIK-NIK−/− → WT, DCNIK-NIK+/− → WT, and NIK−/− → WT BMCs and analyzed the expression of transcription factors in CD4+ SP thymocytes. As shown in Fig. 8 (A and B), DC-restricted NIKWT expression fully restored RORγt, Tbet, and also Foxp3 expression in CD4+ SP thymocytes.
Figure 8.
Restoration of NIK in DCs rescues Foxp3, RORγt, and Tbet expression in developing thymocytes. (A and B) DCNIK-NIK−/− → WT, DCNIK-NIK+/− → WT, and NIK−/− → WT BMCs were generated. Percentage of FoxP3+ cells in the thymi (A) and transcript levels of RORγt, Tbet, and GATA3 in FACS-sorted CD4+GFP− SP thymocytes (B) were analyzed. Shown is fold change of expression levels compared with one of three NIK−/− → WT samples, which was set to 1. Data are representative of two independent experiments (n ≥ 3). Error bars indicate SD. *, P ≤ 0.05.
DISCUSSION
NIK is widely held as a central mediator of noncanonical NF-κB signaling and the activation of NF-κB2. Indeed, both NF-κB2−/− and NIKaly/aly mice show impaired T and B cell responses, while displaying lymphocyte infiltration into various organs similar to that of Aire−/− mice (Anderson et al., 2002; Liston et al., 2003; Kajiura et al., 2004; Zhu et al., 2006). However, the autoimmune phenotype in NIKaly/aly and NF-κB2−/− mice seems to originate from the stromal compartment, as transplantation of NIKaly/aly or NF-κB2−/− thymi into WT mice was sufficient to induce the breakdown in self-tolerance, which is mediated by the loss of Aire function in mTECs (Kajiura et al., 2004; Zhu et al., 2006). In contrast, the impairment of T cell responses in NIKaly/aly mice resulted from disrupted NIK signaling in hematopoietic cells (Greter et al., 2009). Also, NIKaly/aly mice have a defect in the generation of Treg cells, which is not observed in NF-κB2−/− mice (Zhu et al., 2006). These and other observations (Ramakrishnan et al., 2004; Speirs et al., 2004; Zarnegar et al., 2008; Sasaki et al., 2011) suggest that the signaling cascade by which NIK executes its function in cell-mediated immunity may not be exclusive to the p52-dependent noncanonical NF-κB pathway.
As it was widely believed that NIK signaling is predominately involved in the formation of SLTs, the apparent immunodeficiency of the NIKaly/aly strain was held as evidence for the requirement of SLTs in the formation of cell-mediated immunity (Hofmann et al., 2010). In recent years, it has however become evident that NIK signaling is also applied by other cell types such as B cells, osteoclasts, cancer cells, and also by DCs and T cells, suggesting a role of noncanonical NF-κB signaling in adaptive immune responses (Matsumoto et al., 2002; Ishimaru et al., 2006; Tamura et al., 2006; Lind et al., 2008; Greter et al., 2009; Jin et al., 2009). We have recently shown that the inability of NIKaly/aly mice to mount cell-mediated immunity is independent of the developmental lymphoreticular malformations but a result of the interrupted NIK signaling in hematopoietic cells (Greter et al., 2009). Yet the mechanistic consequences of lesioned NIK signaling in DCs and T cells remained poorly understood or might have been wrongly interpreted.
Recently, it was reported that NIK−/− T cells adoptively transferred into Rag2−/− mice failed to develop encephalitogenic properties and to secrete proinflammatory cytokines (Jin et al., 2009). The authors concluded that NIK−/− T cells are intrinsically defective and that NIK signaling in T cells is vital for the acquisition of an effector phenotype. This assumption is corroborated by a previous study showing that NIKaly/aly T cells secrete reduced levels of IL-2 and GM-CSF (Matsumoto et al., 2002). Indeed, we confirmed a reduction in the secretion of proinflammatory cytokines by NIKaly/aly T cells and their failure to homeostatically expand. In addition we found that NIKaly/aly T cells were anergic toward their cognate antigen and thus failed to acquire pathogenic properties in the context of autoimmune disease. However, our observation that T cell function was impaired as a result of the loss of NIK in hematopoietic accessory cells and more specifically in DCs challenges the concept of a T cell–intrinsic defect in NIKaly/aly mice. Three independent experimental setups demonstrated that the T cell defects were the result of a DC-intrinsic utilization of NIK: first, the presence of NIKaly/aly DCs together with WT T cells in vivo was sufficient to significantly diminish EAE development (Fig. 4); second, NIK−/− T cells could differentiate into fully functional, autoaggressive T cells when NIK was restored in accessory cells only (Fig. 2); and third, when the expression of NIK was transgenically restricted to DCs via the CD11c promoter, cell-mediated immunity was fully restored even if T cells were NIK−/− (Fig. 5). One caveat is that CD11c-Cre was also active in a small population of T cells. This small population of transgenic NIK-expressing T cells could potentially be involved in the rescue of immune function in DCNIK-NIK−/− → WT BMCs. However, this is most unlikely because (a) we did not observe a preferential accumulation of NIK-expressing GFP+ T cells in the inflamed CNS at peak disease in DCNIK-NIK−/− → WT BMCs (not depicted), (b) T cell function of mixed Rag1−/− + NIKaly/aly → Rag1−/− BMCs was fully restored even though the entire T cell compartment was NIK deficient, and (c) the transgenic expression of NIKWT in T cells of TNIK-NIK−/−-2d2 → WT BMCs did not render mice EAE susceptible (Fig. S2 B). However, preliminary data suggested that ectopic expression of NIKWT in TNIK mice might affect additional aspects of T cell function.
Interestingly, adoptively transferred adult NIKaly/aly T cells failed to acquire pathogenicity but rather displayed an anergy-like state, although they were primed by NIK-sufficient accessory cells (Fig. 1). Only upon undergoing thymic development in the presence of NIK-sufficient accessory cells could CD4+ T cells give rise to pathogenic effector cells. This suggests that against current belief, NIK is largely dispensable within T cells. In contrast, we suggest that the anergic T cell phenotype observed in NIKaly/aly and NIK−/− mice is caused by defective T cell development caused by dysfunctional thymic DCs. We therefore propose that NIK signaling is critical in DCs to license T cells during thymic development and avoid anergy.
A role for noncanonical NF-κB signaling in DCs has already been suggested, but the precise impact on T cell function has not been addressed before. Although it was claimed that CD40-mediated activation of DCs is not dependent on NIK (Garceau et al., 2000; Yamada et al., 2000; Andreakos et al., 2003), others have demonstrated that peripheral NIKaly/aly DCs show reduced APC capacity, which results in diminished T cell proliferation (Tamura et al., 2006). Furthermore, NIKaly/aly DCs showed a decreased ability to induce the expansion of CD25+ CD4+ T cells in vitro (Tamura et al., 2006) and were unable to cross-prime CD8+ T cells to exogenous antigen, involving multiple defects in antigen-processing pathways (Lind et al., 2008).
We demonstrate that splenic NIKaly/aly DCs produced lower levels of IL-12, IL-23, and IL-6. Furthermore, we show that NIKaly/aly DCs were hampered in the priming of CD4+ autoreactive T cells in vivo (Fig. 4). However, the relevance of NIK signaling in thymic DCs has until now not been addressed, most likely because of the incomplete understanding of the function of thymic DCs in general.
Currently, three phenotypically distinct thymic DC subsets have been described, namely pDCs, CD172α+CD11b+CD8α−/lo migratory cDCs, and CD172α−CD11b−CD8αhi thymic resident cDCs (Wu and Shortman, 2005). One important function of thymic DCs appears to be negative selection at the CD4+ SP stage in T cell development (Brocker et al., 1997; Dakic et al., 2004; Gallegos and Bevan, 2004; Bonasio et al., 2006). In this context, death caused by high-affinity interaction or by no interaction (death by neglect) is not the only fate of developing thymocytes, but also the generation of nTreg cells or induction of anergic T cells (Ramsdell et al., 1989; Ramsdell and Fowlkes, 1990; Bendelac, 2004). The mechanistic underpinnings of these processes are up to today poorly understood. Although the effect of the complete lack of DCs on T cell development and negative selection is discussed controversially (Birnberg et al., 2008; Ohnmacht et al., 2009), a diverted function of thymic DCs is expected to have consequences on T cell development.
The expression of various chemokine receptors and chemokines in thymic DC subsets has been analyzed and compared with expression profiles of splenic DCs (Proietto et al., 2008a). We also profiled thymic DCs of NIKaly/aly mice and found strongly reduced gene expression of CCR2, CCR5, CCR6, and CCR7. It has been proposed that these chemokine receptors are important for the localization and migration of DCs into lymphoid tissues in general including the thymus (Bonasio et al., 2006). We further observed a strong reduction in the expression of the chemokines CCL17, CCL19, and CCL21 in thymic DCs of NIKaly/aly mice, which are important for intrathymic migration of positively selected thymocytes from the cortex to the medulla (Ueno et al., 2004; Proietto et al., 2008a).
Thymic DCs have been proposed to induce FoxP3 expression and the formation of nTreg cells (Proietto et al., 2008b). Recently, it became evident that also other αβ T effector cell lineages such as TH17 cells can at least partially be licensed already during thymic development (Marks et al., 2009). Of note, for γδ T cells, a similar commitment toward IL-17 or IFN-γ production has been shown previously (Ribot et al., 2009), and a very recent study suggested both RelA and RelB to be the critical factors for this process (Powolny-Budnicka et al., 2011). We found that thymic licensing of αβ CD4+ T effector lineages at least to a certain extent relies on the function of NIK and that the expression of NIKWT in DCs alone could rescue not only FoxP3 but also Tbet and RORγt expression in developing NIK-deficient thymocytes. Therefore, we propose that NIK signaling in thymic DCs is crucial to imprint developing T cells to subsequently acquire full effector capabilities and to avoid progression into an anergic state.
MATERIALS AND METHODS
Mice and BM reconstitution.
C57BL/6 (WT) and Rag1−/− mice were purchased from the Jackson Laboratory and bred in house. Alymphoplasia mice (Map3k14aly mice here depicted as NIKaly/aly) were obtained from Clea Laboratories and bred in house. NIK−/− mice on 129Sv/Ev background were provided by R.D. Schreiber (Washington University in St. Louis, St. Louis, MO) and bred onto C57BL/6 background in house for 10 generations. Both NIKaly/aly and NIK−/− mice were maintained by heterozygous breedings. NIKaly/+ and NIK+/− mice are haplo-sufficient (Miyawaki et al., 1994; Yanagawa and Onoé, 2006), justifying the use of heterozygous animals as littermate controls. Furthermore, NIKaly/aly and NIK−/− mice are identical in several aspects of their structural and functional impairments (Yin et al., 2001; Greter et al., 2009; Jin et al., 2009). In all breedings with CD4-cre and CD11c-cre as well as R26NIKWT mice, which are pure C57BL/6, we used NIK−/− animals to avoid the usage of mixed genetic backgrounds. Nonetheless, to ensure consistency between the different strains used, we analyzed NIKaly/aly and NIK−/− mice and heterozygous controls as well as NIK+/+ mice for expression of effector cytokines and the proportion of nTreg cells (Fig. S4). 2d2 (MOG-TCR transgenic) mice were provided by V. Kuchroo (Harvard Medical School, Boston, MA). CD11cDTR mice were provided by S. Jung (Weizmann Institute of Science, Rehovot, Israel). CD11c-cre mice were provided by B. Reizis (Columbia University, New York, NY). R26StopFLNIKWT and all other mice were maintained under specific pathogen-free conditions.
BMCs were generated as described previously (Becher et al., 2002, 2003). In brief, mice were lethally irradiated with a split dose of 1,100 rad. Femur, tibia, and pelvis of donor animals were flushed with PBS to obtain BM stem cells. 10 × 106 cells were injected i.v. per mouse. Mice were treated with 0.2% BORGAL in drinking water for 3 wk to prevent bacterial infections. All experiments involving animals were approved by the Swiss Cantonal Veterinary Office (13/2006, 55/2009; Zurich, Switzerland).
Induction of EAE and DTx treatment.
EAE was induced as described previously (Gutcher et al., 2006; Gyülvészi et al., 2009). In brief, mice were immunized s.c. with 200 µg MOG35–55 (MEVGWYRSPFSRVVHLYRNGK; GenScript) emulsified in CFA (Difco) and two i.p. injections of 200 ng pertussis toxin on days 0 and 2. BMCs did not receive pertussis toxin. For EAE experiments with DTx treatment, mice were injected i.p. with 400 ng DTx (EMD) 1 d before immunization and then 200 ng DTx every second day for the entire length of the experiment.
Isolation of splenic DCs and in vitro stimulation.
Spleens were removed under sterile conditions. Each spleen was injected with a cocktail of 1 mg/ml Liberase and 0.5 mg/ml DNaseI (Roche) in medium and incubated at 37°C for 20 min. Single cell suspensions were prepared by homogenizing the tissue between glass slides and filtering through 70-µm cell strainers followed by erythrocyte lysis. Splenic DCs were isolated with CD11c+ positive magnetic selection according to the manufacturer’s instructions (Miltenyi Biotech).
DCs were plated at a concentration of 106 cells/ml in RPMI 1640 medium supplemented with 10% FCS, 1% l-glutamine, and 1% penicillin-streptavidin (Invitrogen) and stimulated for 6–24 h at 37°C and 5% CO2 with 10 µg/ml α-CD40 (FGK 4.5; BioXCell) and 20 ng/ml IFN-γ (PeproTech). Supernatants were analyzed for IL-6 and IL-12/IL-23p40 using ELISA according to the manufacturer’s instructions (BD).
Isolation of thymic DCs and in vitro co-culture assays.
Thymic DCs were isolated as previously described (Wirnsberger et al., 2009). In brief, thymi were digested in IMDM containing 2% FCS, 25 mM HEPES, 0.4 mg/ml Collagenase D (Roche), and DNase for 40 min at 37°C. Afterward, high-density cells were separated from low-density cells by using a discontinuous Percoll density gradient (ρ = 1.115 and ρ = 1.055; GE Healthcare). After removal from the gradient, cells were washed and stained with antibodies against CD11c, CD8, CD172a, and CD45RA, followed by sorting into migratory DCs (CD11c+CD172a+), resident DCs (CD11c+CD172a−), and pDCs (CD11cintCD45RA+) on a FACSAria (BD). Purity was routinely >95%.
For in vitro culture, 20,000 DCs were cultured with 100,000 sorted and CFSE-labeled CD4+ thymocytes or peripheral T cells in the presence of 10 µg/ml MOG35–55 and 10 ng/ml IL-7. Analysis was performed after 72 h of culture.
Peripheral CD4+ T cell purification, in vitro stimulation, and adoptive transfer.
Splenocyte single cell suspensions were prepared as described in Isolation of splenic DCs and in vitro stimulation. CD4+ T cells were purified with CD4+ negative magnetic selection according to the manufacturer’s instructions (Miltenyi Biotech). The purity was routinely >95% as confirmed by flow cytometry.
For in vitro stimulations of splenic CD4+ T cells, 3 × 106 CD4+ T cells/ml were cultured in RPMI 1640 medium supplemented with 10% FCS, 1% l-glutamine, and 1% penicillin-streptavidin. Polyclonal CD4+ T cell activation was performed with 5 µg/ml plate-bound α-CD3 and α-CD28 for 48 h. For antigen-specific CD4+ T cell activation, whole 2d2 splenocytes were stimulated with 20 µg/ml MOG35–55 and 5 µg/ml soluble α-CD28 for 48 h. Supernatants were harvested, and concentrations of IFN-γ, IL-17, GM-CSF, IL-2, and IL-4 were quantified by ELISA according to the manufacturer’s instructions (BD).
For adoptive transfer experiments, 2 × 106 purified splenic CD4+ T cells in PBS were injected i.v. into Rag1−/− mice. Homeostatic expansion of T cells in Rag1−/− mice was monitored weekly by tail bleeding and flow cytometry, and the percentage of CD4+ T cells of the lymphocyte gate was calculated.
Flow cytometry.
For cell surface staining, we used the following antibodies: CD11c, Iab, CD80, CD86, CD172a, CD45RA, CD4, CD8, IL-12/IL-23p40, Vα3.2, and Vβ11 (BD). Intracellular FoxP3 staining was performed according to the manufacturer’s instructions (eBioscience). Cells were incubated with antibodies at the optimal concentration for 20 min at 4°C, and cells were analyzed either on FACS Canto II or LSRII Fortessa (BD). Postacquisition analysis was performed with either FACS Diva or FlowJo (Tree Star) software. Cytofluorometric analysis of CNS-invading lymphocytes has been described previously (Gutcher et al., 2006). For intracellular cytokine staining, cells were treated with GolgiPlug (BD) for the last 4 h of culture. T cells were additionally stimulated with PMA and ionomycin for the last 4 h of culture. After surface staining, cells were permeabilized with Cytofix/Cytoperm (BD) according to the manufacturer’s recommendations and stained intracellularly with IL-12/IL-23p40–specific antibody (BD) or FoxP3-, IL-17 (eBioscience)–, and IFN-γ–specific antibody (BD).
RNA isolation and qRT-PCR.
Total RNA was isolated according to the manufacturer’s instructions (RNeasy Mini Plus kit; QIAGEN). RT was performed using random hexamer primers and Moloney murine leukemia virus RT (splenic DCs) or Superscript II (thymic DCs; Invitrogen). cDNA was analyzed by quantitative real-time PCR (qRT-PCR; Bio-Rad Laboratories) in duplicates using SYBR Green PCR Mastermix (Invitrogen) or hydrolysis TAMRA probes (Roche). The expression level of each gene was normalized to HPRT or DNA Polymerase II. The following primers purchased from Operon Technologies were used: HPRT (5′-GACCGGTCCCGTCATGC-3′ and 5′-TCATAACCTGGTTCATCATCGC-3′), DNA Polymerase II (5′-CTGGTCCTTCGAATCCGCATC-3′ and 5′-GCTCGATACCCTGCAGGGTCA-3′), IL-12/IL-23p40 (5′-GACCATCACTGTCAAAGAGTTTCTAGAT-3′ and 5′-AGGAAAGTCTTGTTTTTGAAATTTTTTAA-3′), IL-12p35 (5′-TACTAGAGAGACTTCTTCCACAACAAGAG-3′ and 5′-TCTGGTACATCTTCAAGTCCTCATAGA-3′), IL-23p19 (5′-AGCGGGACATATGAATCTACTAAGAGA-3′ and 5′-GTCCTAGTAGGGAGGTGTGAAGTTG-3′), TLR9 (5′-GCCTTCGTGGTGTTCGATAAGG-3′ and 5′-GAGGTTCTCGAAGAGCGTCTGG-3′), CCL17 (5′-TACTTCAAAGGGGCCATTCCT-3′ and 5′-GCCTTGGGTTTTTCACCAATCT-3′), CCL19 (5′-GGCCTGCCTCAGATTATCTGCCAT-3′ and 5′-GGAAGGCTTTCACGATGTTCC-3′), CCL21 (5′-GGACCCAAGGCAGTGATGGAG-3′ and 5′-CTTCCTCAGGGTTTGCACATAG-3′), CCR2 (5′-ACAAGCACTTAGACCAGGCCAT-3′ and 5′-AAACTGGGCACTGTTTGC-3′), CCR5 (5′-ACTGCTGCCTAAACCCTGTCA-3′ and 5′-GTTTTCGGAAGAACACTGAGAGATAA-3′), CCR6 (5′-TCCATCATCATCTCAAGCCCTACA-3′ and 5′-AGGGGTGAAGAACCCAAAGAACA-3′), CCR7 (5′-ACCATGGACCCAGGGAAC-3′ and 5′-GGTATTCTCGCCGATGTAGTCAT-3′), and CCR9 (5′-TGGCTTGTGTTCATTGTGGGCA-3′ and 5′-ATCCATTGACCAGCAGCAGCAA-3′).
Online supplemental material.
Fig. S1 shows in vitro stimulated splenic DCs and their expression of IL-12/IL-23p40, IL-12p35, and IL-12p19 as described in Fig. 3. Fig. S2 shows the transgenic GFP expression of DCNIK-NIK−/− → WT BMCs and the EAE score of TNIK-NIK−/− → WT BMCs. Fig. S3 summarizes the expression of various chemokine receptors of thymic DC subsets as described in Fig. 6 D. Fig. S4 shows a phenotypic comparison of T cells from NIKaly/aly, NIKaly/+, NIK−/−, NIK+/−, and NIK+/+ mice, in particular the expression of FoxP3 and effector cytokines. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110128/DC1.
Acknowledgments
We thank R.D. Schreiber for NIK−/− mice, S. Jung for CD11cDTR mice, B. Reizis for CD11c-cre mice, and V. Tosevski, R. Höppli, D. Haefeli, A. Reiter, J. Jaberg, and S. Hasler for technical assistance.
The project was supported financially by a grant from the Swiss National Science Foundation (to B. Becher), the Swiss Multiple Sclerosis Society (to B. Becher), the David and Betty Koetser Foundation (to B. Becher and J. Hofmann), and a junior research fellowship from the University of Zürich (to F. Mair).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- BMC
- BM chimeric mouse
- cDC
- conventional DC
- CNS
- central nervous system
- DTx
- diphtheria toxin
- EAE
- experimental autoimmune encephalomyelitis
- mTEC
- medullary thymic epithelial cell
- NIK
- NF-κB–inducing kinase
- nTreg cell
- natural regulatory T cell
- pDC
- plasmacytoid DC
- qRT-PCR
- quantitative RT-PCR
- SLT
- secondary lymphoid tissue
- SP
- single positive
References
- Akiyama T., Shimo Y., Yanai H., Qin J., Ohshima D., Maruyama Y., Asaumi Y., Kitazawa J., Takayanagi H., Penninger J.M., et al. 2008. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 29:423–437 10.1016/j.immuni.2008.06.015 [DOI] [PubMed] [Google Scholar]
- Anderson M.S., Venanzi E.S., Klein L., Chen Z., Berzins S.P., Turley S.J., von Boehmer H., Bronson R., Dierich A., Benoist C., Mathis D. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401 10.1126/science.1075958 [DOI] [PubMed] [Google Scholar]
- Andreakos E., Smith C., Monaco C., Brennan F.M., Foxwell B.M., Feldmann M. 2003. Ikappa B kinase 2 but not NF-kappa B-inducing kinase is essential for effective DC antigen presentation in the allogeneic mixed lymphocyte reaction. Blood. 101:983–991 10.1182/blood-2002-06-1835 [DOI] [PubMed] [Google Scholar]
- Annunziata C.M., Davis R.E., Demchenko Y., Bellamy W., Gabrea A., Zhan F., Lenz G., Hanamura I., Wright G., Xiao W., et al. 2007. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 12:115–130 10.1016/j.ccr.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson P.G., Coope H.J., Rowe M., Ley S.C. 2003. Latent membrane protein 1 of Epstein-Barr virus stimulates processing of NF-kappa B2 p100 to p52. J. Biol. Chem. 278:51134–51142 10.1074/jbc.M304771200 [DOI] [PubMed] [Google Scholar]
- Banks T.A., Rouse B.T., Kerley M.K., Blair P.J., Godfrey V.L., Kuklin N.A., Bouley D.M., Thomas J., Kanangat S., Mucenski M.L. 1995. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 155:1685–1693 [PubMed] [Google Scholar]
- Becher B., Durell B.G., Noelle R.J. 2002. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 110:493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Durell B.G., Noelle R.J. 2003. IL-23 produced by CNS-resident cells controls T cell encephalitogenicity during the effector phase of experimental autoimmune encephalomyelitis. J. Clin. Invest. 112:1186–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A. 2004. Nondeletional pathways for the development of autoreactive thymocytes. Nat. Immunol. 5:557–558 10.1038/ni0604-557 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Borthakur A., Dudeja P.K., Tobacman J.K. 2010. Lipopolysaccharide-induced activation of NF-κB non-canonical pathway requires BCL10 serine 138 and NIK phosphorylations. Exp. Cell Res. 316:3317–3327 10.1016/j.yexcr.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnberg T., Bar-On L., Sapoznikov A., Caton M.L., Cervantes-Barragán L., Makia D., Krauthgamer R., Brenner O., Ludewig B., Brockschnieder D., et al. 2008. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 29:986–997 10.1016/j.immuni.2008.10.012 [DOI] [PubMed] [Google Scholar]
- Bonasio R., Scimone M.L., Schaerli P., Grabie N., Lichtman A.H., von Andrian U.H. 2006. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat. Immunol. 7:1092–1100 10.1038/ni1385 [DOI] [PubMed] [Google Scholar]
- Brocker T., Riedinger M., Karjalainen K. 1997. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J. Exp. Med. 185:541–550 10.1084/jem.185.3.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin R.K., Lo J.C., Kim O., Blink S.E., Christiansen P.A., Peterson P., Wang Y., Ware C., Fu Y.X. 2003. Lymphotoxin pathway directs thymic Aire expression. Nat. Immunol. 4:1121–1127 10.1038/ni982 [DOI] [PubMed] [Google Scholar]
- Dakic A., Shao Q.X., D’Amico A., O’Keeffe M., Chen W.F., Shortman K., Wu L. 2004. Development of the dendritic cell system during mouse ontogeny. J. Immunol. 172:1018–1027 [DOI] [PubMed] [Google Scholar]
- Darnay B.G., Ni J., Moore P.A., Aggarwal B.B. 1999. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J. Biol. Chem. 274:7724–7731 10.1074/jbc.274.12.7724 [DOI] [PubMed] [Google Scholar]
- Eliopoulos A.G., Caamano J.H., Flavell J., Reynolds G.M., Murray P.G., Poyet J.L., Young L.S. 2003. Epstein-Barr virus-encoded latent infection membrane protein 1 regulates the processing of p100 NF-kappaB2 to p52 via an IKKgamma/NEMO-independent signalling pathway. Oncogene. 22:7557–7569 10.1038/sj.onc.1207120 [DOI] [PubMed] [Google Scholar]
- Fütterer A., Mink K., Luz A., Kosco-Vilbois M.H., Pfeffer K. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 9:59–70 10.1016/S1074-7613(00)80588-9 [DOI] [PubMed] [Google Scholar]
- Gallegos A.M., Bevan M.J. 2004. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J. Exp. Med. 200:1039–1049 10.1084/jem.20041457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garceau N., Kosaka Y., Masters S., Hambor J., Shinkura R., Honjo T., Noelle R.J. 2000. Lineage-restricted function of nuclear factor kappaB–inducing kinase (NIK) in transducing signals via CD40. J. Exp. Med. 191:381–386 10.1084/jem.191.2.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M., Hofmann J., Becher B. 2009. Neo-lymphoid aggregates in the adult liver can initiate potent cell-mediated immunity. PLoS Biol. 7:e1000109 10.1371/journal.pbio.1000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutcher I., Urich E., Wolter K., Prinz M., Becher B. 2006. Interleukin 18-independent engagement of interleukin 18 receptor-alpha is required for autoimmune inflammation. Nat. Immunol. 7:946–953 10.1038/ni1377 [DOI] [PubMed] [Google Scholar]
- Gyülvészi G., Haak S., Becher B. 2009. IL-23-driven encephalo-tropism and Th17 polarization during CNS-inflammation in vivo. Eur. J. Immunol. 39:1864–1869 10.1002/eji.200939305 [DOI] [PubMed] [Google Scholar]
- Hofmann J., Greter M., Du Pasquier L., Becher B. 2010. B-cells need a proper house, whereas T-cells are happy in a cave: the dependence of lymphocytes on secondary lymphoid tissues during evolution. Trends Immunol. 31:144–153 10.1016/j.it.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Ishimaru N., Kishimoto H., Hayashi Y., Sprent J. 2006. Regulation of naive T cell function by the NF-kappaB2 pathway. Nat. Immunol. 7:763–772 10.1038/ni1351 [DOI] [PubMed] [Google Scholar]
- Jin W., Zhou X.F., Yu J., Cheng X., Sun S.C. 2009. Regulation of Th17 cell differentiation and EAE induction by MAP3K NIK. Blood. 113:6603–6610 10.1182/blood-2008-12-192914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiura F., Sun S., Nomura T., Izumi K., Ueno T., Bando Y., Kuroda N., Han H., Li Y., Matsushima A., et al. 2004. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J. Immunol. 172:2067–2075 [DOI] [PubMed] [Google Scholar]
- Li J., Park J., Foss D., Goldschneider I. 2009. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J. Exp. Med. 206:607–622 10.1084/jem.20082232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G., Zhang M., Harhaj E.W., Sun S.C. 2004. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J. Biol. Chem. 279:26243–26250 10.1074/jbc.M403286200 [DOI] [PubMed] [Google Scholar]
- Lind E.F., Ahonen C.L., Wasiuk A., Kosaka Y., Becher B., Bennett K.A., Noelle R.J. 2008. Dendritic cells require the NF-kappaB2 pathway for cross-presentation of soluble antigens. J. Immunol. 181:354–363 [DOI] [PubMed] [Google Scholar]
- Liston A., Lesage S., Wilson J., Peltonen L., Goodnow C.C. 2003. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4:350–354 10.1038/ni906 [DOI] [PubMed] [Google Scholar]
- Lu L.F., Gondek D.C., Scott Z.A., Noelle R.J. 2005. NF kappa B-inducing kinase deficiency results in the development of a subset of regulatory T cells, which shows a hyperproliferative activity upon glucocorticoid-induced TNF receptor family-related gene stimulation. J. Immunol. 175:1651–1657 [DOI] [PubMed] [Google Scholar]
- Marks B.R., Nowyhed H.N., Choi J.Y., Poholek A.C., Odegard J.M., Flavell R.A., Craft J. 2009. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat. Immunol. 10:1125–1132 10.1038/ni.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Fukushima H., Nakao K., Shin M., Yasuda H., Weih F., Doi T., Aoki K., Alles N., Ohya K., et al. 2010. Processing of the NF-kappa B2 precursor p100 to p52 is critical for RANKL-induced osteoclast differentiation. J. Bone Miner. Res. 25:1058–1067 [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Yamada T., Yoshinaga S.K., Boone T., Horan T., Fujita S., Li Y., Mitani T. 2002. Essential role of NF-kappa B-inducing kinase in T cell activation through the TCR/CD3 pathway. J. Immunol. 169:1151–1158 [DOI] [PubMed] [Google Scholar]
- Miyawaki S., Nakamura Y., Suzuka H., Koba M., Yasumizu R., Ikehara S., Shibata Y. 1994. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol. 24:429–434 10.1002/eji.1830240224 [DOI] [PubMed] [Google Scholar]
- Nadiminty N., Chun J.Y., Hu Y., Dutt S., Lin X., Gao A.C. 2007. LIGHT, a member of the TNF superfamily, activates Stat3 mediated by NIK pathway. Biochem. Biophys. Res. Commun. 359:379–384 10.1016/j.bbrc.2007.05.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C., Pullner A., King S.B., Drexler I., Meier S., Brocker T., Voehringer D. 2009. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 206:549–559 10.1084/jem.20082394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powolny-Budnicka I., Riemann M., Tänzer S., Schmid R.M., Hehlgans T., Weih F. 2011. RelA and RelB transcription factors in distinct thymocyte populations control lymphotoxin-dependent interleukin-17 production in γδ T cells. Immunity. 34:364–374 10.1016/j.immuni.2011.02.019 [DOI] [PubMed] [Google Scholar]
- Proietto A.I., Lahoud M.H., Wu L. 2008a. Distinct functional capacities of mouse thymic and splenic dendritic cell populations. Immunol. Cell Biol. 86:700–708 10.1038/icb.2008.63 [DOI] [PubMed] [Google Scholar]
- Proietto A.I., van Dommelen S., Zhou P., Rizzitelli A., D’Amico A., Steptoe R.J., Naik S.H., Lahoud M.H., Liu Y., Zheng P., et al. 2008b. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. USA. 105:19869–19874 10.1073/pnas.0810268105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietto A.I., van Dommelen S., Wu L. 2009. The impact of circulating dendritic cells on the development and differentiation of thymocytes. Immunol. Cell Biol. 87:39–45 10.1038/icb.2008.86 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan P., Wang W., Wallach D. 2004. Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF-kappaB-inducing kinase. Immunity. 21:477–489 10.1016/j.immuni.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Fowlkes B.J. 1990. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 248:1342–1348 10.1126/science.1972593 [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Lantz T., Fowlkes B.J. 1989. A nondeletional mechanism of thymic self tolerance. Science. 246:1038–1041 10.1126/science.2511629 [DOI] [PubMed] [Google Scholar]
- Ribot J.C., deBarros A., Pang D.J., Neves J.F., Peperzak V., Roberts S.J., Girardi M., Borst J., Hayday A.C., Pennington D.J., Silva-Santos B. 2009. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 10:427–436 10.1038/ni.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Valdepeñas C., Martín A.G., Ramakrishnan P., Wallach D., Fresno M. 2006. NF-kappaB-inducing kinase is involved in the activation of the CD28 responsive element through phosphorylation of c-Rel and regulation of its transactivating activity. J. Immunol. 176:4666–4674 [DOI] [PubMed] [Google Scholar]
- Sánchez-Valdepeñas C., Casanova L., Colmenero I., Arriero M., González A., Lozano N., González-Vicent M., Díaz M.A., Madero L., Fresno M., Ramírez M. 2010. Nuclear factor-kappaB inducing kinase is required for graft-versus-host disease. Haematologica. 95:2111–2118 10.3324/haematol.2010.028829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A.B., Sanchez-Niño M.D., Izquierdo M.C., Jakubowski A., Justo P., Blanco-Colio L.M., Ruiz-Ortega M., Selgas R., Egido J., Ortiz A. 2010. TWEAK activates the non-canonical NFkappaB pathway in murine renal tubular cells: modulation of CCL21. PLoS ONE. 5:e8955 10.1371/journal.pone.0008955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki C.Y., Ghosh P., Longo D.L. 2011. Recruitment of RelB to the Csf2 promoter enhances RelA-mediated transcription of granulocyte-macrophage colony-stimulating factor. J. Biol. Chem. 286:1093–1102 10.1074/jbc.M110.119438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Calado D.P., Derudder E., Zhang B., Shimizu Y., Mackay F., Nishikawa S., Rajewsky K., Schmidt-Supprian M. 2008. NIK overexpression amplifies, whereas ablation of its TRAF3-binding domain replaces BAFF:BAFF-R-mediated survival signals in B cells. Proc. Natl. Acad. Sci. USA. 105:10883–10888 10.1073/pnas.0805186105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senftleben U., Cao Y., Xiao G., Greten F.R., Krähn G., Bonizzi G., Chen Y., Hu Y., Fong A., Sun S.C., Karin M. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 293:1495–1499 10.1126/science.1062677 [DOI] [PubMed] [Google Scholar]
- Shinkura R., Matsuda F., Sakiyama T., Tsubata T., Hiai H., Paumen M., Miyawaki S., Honjo T. 1996. Defects of somatic hypermutation and class switching in alymphoplasia (aly) mutant mice. Int. Immunol. 8:1067–1075 10.1093/intimm/8.7.1067 [DOI] [PubMed] [Google Scholar]
- Shinkura R., Kitada K., Matsuda F., Tashiro K., Ikuta K., Suzuki M., Kogishi K., Serikawa T., Honjo T. 1999. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat. Genet. 22:74–77 10.1038/8780 [DOI] [PubMed] [Google Scholar]
- Speirs K., Lieberman L., Caamano J., Hunter C.A., Scott P. 2004. Cutting edge: NF-kappa B2 is a negative regulator of dendritic cell function. J. Immunol. 172:752–756 [DOI] [PubMed] [Google Scholar]
- Staudt L.M. 2010. Oncogenic activation of NF-kappaB. Cold Spring Harb. Perspect. Biol. 2:a000109 10.1101/cshperspect.a000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.C., Ley S.C. 2008. New insights into NF-kappaB regulation and function. Trends Immunol. 29:469–478 10.1016/j.it.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura C., Nakazawa M., Kasahara M., Hotta C., Yoshinari M., Sato F., Minami M. 2006. Impaired function of dendritic cells in alymphoplasia (aly/aly) mice for expansion of CD25+CD4+ regulatory T cells. Autoimmunity. 39:445–453 10.1080/08916930600833390 [DOI] [PubMed] [Google Scholar]
- Ueno T., Saito F., Gray D.H., Kuse S., Hieshima K., Nakano H., Kakiuchi T., Lipp M., Boyd R.L., Takahama Y. 2004. CCR7 signals are essential for cortex–medulla migration of developing thymocytes. J. Exp. Med. 200:493–505 10.1084/jem.20040643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E., Blankenship J.W., Wayson S.M., Fedorova A.V., Kayagaki N., Garg P., Zobel K., Dynek J.N., Elliott L.O., Wallweber H.J., et al. 2007. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 131:669–681 10.1016/j.cell.2007.10.030 [DOI] [PubMed] [Google Scholar]
- Venanzi E.S., Gray D.H., Benoist C., Mathis D. 2007. Lymphotoxin pathway and Aire influences on thymic medullary epithelial cells are unconnected. J. Immunol. 179:5693–5700 [DOI] [PubMed] [Google Scholar]
- Vince J.E., Wong W.W., Khan N., Feltham R., Chau D., Ahmed A.U., Benetatos C.A., Chunduru S.K., Condon S.M., McKinlay M., et al. 2007. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 131:682–693 10.1016/j.cell.2007.10.037 [DOI] [PubMed] [Google Scholar]
- Weih F., Caamaño J. 2003. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol. Rev. 195:91–105 10.1034/j.1600-065X.2003.00064.x [DOI] [PubMed] [Google Scholar]
- Wirnsberger G., Mair F., Klein L. 2009. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc. Natl. Acad. Sci. USA. 106:10278–10283 10.1073/pnas.0901877106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Shortman K. 2005. Heterogeneity of thymic dendritic cells. Semin. Immunol. 17:304–312 10.1016/j.smim.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Xiao G., Cvijic M.E., Fong A., Harhaj E.W., Uhlik M.T., Waterfield M., Sun S.C. 2001a. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J. 20:6805–6815 10.1093/emboj/20.23.6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G., Harhaj E.W., Sun S.C. 2001b. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell. 7:401–409 10.1016/S1097-2765(01)00187-3 [DOI] [PubMed] [Google Scholar]
- Xiao G., Fong A., Sun S.C. 2004. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J. Biol. Chem. 279:30099–30105 10.1074/jbc.M401428200 [DOI] [PubMed] [Google Scholar]
- Yamada T., Mitani T., Yorita K., Uchida D., Matsushima A., Iwamasa K., Fujita S., Matsumoto M. 2000. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-kappa B-inducing kinase. J. Immunol. 165:804–812 [DOI] [PubMed] [Google Scholar]
- Yanagawa Y., Onoé K. 2006. Distinct regulation of CD40-mediated interleukin-6 and interleukin-12 productions via mitogen-activated protein kinase and nuclear factor kappaB-inducing kinase in mature dendritic cells. Immunology. 117:526–535 10.1111/j.1365-2567.2006.02329.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Wu L., Wesche H., Arthur C.D., White J.M., Goeddel D.V., Schreiber R.D. 2001. Defective lymphotoxin-beta receptor-induced NF-kappaB transcriptional activity in NIK-deficient mice. Science. 291:2162–2165 10.1126/science.1058453 [DOI] [PubMed] [Google Scholar]
- Youssef S., Steinman L. 2006. At once harmful and beneficial: the dual properties of NF-kappaB. Nat. Immunol. 7:901–902 10.1038/ni0906-901 [DOI] [PubMed] [Google Scholar]
- Zarnegar B., Yamazaki S., He J.Q., Cheng G. 2008. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc. Natl. Acad. Sci. USA. 105:3503–3508 10.1073/pnas.0707959105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Chin R.K., Christiansen P.A., Lo J.C., Liu X., Ware C., Siebenlist U., Fu Y.X. 2006. NF-kappaB2 is required for the establishment of central tolerance through an Aire-dependent pathway. J. Clin. Invest. 116:2964–2971 10.1172/JCI28326 [DOI] [PMC free article] [PubMed] [Google Scholar]