Abstract
This review summarizes recent evidence that prenatal exposure to diverse environmental chemicals dysregulates the fetal epigenome, with potential consequences for subsequent developmental disorders and disease manifesting in childhood, over the lifecourse, or even transgenerationally. The primordial germ cells, embryo, and fetus are highly susceptible to epigenetic dysregulation by environmental chemicals, which can thereby exert multiple adverse effects. The data reviewed here on environmental contaminants have potential implications for risk assessment although more data are needed on individual susceptibility to epigenetic alterations and their persistence before this information can be used in formal risk assessments. The findings discussed indicate that identification of environmental chemicals that dysregulate the prenatal epigenome should be a priority in health research and disease prevention.
Keywords: BPA, DNA methylation, Environmental chemicals, Environmental tobacco smoke, Epigenetics, Fetus, Polycyclic aromatic hydrocarbons, Transgenerational
1. Introduction
Following a brief summary of the role of epigenetics in early development and disease, this review focuses on the evidence that the prenatal/fetal period is highly susceptible to epigenomic dysregulation with implications for health, both lifelong and transgenerationally. We then offer examples of developmental exposure to various environmental pollutants shown to induce epigenetic changes and neurodevelopmental deficits and diseases. Interactions between toxic and environmental exposures and genetic, nutritional and social factors that can exacerbate effects are then described. Finally, two case studies are provided to illustrate the strengths and limitations of available epigenetic data and the potential of using epigenetic markers to forge causal links between toxic environmental exposures and neurodevelopmental outcomes. In this section, we summarize evidence that epigenetic alterations in endocrine and immune pathways are directly involved in the adverse neurodevelopmental effects associated with in utero exposure to the two classic endocrine disruptors, polycyclic aromatic hydrocarbons (PAHs) and bisphenol A (BPA).
2. The role of epigenetics in early development and disease: the prenatal/fetal window of susceptibility
Epigenetics is the study of heritable changes in gene expression or phenotype occurring without changes in DNA sequence [1]. For general reviews, see [2–4]. The genetic information in DNA has been likened to the notes of an orchestral score and epigenetics to the conductor who interprets the score and controls the dynamics of the symphonic performance [5]. While new epigenetic mechanisms are being uncovered, the best characterized are DNA methylation, changes in histone proteins around which DNA is packaged, and expression of non-coding RNAs (see [6–9] for review). Interactions between these epigenetic mechanisms generate diversity of cell types during development and then maintain the expression profiles of the different cell types throughout life [6]. The term “environmental epigenomics” reflects the constant interplay between the environment, which includes both endogenous (such as hormone levels or immune status) and exogenous factors (such as nutritional and chemical exposures), and the epigenome. The best characterized epigenetic events in early mammalian development are genomic imprinting (the silencing of one parental allele at a single locus, which occurs in the parental germ stem cells) resulting in monoallelic gene expression and x-chromosome inactivation (silencing of one of the two X chromosomes in mammalian females) occurring in early embryogenesis (reviewed in [6]). Dysregulation of imprinted genes during early development is involved in disorders such as Angelman’s, Prader-Willi and Beckwith-Wiederman Syndromes, certain cancers, and possibly in autism and other neurological syndromes [10].
Gene expression can be regulated by epigenetic processes. Two examples include coordinated epigenetic modifications of chromatin by DNA methylation and post-translational covalent modifications of histone proteins [4,11,12] and micro-RNA-induced suppression of gene expression during development [13]. DNA methylation is the most extensively investigated of the epigenetic mechanisms and involves the addition of a methyl group at the carbon-5 position of cytosine in CpG dinucleotides. While CpG dinucleotides are underrepresented in mammalian genomes overall, and usually exist in a methylated state, proximal gene promoter regions often overlap with CpG rich regions known as “CpG islands” that are typically unmethylated. In these regions, cytosine methylation serves a regulatory function [14]. By extending into the major groove of DNA, the methyl group of 5-methylcytosine (5-mC) interferes with transcription binding proteins, inhibiting transcription, and effectively silencing the gene [7]. More importantly, DNA methylation acts as a docking site for methyl-DNA binding proteins that recruit other chromatin remodeling proteins. The importance of methylation changes at non CpG islands is now being recognized [15]. Both hypermethylation and hypomethylation of DNA can result from exposure to exogenous chemicals. For some genes, even a small change in the level of DNA methylation at a few CpG sites might subtly alter gene expression and increase disease risk [16,17].
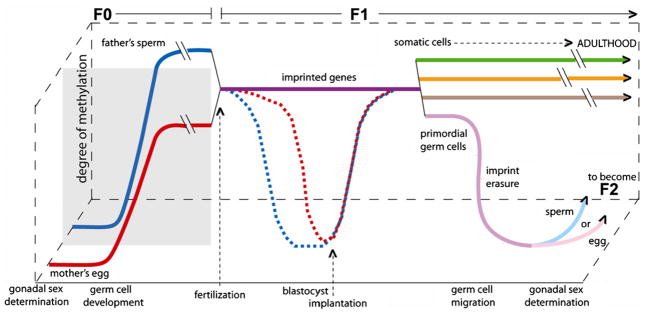
The epigenome is susceptible to dysregulation throughout life; however, it is thought to be most vulnerable to environmental factors during embryogenesis, which is a period of rapid cell division and epigenetic remodeling [16,18]. Following a complex choreography, following fertilization, DNA methylation patterns are largely erased and established early in mammalian development (reviewed in [7,19]). Fig. 1 illustrates the normal timetable for reprogramming of methylation of non-imprinted and imprinted genes during early development, beginning with the primordial germ cells (PGCs) of each of the parents (F0) through gametogenesis, fertilization, the embryonic period of the offspring (F1), followed by the maintenance of methylation in somatic cells and the development of germ cells that will become F2 [7,20,21]. These dynamic stages represent windows of potential vulnerability to epigenetic dysregulation [7]. While the maintenance of imprinted genes throughout the preimplantation period is essential for normal embryonic development, demethylation of other genes is needed to make the genome broadly available to the developing embryo. Thus, after fertilization and prior to implantation, the embryo undergoes genome-wide demethylation, with the exception of imprinted genes (which retain the methylation profile of the parent-of-origin) and some retrotransposable elements [16]. Beginning when the embryo is in the blastocyst stage (starting day 5 post fertilization for humans) and before implantation into the uterine wall (about 7 days post fertilization), methylation patterns in non-imprinted genes are reestablished de novo by the DNA methyltransferases DNMT3a and DNMT3b and their cofactor DNMT3L [20,22]. DNA methylation patterns are maintained by DNMT1, which restores full methylation to hemi-methylated CpG sites following DNA replication; this maintenance is critical for normal development [20,23].
Fig. 1.
There are multiple periods during which environmental exposures could affect the F1 individual’s methylation status, potentially affecting the F1 phenotype. The first window is during F0 (parental) germ cell development when methylation is reprogrammed following imprint erasure in the father’s sperm (solid blue line) and the mother’s egg (solid red line) The second window is post-conception, during F1 embryonic development, when all but imprinted genes are demethylated, with the male germ line (dashed blue line) demethylating more quickly, followed by the female germ line (dashed red line). Imprinted genes (purple line) maintain their methylation marks throughout this reprogramming, allowing for the inheritance of parental-specific monoallelic expression in somatic tissues throughout adulthood [203]. All of the non-imprinted genes are subsequently remethylated once the embryo reaches the early blastocyst stage. During the gonadal sex determination of the F1 embryo, primordial germ cells undergo epigenetic reprogramming, where parental imprinting is erased, as the germ cells of the F1 individual mature (solid light blue or pink line). To determine whether epigenetic alterations are transmitted transgenerationally, the F3 generation must be studied (see text and Fig. 2) [7]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
As noted, imprinted genes do not undergo genome-wide demethylation before implantation but maintain their methylation patterns throughout this period of reprogramming, allowing for the inheritance of parental-specific monoallelic expression in somatic tissues throughout adulthood [20]. Primordial germ cells (PGC) (the precursor cells that develop into spermatogonia and oogonia) have differential methylation by parent-of-origin at imprinted genes until they enter the genital ridge, when their DNA undergoes global demethylation of both imprinted and non-imprinted genes [24,21]. Remethylation of imprinted genes occurs in a sex-specific manner during gametogenesis (the division of gametocytes into haploid sperm and oocytes by meiosis) [19,20]. Imprints are established perinatally in the male germ line and are maintained throughout the mitotic divisions of the spermatogonial stem cells [20,21,48]. In the female germline, imprints are established during oocyte growth while they are arrested during the meiotic prophase I and are erased soon thereafter in the primordial germ cells of the next generation [25,26]. It can be seen from this brief summary that, prior to complete cell differentiation and the persistence of a stable epigenetic pattern, there is an opportunity for prenatal endogenous and exogenous exposures to alter the elaborate DNA methylation patterning required for normal tissue development [18]. Imprinted genes may be a particularly susceptible target for environmentally induced epigenetic effects [27]. The early developmental period is thought to be the most susceptible to epigenetic insults because the DNA synthesis rate is high and the elaborate DNA methylation patterning and chromatin structure required for normal tissue development is established at that time [28]. However, after birth, somatic cell methylation patterns continue to adjust in response to developmental and environmental factors [2,3,29].
In 1992, Barker and colleagues laid the groundwork for the “fetal basis of adult disease” (FEBAD) hypothesis, postulating that, because organs undergo developmental programming in utero that predetermines subsequent physiologic and metabolic adaptation during adult life, prenatal insults such as nutritional deprivation or environmental exposures that disturbed developmental programming could lead to a higher risk of disease in adulthood. They showed that abdominal fatness in adult men, an indicator of increased risk of cardiovascular disease and diabetes independent of body mass, was associated with retarded fetal growth, suggesting a persisting response to adverse conditions in fetal life [30]. Since 1992, the evidence has grown that developmental plasticity allows the fetus to make anticipatory responses to the external environment by altering the course of cellular and organ differentiation in utero in order to gain adaptive advantage for later life challenges [31,32]. However, a mismatch between the prenatal and the postnatal environment or synthetic environmental agents that mimic internal or natural cues can result in disease. The FEBAD hypothesis has been supported by evidence that fetal nutrient availability, other intrauterine factors, and external environmental factors can cause serious consequences in later life by permanently reprogramming the functional capacity of organs. Classical examples include the association of low or lower birth weight with increased risk of adult onset cardiovascular disease [31], type 2 diabetes mellitus, osteoporosis [33], depressive disorders [34] and certain cancers [35].
There is compelling evidence that epigenetic dysregulation underlies the observed associations between adult disease and adverse environmental/nutritional conditions early in development. For example, Heijmans and colleagues reported that individuals who were periconceptionally exposed to famine during the Dutch Hunger Winter in 1944–1945 had, six decades later, significantly less DNA methylation of the imprinted insulin-like growth factor II (IGF2) gene compared to their unexposed same-sex siblings [36]. IGF2 is a key factor in human growth and development and is maternally imprinted. Diseases that have been associated with early gestational exposure to famine include schizophrenia and coronary heart disease – diseases in which IGF2 may play a role.
In addition, a series of elegant studies in mice has shown that prenatal exposure to dietary methyl-donor supplementation with folic acid, Vitamin B12, choline, and betaine not only increased DNA methylation at specific CpG sites but also altered subsequent phenotypes such as coat color and obesity in the Avy mouse model (reviewed in [7]). The fact that CpG sites were altered in tissues derived from the ectodermal, endodermal, and mesodermal lineages indicates that methylation profiles were changed early in embryonic development [7,18,37].
Another often-cited illustration of the importance of methylation changes attributable to environmental factors, albeit one involving neonatal exposure, is the work of Weaver et al. [38,39] showing that maternal stress and subsequent nurturing behaviors alter the epigenotype in rodent offspring, affecting their glucocorticoid receptor (GR) expression and behavior. The epigenetic changes could be reversed in adulthood by administering methionine or histone deacetylatase (HDAC) inhibitor. These epigenetic effects are not germline inherited but are passed on to the offspring directly from the mother through her behavior during the first week of postnatal life [40,29]. In a related study, newborns of mothers who had symptoms of depression during pregnancy had increased methylation of the glucocorticoid receptor gene in umbilical cord blood cells and the infants had elevated salivary cortisol concentrations at three month of age [41].
Bagot and Meaney conclude that epigenetic remodeling can occur both during early and later stages of development in response to environmental events that regulate development and function, with increased risk for psychopathology [42]. Most studies have focused on the influence of the maternal environment and maternal-infant interactions. However, recent evidence suggests that paternal factors (nutritional, toxicological, age, and phenotypic variation) can affect offspring and in some cases grandoffspring [43].
With respect to the lifecourse, a well studied example of an exogenous in utero exposure affecting adult disease is diethylstilbesterol (DES), the estrogenic pharmaceutical agent. This non-genotoxic, epigenetic carcinogen induced reproductive disorders and cancers in daughters exposed in utero and even in their granddaughters (reviewed in [44]). DES has been shown to alter gene methylation in mice exposed in utero suggesting that epigenetic mechanisms are involved [45].
An indirect mechanism by which environmental toxicants may increase propensity to adult disease is through the induction of changes in gene expression in response to IUGR (see review by Joss-Moore and Lane [4,12]. Among the epigenetic environmental exposures that have been associated with IUGR are air pollution [46–50], organochlorine pesticides [51], and possibly trihalomethanes or other water disinfection byproducts [51,52]. IUGR affects organ systems by interrupting developmental processes such as apoptosis or altering levels of homeostatic regulation factors [4,12]. Epigenetic dysregulation is at least partially responsible for these effects, as IUGR can induce changes in gene expression accompanied by changes in levels and activities of chromatin modifying enzymes such as DNMT1 and HDAC1, global DNA hypomethylation, and increased histone H3 acetylation [53,54]. IUGR-related adult morbidities include metabolic disorders (dyslipidemia, fatty liver, obesity) and non-metabolic disorders (chronic lung disease, neurodevelopmental disorders) [4,12]. Recent studies suggest that mechanisms altering epigenetics help drive disease processes. For example, transdifferentiation processes have been implicated in diabetes [55,56].
3. Prenatal exposure to environmental pollutants, related health effects, and epigenetic dysregulation
As reviewed by Baccarelli and Bollati [57], studies in adults have demonstrated epigenetic changes related to environmental exposure to metals, air pollution, benzene and persistent organic pollutants. For example, in a study of adult coke oven workers and controls, global and IL-6 hypermethylation and p53 hypomethylation were associated with PAH exposure [58]. In workers exposed to the leukemogen, benzene, epigenomic data showed effects of benzene on DNA methylation of a number of specific genes [59].
With respect to prenatal exposures, there is an increasing body of evidence that diverse pollutants alter epigenetic programming and disease risk in the F1 and even F2 and F3 generations. These include arsenic, tobacco smoke, air pollutants, and endocrine disrupting chemicals.
3.1. Arsenic
The long-term consequences of in utero and early childhood arsenic exposure in human populations include increased mortality from lung cancer and bronchiectasis in young adulthood [60]. In rodent models, in utero arsenic exposure resulted in a sharp increase in hepatocellular carcinomas in exposed offspring and also changed the expression of genes involved in cell proliferation, stress and cell-to-cell communication. These gene expression changes were evident when the offspring reached adulthood [61].
Extending this experimental work, Fry and colleagues [61] have reported that, among 32 newborns born to arsenic-exposed and arsenic unexposed mothers in Thailand, gene expression changes in cord blood were highly predictive of in utero arsenic exposure. Arsenic exposure was associated with robust activation of an integrated network of pathways involving the gene NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), inflammation, cell proliferation, stress, and apoptosis. This finding is biologically plausible because NF-κB regulates a large number of genes critical for apoptosis as well as inflammation-driven tumor progression.
Few studies have directly linked epigenetic or gene expression changes induced by arsenic to adverse health outcomes in a human population and none has examined the link between prenatal arsenic exposure, methylation, and disease. However, a population-based study of human bladder cancer found that arsenic exposure, measured as toenail arsenic, was associated with promoter methylation of the candidate tumor suppressor gene RASSF1A in human bladder tumors. These results suggest that bladder carcinogens induce epigenetic alterations important in bladder cancer causation [62]. Another study of adults in Bangladesh found that arsenic exposure was associated with increased genomic methylation of leukocyte DNA but that genomic hypomethylation of leukocyte DNA was associated with increased risk for arsenic-induced skin lesions [63]. In a nested case-control study of 274 cases who developed lesions two years after recruitment and 274 controls matched to cases for sex, age, and water arsenic, the odds ratio for development of skin lesions among participants with hypomethylated leukocyte DNA at recruitment was 1.8 (95% confidence intervals (CIs)).
3.2. Tobacco smoke
Prenatal exposure to active or passive maternal tobacco smoking has been associated in some studies with lower pulmonary function, increased risk of asthma [64], cancer [65,66], obesity [67,68], type II diabetes [69], and low birth weight which is associated with coronary heart disease, obesity and type II diabetes [70].
Alterations in DNA methylation patterns in genomic DNA from buccal cells of children were associated with in utero exposure to maternal smoking such that prenatally exposed children had significantly lower levels of global methylation as well as increased methylation of several genes compared to children without exposure [71]. Adjustment for postnatal ETS exposure did not appreciably change the results. The finding of an association between prenatal tobacco smoke exposure and global hypomethylation was observed for ALuY68 but not LINE 1, possibly reflecting their different control mechanisms and transcription patterns in response to cellular stressors [72]. In contrast, Terry et al. [73] reported that prenatal exposure to maternal tobacco smoking was associated with higher levels of global methylation in mononuclear blood cells from adult women. The inconsistencies may reflect the different assays, tissues, and age of subjects. If confirmed, the finding of global hypomethylation in children exposed prenatally to tobacco smoke is of concern since the trend of global hypomethylation with region or gene-specific hypermethylation has been observed previously in cancers [57,74]. Global hypomethylation is thought to result in chromosomal instability and increased mutational events, while promoter hypermethylation can silence expression of tumor suppressor genes [75].
In addition to the observed effects of F0 exposure during embryonic development of the F1 generation, environmental exposures during gestation have been shown to influence disease risk in the F2 generation. For example, grandmaternal smoking during the mother’s fetal period was associated with a greater risk of asthma in the grandchildren (F2 generation), independent of maternal smoking [76]. Risk was further increased if both the grandmother and the mother smoked during pregnancy. Epigenetic mechanisms have been proposed for this phenomenon [76]. Another example of F0 exposure affecting the F2 generation is provided by experimental studies of DES and uterine cancer [77–79].
3.3. Air pollution/PAHs
Benzo[a]pyrene (BaP) and other PAHs exert both genotoxicity (inducing DNA damage, DNA adducts, and mutations) and epigenetic toxicity. Certain PAHs resemble steroid hormones and are considered endocrine disruptors. They are lipid soluble, accumulate in adipose tissue, and are transferred across the placenta and the fetal blood brain barrier (reviewed in [80,81]). In the Columbia Center for Children’s Environmental Health (CCCEH) New York City (NYC) cohort, prenatal exposure to PAHs produced by burning of fossil fuel and other organic material has been associated with multiple adverse effects including fetal growth reduction [82] and IUGR [46] in African Americans, as well as developmental delay [83], reduced IQ [84], and behavioral disorders (in preparation) in both African Americans and Dominicans. In a parallel cohort study of Polish Caucasians, adverse effects were also observed on fetal growth [85], cognitive development [86], and behavioral disorders.
In a subset of the NYC cohort, prenatal PAH exposure was significantly associated with genomic hypomethylation in umbilical cord white blood cell (UCWBC) DNA. Newborns in the highest prenatal PAH exposure group had an average decrease of 0.42 ng/100 mg total DNA compared to newborns in the lowest prenatal PAH exposure group (p < 0.01) [87]. In the same 159 cohort children, the persistence of global methylation pattern was evaluated by comparing methylation measured in cord blood to methylation measured in blood collected at age 3. Global DNA methylation levels in cord and 3-year blood were significantly correlated (r = 0.82, p < 0.01), suggesting that changes in cord blood epigenetic markers due to prenatal PAH exposure may be stable alterations that persist in blood through early childhood [87].
In order to explore the association between prenatal PAH exposure, epigenetic changes and childhood asthma, methylation sensitive restriction fingerprinting was used to analyze umbilical cord white blood cell (UCWBC) DNA of 20 CCCEH cohort children [88]. Over 30 DNA sequences were identified whose methylation status was dependent on the level of maternal PAH exposure. Six of the 30 DNA sequences initially identified were found to be homologous to known genes having one or more 5′-CpG island(s) (5′-CGI). Of these, acyl-CoA synthetase long-chain family member 3 (ACSL3), which belongs to the acyl-CoA synthetase long chain (ACSL) family of genes which encodes key enzymes in fatty acid metabolism, exhibited the highest concordance between the extent of methylation of its 5′-CGI in UCWBCs. The level of gene expression in matched fetal placental tissues in the initial 20 cohort children. In a larger sample of 56 cohort children, hypermethylation of the ACSL3 5′-CGI was found to be significantly associated with maternal airborne PAH exposure exceeding 2.41 ng/m3 (OR = 13.8; p < 0.001) and with a parental report of asthma symptoms in children prior to age 5 (OR = 3.9; p < 0.05). Hypermethylation of this gene in T helper cells or lung tissues is expected to diminish fatty acid utilization and beta-oxidation-energy production, and possibly influence membrane phospholipid composition. Thus, if validated, methylated ACSL3 5′ CGI in UCWBC DNA may be a surrogate endpoint for transplacental PAH exposure and/or a potential biomarker for environmentally related asthma and may provide mechanistic support for the FEBAD hypothesis.
3.4. Phthalates
The phthalates are ubiquitous industrial plasticizers and include agents such as di(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), and butyl benzyl phthalate (BBP), which are classified as endocrine disruptors because of their anti-androgenic or pro-estrogenic effects [89,90]. They are used to soften polyvinyl chloride and are found in adhesives and glues, agricultural adjuvants, building materials, personal care products, medical devices, detergents, packaging, children’s toys, pharmaceuticals, food products, and textiles [91]. Exposure to phthalates can occur through diet, inhalation, or dermal exposure.
While not all studies have been consistent, prenatal exposure to phthalates has been associated with shortened gestational age [92,93] and with a number of adverse reproductive and developmental effects including decreased anogenital distance among newborn boys [90], undescended testis (exposure to a combination of phthalates and anti-androgenic pesticides) [94], and adverse neonatal neurodevelopment among girls [95]. Phthalate exposure has also been associated with elevated body mass index (BMI) during the first three years of life [96].
Phthalates are epigenetically toxic. In MCF7 breast cancer cells, treatment with BBP led to the demethylation of estrogen receptor (ER) alpha promoter-associated CpG islands, suggesting that altered ER mRNA expression by BBP might be related to aberrant DNA methylation in the promoter region of the receptor [89]. Exposure to DEHP during sexual differentiation of rats caused male reproductive tract malformations and abnormal expression of insulin-like growth factor-(IGF-1), c-kit ligand (KITL), and leukemia inhibitory factor (LIF), genes that may contribute to the reproductive toxicity of phthalates [97].
3.5. Bisphenol A (BPA)
BPA is also considered an endocrine disruptor and can accumulate in adipose tissue [98]. BPA is used in the production of plastics and resins which are used in food and drink containers, flame retardants, dental sealants, and in the recycling of thermal paper. Almost all exposure, including that to children, has been thought to occur through diet [99,100]. Recent studies, however, have suggested that non-dietary sources may be important as well [101,102]. In experimental models BPA has been associated with adverse reproductive effects in females [103] and with an increased susceptibility to cancer [104].
Developmental (neonatal) exposure of rats to BPA resulted in increased incidence of prostate intraepithelial neoplasia (PIN) when followed by prolonged estradiol and testosterone exposure in adulthood [35]. The prostate tissues showed consistent methylation changes as a result of neonatal estrogen or BPA exposures. The phosphodiesterase Type 4 variant 4 (PDE4D4) gene showed hypomethylation of the 5′CpG island, resulting in increased PDE4D4 expression in the adult prostate [105]. These findings jointly suggest that the prostate epigenome is permanently altered by early exposure to BPA and that the epigenetic alteration may lead to heightened risk of prostate cancer with aging.
4. Case studies: a proposed epigenetic mechanism for the neurodevelopmental effects of in utero exposure to PAHs and BPA
The prior section has reviewed the evidence that diverse prenatal environmental exposures increase risk of various diseases in the offspring and in some cases their grandchildren, that they also alter the epigenome, and that epigenetic dysregulation may mediate their adverse health effects. In contrast, in the following section we present two case studies in which we propose that epigenetic alterations in endocrine and immune pathways are directly involved in the neurodevelopmental effects associated with in utero exposure to PAHs and BPA. This section illustrates both the limitations of available data and the potential of using epigenetic markers to forge links in the causal chain for a particular exposure and a specific health outcome.
PAHs have been shown to be neurotoxic and affect gene expression in humans. Laboratory studies exposing experimental animals to PAHs during the prenatal and neonatal periods have reported neurodevelopmental and behavioral effects including depression-like symptoms and memory impairment in the absence of other overt toxicological effects [106–111]; others have shown that exposure affects neurotransmitter levels and gene expression patterns in the brain [80,112–114]. For example, prenatal treatment of rats with BaP impaired memory and ability to learn, consistent with alterations in the expression profile of glutamate receptor (GluR) subunits, which are key genes involved in long-term potentiation (LTP), considered the cellular correlate of learning and memory [81,111].
Many studies have shown that PAHs such as BaP are endocrine disruptors, affecting gene expression in hormonal regulatory pathways important in early brain development. Gene targets include the aryl hydrocarbon receptor (AhR) [115–117], CYP1A1 and CYP1B1 and CYP19A1; these genes are expressed in the fetal brain and peripheral lymphocytes [118–123]. In experimental studies, BaP caused alterations in levels of noradrenaline, dopamine, and serotonin and/or their metabolites in discrete brain regions [112–114]. In gestationally exposed rats, BaP caused significant reductions in expression of the N-methyl-D-aspartic acid (NMDA) glutamate receptor subunit NMDAR2B [81,111,124], and reduced LTP across the perforant path granular cells synapses in the hippocampus [125]. These data indicate that BaP and other PAHs disrupt the glutamate pathway and the dopaminergic and serotonergic systems, consistent with observed disturbances of learning and emotional behavior [113,125].
In addition, PAHs are immunotoxic contaminants known to affect expression of pro-inflammatory cytokines including interleukin-1beta, tumor necrosis factor-alpha (TNFα) IFN-γ and the chemokine CCL1 and [126,127]. Cytokines are also produced by CNS tissue and peripheral leukocytes [128]. These cytokines are among those most often implicated in neurodevelopment [129–131].
Finally, exposure to BaP upregulated COX-2, a key enzyme involved in inflammation and associated with reactive oxygen species (ROS) production, in rat astrocytes [132,133] in human cells and rats exposed to BaP or its main metabolite BPDE [133–136]. Residents of cities with severe air pollution had significantly higher expression of COX-2 in the frontal cortex and hippocampus compared to controls at autopsy [137].
Fewer studies have evaluated methylation changes due to PAHs than have looked at gene regulation or expression. However, in the CCCEH cohort study described above, a number of genes in addition to ACSL3 were found to be differentially methylated in UCWBC of newborns with high vs. low prenatal PAH exposure [88]. Several are known to be expressed in leukocytes and brain cells and have functions related to inflammatory and or immune pathways. They include CCL17 (a chemokine also known as TARC) that selectively induces migration of Th2 lymphocytes [138], which is expressed constitutively in thymus and in phytohemagglutinin-stimulated peripheral blood mononuclear cells, and has been found to be overexpressed in autistic brains [139].
The experimental studies described above have drawn an inferential link between PAH-related epigenetic alterations and neurodevelopmental effects, suggesting that alterations in methylation/gene expression mediate the neurodevelopmental effects of PAHs. For example, the observed neurodevelopmental effects of PAHs on learning and memory in humans [83] and the observation that prenatal treatment of rats with BaP impaired their ability to learn [111] are consistent with the observed disruptions of the glutamate pathway [111].
BPA is another endocrine disrupting chemical capable of exerting developmental effects. A recent epidemiological study has linked prenatal exposure to BPA with subtle, gender-specific alterations in behavior of 2-year olds [140]. Experimental evidence indicates that gestational exposure to environmentally relevant doses of BPA abrogates sexual dimorphism in brain structure and behavior and disrupts cognition, social behaviors, and other aspects of brain function [141–143]. Perinatal exposure to BPA altered sex differences in anxiety and depression-like responses in rodents [144–148]. In addition, male mouse offspring of dams treated from mating through weaning with low dose BPA exhibited impairment in memory [149]. Hyperactivity in male mice has also been demonstrated in response to perinatal exposure to BPA (females were not examined) [150]. Importantly, these behavioral changes are induced through low dose exposures to BPA in these experimental models. BPA treatment of pregnant female mice led to disruption in neocortical patterning in offspring during adulthood, possibly by accelerating neuronal differentiation and migration [151], and caused changes in gene expression in the fetal forebrain [152].
As is the case for PAHs, most mechanistic research on BPA, an estrogen-mimicking chemical, has focused on gene expression, rather than DNA methylation. BPA has been shown to interact with estrogen signaling pathways through binding to the estrogen receptors ERα and ERβ [153–155], and is also believed to interfere with non-classical estrogen signaling pathways at very low concentrations [156–158]. Studies in mice have shown that perinatal exposure to BPA can disrupt estrogen signaling in the offspring, with changes in gene expression at low doses [159]. Gestational exposure to BPA caused permanent upregulation of ERβ mRNA in the preoptic area of the hypothalamus in male rat offspring [160] and increased ERα and ERβ levels in the dorsal raphe nucleus of male mouse offspring of mice [161].
Prenatal BPA exposure has also been shown to interfere with other endocrine pathways. Prenatal BPA exposure dramatically increased expression levels of AhR in mouse embryonic cerebra, cerebella, and gonads, showing a U-shaped dose–response curve with extremely high response at the lowest dose tested [162]. Prenatal exposure to BPA also interfered with thyroid hormone, required for normal fetal and neonatal brain development [163–165], and increased expression of RC3/neurogranin in the dentate gyrus brain region, suggesting that BPA differentially affects the beta-thyroid receptor (TRβ) vs. the alpha-TR (TRα) [166] and changes the temporal expression patterns of TRα and TRβ [152]. Cell-based assays suggest that BPA blocks expression of TRβ [167,168].
Prenatal exposure to BPA affects immune cells and the expression of genes involved in inflammation such as IL-4 and INFγ [169,170]. BPA also affects methylation of genes involved in several immune pathways. For example, gestational exposure of rats to BPA caused differential methylation of Cebpα [171], which is involved in macrophage differentiation, cytokine signaling, microglial activation, and neuronal signaling [172–175]. As noted above, studies by Ho et al. found that PDE4D (involved in macrophage differentiation, neutrophil recruitment in inflammation, responses to oxidative stress, and possibly in regulation of dopaminergic neurotransmission [176–179]) was hypomethylated in the prostates of rats treated neonatally with BPA and also showed increased expression [35]. All of the genes mentioned above are known or believed to be expressed in both the brain and the blood cells.
A number of studies have attempted to link BPA-related changes in gene expression to neurodevelopmental outcomes. In utero treatment of mice with BPA eliminated sex differences in the size of the anteroventral periventricular preoptic (AVPV) area, significantly reduced the number of TH (tyrosine hydroxylase) positive neurons in the female AVPV, and abrogated sex differences in the number of TH-positive neurons [143]. In the same study, BPA reduced sexual dimorphism in anxiety-related behaviors in the open-field test. Finally, BPA treatment from mating through weaning both increased levels of neurotransmitter-producing choline acetyltransferase in the hippocampus of male offspring and affected performance on the step-through test, indicating memory impairment as a consequence of BPA exposure [149].
As we have seen, genes in inflammatory/immune and endocrine pathways are targets for PAHs and BPA in the context of neurodevelopmental effects. As discussed by Tian [180], genes involved in inflammation and immune response may be common targets for diverse epigenetic environmental agents; and multiple disease endpoints may be affected. Examples include AhR, a pleiotropic ligand-activated transcription factor whose ligands include many natural and synthetic compounds (such as dioxin and PAHs) and NF-κB, a pleiotropic factor that regulates many physiological and pathophysiological processes. Interactions between AhR and NF-κB pathways are potentially important mechanisms for chemical-induced immune dysfunctions, carcinogenesis, alteration of xenobiotic/pollutant metabolism and other pathological responses induced by environmental insults.
5. Transgenerational effects of prenatal exposures
As we have seen, the role of prenatally acquired somatic epigenetic alterations in disease has been quite widely studied, mostly in experimental models. Less well characterized are epigenetic events that are inherited through the germline from parent to child and transmitted to subsequent generations [181].
There is growing evidence that environmental variations experienced by both fathers and mothers may lead to phenotypic variation in the development and behavior of offspring resulting from transmission through the germline [182]. Transmission can result either from altered programming within germ cells of the epigenome of the retrotransposons and imprinted genes or through altered expression of RNA within gametes. “Transgenerational epigenetic inheritance” refers to the transmission of a biological trait to subsequent generations via epigenetic modifications in the germline [7]. As elaborated by Jirtle and Skinner [7], in order to consider transgenerational effects on the epigenome to be a plausible mechanism for a disease phenotype, the epigenetic changes and the disease phenotype must be observed in the F3 generation. This is because gestational exposure of an F0 female directly exposes both the F1 embryo and the F2 germline. Therefore, phenotypes in the F1 and F2 generations may be due to their direct exposure to the environmental factor rather then germline transmission (see Fig. 2).
Fig. 2.
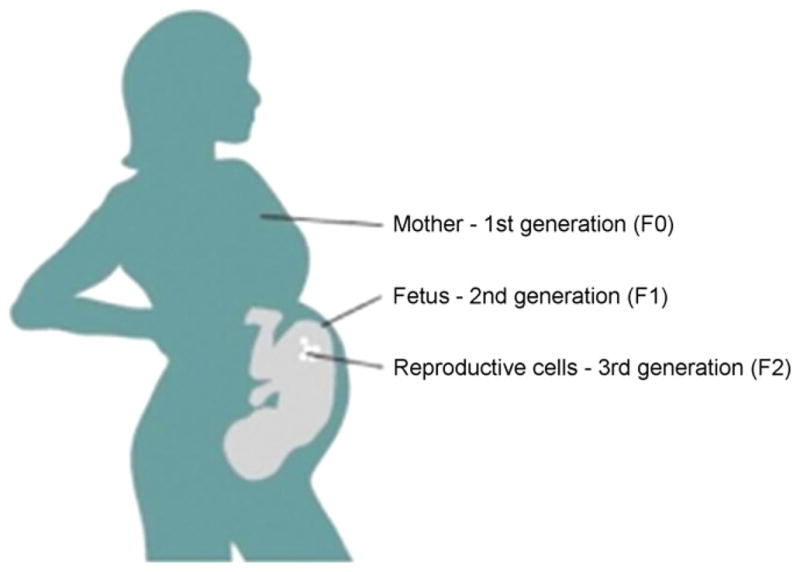
Three generations at once are exposed to the some environmental conditions (diet, toxics, hormones, etc.). In order to provide a convincing case for epigenetic inheritance, an epigenetic change must be observed in the 4th generation.
While multi-generational effects involving direct exposure have been observed for a number of agents [183], there are fewer examples of transgenerational phenotypes occurring in the absence of direct exposure. The best developed example of transgenerational effects of environmental chemicals comes from the classic experiment by Anway et al. [184,185]. These investigators exposed male rats to vinclozolin (an antiandrogenic fungicide) or methoxychlor (an estrogenic organochlorine insecticide) during the period of gonadal sex determination. Exposure resulted in reduced sperm count and viability and increased rates of infertility in adulthood. This loss of fertility was perpetuated through the male germline for four generations. Investigation of the mechanism for the transgenerational phenotype found that endocrine disruptors reprogrammed the male germline during development and induced heritable methylation changes that were stably transmitted through the male germline [186]. Another example is perinatal exposure to BPA shown at environmentally relevant doses of BPA to affect the male germ line, leading to impairment in the fertility of male offspring over three generations [187]. A study of TCDD exposure has demonstrated reduced fertility and an increased incidence of premature birth in F1 mice exposed in utero to this chemical as well as in three subsequent generations [188].
6. Emerging evidence that nutritional, genetic and psychosocial factors may influence DNA methylation by environmental toxicants
Experimental animal studies have shown that the epigenetic reprogramming by behavioral factors is reversible by nutritional factors. For example, Weaver and colleagues showed that the programming of the GR exon 1 promoter associated with low grooming maternal care, as well as the resulting stress response and behavioral phenotypes, were reversible by administration of a methyl donor precursor or a histone deacetylase (HDAC) inhibitor [189]. Other investigators have shown that the effects of maternal exposure to BPA on the offspring, mediated in part by hypomethylation of DNA, are prevented by maternal dietary supplementation [28].
With respect to genetic susceptibility, as noted above, Breton and colleagues observed a significant interaction between the GSTM null genotype of the child and the prenatal exposure to tobacco smoke on global methylation in the child [71]. Foley et al. [16] have reviewed other genetic factors that directly affect DNA methylation. These include the C-to-T substitution at nucleotide 677 of the methylene tetrahydro-folate reductase (MTHFR) gene: TT homozygous individuals have lower levels of DNA methylation than CC homozygous individuals. In addition, variants of the DNMT gene family discussed earlier have been associated with diseases including cancer [190]. As another example, a specific variant (C > T) in the O-6-methylquanine-DNA transferase (MGMT) tumor suppressor gene has been associated with O-6-methylguanine-DNA methyltransferase promoter methylation and gene silencing. Wright [191] has reviewed the evidence that psychosocial stressors and physical environmental toxicants play a joint (synergistic) role in disrupting immune and endocrine pathways involved in respiratory and cognitive development and function. She notes that oxidative stress pathways that may influence health are disrupted by both psychological stressors and environmental pollutants such as tobacco smoke and air pollution, all of which generate reactive oxygen species. Further, by causing dysregulatory behavioral states such as depression and anxiety, psychosocial stressors may produce long-lasting effects on shared physiologic processes and thereby increase risk from pollutant exposures [192]. Wright suggests that both factors may be acting through early life reprogramming of the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system which are particularly susceptible to both stress and physical environmental toxicants.
Examples of joint effects of the social and physical environment include the interaction between traffic-related air pollution (NO2) and elevated social stress on risk for childhood asthma, whereby effects were seen only in children with both exposures [193], and the interaction between traffic related pollution and stress on increased asthma symptoms and inflammatory markers in adolescent asthmatics [194]. Another example of joint effects concerns neurodevelopment and cognition of young children in the CCCEH cohort, in which prenatal material hardship modified the response to maternal ETS exposure during pregnancy, as evidenced by reduced scores on the Bayley Scales of mental development at age 2 [195]. Because social and physical environmental toxicants tend to cluster in the most socially disadvantaged populations, understanding of these complex interdependencies may help explain and ultimately prevent health disparities [196]. Both animal and human studies have also shown that environmental enrichment can reverse the effects of early stress [196].
7. Implications for prevention of childhood, adult and multigenerational disease: conclusions
Most of the research to date has focused on the critical role of epigenetics in mediating the effects of social experience and nutrition [197,198]. However, there is also compelling human and experimental evidence that prenatal environmental exposures to endocrine disruptors and other environmental xenobiotics, acting alone or in combination with genetic, nutritional, or psychosocial factors, adversely affect human development and health in childhood and possibly over the lifecourse, and that a primary mechanism is epigenetic dysregulation. Because epigenetics programming determines the state of expression of genes, epigenetic differences could have the same consequences as genetic polymorphisms [197]. Moreover, there is experimental evidence that exposures during the prenatal window can influence disease risk transgenerationally through epimutations in the germline. The research reviewed here has potential implications for risk assessment; although more data are needed on individual susceptibility to epigenetic alterations and their persistence before this information can be used in formal risk assessments.
Reviewers have tended to emphasize the potential reversibility of epigenetic dysregulation and related phenotypes as encouragement for pharmacological and cognitive-intervention [7,199]. Often cited in this regard are studies with animal models showing that supplementation with folic acid during pregnancy or after weaning alters the phenotype and epigenotype induced by maternal dietary deficiency during gestation [200]. A cautionary note is that pharmacologic or dietary interventions would require a gene-specific approach based on a complete understanding of the epigenetic events involved in fetal adaptation to adverse or suboptional conditions [201]. Global epigenetic modifying agents such as histone deacetylase inhibitors would pose potential risks by modifying epigenetics of multiple genes, with unpredictable consequences. However, the data strongly encourage preventive policies to reduce early life exposure to epigenetically toxic agents as a priority in public health. Such policies could have both immediate and long-term benefits for human health by preventing disease and developmental disorders in childhood, over the lifecourse, and even in future generations [202].
Acknowledgments
The Center’s research is supported by NIEHS, US EPA, and several private foundations and donors including 5PO1ES009600 NIEHS; RD834509 EPA; and 1 P50 ES015905 NIEHS.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest.
References
- 1.Bollati V, Baccarelli A. Environmental epigenetics. Heredity. 2010;105:105–12. doi: 10.1038/hdy.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilera O, Fernandez AF, Munoz A, Fraga MF. Epigenetics and environment: a complex relationship. J Appl Physiol. 2010;109:243–51. doi: 10.1152/japplphysiol.00068.2010. [DOI] [PubMed] [Google Scholar]
- 3.Groom A, Elliott HR, Embleton ND, Relton CL. Epigenetics and child health: basic principles. Arch Dis Child. 2010 doi: 10.1136/adc.2009.165712. [DOI] [PubMed] [Google Scholar]
- 4.Joss-Moore LA, Lane RH. The developmental origins of adult disease. Curr Opin Pediatr. 2009;21:230–4. doi: 10.1097/mop.0b013e328326773b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawrot TS, Adcock I. The detrimental health effects of traffic-related air pollution: a role for DNA methylation? Am J Respir Crit Care Med. 2009;179:523–4. doi: 10.1164/rccm.200812-1900ED. [DOI] [PubMed] [Google Scholar]
- 6.Reamon-Buettner SM, Borlak J. A new paradigm in toxicology and teratology: altering gene activity in the absence of DNA sequence variation. Reprod Toxicol. 2007;24:20–30. doi: 10.1016/j.reprotox.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saetrom P, Snove O, Jr, Rossi JJ. Epigenetics and microRNAs. Pediatr Res. 2007;61:17R–23R. doi: 10.1203/pdr.0b013e318045760e. [DOI] [PubMed] [Google Scholar]
- 9.van Vliet J, Oates NA, Whitelaw E. Epigenetic mechanisms in the context of complex diseases. Cell Mol Life Sci. 2007;64:1531–8. doi: 10.1007/s00018-007-6526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandhu KS. Systems properties of proteins encoded by imprinted genes. Epigenetics. 2010:5. doi: 10.4161/epi.5.7.12883. [DOI] [PubMed] [Google Scholar]
- 11.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 12.Joss-Moore LA, Lane RH. The Developmental Origins of Adult Disease. 2009. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svoboda P, Flemr M. The role of miRNAs and endogenous siRNAs in maternal-to-zygotic reprogramming and the establishment of pluripotency. EMBO Rep. 2010;11:590–7. doi: 10.1038/embor.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Illingworth RS, Bird AP. CpG islands – ‘a rough guide’. FEBS Lett. 2009;583:1713–20. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Fazzari MJ, Greally JM. Introduction to epigenomics and epigenome-wide analysis. Methods Mol Biol. 2010;620:243–65. doi: 10.1007/978-1-60761-580-4_7. [DOI] [PubMed] [Google Scholar]
- 16.Foley DL, Craig JM, Morley R, Olsson CA, Dwyer T, Smith K, et al. Prospects for epigenetic epidemiology. Am J Epidemiol. 2009;169:389–400. doi: 10.1093/aje/kwn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang SC, Oelze B, Schumacher A. Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS One. 2008;3:e2698. doi: 10.1371/journal.pone.0002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards TM, Myers JP. Environmental exposures and gene regulation in disease etiology. Environ Health Perspect. 2007;115:1264–70. doi: 10.1289/ehp.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–32. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet. 2008;9:129–40. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- 22.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 23.Prokhortchouk E, Defossez PA. The cell biology of DNA methylation in mammals. Biochim Biophys Acta. 2008;1783:2167–73. doi: 10.1016/j.bbamcr.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 25.Bourc’his D, Proudhon C. Sexual dimorphism in parental imprint ontogeny and contribution to embryonic development. Mol Cell Endocrinol. 2008;282:87–94. doi: 10.1016/j.mce.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Bourc’his D, Bestor TH. Origins of extreme sexual dimorphism in genomic imprinting. Cytogenet Genome Res. 2006;113:36–40. doi: 10.1159/000090813. [DOI] [PubMed] [Google Scholar]
- 27.Dolinoy DC, Jirtle RL. Environmental epigenomics in human health and disease. Environ Mol Mutagen. 2008;49:4–8. doi: 10.1002/em.20366. [DOI] [PubMed] [Google Scholar]
- 28.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meaney MJ. Epigenetics and the biological definition of gene × environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 30.Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ. Early growth and abdominal fatness in adult life. J Epidemiol Community Health. 1992;46:184–6. doi: 10.1136/jech.46.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, et al. Developmental plasticity and human health. Nature. 2004;430:419–21. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 32.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–7. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 33.Dennison EM, Arden NK, Keen RW, Syddall H, Day IN, Spector TD, et al. Birth-weight, vitamin D receptor genotype and the programming of osteoporosis. Paediatr Perinat Epidemiol. 2001;15:211–9. doi: 10.1046/j.1365-3016.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- 34.Thompson C, Syddall H, Rodin I, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450–5. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- 35.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–9. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–5. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 41.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 42.Bagot RC, Meaney MJ. Epigenetics and the biological basis of gene × environment interactions. J Am Acad Child Adolesc Psychiatry. 2010;49:752–71. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–50. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, et al. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57:4356–9. [PubMed] [Google Scholar]
- 46.Choi H, Rauh V, Garfinkel R, Tu Y, Perera FP. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008;116:658–65. doi: 10.1289/ehp.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction. J Expo Sci Environ Epidemiol. 2007;17:426–32. doi: 10.1038/sj.jes.7500503. [DOI] [PubMed] [Google Scholar]
- 48.Salam MT, Millstein J, Li YF, Lurmann FW, Margolis HG, Gilliland FD. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: results from the Children’s Health Study. Environ Health Perspect. 2005;113:1638–44. doi: 10.1289/ehp.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dejmek J, Solansky I, Benes I, Lenicek J, Sram RJ. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000;108:1159–64. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim OJ, Ha EH, Kim BM, Seo JH, Park HS, Jung WJ, et al. PM10 and pregnancy outcomes: a hospital-based cohort study of pregnant women in Seoul. J Occup Environ Med. 2007;49:1394–402. doi: 10.1097/JOM.0b013e3181594859. [DOI] [PubMed] [Google Scholar]
- 51.Windham G, Fenster L. Environmental contaminants and pregnancy outcomes. Fertil Steril. 2008;89:e111–6. doi: 10.1016/j.fertnstert.2007.12.041. discussion e7. [DOI] [PubMed] [Google Scholar]
- 52.Nieuwenhuijsen MJ, Smith R, Golfinopoulos S, Best N, Bennett J, Aggazzotti G, et al. Health impacts of long-term exposure to disinfection by-products in drinking water in Europe: HIWATE. J Water Health. 2009;7:185–207. doi: 10.2166/wh.2009.073. [DOI] [PubMed] [Google Scholar]
- 53.Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, et al. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics. 2006;25:16–28. doi: 10.1152/physiolgenomics.00093.2005. [DOI] [PubMed] [Google Scholar]
- 54.MacLennan NK, James SJ, Melnyk S, Piroozi A, Jernigan S, Hsu JL, et al. Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol Genomics. 2004;18:43–50. doi: 10.1152/physiolgenomics.00042.2004. [DOI] [PubMed] [Google Scholar]
- 55.Kanaka-Gantenbein C. Fetal origins of adult diabetes. Ann NY Acad Sci. 2010;1205:99–105. doi: 10.1111/j.1749-6632.2010.05683.x. [DOI] [PubMed] [Google Scholar]
- 56.Paris M, Tourrel-Cuzin C, Plachot C, Ktorza A. Review: pancreatic beta-cell neogenesis revisited. Exp Diabesity Res. 2004;5:111–21. doi: 10.1080/15438600490455079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–51. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavanello S, Bollati V, Pesatori AC, Kapka L, Bolognesi C, Bertazzi PA, et al. Global and gene-specific promoter methylation changes are related to anti-B[a]PDE-DNA adduct levels and influence micronuclei levels in polycyclic aromatic hydrocarbon-exposed individuals. Int J Cancer. 2009;125:1692–7. doi: 10.1002/ijc.24492. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, McHale CM, Rothman N, Li G, Ji Z, Vermeulen R, et al. Systems biology of human benzene exposure. Chem Biol Interact. 2010;184:86–93. doi: 10.1016/j.cbi.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114:1293–6. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marsit CJ, Karagas MR, Schned A, Kelsey KT. Carcinogen exposure and epigenetic silencing in bladder cancer. Ann NY Acad Sci. 2006;1076:810–21. doi: 10.1196/annals.1371.031. [DOI] [PubMed] [Google Scholar]
- 63.Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, et al. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ Health Perspect. 2009;117:254–60. doi: 10.1289/ehp.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Pinkerton KE. Detrimental effects of tobacco smoke exposure during development on postnatal lung function and asthma. Birth Defects Res C Embryo Today. 2008;84:54–60. doi: 10.1002/bdrc.20114. [DOI] [PubMed] [Google Scholar]
- 65.Filippini G, Farinotti M, Ferrarini M. Active and passive smoking during pregnancy and risk of central nervous system tumours in children. Paediatr Perinat Epidemiol. 2000;14:78–84. doi: 10.1046/j.1365-3016.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 66.Norman MA, Holly EA, Ahn DK, Preston-Martin S, Mueller BA, Bracci PM. Pre-natal exposure to tobacco smoke and childhood brain tumors: results from the United States West Coast childhood brain tumor study. Cancer Epidemiol Biomarkers Prev. 1996;5:127–33. [PubMed] [Google Scholar]
- 67.Toschke AM, Montgomery SM, Pfeiffer U, von Kries R. Early intrauterine exposure to tobacco-inhaled products and obesity. Am J Epidemiol. 2003;158:1068–74. doi: 10.1093/aje/kwg258. [DOI] [PubMed] [Google Scholar]
- 68.Sharma AJ, Cogswell ME, Li R. Dose–response associations between maternal smoking during pregnancy and subsequent childhood obesity: effect modification by maternal race/ethnicity in a low-income US cohort. Am J Epidemiol. 2008;168:995–1007. doi: 10.1093/aje/kwn223. [DOI] [PubMed] [Google Scholar]
- 69.Montgomery SM, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. BMJ. 2002;324:26–7. doi: 10.1136/bmj.324.7328.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hofhuis W, de Jongste JC, Merkus PJ. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child. 2003;88:1086–90. doi: 10.1136/adc.88.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462–7. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bollati V, Schwartz J, Wright R, Litonjua A, Tarantini L, Suh H, et al. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev. 2009;130:234–9. doi: 10.1016/j.mad.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Terry MB, Ferris JS, Pilsner R, Flom JD, Tehranifar P, Santella RM, et al. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:2306–10. doi: 10.1158/1055-9965.EPI-08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breton MC, Beauchesne MF, Lemiere C, Rey E, Forget A, Blais L. Risk of perinatal mortality associated with asthma during pregnancy. Thorax. 2009;64:101–6. doi: 10.1136/thx.2008.102970. [DOI] [PubMed] [Google Scholar]
- 75.Ohm JE, Baylin SB. Stem cell chromatin patterns: an instructive mechanism for DNA hypermethylation? Cell Cycle. 2007;6:1040–3. doi: 10.4161/cc.6.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005;127:1232–41. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 77.Walker BE, Haven MI. Intensity of multigenerational carcinogenesis from diethylstilbestrol in mice. Carcinogenesis. 1997;18:791–3. doi: 10.1093/carcin/18.4.791. [DOI] [PubMed] [Google Scholar]
- 78.Li SF, Hursting SD, Davis BJ, McLachlan JA, Barrett JC. Epigenetics in cancer prevention: early detection and risk assessment. New York: New York Academy of Sciences; 2003. Environmental exposure, DNA methylation, and gene regulation-lessons from diethylstilbesterol-induced cancers; pp. 161–169. [DOI] [PubMed] [Google Scholar]
- 79.Ruden DM, Xiao L, Garfinkel MD, Lu X. Hsp90 and environmental impacts on epigenetic states: a model for the transgenerational effects of diethylstibesterol on uterine development and cancer. Hum Mol Genet. 2005;14(Spec No 1):R149–55. doi: 10.1093/hmg/ddi103. [DOI] [PubMed] [Google Scholar]
- 80.Hood DB, Nayyar T, Ramesh A, Greenwood M, Inyang F. Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo[a]pyrene following maternal inhalation. Inhal Toxicol. 2000;12:511–35. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- 81.Brown LA, Khousbouei H, Goodwin JS, Irvin-Wilson CV, Ramesh A, Sheng L, et al. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007;28:965–78. doi: 10.1016/j.neuro.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multi-ethnic population. Environ Health Perspect. 2003;111:201–5. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–92. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, et al. Prenatal polycyclic aromatic hydrocarbon exposure and child intelligence at age 5. Pediatrics. 2009;124:e195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi H, Jedrychowski W, Spengler J, Camann DE, Whyatt RM, Rauh V, et al. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ Health Perspect. 2006;114:1744–50. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edwards SC, Jedrychowski W, Li Z, Wang Q, Rauh V, Camann D, et al. Prenatal exposure to PAHs and child intelligence at age 5. ISEE-ISEA joint conference; Pasadena, CA. 2008. [Google Scholar]
- 87.Herbstman JB, Tang D, Zhu D, Perera FP. Prenatal exposure to polycyclic aromatic hydrocarbons and CpG methylation. International society for environmental epidemiology. 21st ISEE conference: food, environment and global health; 2009. [Google Scholar]
- 88.Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, et al. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009;4:e4488. doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang SC, Lee BM. DNA methylation of estrogen receptor alpha gene by phthalates. J Toxicol Environ Health. 2005;68:1995–2003. doi: 10.1080/15287390491008913. [DOI] [PubMed] [Google Scholar]
- 90.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–34. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 92.Latini G, De Lelice C, Presta G, Vecchio AD, Paris I, Ruggieri F, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111:1783–5. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, et al. Prenatal di(2-ethylhexyl)phthalate exposure and length of gestation among an inner-city cohort. Pediatrics. 2009;124:e1213–20. doi: 10.1542/peds.2009-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toppari J, Virtanen H, Skakkebaek NE, Main KM. Environmental effects on hormonal regulation of testicular descent. J Steroid Biochem Mol Biol. 2006;102:184–6. doi: 10.1016/j.jsbmb.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 95.Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, et al. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30:522–8. doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verhulst SL, Nelen V, Hond ED, Koppen G, Beunckens C, Vael C, et al. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect. 2009;117:122–6. doi: 10.1289/ehp.0800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin H, Ge RS, Chen GR, Hu GX, Dong L, Lian QQ, et al. Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc Natl Acad Sci U S A. 2008;105:7218–22. doi: 10.1073/pnas.0709260105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fernandez MF, Arrebola JP, Taoufiki J, Navalon A, Ballesteros O, Pulgar R, et al. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol. 2007;24:259–64. doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 99.Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res. 2007;103:9–20. doi: 10.1016/j.envres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 100.Miyamoto K, Kotake M. Estimation of daily bisphenol a intake of Japanese individuals with emphasis on uncertainty and variability. Environ Sci. 2006;13:15–29. [PubMed] [Google Scholar]
- 101.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. EHP. 2009;117:784–9. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–8. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect. 2009;117:879–85. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, et al. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115:80–6. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prins GS, Tang WY, Belmonte J, Ho SM. Developmental exposure to bisphenol A increases prostate cancer susceptibility in adult rats: epigenetic mode of action is implicated. Fertil Steril. 2008;89:e41. doi: 10.1016/j.fertnstert.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol. 2006;26:427–38. doi: 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- 107.Saunders CR, Ramesh A, Shockley DC. Modulation of neurotoxic behavior in F-344 rats by temporal disposition of benzo(a)pyrene. Toxicol Lett. 2002;129:33–45. doi: 10.1016/s0378-4274(01)00467-2. [DOI] [PubMed] [Google Scholar]
- 108.Saunders CR, Shockley DC, Knuckles ME. Fluoranthene-induced neurobehavioral toxicity in F-344 rats. Int J Toxicol. 2003;22:263–76. doi: 10.1080/10915810305114. [DOI] [PubMed] [Google Scholar]
- 109.Takeda K, Tsukue N, Yoshida S. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ Sci. 2004;11:33–45. [PubMed] [Google Scholar]
- 110.Yokota S, Mizuo K, Moriya N, Oshio S, Sugawara I, Takeda K. Effect of prenatal exposure to diesel exhaust on dopaminergic system in mice. Neurosci Lett. 2009;449:38–41. doi: 10.1016/j.neulet.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 111.Wormley DD, Ramesh A, Hood DB. Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol Appl Pharmacol. 2004;197:49–65. doi: 10.1016/j.taap.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 112.Konstandi M, Harkitis P, Thermos K, Ogren SO, Johnson EO, Tzimas P, et al. Modification of inherent and drug-induced dopaminergic activity after exposure to benzo(alpha)pyrene. Neurotoxicology. 2007;28:860–7. doi: 10.1016/j.neuro.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 113.Stephanou P, Konstandi M, Pappas P, Marselos M. Alterations in central monoaminergic neurotransmission induced by polycyclic aromatic hydrocarbons in rats. Eur J Drug Metab Pharmacokinet. 1998;23:475–81. doi: 10.1007/BF03189998. [DOI] [PubMed] [Google Scholar]
- 114.Tekes K, Tothfalusi L, Hantos M, Csaba G. Effect of neonatal benzpyrene imprinting on the brain serotonin content and nocistatin level in adult male rats. Acta Physiol Hung. 2007;94:183–9. doi: 10.1556/APhysiol.94.2007.3.3. [DOI] [PubMed] [Google Scholar]
- 115.Wu J, Ramesh A, Nayyar T, Hood DB. Assessment of metabolites and AhR and CYP1A1 mRNA expression subsequent to prenatal exposure to inhaled benzo(a)pyrene. Int J Dev Neurosci. 2003;21:333–46. doi: 10.1016/s0736-5748(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 116.Widerak M, Ghoneim C, Dumontier MF, Quesne M, Corvol MT, Savouret JF. The aryl hydrocarbon receptor activates the retinoic acid receptoralpha through SMRT antagonism. Biochimie. 2006;88:387–97. doi: 10.1016/j.biochi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 117.Liu J, Ballaney M, Al-alem U, Quan C, Jin X, Perera F, et al. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci. 2008;102:76–81. doi: 10.1093/toxsci/kfm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang P, Rannug A, Ahlbom E, Hakansson H, Ceccatelli S. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of cytochrome P450 1A1, the aryl hydrocarbon receptor, and the aryl hydrocarbon receptor nuclear translocator in rat brain and pituitary. Toxicol Appl Pharmacol. 2000;169:159–67. doi: 10.1006/taap.2000.9064. [DOI] [PubMed] [Google Scholar]
- 119.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1–4) during development and in different adult tissues. Arch Biochem Biophys. 2005;436:50–61. doi: 10.1016/j.abb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 120.Hakkola J, Pelkonen O, Pasanen M, Raunio H. Xenobiotic-metabolizing cytochrome P450 enzymes in the human feto-placental unit: role in intrauterine toxicity. Crit Rev Toxicol. 1998;28:35–72. doi: 10.1080/10408449891344173. [DOI] [PubMed] [Google Scholar]
- 121.Courter LA, Musafia-Jeknic T, Fischer K, Bildfell R, Giovanini J, Pereira C, et al. Urban dust particulate matter alters PAH-induced carcinogenesis by inhibition of CYP1A1 and CYP1B1. Toxicol Sci. 2007;95:63–73. doi: 10.1093/toxsci/kfl137. [DOI] [PubMed] [Google Scholar]
- 122.Hewitt R, Forero A, Luncsford PJ, Martin FL. Enhanced micronucleus formation and modulation of BCL-2:BAX in MCF-7 cells after exposure to binary mixtures. Environ Health Perspect. 2007;115(Suppl 1):129–36. doi: 10.1289/ehp.9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang JT, Chang H, Chen PH, Lin SL, Lin P. Requirement of aryl hydrocarbon receptor overexpression for CYP1B1 up-regulation and cell growth in human lung adenocarcinomas. Clin Cancer Res. 2007;13:38–45. doi: 10.1158/1078-0432.CCR-06-1166. [DOI] [PubMed] [Google Scholar]
- 124.McCallister MM, Maguire M, Ramesh A, Aimin Q, Liu S, Khoshbouei H, et al. Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology. 2008;29:846–54. doi: 10.1016/j.neuro.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wormley DD, Chirwa S, Nayyar T, Wu J, Johnson S, Brown LA, et al. Inhaled benzo(a)pyrene impairs long-term potentiation in the F1 generation rat dentate gyrus. Cell Mol Biol (Noisy-le-grand) 2004;50:715–21. [PubMed] [Google Scholar]
- 126.N’Diaye M, Le Ferrec E, Lagadic-Gossmann D, Corre S, Gilot D, Lecureur V, et al. Aryl hydrocarbon receptor- and calcium-dependent induction of the chemokine CCL1 by the environmental contaminant benzo[a]pyrene. J Biol Chem. 2006;281:19906–15. doi: 10.1074/jbc.M601192200. [DOI] [PubMed] [Google Scholar]
- 127.Casale GP, Cheng Z, Liu J, Cavalieri EL, Singhal M. Profiles of cytokine mRNAs in the skin and lymph nodes of SENCAR mice treated epicutaneously with dibenzo[a,l]pyrene or dimethylbenz[a]anthracene reveal a direct correlation between carcinogen-induced contact hypersensitivity and epidermal hyperplasia. Mol Carcinog. 2000;27:125–40. doi: 10.1002/(sici)1098-2744(200002)27:2<125::aid-mc8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 128.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition – the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–56. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 129.Jonakait GM. The effects of maternal inflammation on neuronal development: possible mechanisms. Int J Dev Neurosci. 2007;25:415–25. doi: 10.1016/j.ijdevneu.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 130.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–5. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tohmi M, Tsuda N, Watanabe Y, Kakita A, Nawa H. Perinatal inflammatory cytokine challenge results in distinct neurobehavioral alterations in rats: implication in psychiatric disorders of developmental origin. Neurosci Res. 2004;50:67–75. doi: 10.1016/j.neures.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 132.Kontos HA, Wei EP, Povlishock JT, Dietrich WD, Magiera CJ, Ellis EF. Cerebral arteriolar damage by arachidonic acid and prostaglandin G2. Science. 1980;209:1242–5. doi: 10.1126/science.7403881. [DOI] [PubMed] [Google Scholar]
- 133.Weng MW, Hsiao YM, Chen CJ, Wang JP, Chen WC, Ko JL. Benzo[a]pyrene diol epoxide up-regulates COX-2 expression through NF-kappaB in rat astrocytes. Toxicol Lett. 2004;151:345–55. doi: 10.1016/j.toxlet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 134.Chang LW, Chang YC, Ho CC, Tsai MH, Lin P. Increase of carcinogenic risk via enhancement of cyclooxygenase-2 expression and hydroxyestradiol accumulation in human lung cells as a result of interaction between BaP and 17-beta estradiol. Carcinogenesis. 2007;28:1606–12. doi: 10.1093/carcin/bgm013. [DOI] [PubMed] [Google Scholar]
- 135.Miller ME, Holloway AC, Foster WG. Benzo-[a]-pyrene increases invasion in MDA-MB-231 breast cancer cells via increased COX-II expression and prostaglandin E2 (PGE2) output. Clin Exp Metastasis. 2005;22:149–56. doi: 10.1007/s10585-005-6536-x. [DOI] [PubMed] [Google Scholar]
- 136.Tsai KS, Yang RS, Liu SH. Benzo[a]pyrene regulates osteoblast proliferation through an estrogen receptor-related cyclooxygenase-2 pathway. Chem Res Toxicol. 2004;17:679–84. doi: 10.1021/tx0499517. [DOI] [PubMed] [Google Scholar]
- 137.Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–8. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- 138.Hijnen D, De Bruin-Weller M, Oosting B, Lebre C, De Jong E, Bruijnzeel-Koomen C, et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell-attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol. 2004;113:334–40. doi: 10.1016/j.jaci.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 139.Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317–41. doi: 10.1016/s0074-7742(05)71013-8. [DOI] [PubMed] [Google Scholar]
- 140.Raloff J. BPA in the womb shows link to kids’ behavior. Sci News. 2009;176:12. [Google Scholar]
- 141.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–91. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 144.Kalueff AV, Wheaton M, Murphy DL. What’s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 145.Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 146.Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52:307–16. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 147.Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45:345–56. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 148.Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 149.Miyagawa K, Narita M, Akama H, Suzuki T. Memory impairment associated with a dysfunction of the hippocampal cholinergic system induced by prenatal and neonatal exposures to bisphenol-A. Neurosci Lett. 2007;418:236–41. doi: 10.1016/j.neulet.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 150.Mizuo K, Narita M, Miyagawa K, Okuno E, Suzuki T. Prenatal and neonatal exposure to bisphenol-A affects the morphine-induced rewarding effect and hyperlocomotion in mice. Neurosci Lett. 2004;356:95–8. doi: 10.1016/j.neulet.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 151.Nakamura K, Itoh K, Sugimoto T, Fushiki S. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci Lett. 2007;420:100–5. doi: 10.1016/j.neulet.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 152.Nakamura K, Itoh K, Yaoi T, Fujiwara Y, Sugimoto T, Fushiki S. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of Bisphenol A. J Neurosci Res. 2006;84:1197–205. doi: 10.1002/jnr.21020. [DOI] [PubMed] [Google Scholar]
- 153.Naciff JM, Jump ML, Torontali SM, Carr GJ, Tiesman JP, Overmann GJ, et al. Gene expression profile induced by 17alpha-ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat. Toxicol Sci. 2002;68:184–99. doi: 10.1093/toxsci/68.1.184. [DOI] [PubMed] [Google Scholar]
- 154.Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–98. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 155.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee YM, Seong MJ, Lee JW, Lee YK, Kim TM, Nam SY, et al. Estrogen receptor independent neurotoxic mechanism of bisphenol A, an environmental estrogen. J Vet Sci. 2007;8:27–38. doi: 10.4142/jvs.2007.8.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–9. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 158.Tanabe N, Kimoto T, Kawato S. Rapid Ca(2+) signaling induced by Bisphenol A in cultured rat hippocampal neurons. Neuro Endocrinol Lett. 2006;27:97–104. [PubMed] [Google Scholar]
- 159.Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–47. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ramos JG, Varayoud J, Kass L, Rodriguez H, Costabel L, Munoz-De-Toro M, et al. Bisphenol a induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats. Endocrinology. 2003;144:3206–15. doi: 10.1210/en.2002-0198. [DOI] [PubMed] [Google Scholar]
- 161.Kawai K, Murakami S, Senba E, Yamanaka T, Fujiwara Y, Arimura C, et al. Changes in estrogen receptors alpha and beta expression in the brain of mice exposed prenatally to bisphenol A. Regul Toxicol Pharmacol. 2007;47:166–70. doi: 10.1016/j.yrtph.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 162.Nishizawa H, Morita M, Sugimoto M, Imanishi S, Manabe N. Effects of in utero exposure to bisphenol A on mRNA expression of arylhydrocarbon and retinoid receptors in murine embryos. J Reprod Dev. 2005;51:315–24. doi: 10.1262/jrd.16008. [DOI] [PubMed] [Google Scholar]
- 163.Zoeller RT, Crofton KM. Thyroid hormone action in fetal brain development and potential for disruption by environmental chemicals. Neurotoxicology. 2000;21:935–45. [PubMed] [Google Scholar]
- 164.Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145:4037–47. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- 165.Ahmed OM, El-Gareib AW, El-Bakry AM, Abd El-Tawab SM, Ahmed RG. Thyroid hormones states and brain development interactions. Int J Dev Neurosci. 2008;26:147–209. doi: 10.1016/j.ijdevneu.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 166.Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–12. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- 167.Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–90. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- 168.Seiwa C, Nakahara J, Komiyama T, Katsu Y, Iguchi T, Asou H. Bisphenol A exerts thyroid-hormone-like effects on mouse oligodendrocyte precursor cells. Neuroendocrinology. 2004;80:21–30. doi: 10.1159/000080663. [DOI] [PubMed] [Google Scholar]
- 169.Yoshino S, Yamaki K, Li X, Yanagisawa ST, Takano R, Taneda HS, et al. Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology. 2004;112:489–95. doi: 10.1111/j.1365-2567.2004.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yan H, Takamoto M, Sugane K. Exposure to Bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ Health Perspect. 2008;116:514–9. doi: 10.1289/ehp.10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yaoi T, Itoh K, Nakamura K, Ogi H, Fujiwara Y, Fushiki S. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376:563–7. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 172.Mackey SL, Darlington GJ. CCAAT enhancer-binding protein alpha is required for interleukin-6 receptor alpha signaling in newborn hepatocytes. J Biol Chem. 2004;279:16206–13. doi: 10.1074/jbc.M400737200. [DOI] [PubMed] [Google Scholar]
- 173.Feng R, Desbordes SC, Xie H, Tillo ES, Pixley F, Stanley ER, et al. PU. 1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc Natl Acad Sci U S A. 2008;105:6057–62. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Walton M, Saura J, Young D, MacGibbon G, Hansen W, Lawlor P, et al. CCAAT-enhancer binding protein alpha is expressed in activated microglial cells after brain injury. Brain Res Mol Brain Res. 1998;61:11–22. doi: 10.1016/s0169-328x(98)00169-7. [DOI] [PubMed] [Google Scholar]
- 175.Calella AM, Nerlov C, Lopez RG, Sciarretta C, von Bohlen und Halbach O, Bereshchenko O, et al. Neurotrophin/Trk receptor signaling mediates C/EBPalpha, -beta and NeuroD recruitment to immediate-early gene promoters in neuronal cells and requires C/EBPs to induce immediate-early gene transcription. Neural Dev. 2007;2:4. doi: 10.1186/1749-8104-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nishi A, Kuroiwa M, Miller DB, O’Callaghan JP, Bateup HS, Shuto T, et al. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–71. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shepherd MC, Baillie GS, Stirling DI, Houslay MD. Remodelling of the PDE4 cAMP phosphodiesterase isoform profile upon monocyte-macrophage differentiation of human U937 cells. Br J Pharmacol. 2004;142:339–51. doi: 10.1038/sj.bjp.0705770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ariga M, Neitzert B, Nakae S, Mottin G, Bertrand C, Pruniaux MP, et al. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol. 2004;173:7531–8. doi: 10.4049/jimmunol.173.12.7531. [DOI] [PubMed] [Google Scholar]
- 179.Hill EV, Sheppard CL, Cheung YF, Gall I, Krause E, Houslay MD. Oxidative stress employs phosphatidyl inositol 3-kinase and ERK signalling pathways to activate cAMP phosphodiesterase-4D3 (PDE4D3) through multi-site phosphorylation at Ser239 and Ser579. Cell Signal. 2006;18:2056–69. doi: 10.1016/j.cellsig.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 180.Tian Y. Ah receptor and NF-kappaB interplay on the stage of epigenome. Biochem Pharmacol. 2009;77:670–80. doi: 10.1016/j.bcp.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 181.Fleming JL, Huang TH, Toland AE. The role of parental and grand-parental epigenetic alterations in familial cancer risk. Cancer Res. 2008;68:9116–21. doi: 10.1158/0008-5472.CAN-08-2184. [DOI] [PubMed] [Google Scholar]
- 182.Curley JP, Mashoodh R. Parent-of-origin and transgenerational germline influences on behavioral development: the interacting roles of mothers, fathers, and grandparents. Dev Psychobiol. 2010;52:312–30. doi: 10.1002/dev.20430. [DOI] [PubMed] [Google Scholar]
- 183.Skinner MK, Guerrero-Bosagna C. Environmental signals and transgenerational epigenetics. Epigenomics. 2009;1:111–7. doi: 10.2217/epi.09.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–23. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J Androl. 2006;27:868–79. doi: 10.2164/jandrol.106.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chang HS, Anway MD, Rekow SS, Skinner MK. Transgenerational epigenetic imprinting of the male germline by endocrine disruptor exposure during gonadal sex determination. Endocrinology. 2006;147:5524–41. doi: 10.1210/en.2006-0987. [DOI] [PubMed] [Google Scholar]
- 187.Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009;85:742–52. doi: 10.1016/j.lfs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 188.Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2010 doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26:139–62. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 190.Ostler KR, Davis EM, Payne SL, Gosalia BB, Exposito-Cespedes J, Le Beau MM, et al. Cancer cells express aberrant DNMT3B transcripts encoding truncated proteins. Oncogene. 2007;26:5553–63. doi: 10.1038/sj.onc.1210351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wright RJ. Moving towards making social toxins mainstream in children’s environmental health. Curr Opin Pediatr. 2009;21:222–9. doi: 10.1097/MOP.0b013e3283292629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 193.Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115:1140–6. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen E, Schreier HM, Strunk RC, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ Health Perspect. 2008;116:970–5. doi: 10.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. 2004;26:373–85. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wright RJ. Moving towards making social toxins mainstream in children’s environmental health. Curr Opin Pediatr. 2009;21:222–9. doi: 10.1097/MOP.0b013e3283292629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McGowan PO, Szyf M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol Dis. 2010;39:66–72. doi: 10.1016/j.nbd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 198.Champagne FA. Epigenetic influence of social experiences across the lifespan. Dev Psychobiol. 2010;52:299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- 199.Szyf M. The early life environment and the epigenome. Biochim Biophys Acta. 2009;1790:878–85. doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 200.Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–39. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- 201.Joss-Moore LA, Metcalfe DB, Albertine KH, McKnight RA, Lane RH. Epigenetics and fetal adaptation to perinatal events: diversity through fidelity. J Anim Sci. 2010;88:E216–22. doi: 10.2527/jas.2009-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–3. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 203.Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–2. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]