Constitutive NF-κB activation in IECs induces inflammatory cytokines and chemokines in the lamina propria, but does not result in overt tissue damage unless acute inflammatory insults are present, causing TNF-dependent destruction and barrier disruption.
Abstract
Nuclear factor (NF)-κB, activated by IκB kinase (IKK), is a key regulator of inflammation, innate immunity, and tissue integrity. NF-κB and one of its main activators and transcriptional targets, tumor necrosis factor (TNF), are up-regulated in many inflammatory diseases that are accompanied by tissue destruction. The etiology of many inflammatory diseases is poorly understood, but often depends on genetic factors and environmental triggers that affect NF-κB and related pathways. It is unknown, however, whether persistent NF-κB activation is sufficient for driving symptomatic chronic inflammation and tissue damage. To address this question, we generated IKKβ(EE)IEC mice, which express a constitutively active form of IKKβ in intestinal epithelial cell (IECs). IKKβ(EE)IEC mice exhibit NF-κB activation in IECs and express copious amounts of inflammatory chemokines, but only small amounts of TNF. Although IKKβ(EE)IEC mice exhibit inflammatory cell infiltration in the lamina propria (LP) of their small intestine, they do not manifest tissue damage. Yet, upon challenge with relatively mild immune and microbial stimuli, IKKβ(EE)IEC mice succumb to destructive acute inflammation accompanied by enterocyte apoptosis, intestinal barrier disruption, and bacterial translocation. Inflammation is driven by massive TNF production, which requires additional activation of p38 and extracellular-signal–regulated kinase mitogen-activated protein kinases (MAPKs).
NF-κB is a key regulator of inflammation and innate immunity (Ghosh and Karin, 2002). It has long been considered the prototypical proinflammatory signaling pathway, as it is responsive to proinflammatory cytokines such as IL-1 and TNF, and is in charge of their synthesis (Ghosh and Karin, 2002). In addition, NF-κB transcription factors activate expression of genes encoding cytokines, chemokines, adhesion molecules, matrix metalloproteinases, cyclooxygenase 2, inducible nitric oxide synthase, and enzymes and molecules with microbicidal activity (Li and Verma, 2002; Lawrence, 2009; Pasparakis, 2009). Through such genes, NF-κB plays a key role in coordination of inflammation and immune responses. However, by controlling cell proliferation and survival, NF-κB also maintains tissue integrity and might play an antiinflammatory role (Karin and Lin, 2002; Pasparakis, 2009). NF-κB protects cells from death by inducing expression of antiapoptotic proteins, including Bcl-xL, FLICE-like inhibitory protein, and members of the inhibitor of apoptosis (IAP) family (Karin and Lin, 2002; Pasparakis, 2009).
NF-κB activity is of particular importance for maintenance of epithelial barriers (Karin and Lin, 2002; Pasparakis, 2009), but it was also proposed that NF-κB activation in epithelial cells can lead to production of inflammatory chemokines that recruit immune cells to the tissue, thereby initiating an inflammatory amplification cascade (Barnes and Karin, 1997). Indeed, persistent activation of NF-κB signaling pathways is often associated with chronic inflammatory diseases, such as rheumatoid arthritis (Handel et al., 1995; Tak et al., 2001), inflammatory bowel disease (Neurath et al., 1996; Kaser et al., 2010), psoriasis (Lizzul et al., 2005), and asthma (Edwards et al., 2009). NF-κB activity and nuclear localization have been consistently detected in biopsies from such patients, and are accompanied by enhanced recruitment of inflammatory cells and production of proinflammatory mediators such as IL-1, IL-6, and TNF (Barnes and Karin, 1997; Tak and Firestein, 2001; Pasparakis, 2009). Patients with elevated NF-κB also suffer a worse outcome in septic shock/multiple organ failure (Böhrer et al., 1997; Arnalich et al., 2000; Liu and Malik, 2006). The etiology of these inflammatory diseases is unknown, but is likely to depend on a combination of genetic susceptibility and environmental triggers, and single nucleotides polymorphisms in NF-κB or related-pathways have been strongly implicated (Vereecke et al., 2009; Zhang et al., 2011). It is unclear, however, whether the persistent activation of NF-κB in epithelial cells is sufficient for driving chronic inflammation and tissue damage, given its aforementioned protective role.
NF-κB is regulated by the activated by IκB kinase (IKK) complex consisting of the IKKα and IKKβ catalytic subunits and IKKγ/NEMO regulatory subunit (DiDonato et al., 1997; Ghosh and Karin, 2002; Rothwarf and Karin, 1999). To determine whether persistent epithelial cell activation of NF-κB can drive chronic and destructive inflammation, we generated IKKβ(EE)IEC mice that selectively express constitutively activated IKKβ (Delhase et al., 1999) in intestinal epithelial cells (IECs). It is well established that NF-κB can stimulate transcription of genes encoding TNF and other proinflammatory cytokines and chemokines in IECs (Eckmann and Kagnoff, 2005). However, the absence of IKKβ or IKKγ/NEMO in IECs can result in tissue destruction caused by the extensive apoptosis, and this tissue damage can also result in increased inflammation (Chen et al., 2003; Egan et al., 2004; Greten et al., 2004; Chae et al., 2006; Nenci et al., 2007; Spehlmann and Eckmann, 2009). Hence, in IECs, NF-κB can have both pro- and antiinflammatory functions, making it difficult to predict which of these opposing functions would prevail in IKKβ(EE)IEC mice.
As expected, IKKβ(EE)IEC mice expressed copious amounts of inflammatory chemokines and cytokines, but unexpectedly produced only small amounts of TNF. The lamina propria (LP) of IKKβ(EE)IEC mice was infiltrated with immune cells, but this inflammation did not progress over time and the intestinal barrier remained intact. When challenged with pathogenic bacteria, LPS, or TCR agonists, IKKβ(EE)IEC mice succumbed to damaging acute inflammation accompanied by IEC apoptosis and bacterial translocation, that was driven by massive TNF production, found to require additional activation of p38 and extracellular-signal–regulated kinase (ERK) mitogen-activated protein kinases (MAPK) on top of the already active NF-κB. Our results strongly suggest that persistent NF-κB activation in epithelial cells is insufficient for driving symptomatic inflammation and tissue damage, because synthesis of key inflammatory mediators, such as TNF, is subject to intricate transcriptional and posttranscriptional controls.
RESULTS
IKKβ(EE)IEC mice exhibit persistent NF-κB activation and target gene expression, but not tissue damage
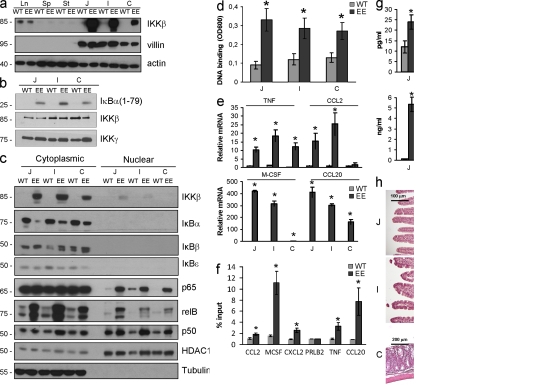
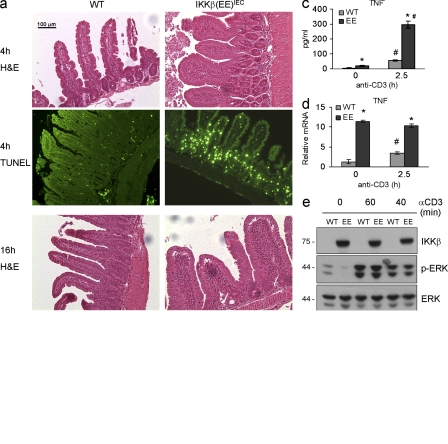
To persistently activate NF-κB in enterocytes, we generated transgenic (Tg) IKKβ(EE)IEC mice, which express a constitutively active IKKβ variant, IKKβ(EE) (Delhase et al., 1999), from the villin control region (Madison et al., 2002). Heterozygous IKKβ(EE)IEC mice were obtained at the expected Mendelian ratio and their general condition and survival did not differ from WT littermates. IKKβ(EE) was expressed throughout the intestine after a decreasing cephalocaudal gradient that paralleled villin expression (Fig. 1 a). Enterocyte IκB kinase activity was enhanced in IKKβ(EE)IEC mice (Fig. 1 b), resulting in nuclear translocation of p65 (RelA), RelB, and p50 (Fig. 1 c) and increased p65 DNA binding (Fig. 1 d).
Figure 1.
IKKβ(EE)IEC mice exhibit persistent NF-κB activation, expression of NF-κB target genes, but no tissue damage. (a) Lysates of WT and IKKβ(EE)IEC IECs were analyzed for villin and IKKβ expression by immunoblotting. Ln, lung; Sp, spleen; St, stomach; J, jejunum; I, ileum; C, colon. Representative of five experiments. (b) IKK activity was measured by immunocomplex kinase assay using GST-IκBα(1–79) as a substrate in lysates of WT and IKKβ(EE)IEC jejunal (J), ileal (I), and colonic (C) enterocytes. Representative of five experiments. (c) Presence of the indicated proteins in cytoplasmic and nuclear extracts of WT and IKKβ(EE)IEC enterocytes was examined by immunoblotting. Representative of five experiments. (d) Nuclear extracts of WT and IKKβ(EE)IEC enterocytes were analyzed for p65 DNA binding by ELISA. Results (absorbance/micrograms protein) are means ± SEM (n = 3). *, P < 0.05 versus WT. Data are representative of three independent experiments. (e) WT or IKKβ(EE)IEC enterocyte RNAs were analyzed in triplicates by Q-RT-PCR for expression of the indicated genes. mRNA amounts were normalized to Hprt mRNA. Results are means ± SEM (n = 4). *, P < 0.05 versus WT. Data are representative of four independent experiments. (f) ChIP was performed with a p65-specific antibody using fixed and sheared chromatin from WT and IKKβ(EE)IEC small bowel enterocytes. Promoter sequences were analyzed by Q-RT-PCR. Results are means ± SEM (n = 3). *, P < 0.05 versus WT. Data are representative of three independent experiments. (g) WT and IKKβ(EE)IEC enterocyte lysates (75 µg protein) were analyzed by ELISA for the indicated proteins. Results are means ± SEM (n = 3). *, P < 0.05 versus WT. Data are representative of four independent experiments. (h) Intestinal histology of WT and IKKβ(EE)IEC mice. Representative of 10 experiments.
To investigate NF-κB–driven gene expression in IECs, we performed microarray analysis and confirmed representative results by quantitative (Q) real time (RT) PCR. Expression of ∼150 genes was increased >2.5-fold in IKKβ(EE)IEC enterocytes relative to WT counterparts (Fig. 1 e; Fig. S1 a; and Table S1). Many up-regulated genes are known NF-κB targets, including Tnfaip3, Birc3, Nfkbia, Ccl20, Ccl2, Cxcl10, Icam1, Tnf, RelB, Csf1 and Bcl3. Chromatin immunoprecipitation (ChIP) demonstrated RelA recruitment to the promoters of several of these genes, including Tnf (Fig. 1 f). However, IKKβ(EE)IEC enterocytes exhibited a very large increase in the secretion of several chemokines and cytokines, including CCL20, CCL2, and M-CSF, whose concentrations reached several nanograms/milliliter; however, the increase in TNF production was very modest and did not exceed 20–25 pg/ml (Fig. 1 g). This small increase in TNF protein secretion was in marked contrast to the 10–20-fold increase in its mRNA amounts (Fig. 1 e). In situ hybridization confirmed that TNF mRNA accumulated in IECs of IKKβ(EE)IEC mice (Fig. S1 b). Consistent with increased chemokine production, histological examination revealed marked infiltration with predominantly mononuclear leukocytes in the LP of the villus regions in jejunum and ileum of IKKβ(EE)IEC mice. This was paralleled by modest epithelial hyperplasia and crypt deepening without apparent loss of goblet cells or any epithelial ulcerations (Fig. 1 h). Most IKKβ(EE)IEC mice displayed no morphological changes in the colon, although a small number of these mice had modest mucosal infiltration with mononuclear cells in the mid-to-distal colon without epithelial ulceration. Submucosa and muscularis layers were not affected in the small intestine or colon of the Tg mice. Infiltration was apparent within 3 wk after birth, but did not progress over time. Even in mice older than 1 yr, small intestinal villi were intact without transmural infiltration or granulomas, and no spontaneous intestinal adenomas or carcinomas were detected. IKKβ(EE)IEC mice did not show any sign of diarrhea, and stool weight and water content did not differ between WT and Tg mice. There was no breakdown of the epithelial barrier as measured by fluorescein-dextran uptake (Fig. S1 c).
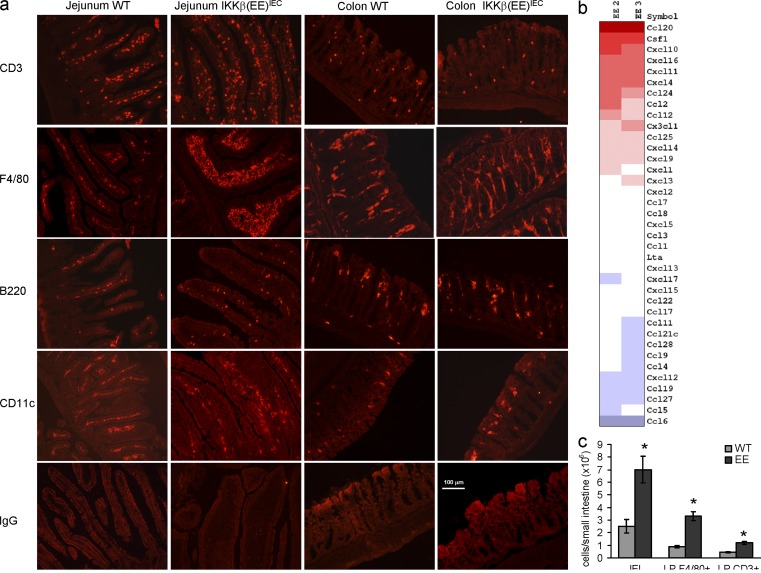
Immunofluorescence studies showed an influx of mainly F4/80+ and CD3+ cells into the small intestinal LP of IKKβ(EE)IEC mice (Fig. 2 a), paralleling increased expression of chemokines and cytokines important for macrophage and T cell recruitment and survival, such as CCL20, CCL2, CXL10, and M-CSF (Fig. 2 b and Fig. 1 e). Small intestinal intraepithelial lymphocytes (IELs) and CD3+ and F4/80+ cells in the LP were increased by 2- and 3.5-fold, respectively, in IKKβ(EE)IEC mice (Fig. 2 c). IKKβ(EE)IEC LP F4/80+ cells showed a mild increase in expression of several inflammatory cytokines (Fig. S2 a). TNF and IL-6 secretion elicited by stimulation with TLR ligands (CpG and LPS) was modestly increased relative to WT cells (Fig. S2 b). Yet, expression of several immunoregulatory molecules, including IL-10 and PD-L1, which have been suggested to maintain mucosal tolerance (Denning et al., 2007), did not differ between WT and Tg mice (Fig. S2, b and c). We also detected a modest increase in expression of other known regulators of inflammation, such as IL-12 or IL-23, in small intestinal extracts. This might be related to the increased number of F4/80+ LP cells in IKKβ(EE)IEC mice (Fig. S2 d). Small intestinal IELs and LP lymphocytes (LPLs) did not show increased cytokine (e.g., IFN-γ) staining (Fig. S2 e), or any major differences in their subpopulation distribution between WT and Tg mice (Table I), including Foxp3+ regulatory T cells, which have also been suggested to play a role in the maintenance of intestinal tolerance (Izcue et al., 2006). Immune responses to oral antigens and oral tolerance were also not altered in IKKβ(EE)IEC mice (Fig. S2, f and g). In summary, persistent NF-κB activation in IECs results in enhanced localized macrophage and T cell infiltration, but does not cause destructive symptomatic intestinal inflammation, massive TNF production, or altered lymphocyte homeostasis or responsiveness.
Figure 2.
The IKKβ(EE)IEC small intestinal LP is infiltrated with F4/80 and CD3+ cells. (a) Frozen sections of WT or IKKβ(EE)IEC jejunum and colon were stained for the indicated markers and visualized by immunofluorescence. Representative of five experiments. (b) RNAs from WT or IKKβ(EE)IEC enterocytes were subjected to gene expression profiling using whole-genome oligonucleotide arrays (Agilent). Shown are the expression levels of chemokine genes. Positive or negative values represent fold changes between IKKβ(EE)IEC mice and littermate controls (n = 2). (c) Small intestinal IELs and LPLs were isolated from WT or IKKβ(EE)IEC mice and counted. Percentages of F4/80 and CD3+ cells were determined by flow cytometry in cells gated for CD45. Results are means ± SEM (n = 6). *, P < 0.05 versus WT. Data are representative of three independent experiments.
Table I.
Sizes of Peyer’s patch, MLN, IEL, and LPL subpopulations
| WT | EE | |
| PP | ||
| CD4+ | 11.25 ± 1.3 | 12 ± 1.4 |
| CD8+ | 3.75 ± 0.5 | 3.4 ± 0.3 |
| B220+ | 86 ± 5.1 | 87 ± 7.2 |
| CD11c+ | 0.5 ± 0.1 | 0.3 ± 0.2 |
| MLN | ||
| CD4+ | 31 ± 2.5 | 30 ± 1.7 |
| CD8+ | 21 ± 1.8 | 20 ± 1.2 |
| B220+ | 26 ± 3.5 | 28 ± 2.1 |
| CD11c+ | 4 ± 0.5 | 3 ± 0.7 |
| IELs | ||
| Percentage within CD3+ cells: | ||
| TCRαβ+ | 45.2 ± 3.4 | 45.1 ± 2.3 |
| TCRγδ+ | 54.8 ± 4.5 | 54.9 ± 4.3 |
| Percentage within TCRαβ+ cells: | ||
| CD8αα+ CD8αβ- | 41.2 ± 2.3 | 37 ± 3.4 |
| CD8αβ+ | 30.1 ± 1.5 | 30.2 ± 2.5 |
| CD4+ | 24 ± 1.3 | 22 ± 1.5 |
| Percentage within TCRγδ+ cells: | ||
| CD8αα+ CD8αβ- | 83 ± 3.2 | 82 ± 2.4 |
| CD8αβ+ | 4.1 ± 1.1 | 4.2 ± 0.9 |
| LPLs | ||
| Percentage within TCRαβ+ cells: | ||
| CD8αβ+ | 15.3 ± 1.4 | 12.7 ± 2.8 |
| CD4+ | 78.2 ± 3.4 | 78.4 ± 2.9 |
| CD4+Foxp3+ | 17.2 ± 2.3 | 18.1 ± 3.2 |
Peyer’s patches (PP), MLN, IEL, and LPL subpopulations. Isolated cells from MLN, small intestinal PP, LPLs and IELs of WT or IKKβ(EE)IEC (n = 4) were stained with labeled antibodies and analyzed by FACS.
Small intestinal apoptosis and enhanced mortality is associated with elevated TNF production in challenged IKKβ(EE)IEC mice
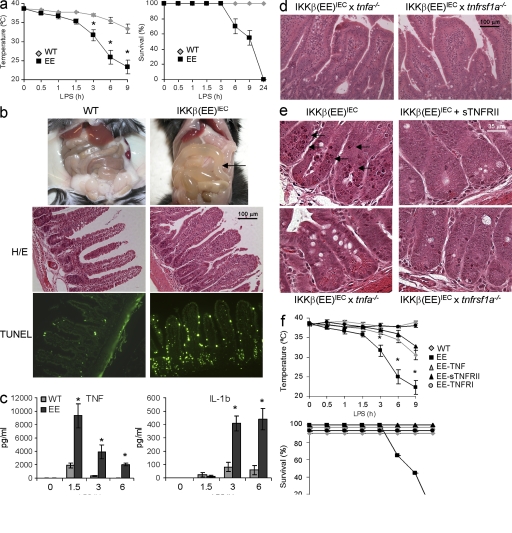
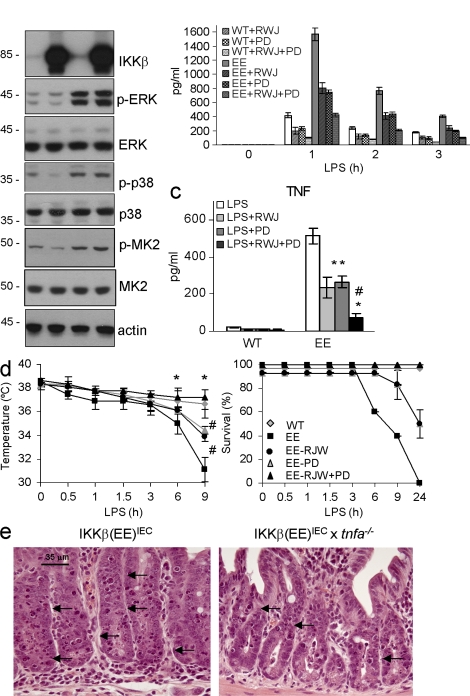
We next examined whether constitutive NF-κB activation protects the epithelium from inflammatory challenge. Unexpectedly, IKKβ(EE)IEC mice were extremely sensitive and exhibited considerable mucosal damage upon challenge with all tested stimuli. Administration of a dose of LPS that did not cause mortality in WT mice led to endotoxic shock, with a large and rapid drop in body temperature, and high mortality in IKKβ(EE)IEC mice (Fig. 3 a). Even a low LPS dose (0.1 mg/kg) induced severe small intestinal injury characterized by fluid-filled dilations (Fig. 3 b), apoptosis, which was most severe in crypts, and shortening of villi (Fig. 3 b). This occurred despite the enhanced expression of antiapoptotic genes in IKKβ(EE)IEC IECs (Fig. S3 a). Inflammatory cytokines, especially TNF, were substantially elevated in LPS-challenged IKKβ(EE)IEC mice (Fig. 3 c). Given the marked increase in TNF, we examined its role in the enhanced susceptibility of IKKβ(EE)IEC mice to LPS challenge. Although IKKβ(EE)IEC mice lacking TNF (IKKβ[EE]IEC x tnf−/−) or TNFR1 (IKKβ[EE]IEC x tnfrsf1−/−) exhibited similar histology and gene expression profiles to unchallenged IKKβ(EE)IEC mice, including immune infiltration of the LP (Fig. 3 d and Fig. S3 b), they were fully protected from LPS-induced tissue damage (Fig. 3 e) and endotoxic shock (Fig. 3 f). Soluble TNFRII (Enbrel) was also protective (Fig. 3, e and f). IL-6 and IL-1β were also elevated in LPS-challenged IKKβ(EE)IEC mice, but neither IL-6 ablation (IKKβ[EE]IEC x il6−/−) nor treatment with an IL-1 receptor antagonist (Anakinra) protected Tg mice from LPS-induced damage (Fig. S3, d and e). Mice were also treated with a cocktail of antibiotics to determine the role of the intestinal microbiota in the exacerbated mucosal responses of IKKβ(EE)IEC mice, but the antibiotics treatment did not protect Tg mice from LPS-induced apoptosis.
Figure 3.
Small intestinal apoptosis and increased endotoxin-induced mortality associated with elevated TNF production in IKKβ(EE)IEC mice. (a) Rectal temperature and survival of WT (gray diamonds) and IKKβ(EE)IEC (black squares) mice after LPS (5 mg/kg E. coli O111:B4) injection. Results are means ± SEM (n = 8). *, P < 0.05 versus WT. Data are pooled from three independent experiments. (b) Abdomens of WT and IKKβ(EE)IEC mice 4 h after LPS (1 mg/kg) injection. Arrow points at fluid-filled dilated small bowel in Tg mice. Jejunal sections were stained with either H&E (top) or by an in situ TUNEL assay (bottom). Representative of 10 experiments. (c) Circulating TNF and IL-1β after LPS administration. Results are means ± SEM (n = 4). *, P < 0.05 versus WT. Data are representative of three independent experiments. (d) Intestinal histology of IKKβ(EE)IEC x tnfa−/− and IKKβ(EE)IEC x tnfrsf1a−/− mice. Jejunal sections were H&E stained. Representative of five experiments. (e) The indicated mouse strains were analyzed 4 h after LPS injection with or without an accompanying dose of Enbrel (sTNFRII) as indicated. Small intestinal sections were stained with H&E. Representative of three experiments. (f) Rectal temperature and survival of WT (gray diamond), IKKβ(EE)IEC (black square), IKKβ(EE)IEC x tnfsrf1−/− (black circle), IKKβ(EE)IEC x tnf−/− (gray triangle), IKKβ(EE)IEC + sTNFRII (black triangle) mice after LPS injection. Results are means ± SEM (n = 3). Survival of WT, IKKβ(EE)IEC x tnfsrf1−/− (black circle), IKKβ(EE)IEC x tnf−/− (gray triangle), and IKKβ(EE)IEC + sTNFRII (black triangle) is 100%. *, P < 0.05 versus IKKβ(EE)IEC. Data are representative of three independent experiments.
Elevated TNF production and mortality in challenged IKKβ(EE)IEC mice depend on MAPK activation
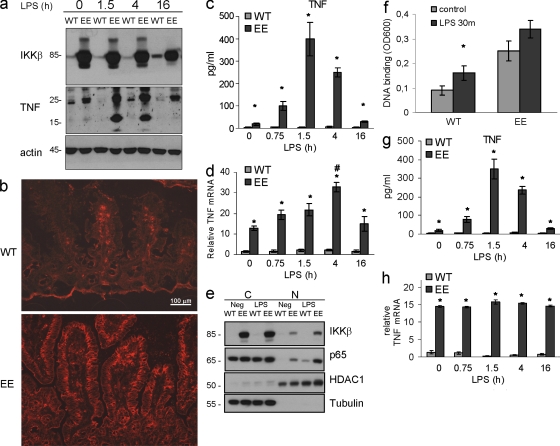
To identify sources of TNF in LPS-challenged IKKβ(EE)IEC mice, we isolated LP F4/80+ cells before and after LPS challenge, and found that their TNF mRNA expression did not differ between Tg and WT mice, although IL-6 and IL-12 mRNAs were higher in Tg cells (Fig. S4). Yet, the number of F4/80+ LP cells was increased in IKKβ(EE)IEC mice, suggesting that they may contribute to total circulating TNF. In contrast, membrane-associated and especially soluble TNF were dramatically increased in IECs of LPS-challenged IKKβ(EE)IEC mice (Fig. 4, a–c). Despite the threefold increase in TNF mRNA (Fig. 4 d), LPS administration led to only a small further increase of p65 nuclear translocation and DNA binding above the basal level of nonstimulated IKKβ(EE)IEC enterocytes (Fig. 4, e and f). A lower LPS dose increased TNF protein production by Tg enterocytes without causing any further increase in its mRNA amounts, but did not elicit TNF production by WT IECs (Fig. 4, g and h).
Figure 4.
Membrane-associated and especially soluble TNF are dramatically increased in IECs of LPS-challenged IKKβ(EE)IEC mice. (a) Lysates of small intestinal enterocytes were prepared when indicated after LPS injection and analyzed for TNF expression by immunoblotting (transmembrane, 26 kD; soluble, 17 kD). Representative of three experiments. (b) Frozen sections of WT or IKKβ(EE)IEC jejuna prepared 2 h after LPS injection were stained with anti-TNF antibody and visualized by immunofluorescence. Representative of three experiments. (c) Lysates of small intestinal enterocytes (75 µg protein) of indicated genotypes were prepared when indicated after LPS injection and analyzed for TNF content by ELISA. Results are means ± SEM (n = 4). *, P < 0.05 versus WT. Data are representative of four independent experiments. (d) RNAs from small intestinal enterocytes of indicated genotypes were prepared when indicated after LPS injection and analyzed in triplicates by Q-RT-PCR for TNF mRNA and normalized to Hprt mRNA. *, P < 0.05 versus WT; #, P < 0.05 versus other time points in IKKβ(EE)IEC mice. Results are means ± SEM (n = 4). Data are representative of four independent experiments. (e and f) LPS administration results in only a mild increase of p65 nuclear translocation and DNA binding in IKKβ(EE)IEC enterocytes. (e) WT and IKKβ(EE)IEC enterocytes were isolated 30 min after LPS injection and separated into cytosolic (C) and nuclear (N) fractions that were analyzed for their content of the indicated proteins by immunoblotting. Representative of two experiments. (f) Nuclear extracts of WT and IKKβ(EE)IEC enterocytes isolated 30 min after LPS injection were analyzed for p65 DNA binding by ELISA. Results are means ± SEM (n = 3). *, P < 0.05 versus control. Data are representative of two independent experiments. (g and h) Low LPS dose administration induces TNF protein production by Tg enterocytes without a further increase in its mRNA amounts. (g) Lysates of small intestinal enterocytes (75 µg protein) of indicated genotypes prepared when indicated after low LPS dose (0.2 mg/kg) injection were analyzed for TNF content by ELISA. *, P < 0.05 versus WT. Results are means ± SEM (n = 3). Data are representative of three independent experiments. (h) RNAs from small intestinal IECs prepared when indicated after low LPS dose (0.2 mg/kg) injection were analyzed in triplicates by Q-RT-PCR for TNF mRNA amounts and normalized to Hprt mRNA. Results are means ± SEM (n = 3). *, P < 0.05 versus WT. Data are representative of three independent experiments.
These results suggested that although NF-κB is activated in Tg enterocytes and is sufficient for increasing basal TNF gene transcription, accumulation of TNF mRNA and especially protein may depend on posttranscriptional events. MAPK signaling pathways, both p38 MAPK and ERK, play important roles in posttranscriptional regulation of TNF production (Kotlyarov et al., 1999; Dumitru et al., 2000; Rousseau et al., 2008; Xu and Derynck, 2010; Gais et al., 2010). LPS administration enhanced p38, MK2, and ERK phosphorylation in IECs of both WT and Tg mice (Fig. 5 a). TNF-α–converting enzyme (TACE) activity was also enhanced after LPS administration, but the extent of elevation was similar in both groups of mice (Fig. S5 a). Oral administration of RWJ 67657, a p38 MAPK inhibitor (Wadsworth et al., 1999), and PD184352, a specific MEK-1/2 inhibitor, decreased phosphorylation of the corresponding MAPKs and their targets (Fig. S5 b), serum TNF (Fig. 5 b), and its release from enterocytes (Fig. 5 c), and protected IKKβ(EE)IEC mice from endotoxic shock (Fig. 5 d). These results suggest that additional activation of both MAPK pathways on top of the already active NF-κB is necessary to induce TNF production and epithelial damage in IKKβ(EE)IEC mice. We also examined whether IKKβ(EE)IEC IECs were more sensitive to TNF-induced killing than WT IECs. Exogenous administration of TNF induced IEC apoptosis in IKKβ(EE)IEC x tnf−/− mice, although to a lesser extent than in IKKβ(EE)IEC mice (Fig. 5 e; 3.6 ± 0.8 vs. 8.7 ± 1.2 apoptotic cells per crypt; P < 0.001), but did not induce apoptosis in tnf−/− and WT mice.
Figure 5.
Elevated TNF production in LPS-challenged IKKβ(EE)IEC mice and mortality depend on MAPK activation. (a) Lysates from small intestinal enterocytes prepared 30 min after LPS challenge were analyzed for expression and phosphorylation of the indicated proteins by immunoblotting. Representative of three experiments. (b) Circulating TNF after LPS injection (0.2 mg/kg) with or without RWJ67657 (RWJ) or PD184352 (PD). Results are means ± SEM (n = 3). *, P < 0.05 versus IKKβ(EE)IEC; #, P < 0.05 versus IKKβ(EE)IEC with RWJ or PD. Data are representative of three independent experiments. (c) Small intestinal enterocyte lysates (75 µg protein) of indicated genotypes were prepared 90 min after LPS injection (0.2 mg/kg) with or without RWJ67657 (RWJ) or PD184352 (PD) and analyzed for TNF content by ELISA. Results are means ± SEM (n = 3). *, P < 0.05 versus IKKβ(EE)IEC; #, P < 0.05 versus IKKβ(EE)IEC with RWJ or PD. Data are representative of three independent experiments. (d) Rectal temperature and survival after LPS injection (0.2 mg/kg) with or without RWJ67657 (RWJ) or PD184352 (PD). Results are means ± SEM (n = 6). WT (gray diamond), IKKβ(EE)IEC (black square), IKKβ(EE)IEC + RWJ (black circle), IKKβ(EE)IEC + PD (gray triangle), and IKKβ(EE)IEC + RWJ + PD (black triangle). Survival of WT (gray diamond) and IKKβ(EE)IEC + RWJ + PD (black triangle) is 100%, and survival of IKKβ(EE)IEC + RWJ (black circle) and IKKβ(EE)IEC + PD (gray triangle) is 100% up to 6 h. *, P < 0.05 for WT and IKKβ(EE)IEC + RWJ + PD versus IKKβ(EE)IEC; #, P < 0.05 for IKKβ(EE)IEC versus IKKβ(EE)IEC + RWJ or PD, and WT and IKKβ(EE)IEC + RWJ + PD versus IKKβ(EE)IEC + RWJ or PD. Data are pooled from two independent experiments. (e) The indicated mouse strains were analyzed 4 h after TNF administration (mice were injected i.v. with 2 µg of recombinant murine TNF). Small intestinal sections were stained with H&E. Arrows point at apoptotic cells. Representative of three experiments.
Citrobacter rodentium infection and CD3 activation cause loss of barrier function in IKKβ(EE)IEC mice
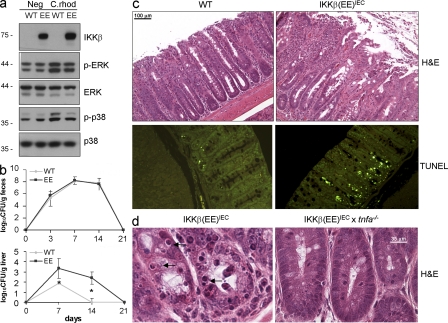
To further investigate the effect of chronic NF-κB activation on maintenance of intestinal barrier function and innate immunity, we challenged IKKβ(EE)IEC mice with the Gram-negative bacterium C. rodentium, an attaching and effacing (A/E) pathogen that colonizes the mouse colon, activates MAPK signaling in colonic IECs (Fig. 6 a), and typically leads to crypt hyperplasia and mucosal inflammation before its clearance from immunocompetent mice (Borenshtein et al., 2008). Tg and WT mice had similar fecal bacterial counts after C. rodentium infection (Fig. 6 b), indicating similar bacterial burden, immune response, and bacterial clearance. However, Tg mice had greater colonic mucosal ulceration and crypt apoptosis (Fig. 6 c) and higher liver bacterial counts than their WT counterparts (Fig. 6 b), suggesting that epithelial barrier disruption enhanced systemic bacterial spread. IKKβ(EE)IEC x Tnf−/− mice were protected from the increase in C. rodentium–induced colon damage (Fig. 6 d) and subsequent bacterial translocation, as the log10CFU/gram liver for WT, IKKβ(EE)IEC, and IKKβ(EE)IEC x Tnf−/− mice at 14 d after infection were 0, 3.2 ± 0.5, and 0.8 ± 0.35, respectively. IKKβ(EE)IEC mice were also extremely sensitive to oral administration of the irritant dextran sulfate salt (DSS), which causes acute intestinal inflammation, as they developed much more severe colonic ulceration and displayed increased mortality compared with WT mice (Fig. S6, a and b). This effect was again dependent on TNF, as IKKβ(EE)IEC x Tnf−/− mice were partially protected against acute DSS-induced colitis, as shown in Fig. S6 c.
Figure 6.
C. rodentium infection causes loss of barrier function and enterocyte apoptosis in IKKβ(EE)IEC mice. (a) Lysates of small intestinal WT and IKKβ(EE)IEC enterocytes prepared 6 d after C. rodentium challenge were analyzed for expression and phosphorylation of the indicated proteins by immunoblotting. Representative of four experiments. (b) C. rodentium counts in stool and liver were determined by CFU assay. Results are means ± SEM (n = 8). *, P < 0.05 versus WT. Data are representative of three independent experiments. (c) WT and IKKβ(EE)IEC mice were analyzed 14 d after C. rodentium infection. Colon sections were stained with either H&E (top) or by an in situ TUNEL assay (bottom). Representative of three experiments. (d) Indicated mouse strains were analyzed 14 d after C. rodentium infection. Colon sections were H&E stained. Arrows point at apoptotic cells. Representative of two experiments.
Intestinal inflammation is associated in some diseases with exaggerated and poorly controlled T cell–mediated immune responses (Kaser et al., 2010). To mimic antigen stimulation of small intestinal T cells, we injected mice with a low dose of a CD3-activating antibody that triggers only minimal enterocyte apoptosis and small intestinal damage in normal mice (Miura et al., 2005). In IKKβ(EE)IEC mice, however, this treatment caused massive enterocyte apoptosis, followed by villous shortening (Fig. 7 a) and a rapid drop in body temperature (Fig. 7 b), as well as increased TNF protein amounts in serum (Fig. S7 a) and release by IECs without a further increase in TNF mRNA (Fig. 7, c and d). As seen with LPS, anti-CD3 administration caused MAPK activation in enterocytes from both WT and Tg mice (Fig. 7 e). No significant differences in intracellular cytokines were found between Tg and WT lymphocytes after anti-CD3 injection (Fig. S7, b and c). Yet, other immune cells that are indirectly activated may also contribute to TNF secretion or IEC apoptosis. Ablation of TNF or TNFR1 or Enbrel administration protected IKKβ(EE)IEC mice from anti-CD3–induced tissue damage and pharmacological inhibition of p38 partially decreased enterocyte TNF production (Fig. 7 f and Fig. S7 d).
Figure 7.
CD3 activation causes loss of barrier function and enterocyte apoptosis in IKKβ(EE)IEC mice. (a) Indicated mouse strains were analyzed 4 and 16 h after anti-CD3 (12.5 µg/mouse) administration. Jejunal sections analyzed at 4 h were stained with either H&E (top) or by in situ TUNEL assay (bottom). Jejunal sections analyzed at 16 h were H&E stained. Representative of four experiments. (b) Rectal temperature of WT (gray diamonds) and IKKβ(EE)IEC (black squares) mice after anti-CD3 injection. Results are means ± SEM (n = 4). *, P < 0.05 versus WT. Data are representative of three independent experiments. (c) Lysates of small intestinal IECs were prepared 2.5 h after CD3 injection and analyzed (75 µg protein) for TNF content by ELISA. Results are means ± SEM (n = 4). *, P < 0.05 versus WT; # P < 0.05 versus time 0. Data are representative of three independent experiments. (d) RNAs from small intestinal WT or IKKβ(EE)IEC IECs were prepared 1.5 h after CD3 injection and analyzed in triplicates by Q-RT-PCR for TNF mRNA amounts that were normalized to Hprt mRNA. Results are means ± SEM (n = 3). *, P < 0.05 versus WT; #, P < 0.05 versus time 0. Data are representative of three independent experiments. (e) WT and IKKβ(EE)IEC enterocytes lysates prepared 40 and 60 min after anti-CD3 injection were analyzed for expression and phosphorylation of the indicated proteins by immunoblotting. Representative of three experiments. (f) Small intestinal enterocyte lysates (75 µg protein) were prepared 90 min after anti-CD3 injection (6.25 µg/mouse) in the absence or presence of RWJ 67657 and analyzed for TNF content by ELISA. Results are means ± SEM (n = 3). *, P < 0.05 versus IKKβ(EE)IEC. Data are representative of three independent experiments.
DISCUSSION
NF-κB has been implicated in the pathogenesis of many inflammation-related diseases (Tak and Firestein, 2001; Pasparakis, 2009), and genetic manipulations that lead to increased NF-κB activity often trigger inflammation-related pathologies (Beg et al., 1995; Klement et al., 1996). Other studies confirmed that NF-κB inhibition has antiinflammatory effects in vivo (Greten et al., 2004; Broide et al., 2005). However, several recent studies have surprisingly shown that NF-κB inhibition in nonimmune, epithelial, or parenchymal cells triggers the spontaneous development of severe inflammatory conditions (Pasparakis, 2009). In IECs, for instance, NF-κB mainly plays a protective and antiinflammatory role. IEC-specific ablation of the NEMO/IKKγ regulatory subunit, or of IKKβ, resulted in complete or partial loss of barrier function, bacterial translocation, and severe inflammation (Chen et al., 2003; Egan et al., 2004; Greten et al., 2004; Chae et al., 2006; Nenci et al., 2007). The ability of NF-κB to maintain barrier function and immune homeostasis may depend on suppression of enterocyte death and expression of barrier function genes and defensins. This function may be essential, as the intestinal epithelium has an important function in the maintenance of intestinal immune homeostasis. By forming a mechanical barrier and producing antimicrobial peptides, IECs prevent bacteria from invading the mucosa, where they could encounter immune cells and induce inflammation.
Our results suggest that persistent NF-κB activation in IECs is sufficient for expression of large amounts of inflammatory chemokines, dramatic mononuclear cell infiltration into the small intestinal LP, and Tnf gene transcription, but is insufficient for substantial production of TNF protein and therefore cannot drive symptomatic intestinal inflammation by itself. Consequently, IKKβ(EE)IEC mice did not show any sign of diarrhea or intestinal bleeding, and their survival did not differ from WT littermates. The noteworthy absence of epithelial barrier breakdown despite the overall elevated mucosal cytokine levels could support the concept of a protective role of intestinal epithelial NF-κB. Increased expression of antiapoptotic genes together with the low production of TNF protein by the unchallenged IKKβ(EE)IEC IECs could shift the balance toward maintenance of the barrier and intestinal homeostasis.
However, IKKβ(EE)IEC mice exposed to relatively mild inflammatory and immune stimuli, at doses that trigger only modest responses in WT mice, showed epithelial apoptosis, intestinal barrier disruption, and subsequent bacterial translocation. These unexpected results are counterintuitive, given the role of NF-κB in maintenance of barrier function and control of bacterial translocation. These stimuli, which activate additional signaling pathways, especially MAPK, greatly enhanced TNF production in IKKβ(EE)IEC mice and led to a widespread epithelial apoptosis and disruption of intestinal integrity. However, neither LPS, which signals via TLR4, which is expressed mainly on macrophages and dendritic cells, nor anti-CD3, which activates TCR, can directly activate MAPKs in IECs. Most likely, they induce production of a cytokine, possibly TNF itself, which acts on IECs to activate MAPKs. Curiously, none of the cytokines produced by IKKβ(EE)IEC enterocytes are sufficient for autocrine or paracrine activation of MAPKs in these cells. IKKβ(EE)IEC enterocytes were also sensitive to circulating TNF, suggesting that persistent NF-κB activation up-regulates other proapoptotic pathways that, together with the increase in TNF production, shift the balance toward apoptosis.
We speculate that a similar mechanism may underlie the pathogenesis of chronic inflammatory disorders with relapsing acute flare-ups, such as inflammatory bowel diseases, psoriasis, or rheumatoid arthritis, in which relatively quiescent periods without overt symptomatic inflammation erupt into acute inflammatory flare-ups upon encounter of poorly defined environmental challenges. In these disorders, either direct or indirect chronic NF-κB activation was observed in nonimmune cells such as IECs, keratinocytes, and synoviocytes (Li and Verma, 2002), single nucleotide polymorphisms in NF-κB pathway components were found (Vereecke et al., 2009). In addition, these disorders respond to anti-TNF therapy (Sfikakis, 2010). The new findings may also explain microbial translocation in patients and animals experiencing gut insults that increase NF-κB and cause massive TNF production, such as intestinal ischemia, necrotizing enterocolitis and obstruction (Chen et al., 2003). Our results may also be relevant to the increased translocation of enteric bacteria seen in AIDS patients, who are known to have elevated TNF (Stockmann et al., 2000). Given the increase in IL-1β–driven inflammation caused by IKKβ inhibition (Greten et al., 2007; Hsu et al., 2011), inhibition of MAPKs (ERK and p38) or anti-TNF therapy may be the preferred treatments for such disorders.
MATERIALS AND METHODS
Mice.
IKKβEE cDNA (Delhase et al., 1999) was PCR-amplified and inserted into a plasmid containing the 12.4-kb Villin promoter (D. Gumucio, University of Michigan, Ann Arbor, MI; Madison et al., 2002). The 15.7-kb expression cassette was excised by a PmeI digest, purified, and injected into fertilized C57BL/6 oocytes to derive founder mice, one of which transmitted the IkkβEE transgene. Tg mice were crossed to tnf−/−, tnfrsf1−/−, and il6−/− mutants, also in the C57BL/6 background, purchased from The Jackson Laboratory. Mice used in the experiments were 8–12 wk old and were maintained under standard conditions at a University of California at San Diego animal facility accredited by the American Association for Accreditation of Laboratory Animal Care. All animal protocols were approved by the institutional review board, following National Institutes of Health guidelines.
Reagents.
LPS (Escherichia coli O111:B4; Sigma-Aldrich) was injected i.p. at a dose of 5 mg/kg unless otherwise indicated. Recombinant mouse TNF from R&D Systems was injected i.v. at dose of 2 µg/mouse. Antibody against CD3 (clone 145-2C11; BD) was injected i.p. at a dose of 12.5 µg/mouse, unless otherwise indicated. RWJ 67657 (Wadsworth et al., 1999; p38 MAPK inhibitor) and PD184352 (MEK-1/2 inhibitor) were purchased from Tocris Bioscience and Selleck Chemicals LLC, respectively, diluted in 0.1 N HCl, and administrated by oral gavage (50 mg/kg). Enbrel (sTNFRII; Amgen) was administrated i.v. at 5 mg/kg. IL-1 receptor antagonist (IL-1Ra or Anakinra; Amgen) was administered i.v. at 300 mg/kg. Drugs were administrated 30 min before challenge.
Flow cytometry, intracellular cytokine staining, and cell sorting.
For intracellular cytokine staining, surface staining was performed for 25 min with the corresponding cocktail of fluorescently labeled antibodies. After surface staining, cells were resuspended in Fixation/Permeabilization solution (Cytofix/Cytoperm kit; BD), and intracellular cytokine and FoxP3 staining was performed according to the manufacturer’s protocol. For cell sorting, a FACS Diva cell sorter (BD) was used. Data were analyzed using FlowJo software (Tree Star).
Histological analysis and TUNEL assay.
Intestines were removed, opened longitudinally, cleaned, processed as “Swiss rolls,” and fixed in 10% phosphate-buffered formalin for 24 h. Fixed tissues were embedded in paraffin, and 5-µm sections were prepared and stained with hematoxylin and eosin (H&E). For immunofluorescence studies, tissues were frozen in OCT compound, cut on a cryomicrotome, air-dried, and fixed in acetone. The sections were incubated overnight at 4°C with antibodies against F4/80 (AbD Serotec), CD11c, B220 (BD), CD3, and TNF (Abcam). In situ TUNEL assay was performed using ApoAlert DNA fragmentation assay kit (Takara Bio Inc.). Samples were examined under a microscope (Axioplan 2; Carl Zeiss, Inc.).
Expression array profiling.
Total RNA (isolated by RNeasy kit; QIAGEN) was prepared from enterocytes obtained from small intestines of IKKβ(EE)IEC and WT littermates. Purified RNA, 0.5 µg per sample, was labeled using the LRILAK PLUS two-color low RNA input Linear Amplification kit, and then hybridized to a mouse whole-genome microarray 4 × 44K 60 mer slide according to the manufacturer’s instructions (Agilent). Two biological replicates were performed for each experimental condition. GEO submission number is GSE29701.
Isolation of IELs and LPLs.
IELs and LPLs were isolated as previously described (Mucida et al., 2007).
Immunoblotting.
Whole enterocyte extracts were obtained by lysing cells in 1% SDS and 10 mM Tris-HCl (pH 7.4). Cytoplasmic and nuclear extracts were obtained as previously described (Rius et al., 2008). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes that were incubated with antibodies against IKKβ (Millipore); IκBα, IκBβ, IκBε, p65, RelB, p50, HDAC1, p38, villin (Santa Cruz Biotechnology, Inc.); TNF (R&D Systems); phospho-MK2, MK2, phospho-p38, phospho-ERK, and ERK1/2 (Cell Signaling Technology); and actin and tubulin (Sigma-Aldrich).
p65 DNA binding.
Nuclear extracts were obtained as previously described (Rius et al., 2008) and analyzed for p65 DNA binding by ELISA (Cayman Chemical) according to the manufacturer’s instructions.
ChIP.
Epithelium from small intestine was scraped into 1% formaldehyde solution and fixed for 10 min at room temperature. Chromatin was precipitated with a p65 antibody (Santa Cruz Biotechnology Inc., San Jose, CA, sc-372) as described (Lee et al., 2006). Precipitated material was analyzed by Q-RT-PCR. Primer sequences are available upon request. Although not shown, we have confirmed the results of the anti-p65 ChIP with an anti-PolII antibody (Santa Cruz Biotechnology Inc., San Jose, CA, sc-899).
In situ hybridization.
Intestinal paraffin sections were hybridized to anti-TNF probe (IMAGE clone #8860739) at 65°C for 72 h as described (Gregorieff et al., 2005).
C. rodentium infection.
Mice were infected (5 × 108 bacteria) by oral gavage. Bacterial counts were determined as previously described (Spehlmann et al., 2009).
Intestinal permeability in vivo.
In vivo permeability assay to assess barrier function was performed using a FITC-labeled dextran method. Food and water were withdrawn for 4 h, and mice were gavaged with permeability tracer (60 mg/100 g body weight of FITC-labeled dextran; FD-4; Sigma-Aldrich). Serum was collected retroorbitally 4 h after FD-4 gavage, and fluorescence intensity of each sample was measured (excitation, 492 nm; emission, 525 nm).
Kinase assays.
Enterocytes were lysed and immunoprecipitated with an anti-IKKγ antibody (BD). The immune complexes were incubated with GST-IκBα(1–79) in the presence of γ[32P]ATP. Proteins were resolved by SDS-PAGE and transferred to a nitrocellulose membrane that was exposed to x-ray film and hybridized with antibodies against IKKβ (Millipore) and IKKγ (Santa Cruz Biotechnology, Inc.).
Intestinal antigen uptake and processing.
Naive CD4+ T cells were isolated from spleens and mesenteric LNs (MLNs) of OT-2/CD90.1+ mice via negative selection (MACS; Miltenyi Biotec). The purity of CD4+ cells exceeded 80%. Cells were labeled with 5 µM CFSE for 5 min at room temperature, washed twice with PBS, and i.v. injected into either IKKβ(EE)IEC or WT recipient mice. Mice were fed with oral OVA: high dose (20% OVA diet; Research Diets Inc.) or low dose (0.1% grade II OVA; Sigma-Aldrich) in nonacidified drinking water. On day 5, MLNs were analyzed for the presence of proliferating CD90.1/CD4+ T cells.
OVA oral tolerance.
Both WT and IKKβ(EE)IEC mice were fed with 20% OVA diet (Research Diets Inc.) for 2 d. On day 7, mice were immunized by a s.c. tail-base injection of 50 µg of grade V OVA in 100 µl of complete Freud’s adjuvant (Sigma-Aldrich). On day 22, anti-OVA antibody concentrations in serum were determined by ELISA.
TACE activity.
Enterocytes were lysed and analyzed for TACE activity by SensoLyte 520 TACE (α-Secretase) Activity Assay kit Fluorometric (Anaspec) according to the manufacturer’s instructions.
Statistical analysis.
Data are expressed as mean ± SEM. The analysis used unpaired Student’s t test for comparing two groups and ANOVA for multiple group comparisons. Bonferroni post-hoc tests were used for multiple pairwise comparisons. All statistical analyses were performed using the SPSS 15.0 (SPSS) statistical package. Results were considered significant at P < 0.05.
Online supplemental material.
Fig. S1 shows that IKKβ(EE)IEC mice exhibit persistent NF-κB target gene expression, but no tissue damage. Fig. S2 shows that persistent NF-κB activation in IECs does not cause altered lymphocyte homeostasis and responsiveness. Fig. S3 shows that TNF or TNFR1 deficiencies in IKKβ(EE)IEC mice do not alter intestinal histology or gene expression, and IL6 ablation and IL-1 receptor antagonist (Anakinra) treatment does not protect IKKβ(EE)IEC mice from intestinal apoptosis after LPS injection. Fig. S4 shows that TNF expression in small intestinal F4/80+ LP cells after LPS injection does not differ between WT and IKKβ(EE)IEC. Fig. S5 shows that TACE activity does not differ between WT and IKKβ(EE)IEC. Fig. S6 shows that DSS exposure cause an increase of colonic damage and high mortality in IKKβ(EE)IEC mice. Fig. S7 shows that WT or IKKβ(EE)IEC small intestinal T cells do not exhibit significant differences in cytokine production after anti-CD3 injection, and anti-TNF therapy protects IKKβ(EE)IEC mice from anti-CD3–induced tissue damage. Table S1 shows the expression profiles of WT and IKKβ(EE)IEC IECs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110242/DC1.
Acknowledgments
We are grateful to Dr. D. Gumucio from the University of Michigan for the plasmid containing the 12.4 kb Villin promoter.
M. Guma was supported by the Arthritis Foundation. M.E. Spehlmann was supported by postdoctoral fellowships from the German Research Foundation and Mucosaimmunologie gemeinnützige Forschungsgesellschaft Educative Science. Work in M. Karin, M.F. Kagnoff, H. Cheroutre, and L. Eckmann’s laboratories was supported by grants from the National Institutes of Health and Wm. K. Warren Foundation. M. Karin is an American Cancer Society Research Professor.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- ChIP
- chromatin immunoprecipitation
- DSS
- dextran sulfate salt
- IEC
- intestinal epithelial cell
- IEL
- intestinal intraepithelial lymphocyte
- IKK
- activated by IκB kinase
- LP
- lamina propria
- LPL
- LP lymphocyte
- MAPK
- mitogen-activated protein kinase
- MLN
- mesenteric LN
- Q-RT-PCR
- quantitative real-time PCR
- TACE
- TNF-α–converting enzyme
References
- Arnalich F., Garcia-Palomero E., López J., Jiménez M., Madero R., Renart J., Vázquez J.J., Montiel C. 2000. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect. Immun. 68:1942–1945 10.1128/IAI.68.4.1942-1945.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P.J., Karin M. 1997. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336:1066–1071 [DOI] [PubMed] [Google Scholar]
- Beg A.A., Sha W.C., Bronson R.T., Baltimore D. 1995. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 9:2736–2746 10.1101/gad.9.22.2736 [DOI] [PubMed] [Google Scholar]
- Böhrer H., Qiu F., Zimmermann T., Zhang Y., Jllmer T., Männel D., Böttiger B.W., Stern D.M., Waldherr R., Saeger H.D., et al. 1997. Role of NFkappaB in the mortality of sepsis. J. Clin. Invest. 100:972–985 10.1172/JCI119648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenshtein D., McBee M.E., Schauer D.B. 2008. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr. Opin. Gastroenterol. 24:32–37 10.1097/MOG.0b013e3282f2b0fb [DOI] [PubMed] [Google Scholar]
- Broide D.H., Lawrence T., Doherty T., Cho J.Y., Miller M., McElwain K., McElwain S., Karin M. 2005. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc. Natl. Acad. Sci. USA. 102:17723–17728 10.1073/pnas.0509235102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae S., Eckmann L., Miyamoto Y., Pothoulakis C., Karin M., Kagnoff M.F. 2006. Epithelial cell I kappa B-kinase beta has an important protective role in Clostridium difficile toxin A-induced mucosal injury. J. Immunol. 177:1214–1220 [DOI] [PubMed] [Google Scholar]
- Chen L.W., Egan L., Li Z.W., Greten F.R., Kagnoff M.F., Karin M. 2003. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat. Med. 9:575–581 10.1038/nm849 [DOI] [PubMed] [Google Scholar]
- Delhase M., Hayakawa M., Chen Y., Karin M. 1999. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 284:309–313 10.1126/science.284.5412.309 [DOI] [PubMed] [Google Scholar]
- Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8:1086–1094 10.1038/ni1511 [DOI] [PubMed] [Google Scholar]
- DiDonato J.A., Hayakawa M., Rothwarf D.M., Zandi E., Karin M. 1997. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 388:548–554 10.1038/41493 [DOI] [PubMed] [Google Scholar]
- Dumitru C.D., Ceci J.D., Tsatsanis C., Kontoyiannis D., Stamatakis K., Lin J.H., Patriotis C., Jenkins N.A., Copeland N.G., Kollias G., Tsichlis P.N. 2000. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 103:1071–1083 10.1016/S0092-8674(00)00210-5 [DOI] [PubMed] [Google Scholar]
- Eckmann L., Kagnoff M.F. 2005. Intestinal mucosal responses to microbial infection. Springer Semin. Immunopathol. 27:181–196 10.1007/s00281-005-0207-5 [DOI] [PubMed] [Google Scholar]
- Edwards M.R., Bartlett N.W., Clarke D., Birrell M., Belvisi M., Johnston S.L. 2009. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol. Ther. 121:1–13 10.1016/j.pharmthera.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan L.J., Eckmann L., Greten F.R., Chae S., Li Z.W., Myhre G.M., Robine S., Karin M., Kagnoff M.F. 2004. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc. Natl. Acad. Sci. USA. 101:2452–2457 10.1073/pnas.0306734101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais P., Tiedje C., Altmayr F., Gaestel M., Weighardt H., Holzmann B. 2010. TRIF signaling stimulates translation of TNF-alpha mRNA via prolonged activation of MK2. J. Immunol. 184:5842–5848 10.4049/jimmunol.0902456 [DOI] [PubMed] [Google Scholar]
- Ghosh S., Karin M. 2002. Missing pieces in the NF-kappaB puzzle. Cell. 109(Suppl):S81–S96 10.1016/S0092-8674(02)00703-1 [DOI] [PubMed] [Google Scholar]
- Gregorieff A., Pinto D., Begthel H., Destrée O., Kielman M., Clevers H. 2005. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 129:626–638 [DOI] [PubMed] [Google Scholar]
- Greten F.R., Eckmann L., Greten T.F., Park J.M., Li Z.W., Egan L.J., Kagnoff M.F., Karin M. 2004. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 118:285–296 10.1016/j.cell.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Greten F.R., Arkan M.C., Bollrath J., Hsu L.C., Goode J., Miething C., Göktuna S.I., Neuenhahn M., Fierer J., Paxian S., et al. 2007. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 130:918–931 10.1016/j.cell.2007.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel M.L., McMorrow L.B., Gravallese E.M. 1995. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 38:1762–1770 10.1002/art.1780381209 [DOI] [PubMed] [Google Scholar]
- Hsu L.C., Enzler T., Seita J., Timmer A.M., Lee C.Y., Lai T.Y., Yu G.Y., Lai L.C., Temkin V., Sinzig U., et al. 2011. IL-1β-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKβ. Nat. Immunol. 12:144–150 10.1038/ni.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A., Coombes J.L., Powrie F. 2006. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 212:256–271 10.1111/j.0105-2896.2006.00423.x [DOI] [PubMed] [Google Scholar]
- Karin M., Lin A. 2002. NF-kappaB at the crossroads of life and death. Nat. Immunol. 3:221–227 10.1038/ni0302-221 [DOI] [PubMed] [Google Scholar]
- Kaser A., Zeissig S., Blumberg R.S. 2010. Inflammatory bowel disease. Annu. Rev. Immunol. 28:573–621 10.1146/annurev-immunol-030409-101225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement J.F., Rice N.R., Car B.D., Abbondanzo S.J., Powers G.D., Bhatt P.H., Chen C.H., Rosen C.A., Stewart C.L. 1996. IkappaBalpha deficiency results in a sustained NF-kappaB response and severe widespread dermatitis in mice. Mol. Cell. Biol. 16:2341–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyarov A., Neininger A., Schubert C., Eckert R., Birchmeier C., Volk H.D., Gaestel M. 1999. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell Biol. 1:94–97 10.1038/10061 [DOI] [PubMed] [Google Scholar]
- Lawrence T. 2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1:a001651 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.I., Johnstone S.E., Young R.A. 2006. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protoc. 1:729–748 10.1038/nprot.2006.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Verma I.M. 2002. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2:725–734 10.1038/nri910 [DOI] [PubMed] [Google Scholar]
- Liu S.F., Malik A.B. 2006. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L622–L645 10.1152/ajplung.00477.2005 [DOI] [PubMed] [Google Scholar]
- Lizzul P.F., Aphale A., Malaviya R., Sun Y., Masud S., Dombrovskiy V., Gottlieb A.B. 2005. Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept. J. Invest. Dermatol. 124:1275–1283 10.1111/j.0022-202X.2005.23735.x [DOI] [PubMed] [Google Scholar]
- Madison B.B., Dunbar L., Qiao X.T., Braunstein K., Braunstein E., Gumucio D.L. 2002. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277:33275–33283 10.1074/jbc.M204935200 [DOI] [PubMed] [Google Scholar]
- Miura N., Yamamoto M., Fukutake M., Ohtake N., Iizuka S., Ishige A., Sasaki H., Fukuda K., Yamamoto T., Hayakawa S. 2005. Anti-CD3 induces bi-phasic apoptosis in murine intestinal epithelial cells: possible involvement of the Fas/Fas ligand system in different T cell compartments. Int. Immunol. 17:513–522 10.1093/intimm/dxh231 [DOI] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 317:256–260 10.1126/science.1145697 [DOI] [PubMed] [Google Scholar]
- Nenci A., Becker C., Wullaert A., Gareus R., van Loo G., Danese S., Huth M., Nikolaev A., Neufert C., Madison B., et al. 2007. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 446:557–561 10.1038/nature05698 [DOI] [PubMed] [Google Scholar]
- Neurath M.F., Pettersson S., Meyer zum Büschenfelde K.H., Strober W. 1996. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat. Med. 2:998–1004 10.1038/nm0996-998 [DOI] [PubMed] [Google Scholar]
- Pasparakis M. 2009. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat. Rev. Immunol. 9:778–788 10.1038/nri2655 [DOI] [PubMed] [Google Scholar]
- Rius J., Guma M., Schachtrup C., Akassoglou K., Zinkernagel A.S., Nizet V., Johnson R.S., Haddad G.G., Karin M. 2008. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 453:807–811 10.1038/nature06905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwarf D.M., Karin M. 1999. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci. STKE. 1999:re1 10.1126/stke.1999.5.re1 [DOI] [PubMed] [Google Scholar]
- Rousseau S., Papoutsopoulou M., Symons A., Cook D., Lucocq J.M., Prescott A.R., O’Garra A., Ley S.C., Cohen P. 2008. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. J. Cell Sci. 121:149–154 10.1242/jcs.018671 [DOI] [PubMed] [Google Scholar]
- Sfikakis P.P. 2010. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr. Dir. Autoimmun. 11:180–210 10.1159/000289205 [DOI] [PubMed] [Google Scholar]
- Spehlmann M.E., Eckmann L. 2009. Nuclear factor-kappa B in intestinal protection and destruction. Curr. Opin. Gastroenterol. 25:92–99 10.1097/MOG.0b013e328324f857 [DOI] [PubMed] [Google Scholar]
- Spehlmann M.E., Dann S.M., Hruz P., Hanson E., McCole D.F., Eckmann L. 2009. CXCR2-dependent mucosal neutrophil influx protects against colitis-associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J. Immunol. 183:3332–3343 10.4049/jimmunol.0900600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmann M., Schmitz H., Fromm M., Schmidt W., Pauli G., Scholz P., Riecken E.O., Schulzke J.D. 2000. Mechanisms of epithelial barrier impairment in HIV infection. Ann. N. Y. Acad. Sci. 915:293–303 10.1111/j.1749-6632.2000.tb05257.x [DOI] [PubMed] [Google Scholar]
- Tak P.P., Firestein G.S. 2001. NF-kappaB: a key role in inflammatory diseases. J. Clin. Invest. 107:7–11 10.1172/JCI11830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak P.P., Gerlag D.M., Aupperle K.R., van de Geest D.A., Overbeek M., Bennett B.L., Boyle D.L., Manning A.M., Firestein G.S. 2001. Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum. 44:1897–1907 [DOI] [PubMed] [Google Scholar]
- Vereecke L., Beyaert R., van Loo G. 2009. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 30:383–391 10.1016/j.it.2009.05.007 [DOI] [PubMed] [Google Scholar]
- Wadsworth S.A., Cavender D.E., Beers S.A., Lalan P., Schafer P.H., Malloy E.A., Wu W., Fahmy B., Olini G.C., Davis J.E., et al. 1999. RWJ 67657, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. J. Pharmacol. Exp. Ther. 291:680–687 [PubMed] [Google Scholar]
- Xu P., Derynck R. 2010. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol. Cell. 37:551–566 10.1016/j.molcel.2010.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.L., Zou Y.F., Feng X.L., Shi H.J., Du X.F., Shao M.H., Gu Y., Zhou Q. 2011. Association of the NFKBIA gene polymorphisms with susceptibility to autoimmune and inflammatory diseases: a meta-analysis. Inflamm. Res. 60:11–18 10.1007/s00011-010-0216-2 [DOI] [PubMed] [Google Scholar]