Abstract
The repair of articular cartilage defects remains a significant challenge in orthopedic medicine. Hydrogels, three-dimensional polymer networks swollen in water, offer a unique opportunity to generate a functional cartilage substitute. Hydrogels can exhibit similar mechanical, swelling, and lubricating behavior to articular cartilage, and promote the chondrogenic phenotype by encapsulated cells. Hydrogels have been prepared from naturally derived and synthetic polymers, as cell-free implants and as tissue engineering scaffolds, and with controlled degradation profiles and release of stimulatory growth factors. Using hydrogels, cartilage tissue has been engineered in vitro that has similar mechanical properties to native cartilage. This review summarizes the advancements that have been made in determining the potential of hydrogels to replace damaged cartilage or support new tissue formation as a function of specific design parameters, such as the type of polymer, degradation profile, mechanical properties and loading regimen, source of cells, cell-seeding density, controlled release of growth factors, and strategies to cause integration with surrounding tissue. Some key challenges for clinical translation remain, including limited information on the mechanical properties of hydrogel implants or engineered tissue that are necessary to restore joint function, and the lack of emphasis on the ability of an implant to integrate in a stable way with the surrounding tissue. Future studies should address the factors that affect these issues, while using clinically relevant cell sources and rigorous models of repair.
Introduction
Cartilage damage and cartilage-related diseases are the most common cause of disability in the United States today, occurring in approximately 6% of people of 30 years of age and older, at a cost to the economy of $128 billion.1–3 The prevalence of arthritis is projected to reach 25% of the population, or 67 million people, by the year 2030, as a result of the aging population and the obesity epidemic.4
The three types of cartilage include hyaline cartilage, found in articulating joints, nose, trachea, intervertebral disks, and vertebral endplates; elastic cartilage, found in tendon and ligament insertion sites and the meniscus; and fibrocartilage of the ear. Fibrocartilage has a higher collagen content and lower proteoglycan content than hyaline cartilage, and elastic cartilage contains elastic fibers in addition to collagen and proteoglycans.5,6 Although the focus of this review is the repair of hyaline cartilage, and more specifically the cartilage in articulating joints, much of the information can be extended to apply to the repair of other types of cartilage.
Articular cartilage is a multiphasic tissue consisting of <5% chondrocytes, 60%–85% interstitial fluid, and a solid extracellular matrix (ECM), composed of about 15%–22% type II collagen, 4%–7% proteoglycans, and other protein macromolecules.7–9 Cartilage can be modeled as a material having three layers that are consistent with depth-dependent variations in the mechanical environment. The superficial layer is in contact with the articulating surfaces; collagen fibrils are arranged parallel to the surface; fluid flow is high and compressive strains can reach up to 50%.8,10 Chondrocytes in this layer, subjected to fluid flow and matrix consolidation, are flattened parallel to the surface.10 Chondrocytes in the middle zone experience little strain or fluid flow and are loaded primarily under hydrostatic pressure. They are rounded and produce high amounts of glycosaminoglycans and type II collagen in random orientation.10 In the deep zone the fibers form thicker bundles that are perpendicular to the joint surface, which anchor the cartilage to bone at the tidemark, the transition to calcified cartilage and subchondral bone.8 The cell density in mature cartilage decreases from about 60 cells/cm3 in the superficial layer to about 10 cells/cm3 in the deep zone, a result of high rates of synthesis of ECM.11,12
Cartilage damage typically begins as a focal defect, the result of trauma, mechanical injury, or wear and tear. The treatment of damaged articular cartilage in the knee has been a challenging endeavor because of its limited capacity for self-repair and its complex structure that imparts unique functional properties to the tissue. It is aneural, alymphatic, and avascular, severely limiting spontaneous repair that requires access to reparative stem cells. Cartilage also withstands some of the highest loads in the body; articular cartilage in the hip joint can endure stresses of up to 18 MPa during daily activities such as rising from a chair.9,13
If a cartilage defect is in contact with the underlying bone marrow, known as a full-thickness defect, spontaneous repair is possible but the resultant tissue is fibrotic and mechanically and structurally inferior to the native tissue, and eventually degenerates under the high loads found in the knee.14 Surgical procedures directed at trying to induce repair include subchondral microfracture, the transplantation of cartilage plugs, and autologous chondrocyte implantation.15–18 Biomaterials intended to function as a synthetic cartilage replacement or to encourage new tissue regeneration have been developed to address the shortfalls of currently used techniques.19–22 Cartilage is composed of long-chain polymers of glycosaminoglycans and collagen swollen in a large amount of water, giving it properties akin to hydrogels, three-dimensional hydrophilic polymer networks that are highly swollen in water.23,24 Since the development of methacrylate-based hydrogels for biological applications in the early 1960s,25 hydrogels have been utilized in drug delivery, surface modification of biomedical implants, diagnostic devices, and tissue engineering of multiple tissue types.26 Hydrogels are considered biocompatible, although the immune response depends on numerous properties, such as the type of polymer, crosslinking methods, degradation rate, and byproducts, whether cells are included, and the release of bioactive factors.27 The material properties of hydrogels can be tailored by varying parameters such as polymer composition, crosslinking density, network morphology, and degradability.24,26
Hydrogels have been used in two forms for the purpose of replacing articular cartilage—as permanent implants to replace damaged cartilage, or as cell carrier materials to encourage tissue regeneration. As cell-free implants, hydrogels can be structurally and mechanically similar to cartilage and allow efficient load transfer.28,29 As cell-seeded tissue engineering scaffolds, hydrogels are also extremely useful: they promote chondrocyte attachment in a manner that is similar to the cartilage ECM,30 they maintain the chondrocyte phenotype in a way that is impossible in monolayer culture,31–33 and their viscoelastic nature permits effective transfer of loads to the chondrocytes, which depend on mechanical signals for survival.34,35
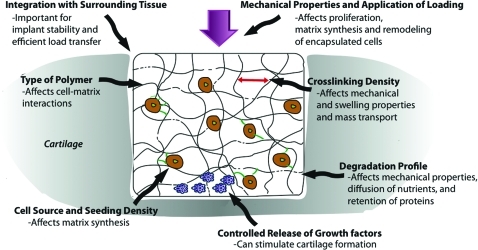
There are several design variables that can be modified with resulting effects on the potential of a hydrogel to function as a cartilage substitute or tissue engineering scaffold (Fig. 1). The main parameters are type of polymer, crosslinking density, degradation profile, mechanical properties and loading regimen, source of cells, cell-seeding density, controlled release of growth factors, and strategies to cause integration with surrounding tissue.
FIG. 1.
Schematic representation of how design variables can affect hydrogel properties and response of encapsulated cells. Color images available online at www.liebertonline.com/teb
Design Variables
Type of polymer
Hydrogels can be composed of naturally derived materials, such as agarose and collagen, or of synthetic polymers like poly(ethylene glycol) (PEG) and poly(vinyl alcohol) (PVA), and can be physically, ionically, or covalently crosslinked.
Naturally derived polymers
Hydrogels formed from naturally derived polymers, such as agarose, alginate, chitosan, hyaluronan, collagen, fibrin, and polysaccharides, are attractive biomaterials because they are biochemically similar to cartilage and can be degraded by cell-secreted enzymes. Scaffolds prepared from naturally derived polymers have been used in matrix-assisted autologous chondrocyte implantation, including collagen type I/III, hyaluronan (Hyalograft®-C, HYAFF®11; Fidia Advanced Biopolymers, Abano Terme, Italy), and fibrin (Tissucol, Baxter, Austria).36,37 Although naturally derived hydrogels lack the mechanical properties to withstand physiological loads, they have the potential to support the formation of healthy cartilage, and some commercially available products are based on these polymers (Table 1).
Table 1.
Types of Naturally Derived Hydrogels
| Polymer | Prepared from | Strengths | Commercially available products | Clinical results (all in combination with autologous chondrocytes) | References |
|---|---|---|---|---|---|
| Alginate, agarose | Marine algae | Ease of preparation; maintains chondrocytic phenotype | CARTIPATCH (Tissue Bank of France, Lyon, France) | Improvement in IKDC score at 2 years in phase II (ref.38) | 32, 34, 38–42 |
| Collagen | Animal cartilaginous tissue | Biochemically similar to cartilage | CaReS® (Arthro Kinetics Biotechnology, Krems, Austria); PureCol/VitroCol/Nutragen (Glycosan Biosystems, Salt Lake City, UT) | Improvement in IKDC, Brittberg, and Lysholm scores at 24 and 25 months in phase II (ref.43–45) | 32, 43, 45–62 |
| Fibrin | Blood | Can be prepared autologously; good adhesion to surrounding tissue | Tissucol (Baxter, Deerfield, IL) | Improvement in IKDC and Lysholm scores at 12 months in phase II (ref.63) | 63–67 |
| Hyaluronan | Animal tissue or microbial fermentation | Promotes cartilage formation by encapsulated cells; can be prepared without the risk of animal pathogens | HyStem/Extracel/Glycosil (Glycosan Biosystems, Salt Lake City, UT) | In preclinical trials | 68–83 |
| Chitosan | Arthropod exoskeletons | Structurally analogous to cartilage glycosaminoglycans | BST-CarGel (Biosyntech, Quebec, Canada) (chitosan, glycerol phosphate) | In phase II trials | 84–96 |
| Self-assembling peptide | Oligopeptides | Can be designed for specific cell–matrix interactions | Puramatrix™ (3DM Inc., Cambridge, MA) | In preclinical trials | 97–106 |
IKDC, International Knee Documentation Committee.
Agarose and alginate
Agarose and alginate, both derived from marine algae, were among the first hydrogels studied for tissue engineering due to ease of gelation and cell encapsulation.41 Alginate gels in the presence of divalent ions and agarose undergo spontaneous gelation under mild conditions due to hydrogen bonding.34 They have been used extensively as model systems to study the effects of dynamic loading and other conditions on cell behavior,34,40,107 and have supported the formation of cartilage tissue with similar mechanical properties to native cartilage.108 In a clinical trial involving 17 patients, chondrocytes suspended in alginate-agarose hydrogels repaired chondral defects, achieving a mean score at 2 years of 77.8 out of 100 from a mean score of 37 preoperatively, using the International Knee Documentation Committee scoring system.38 Alginate has also been used as the substrate in the in situ biofabrication of a geometrically matched hydrogel ex vivo into a cartilage defect using additive printing methods.109 Hydrogels based on alginate and agarose are available commercially (CARTIPATCH; Tissue Bank of France, Lyon, France).38
Collagen
Hydrogels can be formed from collagen of type I or type II, the latter being the dominant component of articular cartilage. Collagen hydrogels are chemically biomimetic, have high swelling ratios, and promote cartilage formation by encapsulated cells.32 Because biomaterials prepared from animal byproducts may induce immune responses, collagen gels have been prepared from atelopeptides, in which potentially immunogenic residues are removed, and are crosslinked physically by spontaneous gelation or chemically through aldehyde or carbodiimide chemistry.54 Chondrocytes interact with collagen gels via integrins, which promotes proliferation and production of ECM components, and can remodel collagen through the secretion of collagenase.32
Type II collagen hydrogels have been shown to promote more efficient chondrogenic differentiation of embedded mesenchymal stem cells (MSCs) than type I collagen gels because they invoke a more round cell shape.61,62 In 1994, the landmark study by Wakitani et al. reported that MSCs embedded in type I collagen gels differentiated into chondrocytes and repaired cartilage defects in rabbits with hyaline-like cartilage, but with areas of incomplete integration with the surrounding cartilage.48 When type I collagen gels containing autologous MSCs were implanted into cartilage defects in a sheep model for 6 months, the MSCs differentiated into chondrocytes and repaired the defects with zonally organized cartilage, although there were areas of incomplete integration as well as high variability within groups.46 In addition, cysts formed in the subchondral bone, which the authors attributed to a lack of mechanical support from the collagen gels.46 Type I collagen gels have been used as cell delivery vehicles for the transplantation of bone marrow stem cells into cartilage defects in humans,51 with some instances of impressive repair in otherwise healthy knees47,50,110 but less success in osteoarthritic knees.49 Autologous chondrocytes embedded in type I collagen gels repaired chondral defects in 26 patients, achieving mean Lysholm scores of 96.7 out of 100 at 25 months from 70.7 preoperatively.43 Collagen hydrogels have also been useful in studying the behavior of chondrocytes and stem cells in vitro under various conditions, such as compression and the presence of growth factors.56–60 Type I collagen gels are available commercially as CaReS® (Arthro Kinetics Biotechnology, Krems, Austria)45 and as PureCol/VitroCol/Nutragen (Glycosan Biosystems, Salt Lake City, UT).
Fibrin
Fibrin hydrogels can be prepared from fibrinogen in the presence of thrombin, isolated from a patient's own blood, reducing the risk of a foreign body reaction, and exhibit excellent adhesion to surrounding tissue.64,66 MSCs differentiated into chondrocytes and produced more cartilage tissue when encapsulated in fibrin hydrogels when compared to alginate hydrogels.67 Fibrin hydrogels have poor mechanical properties,64,65 but they have been investigated as carriers in autologous chondrocyte transplantation in clinical trials in Europe (Tissucol).63
Hyaluronan
Hyaluronan is a natural glycosaminoglycan found in articular cartilage and synovial fluid and is also involved in the regulation of wound healing, cell motility, ECM organization, and cell differentiation.74 Hyaluronan can be prepared from a variety of animal tissues or from microbial fermentation, bypassing the risk of animal-derived pathogens.75 Aqueous solutions of hyaluronan can be crosslinked to form hydrogels through a variety of methods. Most commonly, photocrosslinkable hyaluronan hydrogels are prepared through the addition of a methacrylate group to the hyaluronan backbone and subsequent crosslinking by optical trigger.76,77 Hyaluronan is degraded by cell-secreted hyaluronidase, and the rate of degradation can be tuned by adding hydrolytically degradable moieties such as lactic acid for faster degradation78 and poly(caprolactone) for slower degradation.69 Cartilage formation by chondrocytes and by MSCs was enhanced in hyaluronan hydrogels in comparison to fibrin and to PEG hydrogels, emphasizing the role of biochemical cues in cartilage formation.68,111 Hyaluronan hydrogels supported cartilage formation by human embryonic stem cells in non-weight-bearing defects in a rat model, with good integration with the surrounding cartilage after 12 weeks.71 Hydrogels based on hyaluronan are available commercially (HyStem/Extracel/Glycosil; Glycosan Biosystems).80 MSCs encapsulated in these hydrogels repaired non-weight-bearing defects in rabbits after 8 weeks with a continuous joint surface but some areas of incomplete integration.80
Hyaluronan may stimulate chondrogenesis through interactions with cell surface receptors,69,81 although conflicting results regarding the combination of hyaluronan with other types of hydrogels indicate a more complex system.72 For example, the addition of hyaluronan to collagen I hydrogels increased markers of cartilage formation by encapsulated chondrocytes in a subcutaneous implantation model.73 The addition of hyaluronan to cell culture medium increased deposition of type II collagen and glycosaminoglycans, markers of the cartilage phenotype, by chondrocytes encapsulated in alginate hydrogels,82 but decreased such markers by chondrocytes encapsulated in collagen hydrogels.83 Other studies have shown that the addition of hyaluronan to alginate hydrogels caused an increase in the expression of type I collagen, a marker of fibrous scar-like cartilage formation.72 Interestingly, this trend was reversed in the presence of exogenous insulin-like growth factor-1 (IGF-1), suggesting a role for hyaluronan in the modulation of IGF-1 signaling by entrapped chondrocytes.72
Chitosan
Chitosan is prepared by partial N-deacetylation of chitin, derived from the exoskeleton of arthropods, and is structurally similar to the glycosaminoglycans found in cartilage.87,96 The gelation of chitosan can be induced by ionic or chemical crosslinking.89,93 The kinetics of degradation of chitosan by cell-secreted lysozyme can be controlled through hydrogel properties such as crystallinity and through chitosan's degree of acetylation.90,92 Chitosan has been modified to increase biochemical similarity to cartilage85,88,94,112 and to prepare injectable scaffolds.84–86,91,95 Chondrocytes encapsulated in injectable chitosan hydrogels repaired non-weight-bearing defects in sheep, with good integration with the surrounding tissue.86 In situ-gelling hydrogels based on chitosan and glycerol phosphate (BST-CarGel; Biosyntech, Quebec, Canada) mixed with blood repaired 1 cm3 weight-bearing cartilage defects in sheep with more hyaline cartilage than microfracture controls.113 This strategy is currently being investigated in phase II clinical trials.
Peptides
Hydrogels can also be prepared from self-assembling peptide sequences, which are composed of amino acids containing alternating hydrophobic and hydrophilic side groups.99 The peptides self-assemble into β-sheets when dissolved in water and form injectable, nanofibrous hydrogels when exposed to electrolytes.98 Unlike other naturally derived hydrogels, however, peptide hydrogels can be synthesized without the risk of animal-derived pathogens.97 They also have much smaller fibers than polymer hydrogels, potentially allowing investigations into the length scales of cell–matrix interactions.98
Hydrogels composed of repeating units of the peptide sequence lysine-leucine-aspartic acid and of arginine-alanine-aspartic acid supported formation of cartilage by encapsulated chondrocytes that was comparable to that formed in agarose gels.98,101 These hydrogels have been useful for evaluation of cartilage formation by a number of different cell types and in the presence of growth factors or dynamic loading.98,104–106 A commercially available hydrogel formed from self-assembling peptides is PuraMatrix™ (3DM Inc., Cambridge, MA), which has been shown to support cartilage formation by encapsulated chondrocytes and MSCs.70 Chondrocytes cultured in PuraMatrix™ also generated sufficient cartilage tissue to close a 0.25 mm gap in a cartilage-gap model in vitro.103 The peptide sequences theoretically can be designed for specific properties or cellular interactions. For example, peptide gels with reminiscent chemistry of hyaluronan were designed to increase lubrication between articulating cartilage surfaces.100 Peptide gels were also designed with binding sites for the growth factor transforming growth factor β-1 (TGFβ1), allowing its controlled release based on hydrogel chemistry.102 Controlled release of TGFβ1 using this system caused encapsulated MSCs to differentiate into chondrocytes over 4 weeks in vitro.102
In general, the lack of mechanical integrity of hydrogels prepared from naturally derived materials presents difficulties as weight-bearing implants. In addition, the potential for disease transmission through materials derived from animals has led to investigations into synthetic hydrogels.
Synthetic polymers
Poly(vinyl alcohol): In 1973, Bray and Merrill suggested the use of synthetic hydrogels as cartilage replacements, describing the structural and mechanical similarities between cartilage and PVA hydrogels.114 Aqueous solutions of PVA can be chemically crosslinked into hydrogels through the formation of acetal linkages using difunctional crosslinking agents like formaldehyde and glutaraldehyde, or by electron beam or gamma irradiaton.26 PVA hydrogels can also be physically crosslinked through the formation of crystallites during annealing and dehydration, freeze–thaw cycling, or by phase separation from theta-solutions.24,115–117
PVA hydrogels have been extensively characterized and compared to articular cartilage in terms of their mechanical, fluid flow, and frictional properties (Table 2). Values of compressive modulus, shear modulus, tensile modulus, and permeability were similar to articular cartilage.28,29,118–120 PVA hydrogels have also been used as model systems to study the lubricating and swelling properties of articular cartilage.23,121 They have similar frictional and lubricating behavior to articular cartilage, directing fluid to contact surfaces according to the squeeze-film lubrication model under low loads and the boundary lubrication model under high loads, with similar coefficients of friction.121–125
Table 2.
Comparisons Between Poly(Vinyl Alcohol) Hydrogels (All Prepared by Freeze–Thaw) and Articular Cartilage
| Property | Test | Polymer concentration | Value for hydrogel | Value for mature cartilage | References |
|---|---|---|---|---|---|
| Compressive modulus | Unconfined compression | 20%, w/w | 0.25 MPa | 0.3–0.8 MPa (bovine) | 126–128 |
| Aggregate modulus | Confined compression | 10%, w/w | 0.11 MPa | 0.33 MPa (bovine) | 120 |
| Shear modulus | Torsion | 25%, w/w | 0.17–0.43 | 0.13–0.22 (canine) | 29, 129 |
| Permeability | Confined compression | 10%, w/v | 3.2×l0−14 m4/Ns | 0.546×l0−14 m4/Ns (bovine) | 120 |
| Coefficient of friction | Continuous sliding versus cartilage | 15%, w/w | 0.152–0.171 | 0.029 (human) | 125 |
| Coefficient of friction | Cyclic load versus cartilage | 15%, w/w | 0.046–0.058 | 0.029 (human) | 125 |
Although the mechanical properties of PVA hydrogels are closer to those of cartilage than other hydrogels, the lack of integration between the hydrogels and the surrounding cartilage tissue has so far prevented their use as cartilage replacements.130,131 PVA hydrogels (SaluCartilage; Salumedica, Smyrna, GA) were press-fit into 49 stage IV chondral lesions in 18 patients.132 Although initial results were positive, the average McDermott knee scores decreased after 12 months, and the hydrogel implants were surrounded by fluid. The authors attributed the failures to dislocation due to inadequate fixation to surrounding tissue.132
PVA hydrogels have also been used as tissue engineering substrates, but the harsh manufacturing techniques of traditional PVA hydrogel fabrication prevent the addition of cells before crosslinking.133 PVA hydrogels must therefore be rendered porous to seed cells after preparation134,135 or must be crosslinkable under gentle conditions to encapsulate cells.136 The abundance of pendant hydroxy groups on PVA simplifies chemical modification to vary hydrogel properties. PVA hydrogels have been modified with methacrylate groups to allow photoencapsulation of cells.136 Depending on the concentration of PVA, the compressive moduli ranged from 5 to 2600 kPa, allowing mechanical similarity to cartilage. Encapsulated chondrocytes maintained their spherical morphology over 3 days in vitro.136 These hydrogels were also modified with chondroitin sulfate, providing control over the swelling properties, greater biochemical similarity to cartilage, and degradation by cell-secreted chondroitinase.136 Cell–matrix interactions have also been varied by conjugating the adhesive peptide arginine-glycine aspartic acid (RGD) to the PVA chains.137
Poly(ethylene glycol): Hydrogels prepared from PEG, also known as poly(ethylene oxide), modified with methacrylate groups to allow photo-crosslinking, were first introduced by Elisseeff et al. as an injectable and transdermally photopolymerizable hydrogel for cartilage tissue engineering.138 Chondrocytes, MSCs, and embryonic stem cells have been encapsulated in these hydrogels and induced to form cartilage tissue in the presence of growth factors.138–140 Like PVA, PEG hydrogels can be easily modified and have been useful in studying the effects of hydrogel properties on cartilage formation.141–145
PEG macromers have also been modified with moieties that made the hydrogels more biomimetic. The addition of collagen-mimetic peptide, –(Pro-Hyp-Gly)x–, resulted in enhanced retention of cell-secreted collagen and increased production of both collagen and proteoglycans by chondrocytes and by MSCs.146,147 The incorporation of chondroitin sulfate to PEG hydrogels resulted in enhanced ECM deposition by encapsulated chondrocytes when compared to pure chondroitin sulfate hydrogels and increased expression of chondrogenic markers by encapsulated MSCs.148,149 PEG hydrogels are currently being investigated in clinical trials as a system in conjunction with a bioadhesive based on chondroitin sulfate (ChonDux, Cartilix, Foster City, CA).150
Other synthetic hydrogels
The PEG macromer has also been modified with fumaric acid to form hydrogels made of oligo(poly(ethylene glycol) fumarate) (OPF), which are photocrosslinkable, injectable, and can be prepared with compressive moduli as high as cartilage.151 In a rabbit osteochondral defect, cell-free OPF hydrogels allowed migration and fibrocartilage formation by host MSCs.152 When MSCs were encapsulated in the hydrogels, the quality of the repair tissue was improved.152 These hydrogels have also been used to evaluate the effects of the controlled release of various growth factors on cartilage formation in vitro and in vivo, and the main results of these studies are described later.79,151,153–155
Pluronic® (BASF, Ludwigshafen, Germany) hydrogels are composed of poloxamers or triblock copolymers of PEG and poly(propylene oxide). Hydrogels prepared from Pluronics are thermoreversible and injectable, but generally require chemical modifications to improve mechanical stability and cell viability.156–159 For example, these properties of Pluronic F127 hydrogels were improved with the addition of crosslinked hyaluronic acid and evaluated in osteochondral defects in rabbits.156 The conjugation of TGF-β1 was necessary to maintain the hydrogel in the defect site, probably because of the effects on encapsulated adipose-derived stem cells, which underwent chondrogenic differentiation and produced some cartilaginous tissue.156 Pluronic hydrogels have also been combined with chitosan and with the adhesive peptide RGD, with positive effects on cartilage formation by encapsulated chondrocytes.94
Other thermoreversible and injectable hydrogels are based on poly(N-isoproylacrylamide) (PNIPAAm). Aqueous solutions undergo gelation at a lower critical solution temperature that can be increased or decreased through the modification of the PNIPAAm polymer with more hydrophobic or hydrophilic polymers, respectively.160 These hydrogels are nondegradable and have been investigated in vitro for cartilage formation by chondrocytes and MSCs under several conditions, such as the presence of growth factors or in coculture systems.160,161 PNIPAAm hydrogels have also been modified with chitosan and with gelatin, allowing control over cell–matrix interactions.162,163
Composite hydrogels have also been prepared with entangled hydrogel networks of poly(2-acrylamido-2-methylpropane sulfonic acid) and poly(N,N′-dimethyl acrylamide).164 The two hydrogel networks combine to form one material, theoretically mimicking the collagen and glycosaminoglycan phases of cartilage.165 These degradation-resistant hydrogels exhibit similar compressive moduli to articular cartilage and lower coefficients of friction against normal cartilage than cartilage articulating against cartilage.164,165 Implantation into weight-bearing defects or analysis of integration with surrounding tissue has not yet been performed.
Physical properties of hydrogels
Crosslinking density
The network crosslinking density of a hydrogel controls many of its properties, such as diffusion coefficients, mechanical behavior, and rate of degradation, with significant effects on the behavior of entrapped cells.24,178 The swelling ratio of a hydrogel, the ratio of its swollen weight to its dry weight, is related to the crosslinking density and is a measure of how much water is retained by the hydrogel.143 Less crosslinked hydrogels have a larger mesh size, or the distance between crosslinks, which allows faster diffusion of nutrients and waste to and from encapsulated cells.141 The crosslinking density of PEG hydrogels can be increased by increasing the concentration of the PEG solutions, decreasing the molecular weight of the PEG macromers, or by using branched PEG structures instead of linear structures, with corresponding increases in compressive modulus.166,167,179 Because of the facility of modulating the properties of PEG hydrogels, most studies on the effects of hydrogel properties have been performed using PEG hydrogels.
Chondrocytes encapsulated in PEG hydrogels with lower crosslinking densities and higher swelling ratios produced more ECM components that were more homogenously dispersed than hydrogels with lower swelling ratios.141,143,170,175 These results were confirmed using MSCs encapsulated in PEG-based hydrogels.147,180 The more diffuse distribution of ECM components then led to higher levels of ECM production.167 Less crosslinked hydrogels may allow the formation of a thicker pericellular matrix, the ECM components immediately surrounding the cells, which affect cell–matrix interactions.170 Bryant and Anseth also noted that the mesh size of the hydrogels should be larger than the size of a proteoglycan aggregate to ensure sufficient diffusion.143 Collagen fibrils are significantly larger than proteoglycans, providing another parameter that would affect the distribution of ECM components.166
Degradation
Higher swelling ratios are beneficial for cartilage matrix production but decrease the mechanical properties of the hydrogels. Degradable hydrogels, which experience increases in swelling ratio with degradation, allow initial mechanical support that is transferred to the evolving cartilage matrix over time.143 Control over hydrogel degradation (Fig. 2) also allows control over nutrient and waste diffusion in a growing cartilage construct.181 PEG hydrogels, modified with hydrolytically labile poly(lactic acid), with higher percentages of degradable moieties supported greater production of ECM components by encapsulated chondrocytes, which was attributed to enhanced diffusion as the hydrogels degraded.142,143,167
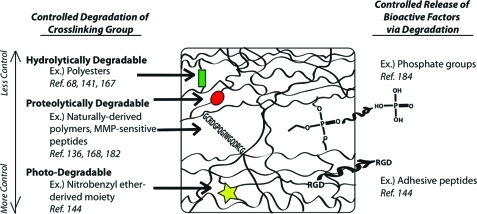
FIG. 2.
Schematic representation of ways to control the degradation of synthetic hydrogels. MMP, matrix metalloproteinases. Color images available online at www.liebertonline.com/teb
To tie the kinetics of hydrogel degradation to cartilage formation, PEG-based hydrogels have been modified with groups that are sensitive to matrix metalloproteinases (MMP).168,182 Compared to nondegradable hydrogels, the increased diffusion of ECM components secreted by encapsulated chondrocytes led to a more dispersed matrix, which in turn led to more ECM production.168 When human MSCs were encapsulated in PEG hydrogels that were sensitive to MMP7, first identified as an enzyme with a temporal profile that corresponded with cartilage development, the engineered cartilage tissue achieved a higher dynamic modulus after 12 weeks in vitro than that of corresponding nondegradable hydrogels.182 This increase in mechanical properties occurred in spite of less proteoglycan retention in the hydrogels, which may indicate that the retained matrix components were more functionally similar to native cartilage. The degradation of PEG hydrogels has also been varied by crosslinking with biodegradable genipin,183 phosphate-releasing groups,184 and by combining with dextran-based hydrogels.185
In addition to the amount of degradation, the rate is also important. PEG hydrogels with an intermediate rate of degradation supported more cartilage formation by encapsulated chondrocytes than hydrogels with slower or faster degradation.141 Similar results were obtained when the degradation rate of hyaluronan hydrogels, which are degraded by cell-secreted hyaluronidase, was manipulated through the addition of hydrolytically degradable units.69,78 A balance exists between a degradation rate that is fast enough to allow room for newly synthesized ECM components and a rate that is slow enough to sufficiently retain the proteins.69,78
The degradation properties of hydrogels are also important because they determine the changing structural and physical properties of the hydrogels. Kloxin et al. precisely controlled hydrogel degradation by incorporating a photodegradable nitro-benzyl-derived moiety into PEG-based hydrogels, so that their degradation could be controlled by the application of light.144 The degradation of these hydrogels was manipulated externally by varying the duration and intensity of the light, and the structures of the hydrogels were controlled by focusing light on specific areas of the hydrogels through photomasks, which has potential applications in directing cell behavior.144
Mechanical considerations
The mechanical properties of hydrogels are controlled by their polymer concentration and crosslinking density, and diminish as the polymer degrades. In the absence of load, the dominant parameter that determines cartilage formation within tissue engineering scaffolds appears to be crosslinking density, because it regulates the diffusion of the ECM components. Cells proliferate more and produce more ECM in less crosslinked hydrogels, creating difficulties for analyzing the effects of stiffness using hydrogels of increasing crosslinking densities. However, hydrogel stiffness did affect the aggregation of MSCs, a critical step in chondrogenesis, which was more pronounced in peptide hydrogels than agarose hydrogels, probably because the peptide gels were considerably weaker and easier to displace than agarose.104
Mechanotransduction, the process in which external mechanical signals lead to intracellular signaling cascades, is an important regulator of chondrocyte metabolism and cartilage homeostasis.169,175,186 Cells deform to different extents in hydrogels with different mechanical properties, resulting in differences in cell proliferation, proteoglycan synthesis, and gene expression.169,170 The application of 15% static strain to PEG hydrogels containing chondrocytes caused an inhibition of proteoglycan production in weaker gels but stimulation in stiffer gels, in which cells deformed more due to network heterogeneities.169 In another study, a thick pericellular matrix formed around chondrocytes in less crosslinked PEG gels because of enhanced diffusion, forming a protective layer around the cells and reducing cellular strain, so that they responded to dynamic loading to roughly the same extent as stiffer gels.170,178,187 Further studies are still required to elucidate the effects of mechanical properties of the hydrogel on chondrocyte response in the presence of loading, without the confounding effects of mass transport limitations.
In general, static loading inhibits cartilage synthesis in cartilage explants and chondrocytes encapsulated in several types of hydrogels.171,173,174,188,189 Cyclic loading regimens, applied for a few hours per day for several weeks, generally result in net increases in ECM synthesis by encapsulated chondrocytes or chondrogenically induced MSCs.34,107,174,176,190–197 These increases in ECM synthesis often corresponded to upregulation of catabolic matrix-metalloproteinases.106,175,198,199 In some cases in which the net increase in ECM retention was higher than free-swelling controls, the amount of ECM components lost to the surrounding medium, a result of enzymatic degradation, was also higher.177 These results suggest that matrix remodeling by metalloproteinases is beneficial for cartilage production.106 Furthermore, given that static compression has been shown to decrease biosynthetic activities and increase catabolic activities of chondrocytes, resulting in a decrease in ECM production,200 a critical balance exists between synthesis and degradation in response to mechanical loading to achieve maximal ECM synthesis.
The magnitude of increased ECM synthesis depends on the type of hydrogel, cell-seeding density, magnitude and frequency of dynamic loading, and timing of the initiation of loading.174,190,192,196,198 The distribution of proteoglycan accumulation may also vary depending on the structure of the hydrogel and the distribution of fluid flows, resulting in greater deposition in a cell's pericellular vicinity170,201 and around the periphery of the hydrogel where flow during unconfined compression is higher.107,173,194 In addition, several studies showed opposite effects of dynamic loading on proteoglycan and collagen synthesis for a given cell–hydrogel system,107,175–177,196 which may be related to increased aggrecanase activity and the fact that proteoglycans are primarily responsible for resisting compression.106,177 The results of these studies show that the response of chondrocytes to their mechanical environment is a complicated system of factors contributed from the structure and stiffness of the hydrogels, the loading regimen, and the biosynthetic and catabolic behavior of the chondrocytes.
Hydrogels have also been useful as model systems to study the response of cells to applied loads in a number of specific conditions.40,108,202 For example, the application of intermittent dynamic loading to chondrocytes cultured in agarose gels increased the biosynthetic response to insulin-like growth factor-1 (IGF1) and to TGFβ1, so that the increases in ECM production and compressive modulus were greater than those due to either growth factor alone.40 Such synergistic effects were also observed with TGFβ3.108 The application of TGFβ1 or TGFβ3 also enhanced chondrogenesis of MSCs under the application of dynamic loading.195,196
Although the ideal mechanical properties for a cartilage replacement are not known, understanding the effects of mechanical properties and loading regimens is important for predicting cartilage-hydrogel behavior in vivo and also for designing bioreactors to maximize cartilage production. More studies are required to determine the effects of hydrogel stiffness on cartilage tissue engineering in the presence of load, utilizing a variety of hydrogels. If cell–hydrogel constructs are to be immediately implanted into cartilage defects without prior cultivation, they must be stiff enough to support physiological loads or they will collapse.131 Because the mechanical properties must be balanced with crosslinking density, it may be beneficial for hydrogels to begin with appropriately high compressive moduli and degrade into less crosslinked networks that are also weaker, whereas the new cartilage tissue takes over more of the load.143 On the other hand, if the hydrogels are to be cultured in bioreactors before implantation, then the loading regimen can be manipulated to optimize cartilage production by a variety of cell types. In this case, more investigations that examine the temporal profile of gene expression and protein synthesis, especially of the catabolic enzymes, are necessary to definitively delineate the differences in the effects of different loading regimens on cartilage tissue engineering. Observations on the effects of various hydrogel properties on the response of encapsulated cells are summarized in Table 3.
Table 3.
Effects of Hydrogel Properties on Cartilage Tissue Engineering
| Property | Type of hydrogel tested | Observation | References |
|---|---|---|---|
| Crosslinking density | PEG-poly(lactic acid), PEG-biodendrimer | Less crosslinked resulted in greater production of ECM components that are more dispersed | 141, 143, 166, 167 |
| Amount of degradation | PEG-based | Level of degradation that allowed increasing space and load transfer to evolving cartilage matrix resulted in better cartilage formation | 142, 143, 168 |
| Degradation rate | Hyaluronan, PEG | Rate that matched ECM formation led to more ECM production | 69, 78, 141 |
| Compressive modulus | PVA, PEG | Has not yet been studied without the effects of crosslinking density, so that stiffer gels have lower ECM production; stiffer gels had higher ECM production under static strain | 131, 143, 169, 170 |
| Loading regime | Agarose, collagen, fibrin, peptide, PEG | Static strain: generally inhibits ECM synthesis Dynamic strain: increases ECM synthesis and catabolic gene expression | 34, 106, 169–177 |
PEG, poly(ethylene glycol); PVA, poly(vinyl alcohol); ECM, extracellular matrix.
Cell source and seeding density
Many preliminary analyses of tissue engineering strategies employ immature chondrocytes due to the ease of cell isolation and expansion. However, compared to mature chondrocytes, immature chondrocytes tend to proliferate more, produce more ECM components, and respond more to the application of growth factors.203,204 Adult chondrocytes were used successfully to generate cartilage in agarose hydrogels that repaired non-weight-bearing defects in a canine model with good integration with the surrounding tissue.205 Chondrocytes can also be isolated from a patient's auricular, nasoseptal, and costal cartilages, with cells from each source differing slightly in behavior, response to external forces, and potential to produce new cartilage tissue. For example, nasal and auricular chondrocytes proliferate much faster than articular chondrocytes.206,207 Auricular chondrocytes encapsulated in hyaluronic acid or fibrin hydrogels experienced increased ECM production and mechanical properties compared to articular chondrocytes after 12 weeks of subcutaneous implantation.208,209 However, articular chondrocytes upregulated expression of type II collagen and aggrecan to greater extents in response to stimulation by dynamic loading.208 In another study, the potential of chondrocytes suspended in fibrin gels to integrate with native articular cartilage did not depend on the source of the chondrocytes.210 However, many of these studies utilized a subcutaneous implantation model, which more closely resembles the auricular environment than the articular environment. More studies are required to determine the effectiveness of the chondrocyte sources in repairing articular cartilage defects.
In any case, the isolation of chondrocytes from any of the cartilages causes considerable damage to the donor site. In contrast, stem cells isolated from mesenchymal tissues can produce cartilage tissue under the right conditions. Whereas immature chondrocytes produced greater levels of cartilage tissue than immature bone marrow-derived MSCs encapsulated in hyaluronic acid, agarose, and peptide hydrogels,70 MSCs derived from skeletally mature horses produced superior cartilage tissue compared to age-matched chondrocytes in peptide hydrogels.203 The MSCs also proliferated in response to TGFβ1, whereas the mature chondrocytes did not.203 It is important that future studies utilize cells from clinically relevant sources to determine the effectiveness of tissue engineering strategies.
The source of MSCs also affects cartilage tissue engineering. Mature MSCs isolated from bone marrow and synovium and encapsulated in type I collagen gels produced superior cartilage tissue when implanted in articular cartilage defects in rabbits than MSCs from adipose or muscle tissues.60 Adult bone marrow-derived MSCs accumulated proteoglycans at a higher rate than adipose-derived MSCs in agarose hydrogels in response to TGFβ1, although there were no differences in the absence of TGFβ1.104 When encapsulated in peptide hydrogels, however, adipose-derived MSCs did accumulate ECM components in response to TGFβ1, indicating that cell–matrix interactions affect chondrogenesis.104 Bone marrow-derived MSCs produced more aggrecan, whereas adipose-derived MSCs produced more collagen. The presence of TGFβ1 was also necessary for chondrogenesis of MSCs derived from human embryoid bodies in response to mechanical stimulation, which down-regulated chondrogenesis in the absence of TGFβ1.195 In contrast, growth factors are not required for the stimulation of chondrogenesis of bone marrow-derived MSCs by mechanical loading, which occurs through upregulation of TGFβ production.107,195,211,212 The combination of mechanical loading and adenoviral transduction of the cartilage transcription factor Sox9 resulted in increased chondrogenesis of bone marrow-derived MSCs in a fibrin-polyurethane hydrogel–scaffold composite, although the expression of the cartilage markers collagen type II and aggrecan was much lower than with the addition of exogenous growth factors.213 These studies suggest that MSCs derived from a variety of sources are suitable for cartilage tissue engineering when cultured under specific conditions.
The seeding density of chondrocytes or MSCs in hydrogels also affects the quality of engineered cartilage tissue. In free-swelling culture, increases in the density of chondrocytes seeded in agarose, alginate, and peptide gels over a range from 4 to 64 million cells/ml resulted in increases in ECM production and mechanical properties.103,190,214,215 However, in the presence of mechanical loading, there were no differences in ECM accumulation and mechanical properties between hydrogels seeded with 20 or 60 million cells/mL.190 Chondrogenic gene expression of MSCs co-encapsulated with TGFβ1 was greater for a seeding density of 10 million cells/mL than 20 million cells/mL in OPF hydrogels, possibly because of a higher dose of TGFβ1 per cell.79 These studies suggest that stimulation by mechanical loading or growth factors can result in greater ECM production from fewer cells.
Controlled release of growth factors
The use of controlled release of growth factors in combination with carefully designed biomaterials may result in improved cartilage tissue engineering. Some commonly investigated growth factors include IGF1, TGFβ1, -2, and -3, and basic fibroblast-derived growth factor, because of their roles in stimulating chondrocyte differentiation, proliferation, and matrix synthesis (Table 4). These growth factors have also been shown to improve the quality of engineered cartilage when added to the culture media of chondrocytes encapsulated in hydrogels in vitro.40,108,216,217 However, their short half-lives, in addition to rapid diffusion or clearance from the defect site, prevent their therapeutic potential as injections.22 Therefore, many have turned to drug delivery systems to provide controlled, sustained release of growth factors, eliminating the need for repeated administration. Furthermore, the chemoattractive effects of these growth factors may enhance integration with surrounding tissue by further stimulating chondrocytes to migrate toward the scaffolds.218–220
Table 4.
Growth Factors Investigated for Controlled Release from Hydrogels for Cartilage Tissue Engineering
| Growth factor | Hydrogels | Cell type | Main effects | References |
|---|---|---|---|---|
| IGF1 | OPF, fibrin, PNIPAAm-PEG | Chondrocytes | Increased ECM synthesis; enhanced repair of chondral defects in horses | 153, 160, 221, 222 |
| IGF1 and TGFβ1 | PEG, OPF | Chondrocytes | Synergistic results in vitro, IGF1 alone more effective in repair of rabbit osteochondral defects | 153, 160, 223 |
| TGFβ1 | Agarose, peptide, Pluronic, OPF, Collagen | Chondrocytes, MSCs | Induced MSC chondrogenesis and enhanced repair of rabbit osteochondral defects | 79, 102, 156, 224–226 |
| TGFβ2 | PNIPAAm-PEG | Chondrocytes | Increased collagen synthesis in vitro | 160 |
| TGFβ3 | PNIPPAm-AAc, OPF | Chondrocytes, MSCs | Increased ECM synthesis by chondrocytes in vitro; Induced chondrogenesis of MSCs and stimulated proteoglycan synthesis in subcutaneous space in vivo | 139, 152, 227 |
IGF1, insulin-like growth factor-1; TGFβ, transforming growth factor β; OPF, oligo(poly(ethylene glycol) fumarate); PNIPAAm, poly(N-isoproylacrylamide); MSCs, mesenchymal stem cells.
Hydrogels have long been popular as controlled delivery systems for proteins, because release profiles can be modulated by changing the hydrogel crosslinking density, which changes the free space available for diffusion.228 The mechanisms of release can also be varied by manipulating interactions between encapsulated proteins and the hydrogel polymer, such as charge interactions, or by changing the degradation profiles of the hydrogels.228,229 Another useful tool for controlling the release of growth factors is the encapsulation of growth factor-loaded microparticles, which allows delayed or tempered release profiles,230–232 spatial control over delivery,233 and greater stability and bioactivity of the encapsulated protein.231 The controlled release of growth factors from hydrogels has been used to stimulate repair of cartilage defects by cells from the surrounding tissue, to enhance cartilage production by encapsulated chondrocytes, and to induce chondrogenesis of MSCs. Delivery of IG1 from cell-free fibrin hydrogels over a few weeks enhanced repair of cartilage defects in adult rabbits and in horses.234 When chondrocytes were co-encapsulated with IGF1 in the gels, the repair of chondral defects in horses was greater than those treated with gels containing chondrocytes alone.221 Fibrin hydrogels loaded with TGFβ1 recruited MSCs and induced chondrogenic differentiation when implanted subcutaneously in rabbits.224 When OPF hydrogels loaded with gelatin microparticles containing IGF1, TGFβ1, or a combination of the two, were implanted into osteochondral defects in rabbits, IGF1-releasing hydrogels were the most effective in improving cartilage repair, with little to no improvements in hydrogels releasing TGFβ1 only or a combination of TGFβ1 and IGF1.153 These results were in contrast to previous studies in vitro, which indicated that TGFβ1 alone or a synergistic combination of TGFβ1 and IGF1 improved cartilage tissue generation in hydrogels.79,160,223 These results suggest that caution should be exercised when translating in vitro results to in vivo repair.
The inclusion of TGFβ1 to agarose, peptide, and collagen hydrogels was sufficient to induce chondrogenesis of encapsulated MSCs,225,226 which was further enhanced by including dexamethasone.226 The delivery of TGFβ3 and hyaluronic acid alone or in combination enhanced chondrogenesis of MSCs in PEG hydrogels; TGFβ3 was required for proteoglycan production and hyaluronic acid reduced the production of type I collagen.139 The release of TGFβ1 or TGFβ3 from OPF hydrogels was also used to stimulate chondrogenic differentiation of MSCs in vitro, which was enhanced by co-culture with osteogenic cells in a subchondral layer of the hydrogel.154,235 The rate of release of dexamethasone was controlled from hydrogels composed of Pluronic and hyaluronic acid through the use of porous and nonporous microparticles of poly(lactic-co-glycolic acid).236 The faster release profile, which was 100% release in 4 weeks, resulted in greater chondrogenesis by encapsulated MSCs after 4 weeks of subcutaneous implantation.236 Heparin was bound to TGFβ3 to retard its release profile, resulting in enhanced cartilage production by chondrocytes in PNIPAAm-based hydrogels compared to the faster release of TGFβ3.227 Although the TGFβ1, -2, and -3 isoforms have different functions in embryonic chondrogenesis,237 their effects have not been directly compared in a cartilage tissue engineering application. More studies investigating the effects of release rates of these and other growth factors on chondrogenesis and cartilage formation are still required.
Hydrogels have also been modified to control the release bioactive molecules and growth factors that are bound directly to hydrogel polymer. The release of the adhesive peptide RGD from PEG hydrogels was controlled through conjugation to a photolabile group.144 By controlling the release using the application of light to match the temporal presentation experienced by MSCs natively, the encapsulated cells secreted a four-fold higher amount of ECM components than hydrogels that persistently expressed RGD.144 The conjugation of heparin, which has a growth factor-binding domain, to Pluronic F127 hydrogels allowed the controlled release of TGFβ1 over the course of three weeks, resulting in chondrogenesis by encapsulated adipose-derived MSCs and enhanced repair of chondral defects in rabbits compared to hydrogels without TGFβ1.156 Peptide gels were formed using a sequence that had a binding affinity for TGFβ1, allowing its controlled release over the course of a few days.102 When these hydrogels were used to fill non-weight-bearing cartilage defects in rabbits in combination with microfracture, MSCs from the subchondral bone filled the defects and differentiated into zonally organized hyaline cartilage with excellent integration with the surrounding tissue.102 These results were also positive without the addition of exogenous growth factor, suggesting that prolonged presence of cell-secreted growth factor also contributed to healing.102
Considerable progress has been made using growth factors to augment tissue engineering, but more in vivo investigations are required to determine their efficacy. Another complicating factor will be the diminished response of arthritic chondrocytes and aged MSCs to growth factors, which will complicate dosage calculations and further increase the disparity between in vitro results, animal models, and repair in humans.238
Integration with surrounding tissue
Even if the properties of a hydrogel are optimized to regenerate healthy hyaline cartilage, if an implant does not integrate well with the surrounding tissue, loads cannot be transferred effectively, and new defects will form around the periphery. The complexity of the joint surface and the limited regenerative potential of chondrocytes result in discontinuities between implants and surrounding cartilage, which ultimately lead to cartilage fibrillation and implant failure.46,239 Even cartilage explants that are implanted back into the defect from which they were removed do not integrate well with the surrounding tissue.240,241
Synthetic hydrogels are generally too hydrophilic to allow protein adsorption or cell attachment,242–244 so hydrogel implants are unlikely to integrate with surrounding tissue. When PVA hydrogels were implanted into cartilage defects in several animal models and in humans, there was no integration between the hydrogels and the surrounding tissue.124,130,131 Oka and coworkers combined PVA hydrogels with titanium fiber mesh to anchor into the subchondral bone, but gaps were still visible around the PVA hydrogels after 24 weeks in canine osteochondral defects.130 When discs of PVA hydrogels were sutured to cartilage discs and implanted subcutaneously into nude mice for 12 weeks, no integration was observed between the hydrogels and cartilage.135 However, when chondrocytes suspended in fibrin hydrogel were injected into the pores of the hydrogels, neocartilage tissue formed in the pores and integrated firmly with the cartilage discs.135,245 Other studies in which the encapsulation of cells significantly enhanced integration between hydrogels and surrounding cartilage confirm that tissue engineering approaches can be useful mechanisms of integration.48,80,103,210 Adhesion strength was also shown to increase when the surrounding tissue was treated with enzymes to remove glycosaminoglycans, which stimulated chondrocytes to proliferate.240,241 Strategies that aim to maximize synthetic activity of chondrocytes at the interface may result in maximal integration.
Elisseeff and coworkers have investigated the chemical attachment of PEG hydrogels to surrounding tissue through tissue-initiated photopolymerization.246 In this method, the proteoglycans in the tissue surrounding a cartilage defect are digested, tyrosyl radicals are generated in the collagen by photooxidation of tyrosine residues, and a PEG macromer solution is injected into the defect and photopolymerized via tyrosyl radical initiation.246 Photopolymerizable PEG gels can thus be formed in situ and crosslinked to collagen fibrils in the surrounding tissue, resulting in tight integration of the hydrogel to the cartilage. A similar approach was used in the design of an adhesive bridge between PEG hydrogels and surrounding tissue using a bioadhesive based on chondroitin sulphate.150,247 The polysaccharide was modified with methacrylate groups to bind to photopolymerizable PEG hydrogels and with aldehyde groups to react with amines in surrounding tissue via a Schiff-base reaction. Hydrogels encapsulating chondrocytes were attached to cartilage explants and implanted subcutaneously in athymic mice for 5 weeks; cells remained viable and the hydrogels remained firmly attached to the cartilage.150 Adhesion to articular cartilage was 10 times stronger than that of fibrin glue,247 and mechanical evaluation indicated that the hydrogel failed before the interface did.150 These hydrogels are currently being investigated in phase II trials (ChonDux; Cartilix).
These studies show that integration between tissue-engineered constructs and the surrounding tissue can be accomplished through chemical crosslinking or through biological interactions between the tissues. Future studies of any tissue engineering strategy should address the strengths of interfaces between tissue engineered cartilage or hydrogel implants with surrounding tissue. Useful in vitro models to quantitatively determine the strength of the interface include the cartilage gap model,103 the sandwich model,210,218 the disc-ring model,241 and the defect repair model,248 although analysis of the integration in vivo is also necessary. More studies are required that investigate the optimal timing and conditions surrounding implantation of developing neocartilage.
Summary and Future Directions
A multitude of hydrogels have been investigated for the repair of articular cartilage defects. Hydrogels prepared from naturally derived polymers have the benefits of biodegradability, biocompatibility, and control over cell–matrix interactions. However, synthetic hydrogels allow precise control over their material properties, which is important if the implants are expected to take over loads found in the knee joint. Studies on the effects of hydrogel properties on cartilage tissue engineering have shown that less crosslinked gels that degrade at moderate rates allow maximal production of ECM components because of increased nutrient transport. Most studies were conducted using PEG hydrogels due to ease of manipulation, so it is unclear if these results will extend to hydrogels with diverse properties and degrees of cell–matrix interactions. Dynamic loading protocols have been developed that aim to maximize cartilage production by encapsulated chondrocytes or MSCs. More studies investigating the anabolic and catabolic response of cells to loading will provide further insight into the optimal loading regimen.
Controlled release of growth factors can augment tissue engineering strategies, but a significant challenge remains in securing integration between tissue-engineered implants and the surrounding tissue. It is possible that perfect hyaline cartilage can be engineered but will not integrate upon implantation, considering that cartilage explants do not even integrate with the surrounding tissue when implanted into the defect from which they were removed. Future studies must focus on strategies that address integration.
An ideal hydrogel for cartilage tissue engineering should initially support the loads found in the joint and gradually degrade, transferring the loads to the evolving cartilage matrix. The hydrogel should encourage chondrogenesis by encapsulated stem cells, such as through the controlled release of growth factors like TGFβ1, which would reduce the time needed for in vitro cultivation before implantation. Most importantly, the hydrogel system should have a strategy for integrating the engineered tissue with the surrounding tissue, such as by encouraging encapsulated cells to migrate out or cells from the surrounding space to migrate in.
Finally, although some have begun to investigate the engineering of zonally organized cartilage using layered hydrogels and chondrocytes isolated from the different zones of cartilage,249 there is still little understanding of how to generate cartilage with appropriately organized ECM. Future studies should address methods to generate zonal organization in cartilage tissue engineering strategies and to determine if it is even necessary.
Acknowledgments
Work in our laboratories was supported by grant UL1 RR024996 from the Clinical and Translation Science Center at Weill Cornell Medical College, and by the Widgeon Point Foundation. We are grateful to the National Science Foundation Graduate Research Fellowship Program and the Institute of International Education Fulbright Program for grants to K.L.S.
Disclosure Statement
No competing financial interests exist.
References
- 1.Felson D.T. Lawrence R.C. Dieppe P.A. Hirsch R. Helmick C.G. Jordan J.M., et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.National, state medical expenditures, lost earnings attributable to arthritis, other rheumatic conditions—United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56:4. [PubMed] [Google Scholar]
- 3.Felson D.T. Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Hootman J.M. Helmick C.G. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 5.Stockwell R. Biology of Cartilage Cells. Cambridge: Cambridge University Press; 1979. [Google Scholar]
- 6.Verma G.P. Cartilage and Bone. Fundamentals of Histology. New Dehli: New Age International Limited; 2001. [Google Scholar]
- 7.Ma P. Langer R. Morphology and mechanical function of long-term in vitro engineered cartilage. Journal of Biomedical Materials Research. 1999;44:217. doi: 10.1002/(sici)1097-4636(199902)44:2<217::aid-jbm12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Mow V. Holmes M. Lai W. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17:377. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 9.Mow V. Ratcliffe A. Poole A. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 10.Wong M. Carter D. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1. doi: 10.1016/s8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 11.Eggli P.S. Hunziker E.B. Schenk R.K. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat Rec. 1988;222:217. doi: 10.1002/ar.1092220302. [DOI] [PubMed] [Google Scholar]
- 12.Stockwell R.A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411. [PMC free article] [PubMed] [Google Scholar]
- 13.Hodge W.A. Fijan R.S. Carlson K.L. Burgess R.G. Harris W.H. Mann R.W. Contact Pressures in the Human Hip-Joint Measured Invivo. P Natl Acad Sci USA. 1986;83:2879. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed T.A. Hincke M.T. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16:305. doi: 10.1089/ten.TEB.2009.0590. [DOI] [PubMed] [Google Scholar]
- 15.Goldring M.B. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20:1003. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2002;10:432. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 17.Brittberg M. Autologous chondrocyte implantation—technique and long-term follow-up. Injury. 2008;39(Suppl 1):S40. doi: 10.1016/j.injury.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Peterson L. Minas T. Brittberg M. Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85-A(Suppl 2):17. doi: 10.2106/00004623-200300002-00003. [DOI] [PubMed] [Google Scholar]
- 19.Frenkel S.R. Cesare P.E.D. Scaffolds for articular cartilage repair. Ann Biomed Eng. 2004;32:26. doi: 10.1023/b:abme.0000007788.41804.0d. [DOI] [PubMed] [Google Scholar]
- 20.Lu L. Zhu X. Valenzuela R.G. Currier B.L. Yaszemski M.J. Biodegradable polymer scaffolds for cartilage tissue engineering. Clin Orthop Relat Res. 2001;S251 doi: 10.1097/00003086-200110001-00024. [DOI] [PubMed] [Google Scholar]
- 21.Li W.J. Tuan R.S. Polymeric scaffolds for cartilage tissue engineering. Macromol Symp. 2005;227:65. [Google Scholar]
- 22.Melrose J. Chuang C. Whitelock J. Tissue engineering of cartilages using biomatrices. J Chem Technol Biotechnol. 2008;83:444. [Google Scholar]
- 23.Broom N.D. Oloyede A. The importance of physicochemical swelling in cartilage illustrated with a model hydrogel system. Biomaterials. 1998;19:1179. doi: 10.1016/s0142-9612(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 24.Lowman A. Peppas N. Hydrogels. In: Mathiowitz E., editor. Encyclopedia of Controlled Drug Delivery. New York: John Wiley and Sons; 1999. [Google Scholar]
- 25.Wichterle O. Lim D. Hydrophilic gels for biologic use. Nature. 1960;185:117. [Google Scholar]
- 26.Peppas N. Hilt J.Z. Khademhosseini A. Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18:1345. [Google Scholar]
- 27.Badylak S.F. Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi M. Oka M. Characterization of a polyvinyl alcohol-hydrogel artificial articular cartilage prepared by injection molding. J Biomater Sci. 2004;15:741. doi: 10.1163/156856204774196135. [DOI] [PubMed] [Google Scholar]
- 29.Stammen J.A. Williams S. Ku D.N. Guldberg R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials. 2001;22:799. doi: 10.1016/s0142-9612(00)00242-8. [DOI] [PubMed] [Google Scholar]
- 30.Cushing M.C. Anseth K.S. Materials science. Hydrogel cell cultures. Science. 2007;316:1133. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 31.Benya P.D. Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 32.Yamaoka H. Asato H. Ogasawara T. Nishizawa S. Takahashi T. Nakatsuka T., et al. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. J Biomed Mater Res A. 2006;78:1. doi: 10.1002/jbm.a.30655. [DOI] [PubMed] [Google Scholar]
- 33.Passaretti D. Silverman R.P. Huang W. Kirchhoff C.H. Ashiku S. Randolph M.A., et al. Cultured chondrocytes produce injectable tissue-engineered cartilage in hydrogel polymer. Tissue Eng. 2001;7:805. doi: 10.1089/107632701753337744. [DOI] [PubMed] [Google Scholar]
- 34.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B., et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 35.Lammi M.J. Current perspectives on cartilage and chondrocyte mechanobiology. Biorheology. 2004;41:593. [PubMed] [Google Scholar]
- 36.Marcacci M. Berruto M. Brocchetta D. Delcogliano A. Ghinelli D. Gobbi A., et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;96 doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 37.Nehrer S. Domayer S. Dorotka R. Schatz K. Bindreiter U. Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57:3. doi: 10.1016/j.ejrad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Selmi T.A. Verdonk P. Chambat P. Dubrana F. Potel J.F. Barnouin L., et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg. 2008;90:597. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 39.Mauck R.L. Wang C.C. Oswald E.S. Ateshian G.A. Hung C.T. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2003;11:879. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Mauck R.L. Nicoll S.B. Seyhan S.L. Ateshian G.A. Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 41.Guo J.F. Jourdian G.W. MacCallum D.K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- 42.Wong M. Siegrist M. Wang X. Hunziker E. Development of mechanically stable alginate/chondrocyte constructs: effects of guluronic acid content and matrix synthesis. J Orthop Res. 2001;19:493. doi: 10.1016/S0736-0266(00)90023-8. [DOI] [PubMed] [Google Scholar]
- 43.Ochi M. Uchio Y. Kawasaki K. Wakitani S. Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. Journal of Bone Joint Surg. 2002;84:571. doi: 10.1302/0301-620x.84b4.11947. [DOI] [PubMed] [Google Scholar]
- 44.Andereya S. Maus U. Gavenis K. Gravius S. Stanzel S. Muller-Rath R., et al. [Treatment of patellofemoral cartilage defects utilizing a 3D collagen gel: two-year clinical results] Z Orthop Unfall. 2007;145:139. doi: 10.1055/s-2007-965181. [DOI] [PubMed] [Google Scholar]
- 45.Andereya S. Maus U. Gavenis K. Muller-Rath R. Miltner O. Mumme T., et al. [First clinical experiences with a novel 3D-collagen gel (CaReS) for the treatment of focal cartilage defects in the knee] Z Orthop Ihre Grenzgeb. 2006;144:272. doi: 10.1055/s-2006-933445. [DOI] [PubMed] [Google Scholar]
- 46.Zscharnack M. Hepp P. Richter R. Aigner T. Schulz R. Somerson J., et al. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am J Sports Med. 2010;38:1857. doi: 10.1177/0363546510365296. [DOI] [PubMed] [Google Scholar]
- 47.Kuroda R. Ishida K. Matsumoto T. Akisue T. Fujioka H. Mizuno K., et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Society. 2007;15:226. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Wakitani S. Goto T. Pineda S.J. Young R.G. Mansour J.M. Caplan A.I., et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Wakitani S. Imoto K. Yamamoto T. Saito M. Murata N. Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2002;10:199. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 50.Wakitani S. Kawaguchi A. Tokuhara Y. Takaoka K. Present status of and future direction for articular cartilage repair. J Bone Miner Metab. 2008;26:115. doi: 10.1007/s00774-007-0802-8. [DOI] [PubMed] [Google Scholar]
- 51.Wakitani S. Okabe T. Horibe S. Mitsuoka T. Saito M. Koyama T., et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5:146. doi: 10.1002/term.299. [DOI] [PubMed] [Google Scholar]
- 52.Lynn A.K. Yannas I.V. Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71:343. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 53.Pieper J.S. van der Kraan P.M. Hafmans T. Kamp J. Buma P. van Susante J.L., et al. Crosslinked type II collagen matrices: preparation, characterization, and potential for cartilage engineering. Biomaterials. 2002;23:3183. doi: 10.1016/s0142-9612(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 54.Lee C.R. Grodzinsky A.J. Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials. 2001;22:3145. doi: 10.1016/s0142-9612(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 55.Lee K.Y. Mooney D.J. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 56.Galois L. Hutasse S. Cortial D. Rousseau C.F. Grossin L. Ronziere M.C., et al. Bovine chondrocyte behaviour in three-dimensional type I collagen gel in terms of gel contraction, proliferation and gene expression. Biomaterials. 2006;27:79. doi: 10.1016/j.biomaterials.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 57.Li Y. Qin J. Lin B. Zhang W. The effects of insulin-like growth factor-1 and basic fibroblast growth factor on the proliferation of chondrocytes embedded in the collagen gel using an integrated microfluidic device. Tissue Eng Part C Methods. 2010;16:1267. doi: 10.1089/ten.TEC.2009.0682. [DOI] [PubMed] [Google Scholar]
- 58.Hirano Y. Ishiguro N. Sokabe M. Takigawa M. Naruse K. Effects of tensile and compressive strains on response of a chondrocytic cell line embedded in type I collagen gel. J Biotechnol. 2008;133:245. doi: 10.1016/j.jbiotec.2007.07.955. [DOI] [PubMed] [Google Scholar]
- 59.Hui T.Y. Cheung K.M. Cheung W.L. Chan D. Chan B.P. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials. 2008;29:3201. doi: 10.1016/j.biomaterials.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Koga H. Muneta T. Nagase T. Nimura A. Ju Y.J. Mochizuki T., et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 61.Lu Z. Doulabi B.Z. Huang C. Bank R.A. Helder M.N. Collagen type II enhances chondrogenesis in adipose tissue-derived stem cells by affecting cell shape. Tissue Eng Part A. 2010;16:81. doi: 10.1089/ten.TEA.2009.0222. [DOI] [PubMed] [Google Scholar]
- 62.Bosnakovski D. Mizuno M. Kim G. Takagi S. Okumura M. Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 63.Visna P. Pasa L. Cizmar I. Hart R. Hoch J. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques—a randomized controlled study. Acta Chir Belg. 2004;104:709. doi: 10.1080/00015458.2004.11679648. [DOI] [PubMed] [Google Scholar]
- 64.Dare E.V. Griffith M. Poitras P. Wang T. Dervin G.F. Giulivi A., et al. Fibrin sealants from fresh or fresh/frozen plasma as scaffolds for in vitro articular cartilage regeneration. Tissue Eng Part A. 2009;15:2285. doi: 10.1089/ten.tea.2008.0228. [DOI] [PubMed] [Google Scholar]
- 65.van Susante J.L. Buma P. Schuman L. Homminga G.N. van den Berg W.B. Veth R.P. Resurfacing potential of heterologous chondrocytes suspended in fibrin glue in large full-thickness defects of femoral articular cartilage: an experimental study in the goat. Biomaterials. 1999;20:1167. doi: 10.1016/s0142-9612(97)00190-7. [DOI] [PubMed] [Google Scholar]
- 66.Chang F. Ishii T. Yanai T. Mishima H. Akaogi H. Ogawa T., et al. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J Orthop Res. 2008;26:18. doi: 10.1002/jor.20470. [DOI] [PubMed] [Google Scholar]
- 67.Ho S.T. Cool S.M. Hui J.H. Hutmacher D.W. The influence of fibrin based hydrogels on the chondrogenic differentiation of human bone marrow stromal cells. Biomaterials. 2010;31:38. doi: 10.1016/j.biomaterials.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 68.Chung C. Burdick J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung C. Beecham M. Mauck R.L. Burdick J.A. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30:4287. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erickson I.E. Huang A.H. Chung C. Li R.T. Burdick J.A. Mauck R.L. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toh W.S. Lee E.H. Guo X.M. Chan J.K. Yeow C.H. Choo A.B., et al. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 72.Yoon D.M. Curtiss S. Reddi A.H. Fisher J.P. Addition of hyaluronic acid to alginate embedded chondrocytes interferes with insulin-like growth factor-1 signaling in vitro and in vivo. Tissue Eng Part A. 2009;15:3449. doi: 10.1089/ten.TEA.2009.0069. [DOI] [PubMed] [Google Scholar]
- 73.Liao E. Yaszemski M. Krebsbach P. Hollister S. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 2007;13:537. doi: 10.1089/ten.2006.0117. [DOI] [PubMed] [Google Scholar]
- 74.Fraser J.R. Laurent T.C. Laurent U.B. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 75.Chong B.F. Blank L.M. McLaughlin R. Nielsen L.K. Microbial hyaluronic acid production. Appl Microbiol Biotechnol. 2005;66:341. doi: 10.1007/s00253-004-1774-4. [DOI] [PubMed] [Google Scholar]
- 76.Smeds K.A. Pfister-Serres A. Miki D. Dastgheib K. Inoue M. Hatchell D.L., et al. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54:115. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 77.Nettles D.L. Vail T.P. Morgan M.T. Grinstaff M.W. Setton L.A. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004;32:391. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 78.Sahoo S. Chung C. Khetan S. Burdick J.A. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules. 2008;9:1088. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park H. Temenoff J.S. Holland T.A. Tabata Y. Mikos A.G. Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26:7095. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y. Shu X.Z. Prestwich G.D. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 81.Yamane S. Iwasaki N. Majima T. Funakoshi T. Masuko T. Harada K., et al. Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering. Biomaterials. 2005;26:611. doi: 10.1016/j.biomaterials.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Akmal M. Singh A. Anand A. Kesani A. Aslam N. Goodship A., et al. The effects of hyaluronic acid on articular chondrocytes. J Bone Joint Surg. 2005;87:1143. doi: 10.1302/0301-620X.87B8.15083. [DOI] [PubMed] [Google Scholar]
- 83.Nishimoto S. Takagi M. Wakitani S. Nihira T. Yoshida T. Effect of chondroitin sulfate and hyaluronic acid on gene expression in a three-dimensional culture of chondrocytes. J Biosci Bioeng. 2005;100:123. doi: 10.1263/jbb.100.123. [DOI] [PubMed] [Google Scholar]
- 84.Hoemann C.D. Sun J. Legare A. McKee M.D. Buschmann M.D. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2005;13:318. doi: 10.1016/j.joca.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Tan H. Chu C.R. Payne K.A. Marra K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2499. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hao T. Wen N. Cao J.K. Wang H.B. Lu S.H. Liu T., et al. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2010;18:257. doi: 10.1016/j.joca.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 87.Berger J. Reist M. Mayer J.M. Felt O. Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57:35. doi: 10.1016/s0939-6411(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y.L. Lee H.P. Chan H.Y. Sung L.Y. Chen H.C. Hu Y.C. Composite chondroitin-6-sulfate/dermatan sulfate/chitosan scaffolds for cartilage tissue engineering. Biomaterials. 2007;28:2294. doi: 10.1016/j.biomaterials.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 89.Chenite A. Chaput C. Wang D. Combes C. Buschmann M.D. Hoemann C.D., et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155. doi: 10.1016/s0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- 90.Ganji F. Abdekhodaie M.J. Ramzani A. Gelation time and degradation rate of chitosan-based injectable hydrogel. J Sol-Gel Sci Technol. 2007;42:47. [Google Scholar]
- 91.Jin R. Moreira Teixeira L.S. Dijkstra P.J. Karperien M. van Blitterswijk C.A. Zhong Z.Y., et al. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2544. doi: 10.1016/j.biomaterials.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 92.Lee K.Y. Ha W.S. Park W.H. Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials. 1995;16:1211. doi: 10.1016/0142-9612(95)98126-y. [DOI] [PubMed] [Google Scholar]
- 93.Mi F.L. Kuan C.Y. Shyu S.S. Lee S.T. Chang S.F. The study of gelation kinetics and chain-relaxation properties of glutaraldehyde-cross-linked chitosan gel and their effects on microsphere preparation and drug release. Carbohydr Polym. 2000;41:389. [Google Scholar]
- 94.Park K. Joung Y.K. Park K.D. Lee S.Y. Lee M.C. RGD-conjugated chitosan-pluronic hydrogels as a cell supported scaffold for articular cartilage regeneration. Macromol Res. 2008;16:517. [Google Scholar]
- 95.Park K.M. Lee S.Y. Joung Y.K. Na J.S. Lee M.C. Park K.D. Thermosensitive chitosan-Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta biomaterialia. 2009;5:1956. doi: 10.1016/j.actbio.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 96.Sechriest V.F. Miao Y.J. Niyibizi C. Westerhausen-Larson A. Matthew H.W. Evans C.H., et al. GAG-augmented polysaccharide hydrogel: a novel biocompatible and biodegradable material to support chondrogenesis. J Biomed Mater Res. 2000;49:534. doi: 10.1002/(sici)1097-4636(20000315)49:4<534::aid-jbm12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 97.Holmes T.C. Novel peptide-based biomaterial scaffolds for tissue engineering. Trends Biotechnol. 2002;20:16. doi: 10.1016/s0167-7799(01)01840-6. [DOI] [PubMed] [Google Scholar]
- 98.Kisiday J. Jin M. Kurz B. Hung H. Semino C. Zhang S., et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang S. Holmes T. Lockshin C. Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci U S A. 1993;90:3334. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bell C.J. Carrick L.M. Katta J. Jin Z. Ingham E. Aggeli A., et al. Self-assembling peptides as injectable lubricants for osteoarthritis. J Biomed Mater Res A. 2006;78:236. doi: 10.1002/jbm.a.30672. [DOI] [PubMed] [Google Scholar]
- 101.Liu J. Song H. Zhang L. Xu H. Zhao X. Self-assembly-peptide hydrogels as tissue-engineering scaffolds for three-dimensional culture of chondrocytes in vitro. Macromol Biosci. 2010;10:1164. doi: 10.1002/mabi.200900450. [DOI] [PubMed] [Google Scholar]
- 102.Shah R.N. Shah N.A. Del Rosario Lim M.M. Hsieh C. Nuber G. Stupp S.I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci U S A. 2010;107:3293. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maher S.A. Mauck R.L. Rackwitz L. Tuan R.S. A nanofibrous cell-seeded hydrogel promotes integration in a cartilage gap model. J Tissue Eng Regen Med. 2010;4:25. doi: 10.1002/term.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kisiday J.D. Kopesky P.W. Evans C.H. Grodzinsky A.J. McIlwraith C.W. Frisbie D.D. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008;26:322. doi: 10.1002/jor.20508. [DOI] [PubMed] [Google Scholar]
- 105.Kisiday J.D. Kurz B. DiMicco M.A. Grodzinsky A.J. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 2005;11:141. doi: 10.1089/ten.2005.11.141. [DOI] [PubMed] [Google Scholar]
- 106.Kisiday J.D. Lee J.H. Siparsky P.N. Frisbie D.D. Flannery C.R. Sandy J.D., et al. Catabolic responses of chondrocyte-seeded peptide hydrogel to dynamic compression. Ann Biomed Eng. 2009;37:1368. doi: 10.1007/s10439-009-9699-9. [DOI] [PubMed] [Google Scholar]
- 107.Mauck R.L. Byers B.A. Yuan X. Tuan R.S. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 108.Lima E.G. Bian L. Mauck R.L. Byers B.A. Tuan R.S. Ateshian G.A., et al. The effect of applied compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Conf Proc IEEE Eng Med Biol Soc. 2006;1:779. doi: 10.1109/IEMBS.2006.259313. [DOI] [PubMed] [Google Scholar]
- 109.Cohen D.L. Lipton J.I. Bonassar L.J. Lipson H. Additive manufacturing for in situ repair of osteochondral defects. Biofabrication. 2010;2:1. doi: 10.1088/1758-5082/2/3/035004. [DOI] [PubMed] [Google Scholar]
- 110.Wakitani S. Mitsuoka T. Nakamura N. Toritsuka Y. Nakamura Y. Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13:595. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 111.Park S.H. Park S.R. Chung S.I. Pai K.S. Min B.H. Tissue-engineered cartilage using fibrin/hyaluronan composite gel and its in vivo implantation. Artif Organs. 2005;29:838. doi: 10.1111/j.1525-1594.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 112.Sechriest V.F. Miao Y.J. Niyibizi C. Westerhausen-Larson A. Matthew H.W. Evans C.H., et al. GAG-augmented polysaccharide hydrogel: a novel biocompatible and biodegradable material to support chondrogenesis. J Biomed Mater Res. 1999;49:534. doi: 10.1002/(sici)1097-4636(20000315)49:4<534::aid-jbm12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 113.Hoemann C.D. Hurtig M. Rossomacha E. Sun J. Chevrier A. Shive M.S., et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87:2671. doi: 10.2106/JBJS.D.02536. [DOI] [PubMed] [Google Scholar]
- 114.Bray J.C. Merrill E.W. Poly(vinyl alcohol) hydrogels for synthetic articular cartilage material. J Biomed Mater Res. 1973;7:431. doi: 10.1002/jbm.820070506. [DOI] [PubMed] [Google Scholar]
- 115.Bodugoz-Senturk H. Macia C.E. Kun J.H. Muratoglu O.K. Poly(vinyl alcohol)-acrylamide hydrogels as load-bearing cartilage substitutes. Biomaterials. 2009;30:589. doi: 10.1016/j.biomaterials.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 116.Stauffer S.R. Peppas N. Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer. 1992;33:3932. [Google Scholar]
- 117.Peppas N. Crystallization of pol yvinyl alcohol-water films by slow dehydration. Eur Polym J. 1976;12:495. [Google Scholar]
- 118.Lee S.Y. Pereira B.P. Yusof N. Selvaratnam L. Yu Z. Abbas A.A., et al. Unconfined compression properties of a porous poly(vinyl alcohol)-chitosan-based hydrogel after hydration. Acta biomaterialia. 2009;5:1919. doi: 10.1016/j.actbio.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 119.Grant C. Twigg P. Egan A. Moody A. Smith A. Eagland D., et al. Poly(vinyl alcohol) hydrogel as a biocompatible viscoelastic mimetic for articular cartilage. Biotechnol Prog. 2006;22:1400. doi: 10.1021/bp060181u. [DOI] [PubMed] [Google Scholar]
- 120.Spiller K.L. Laurencin S.J. Charlton D. Maher S.A. Lowman A.M. Superporous hydrogels for cartilage repair: evaluation of the morphological and mechanical properties. Acta biomaterialia. 2008;4:17. doi: 10.1016/j.actbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 121.Du M. Gong J.P. Surface friction and lubrication of polymer gels. In: Biresaw G., editor; Mittal KL, editor. Surfactants in Tribology. Boca Raton: CRC Press, Taylor and Francis Group; 2008. pp. 223–246. [Google Scholar]
- 122.Greene G.W. Zappone B. Soderman O. Topgaard D. Rata G. Zeng H., et al. Anisotropic dynamic changes in the pore network structure, fluid diffusion and fluid flow in articular cartilage under compression. Biomaterials. 2010;31:3117. doi: 10.1016/j.biomaterials.2010.01.102. [DOI] [PubMed] [Google Scholar]
- 123.Murakami T. Sawae Y. Higaki H. Ohtsuki N. Moriyama S. An adaptive multimode lubrication in knee prostheses with artificial cartilage during walking. In: Dowson D., editor; Taylor C.M., editor; Childs T.H.C., editor; Dalmaz G., editor; Berthier Y., editor; Flamand L., et al., editors. Elastohydrodynamics- '96: Fundamentals and Applications in Lubrication and Traction. Amsterdam: Elsevier; 1997. [Google Scholar]
- 124.Oka M. Noguchi T. Kumar P. Ikeuchi K. Yamamuro T. Hyon S.H., et al. Development of an artificial articular cartilage. Clin Mater. 1990;6:361. doi: 10.1016/0267-6605(90)90053-x. [DOI] [PubMed] [Google Scholar]
- 125.Li F. Su Y. Wang J. Wu G. Wang C. Influence of dynamic load on friction behavior of human articular cartilage, stainless steel and polyvinyl alcohol hydrogel as artificial cartilage. J Mater Sci Mater Med. 2010;21:147. doi: 10.1007/s10856-009-3863-5. [DOI] [PubMed] [Google Scholar]
- 126.Holloway J.L. Lowman A.M. Palmese G.R. Mechanical evaluation of poly(vinyl alcohol)-based fibrous composites as biomaterials for meniscal tissue replacement. Acta Biomater. 2010;6:4716. doi: 10.1016/j.actbio.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 127.Jurvelin J.S. Buschmann M.D. Hunziker E.B. Optical and mechanical determination of Poisson's ratio of adult bovine humeral articular cartilage. J Biomech. 1997;30:235. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- 128.Korhonen R. Laasanen M. Toyras J. Rieppo J. Hirvonen H. Helminen H., et al. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression, and indentation. J Biomech. 2002;35:903. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 129.Setton L.A. Mow V.C. Howell D.S. Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament. J Orthop Res. 1995;13:473. doi: 10.1002/jor.1100130402. [DOI] [PubMed] [Google Scholar]
- 130.Chang Y.S. Gu H.O. Kobayashi M. Oka M. Comparison of the bony ingrowth into an osteochondral defect and an artificial osteochondral composite device in load-bearing joints. Knee. 1998;5:205. [Google Scholar]
- 131.Maher S.A. Doty S.B. Torzilli P.A. Thornton S. Lowman A.M. Thomas J.D., et al. Nondegradable hydrogels for the treatment of focal cartilage defects. J Biomed Mater Res A. 2007;83:145. doi: 10.1002/jbm.a.31255. [DOI] [PubMed] [Google Scholar]
- 132.Lange J. Follak N. Nowotny T. Merk H. [Results of SaluCartilage implantation for stage IV chondral defects in the knee joint area] Unfallchirurg. 2006;109:193. doi: 10.1007/s00113-005-1025-x. [DOI] [PubMed] [Google Scholar]
- 133.Vrana N.E. O'Grady A. Kay E. Cahill P.A. McGuinness G.B. Cell encapsulation within PVA-based hydrogels via freeze-thawing: a one-step scaffold formation and cell storage technique. J Tissue Eng Regen Med. 2009;3:567. doi: 10.1002/term.193. [DOI] [PubMed] [Google Scholar]
- 134.Spiller K.L. Holloway J.L. Gribb M.E. Lowman A.M. Design of semi-degradable hydrogels based on poly(vinyl alcohol) and poly(lactic-co-glycolic acid) for cartilage tissue engineering. J Tissue Eng Regen Med. 2010 doi: 10.1002/term.356. [Epub ahead of print]; [DOI] [PubMed] [Google Scholar]
- 135.Bichara D.A. Zhao X. Bodugoz-Senturk H. Ballyns F.P. Oral E. Randolph M.A., et al. Porous poly(vinyl alcohol)-hydrogel matrix-engineered bio-synthetic cartilage. Tissue Eng Part A. 2011;17:301. doi: 10.1089/ten.TEA.2010.0322. [DOI] [PubMed] [Google Scholar]
- 136.Bryant S.J. Davis-Arehart K.A. Luo N. Shoemaker R.K. Arthur J.A. Anseth K.S. Synthesis and characterization of photopolymerized multifunctional hydrogels: water-soluble poly(vinyl alcohol) and chondroitin sulfate macromers for chondrocyte encapsulation. Macromolecules. 2004;37:6726. [Google Scholar]
- 137.Schmedlen R.H. Masters K.S. West J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials. 2002;23:4325. doi: 10.1016/s0142-9612(02)00177-1. [DOI] [PubMed] [Google Scholar]
- 138.Elisseeff J. Anseth K. Sims D. McIntosh W. Randolph M. Yaremchuk M., et al. Transdermal photopolymerization of poly(ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage. Plast Reconstr Surg. 1999;104:1014. doi: 10.1097/00006534-199909040-00017. [DOI] [PubMed] [Google Scholar]
- 139.Sharma B. Williams C.G. Khan M. Manson P. Elisseeff J.H. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast Reconstr Surg. 2007;119:112. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]
- 140.Hwang N.S. Varghese S. Theprungsirikul P. Canver A. Elisseeff J. Enhanced chondrogenic differentiation of murine embryonic stem cells in hydrogels with glucosamine. Biomaterials. 2006;27:6015. doi: 10.1016/j.biomaterials.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 141.Bryant S.J. Bender R.J. Durand K.L. Anseth K.S. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol Bioeng. 2004;86:747. doi: 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- 142.Bryant S. Anseth K. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering. J Biomed Mater Res. 2003;64A:70. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 143.Bryant S. Anseth K. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 144.Kloxin A.M. Kasko A.M. Salinas C.N. Anseth K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martens P.J. Bryant S.J. Anseth K.S. Tailoring the degradation of hydrogels formed from multivinyl poly(ethylene glycol) and poly(vinyl alcohol) macromers for cartilage tissue engineering. Biomacromolecules. 2003;4:283. doi: 10.1021/bm025666v. [DOI] [PubMed] [Google Scholar]
- 146.Lee H.J. Lee J.S. Chansakul T. Yu C. Elisseeff J.H. Yu S.M. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27:5268. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 147.Liu S.Q. Tian Q. Hedrick J.L. Po Hui J.H. Ee P.L. Yang Y.Y. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials. 2010;31:7298. doi: 10.1016/j.biomaterials.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 148.Bryant S.J. Arthur J.A. Anseth K.S. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomaterialia. 2005;1:243. doi: 10.1016/j.actbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 149.Varghese S. Hwang N.S. Canver A.C. Theprungsirikul P. Lin D.W. Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 150.Wang D.A. Varghese S. Sharma B. Strehin I. Fermanian S. Gorham J., et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Suggs L.J. Kao E.Y. Palombo L.L. Krishnan R.S. Widmer M.S. Mikos A.G. Preparation and characterization of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomater Sci. 1998;9:653. doi: 10.1163/156856298x00073. [DOI] [PubMed] [Google Scholar]
- 152.Guo X. Park H. Young S. Kretlow J.D. van den Beucken J.J. Baggett L.S., et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Holland T.A. Bodde E.W. Cuijpers V.M. Baggett L.S. Tabata Y. Mikos A.G., et al. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2007;15:187. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 154.Guo X. Park H. Liu G. Liu W. Cao Y. Tabata Y., et al. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials. 2009;30:2741. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park H. Temenoff J.S. Tabata Y. Caplan A.I. Mikos A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jung H.H. Park K. Han D.K. Preparation of TGF-beta1-conjugated biodegradable pluronic F127 hydrogel and its application with adipose-derived stem cells. J Control Release. 2010;147:84. doi: 10.1016/j.jconrel.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 157.Khattak S.F. Bhatia S.R. Roberts S.C. Pluronic F127 as a cell encapsulation material: utilization of membrane-stabilizing agents. Tissue Eng. 2005;11:974. doi: 10.1089/ten.2005.11.974. [DOI] [PubMed] [Google Scholar]
- 158.Cellesi F. Tirelli N. Hubbell J.A. Towards a fully-synthetic substitute of alginate: development of a new process using thermal gelation and chemical cross-linking. Biomaterials. 2004;25:5115. doi: 10.1016/j.biomaterials.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 159.Saim A.B. Cao Y. Weng Y. Chang C.N. Vacanti M.A. Vacanti C.A., et al. Engineering autogenous cartilage in the shape of a helix using an injectable hydrogel scaffold. Laryngoscope. 2000;110:1694. doi: 10.1097/00005537-200010000-00023. [DOI] [PubMed] [Google Scholar]
- 160.Yasuda A. Kojima K. Tinsley K.W. Yoshioka H. Mori Y. Vacanti C.A. In vitro culture of chondrocytes in a novel thermoreversible gelation polymer scaffold containing growth factors. Tissue Eng. 2006;12:1237. doi: 10.1089/ten.2006.12.1237. [DOI] [PubMed] [Google Scholar]
- 161.Park J.S. Yang H.N. Woo D.G. Kim H. Na K. Park K.H. Multi-lineage differentiation of hMSCs encapsulated in thermo-reversible hydrogel using a co-culture system with differentiated cells. Biomaterials. 2010;31:7275. doi: 10.1016/j.biomaterials.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 162.Cho J.H. Kim S.H. Park K.D. Jung M.C. Yang W.I. Han S.W., et al. Chondrogenic differentiation of human mesenchymal stem cells using a thermosensitive poly(N-isopropylacrylamide) and water-soluble chitosan copolymer. Biomaterials. 2004;25:5743. doi: 10.1016/j.biomaterials.2004.01.051. [DOI] [PubMed] [Google Scholar]
- 163.Ibusuki S. Fujii Y. Iwamoto Y. Matsuda T. Tissue-engineered cartilage using an injectable and in situ gelable thermoresponsive gelatin: fabrication and in vitro performance. Tissue Eng. 2003;9:371. doi: 10.1089/107632703764664846. [DOI] [PubMed] [Google Scholar]
- 164.Yasuda K. Ping Gong J. Katsuyama Y. Nakayama A. Tanabe Y. Kondo E., et al. Biomechanical properties of high-toughness double network hydrogels. Biomaterials. 2005;26:4468. doi: 10.1016/j.biomaterials.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 165.Arakaki K. Kitamura N. Fujiki H. Kurokawa T. Iwamoto M. Ueno M., et al. Artificial cartilage made from a novel double-network hydrogel: In vivo effects on the normal cartilage and ex vivo evaluation of the friction property. J Biomed Mater Res A. 2010;93:1160. doi: 10.1002/jbm.a.32613. [DOI] [PubMed] [Google Scholar]
- 166.Bryant S.J. Durand K.L. Anseth K.S. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res A. 2003;67:1430. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 167.Sontjens S.H. Nettles D.L. Carnahan M.A. Setton L.A. Grinstaff M.W. Biodendrimer-based hydrogel scaffolds for cartilage tissue repair. Biomacromolecules. 2006;7:310. doi: 10.1021/bm050663e. [DOI] [PubMed] [Google Scholar]
- 168.Park Y. Lutolf M. Hubbell J. Hunziker E. Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10:515. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 169.Villanueva I. Hauschulz D.S. Mejic D. Bryant S.J. Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different crosslinking densities. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2008;16:909. doi: 10.1016/j.joca.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nicodemus G.D. Bryant S.J. The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation. J Biomech. 2008;41:1528. doi: 10.1016/j.jbiomech.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hunter C.J. Imler S.M. Malaviya P. Nerem R.M. Levenston M.E. Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials. 2002;23:1249. doi: 10.1016/s0142-9612(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 172.Hunter C.J. Mouw J.K. Levenston M.E. Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2004;12:117. doi: 10.1016/j.joca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 173.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Hunziker E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 174.Lee D.A. Bader D.L. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 175.Bryant S.J. Nicodemus G.D. Villanueva I. Designing 3D photopolymer hydrogels to regulate biomechanical cues and tissue growth for cartilage tissue engineering. Pharm Res. 2008;25:2379. doi: 10.1007/s11095-008-9619-y. [DOI] [PubMed] [Google Scholar]
- 176.Schmidt O. Mizrahi J. Elisseeff J. Seliktar D. Immobilized fibrinogen in PEG hydrogels does not improve chondrocyte-mediated matrix deposition in response to mechanical stimulation. Biotechnol Bioeng. 2006;95:1061. doi: 10.1002/bit.21072. [DOI] [PubMed] [Google Scholar]
- 177.Kisiday J.D. Jin M. DiMicco M.A. Kurz B. Grodzinsky A.J. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 178.Bryant S.J. Anseth K.S. Lee D.A. Bader D.L. Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. J Orthop Res. 2004;22:1143. doi: 10.1016/j.orthres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 179.Bryant S.J. Chowdhury T.T. Lee D.A. Bader D.L. Anseth K.S. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004;32:407. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 180.Park H. Guo X. Temenoff J.S. Tabata Y. Caplan A.I. Kasper F.K., et al. Effect of swelling ratio of injectable hydrogel composites on chondrogenic differentiation of encapsulated rabbit marrow mesenchymal stem cells in vitro. Biomacromolecules. 2009;10:541. doi: 10.1021/bm801197m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bryant S.J. Nuttelman C.R. Anseth K.S. The effects of crosslinking density on cartilage formation in photocrosslinkable hydrogels. Biomed Sci Instrum. 1999;35:309. [PubMed] [Google Scholar]
- 182.Bahney C.S. Hsu C.W. Yoo J.U. West J.L. Johnstone B. A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells. FASEB J. 2011;25:1486. doi: 10.1096/fj.10-165514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ferretti M. Marra K.G. Kobayashi K. Defail A.J. Chu C.R. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng. 2006;12:2657. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 184.Wang D.A. Williams C.G. Li Q. Sharma B. Elisseeff J.H. Synthesis and characterization of a novel degradable phosphate-containing hydrogel. Biomaterials. 2003;24:3969. doi: 10.1016/s0142-9612(03)00280-1. [DOI] [PubMed] [Google Scholar]
- 185.Jukes J.M. van der Aa L.J. Hiemstra C. van veen T. Dijkstra P.J. Zhong Z.Y., et al. A newly developed chemically crosslinked dextran-poly(ethylene glycol) hydrogel for cartilage tissue engineering. Tissue Eng Part A. 2010;16:565. doi: 10.1089/ten.TEA.2009.0173. [DOI] [PubMed] [Google Scholar]
- 186.Millward-Sadler S.J. Salter D.M. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32:435. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 187.Knight M.M. Lee D.A. Bader D.L. The influence of elaborated pericellular matrix on the deformation of isolated articular chondrocytes cultured in agarose. Biochim Biophys Acta- Molec Cell Res. 1998;1405:67. doi: 10.1016/s0167-4889(98)00102-5. [DOI] [PubMed] [Google Scholar]
- 188.Grodzinsky A.J. Levenston M.E. Jin M. Frank E.H. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 189.Lee C.R. Grodzinsky A.J. Spector M. Biosynthetic response of passaged chondrocytes in a type II collagen scaffold to mechanical compression. J Biomed Mater Res. 2003;64A:560. doi: 10.1002/jbm.a.10443. [DOI] [PubMed] [Google Scholar]
- 190.Mauck R.L. Seyhan S.L. Ateshian G.A. Hung C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 191.Appelman T.P. Mizrahi J. Elisseeff J.H. Seliktar D. The differential effect of scaffold composition and architecture on chondrocyte response to mechanical stimulation. Biomaterials. 2009;30:518. doi: 10.1016/j.biomaterials.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 192.Bian L. Fong J.V. Lima E.G. Stoker A.M. Ateshian G.A. Cook J.L., et al. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16:1781. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chowdhury T.T. Bader D.L. Shelton J.C. Lee D.A. Temporal regulation of chondrocyte metabolism in agarose constructs subjected to dynamic compression. Arch Biochem Biophys. 2003;417:105. doi: 10.1016/s0003-9861(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 194.Kelly T.A. Ng K.W. Wang C.C. Ateshian G.A. Hung C.T. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 195.Terraciano V. Hwang N. Moroni L. Park H.B. Zhang Z. Mizrahi J., et al. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 196.Huang A.H. Farrell M.J. Kim M. Mauck R.L. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur Cell Mater. 2010;19:72. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thorpe S.D. Buckley C.T. Vinardell T. O'Brien F.J. Campbell V.A. Kelly D.J. Dynamic compression can inhibit chondrogenesis of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;377:458. doi: 10.1016/j.bbrc.2008.09.154. [DOI] [PubMed] [Google Scholar]
- 198.Nicodemus G.D. Bryant S.J. Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2010;18:126. doi: 10.1016/j.joca.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 199.De Croos J.N. Dhaliwal S.S. Grynpas M.D. Pilliar R.M. Kandel R.A. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol. 2006;25:323. doi: 10.1016/j.matbio.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 200.Fitzgerald J.B. Jin M. Dean D. Wood D.J. Zheng M.H. Grodzinsky A.J. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004;279:19502. doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- 201.Nicodemus G.D. Skaalure S.C. Bryant S.J. Gel structure has an impact on pericellular and extracellular matrix deposition, which subsequently alters metabolic activities in chondrocyte-laden PEG hydrogels. Acta Biomater. 2010;7:492. doi: 10.1016/j.actbio.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S., et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kopesky P.W. Lee H.Y. Vanderploeg E.J. Kisiday J.D. Frisbie D.D. Plaas A.H., et al. Adult equine bone marrow stromal cells produce a cartilage-like ECM mechanically superior to animal-matched adult chondrocytes. Matrix Biol. 2010;29:427. doi: 10.1016/j.matbio.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tran-Khanh N. Hoemann C.D. McKee M.D. Henderson J.E. Buschmann M.D. Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J Orthop Res. 2005;23:1354. doi: 10.1016/j.orthres.2005.05.009.1100230617. [DOI] [PubMed] [Google Scholar]
- 205.Ng K.W. Lima E.G. Bian L. O'Conor C.J. Jayabalan P.S. Stoker A.M., et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16:1041. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kafienah W. Jakob M. Demarteau O. Frazer A. Barker M.D. Martin I., et al. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8:817. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- 207.Van Osch G.J. Mandl E.W. Jahr H. Koevoet W. Nolst-Trenite G. Verhaar J.A. Considerations on the use of ear chondrocytes as donor chondrocytes for cartilage tissue engineering. Biorheology. 2004;41:411. [PubMed] [Google Scholar]
- 208.Chung C. Erickson I.E. Mauck R.L. Burdick J.A. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Eng Part A. 2008;14:1121. doi: 10.1089/ten.tea.2007.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xu J.W. Zaporojan V. Peretti G.M. Roses R.E. Morse K.B. Roy A.K., et al. Injectable tissue-engineered cartilage with different chondrocyte sources. Plast Reconstr Surg. 2004;113:1361. doi: 10.1097/01.prs.0000111594.52661.29. [DOI] [PubMed] [Google Scholar]
- 210.Johnson T.S. Xu J.W. Zaporojan V.V. Mesa J.M. Weinand C. Randolph M.A., et al. Integrative repair of cartilage with articular and nonarticular chondrocytes. Tissue Eng. 2004;10:1308. doi: 10.1089/ten.2004.10.1308. [DOI] [PubMed] [Google Scholar]
- 211.Li Z. Kupcsik L. Yao S.J. Alini M. Stoddart M.J. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-beta pathway. J Cell Mol Med. 2010;14:1338. doi: 10.1111/j.1582-4934.2009.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li Z. Yao S.J. Alini M. Stoddart M.J. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A. 2010;16:575. doi: 10.1089/ten.TEA.2009.0262. [DOI] [PubMed] [Google Scholar]
- 213.Kupcsik L. Stoddart M.J. Li Z. Benneker L.M. Alini M. Improving chondrogenesis: potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng Part A. 2010;16:1845. doi: 10.1089/ten.TEA.2009.0531. [DOI] [PubMed] [Google Scholar]
- 214.Williams G.M. Klein T.J. Sah R.L. Cell density alters matrix accumulation in two distinct fractions and the mechanical integrity of alginate-chondrocyte constructs. Acta Biomater. 2005;1:625. doi: 10.1016/j.actbio.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 215.Jiang J. Tang A. Ateshian G. Guo X.E. Hung C.T. Lu H.H. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng. 2010;38:2183. doi: 10.1007/s10439-010-0038-y. [DOI] [PubMed] [Google Scholar]
- 216.Loeser R.F. Pacione C.A. Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 217.Worster A.A. Brower-Toland B.D. Fortier L.A. Bent S.J. Williams J. Nixon A.J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 218.Pabbruwe M.B. Esfandiari E. Kafienah W. Tarlton J.F. Hollander A.P. Induction of cartilage integration by a chondrocyte/collagen-scaffold implant. Biomaterials. 2009;30:4277. doi: 10.1016/j.biomaterials.2009.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chang C. Lauffenburger D.A. Morales T.I. Motile chondrocytes from newborn calf: migration properties and synthesis of collagen II. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2003;11:603. doi: 10.1016/s1063-4584(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 220.Fiedler J. Brill C. Blum W.F. Brenner R.E. IGF-I and IGF-II stimulate directed cell migration of bone-marrow-derived human mesenchymal progenitor cells. Biochem Biophys Res Commun. 2006;345:1177. doi: 10.1016/j.bbrc.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 221.Fortier L.A. Mohammed H.O. Lust G. Nixon A.J. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg. 2002;84:276. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 222.Nixon A.J. Fortier L.A. Williams J. Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17:475. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- 223.Elisseeff J. McIntosh W. Fu K. Blunk B.T. Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 224.Huang Q. Goh J.C. Hutmacher D.W. Lee E.H. In Vivo mesenchymal cell recruitment by a scaffold loaded with transforming growth factor b1 and the potential for in situ chondrogenesis. Tissue Eng. 2002;8:469. doi: 10.1089/107632702760184727. [DOI] [PubMed] [Google Scholar]
- 225.Kopesky P.W. Vanderploeg E.J. Kisiday J.D. Frisbie D.D. Sandy J.D. Grodzinsky A.J. Controlled delivery of transforming growth factor beta1 by self-assembling peptide hydrogels induces chondrogenesis of bone marrow stromal cells and modulates Smad2/3 signaling. Tissue Eng Part A. 2011;17:83. doi: 10.1089/ten.tea.2010.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zheng L. Fan H.S. Sun J. Chen X.N. Wang G. Zhang L., et al. Chondrogenic differentiation of mesenchymal stem cells induced by collagen-based hydrogel: an in vivo study. J Biomed Mater Res A. 2010;93:783. doi: 10.1002/jbm.a.32588. [DOI] [PubMed] [Google Scholar]
- 227.Park J.S. Woo D.G. Yang H.N. Na K. Park K.H. Transforming growth factor b-3 bound with sulfate polysaccharide in synthetic extracellular matrix enhanced the biological activities for neocartilage formation in vivo. J Biomed Mater Res. 2009;91A:408. doi: 10.1002/jbm.a.32271. [DOI] [PubMed] [Google Scholar]
- 228.Drury J.L. Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 229.Holland T.A. Tabata Y. Mikos A.G. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 230.Holland T.A. Tessmar J.K. Tabata Y. Mikos A.G. Transforming growth factor-beta 1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Release. 2004;94:101. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 231.Wenk E. Meinel A.J. Wildy S. Merkle H.P. Meinel L. Microporous silk fibroin scaffolds embedding PLGA microparticles for controlled growth factor delivery in tissue engineering. Biomaterials. 2009;30:2571. doi: 10.1016/j.biomaterials.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 232.DeFail A.J. Chu C.R. Izzo N. Marra K.G. Controlled release of bioactive TGF-beta 1 from microspheres embedded within biodegradable hydrogels. Biomaterials. 2006;27:1579. doi: 10.1016/j.biomaterials.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 233.Wang X. Wenk E. Zhang X. Meinel L. Vunjak-Novakovic G. Kaplan D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release. 2009;134:81. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nixon A.J. Fortier L.A. Williams J. Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 2005;17:475. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- 235.Guo X. Liao J. Park H. Saraf A. Raphael R.M. Tabata Y., et al. Effects of TGF-beta3 and preculture period of osteogenic cells on the chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in a bilayered hydrogel composite. Acta Biomater. 2010;6:2920. doi: 10.1016/j.actbio.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bae S.E. Choi D.H. Han D.K. Park K. Effect of temporally controlled release of dexamethasone on in vivo chondrogenic differentiation of mesenchymal stromal cells. J Control Release. 2010;143:23. doi: 10.1016/j.jconrel.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 237.Chimal-Monroy J. Diaz de Leon L. Differential effects of transforming growth factors beta 1, beta 2, beta 3 and beta 5 on chondrogenesis in mouse limb bud mesenchymal cells. Int J Dev Biol. 1997;41:91. [PubMed] [Google Scholar]
- 238.Loeser R.F. Shanker G. Carlson C.S. Gardin J.F. Shelton B.J. Sonntag W.E. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: Studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2001;43:2110. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 239.Akens M.K. von Rechenberg B. Bittmann P. Nadler D. Zlinszky K. Auer J.A. Long term in-vivo studies of a photo-oxidized bovine osteochondral transplant in sheep. BMC Musculoskelet Disord. 2001;2:9. doi: 10.1186/1471-2474-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bos P.K. DeGroot J. Budde M. Verhaar J. Van Osch G.J. Specific enzymatic rreatment of bovine and human articular cartilage. Arthritis Rheum. 2002;46:976. doi: 10.1002/art.10208. [DOI] [PubMed] [Google Scholar]
- 241.Obradovic B. Martin I. Padera R.F. Treppo S. Freed L.E. Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19:1089. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 242.Nuttelman C.R. Mortisen D.J. Henry S.M. Anseth K.S. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res. 2001;57:217. doi: 10.1002/1097-4636(200111)57:2<217::aid-jbm1161>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 243.Hutcheon G.A. Messiou C. Wyre R.M. Davies M.C. Downes S. Water absorption and surface properties of novel poly(ethylmethacrylate) polymer systems for use in bone and cartilage repair. Biomaterials. 2001;22:667. doi: 10.1016/s0142-9612(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 244.Lee C.T. Kung P.H. Lee Y.D. Preparation of poly(vinyl alcohol)-chondroitin sulfate hydrogel as matrices in tissue engineering. Carbohydr Polym. 2005;61:348. [Google Scholar]
- 245.Silverman R.P. Bonasser L. Passaretti D. Randolph M.A. Yaremchuk M.J. Adhesion of tissue-engineered cartilate to native cartilage. Plast Reconstr Surg. 2000;105:1393. doi: 10.1097/00006534-200004040-00019. [DOI] [PubMed] [Google Scholar]
- 246.Wang D. Williams C.G. Yang F. Elisseeff J.H. Enhancing the tissue-biomaterial interface: tissue-initiated integration of biomaterials. Adv Funct Mater. 2004;14:1152. [Google Scholar]
- 247.Strehin I. Nahas Z. Arora K. Nguyen T. Elisseeff J. A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials. 2010;31:2788. doi: 10.1016/j.biomaterials.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hunter C.J. Levenston M.E. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng. 2004;10:736. doi: 10.1089/1076327041348310. [DOI] [PubMed] [Google Scholar]
- 249.Klein T.J. Rizzi S.C. Reichert J.C. Georgi N. Malda J. Schuurman W., et al. Strategies for zonal cartilage repair using hydrogels. Macromol Biosci. 2009;9:1049. doi: 10.1002/mabi.200900176. [DOI] [PubMed] [Google Scholar]