Abstract
In the early postnatal hippocampus, the first synapses to appear on excitatory pyramidal neurons are formed directly on dendritic shafts. Very few dendritic spines are present at this time. By adulthood, however, the overwhelming majority of synapses are located at the tips of dendritic spines. Several models have been proposed to account for the transition from mostly shaft to mostly spinous synapses but none have been demonstrated conclusively. To investigate the cellular mechanism underlying the shaft-to-spinous synapse transition, we designed live imaging experiments to directly observe the dynamics of shaft and spinous synapses on developing dendrites. Immunofluorescent synaptic labeling of GFP-filled neurons showed that the shaft-to-spinous synapse transition in dissociated culture mirrors that in vivo. Along with electron microscopy, the fluorescent labeling also showed that veritable shaft synapses are abundant in dissociated culture and that shaft synapses are frequently adjacent to spines or other dendritic protrusions, a configuration previously observed in vivo by others. We used live long term time lapse confocal microscopy of GFP-filled dendrites and VAMP2-DsRed-labeled boutons to record the fate of shaft synapses and associated dendritic protrusions and boutons with images taken hourly for up to 31 continuous hours. Inspection of the time lapse imaging series revealed that shaft synapses can persist adjacent to either existing or newly grown dendritic protrusions. Alternatively, a shaft synapse bouton can redistribute to contact an adjacent dendritic protrusion. However, we never observed shaft synapses transforming themselves into spines or any type of dendritic protrusions. We conclude that repeated iterations of dendritic protrusion or spine outgrowth adjacent to shaft synapses is very likely to be a critical component of the shaft-to-spinous synapse transition during CNS development.
Keywords: synaptogenesis, spinogenesis, synaptic plasticity, correlative light and electron microscopy (CLEM), laser scanning confocal microscopy (LSCM)
1. Introduction
In the adult hippocampus and other brain regions, the vast majority of excitatory synapses are located on the tips of dendritic spines protruding from the dendrites of pyramidal cells and other types of spiny neurons. Only a very small minority of excitatory synapses on spiny neurons are formed directly on the cell bodies or dendritic shafts. Thus, spinous synapses are the principle unit of synaptic transmission in the majority of the cortex, hippocampus, cerebellum, and striatum. The developmental processes that generate spinous synapses are still not completely understood.
Prior to the emergence of dendritic spines, axons in the developing neuropil contact dendrites and form synapses directly on dendritic shafts (Ahmari et al., 2000; Boyer et al., 1998; Fiala et al., 1998; Ziv and Smith, 1996). It is not until after these shaft synapses have formed that dendritic spines begin to emerge and form synapses, eventually constituting the majority of synapses. In rats, the first dendritic spines begin to emerge after postnatal week 1. From postnatal day 1 to 12 in the rat hippocampus, the number of shaft synapses decreases from over 50% to approximately 30% while the number of spinous synapses increases from approximately 5% to approximately 40% (Fiala et al., 1998). A similar trend has been shown to occur in dissociated hippocampal neurons in culture (Boyer et al., 1998).
Studies of shaft synaptogenesis conducted with cultured neurons have shown that contact between the axon and dendrite leads to vesicle stabilization within the axon and subsequent synapse formation (Ahmari et al., 2000; Sabo et al., 2006; Ziv and Smith, 1996). By the time dendritic spines have emerged, however, initial axo-dendritic contact has already been made and shaft synapses have already been formed. Therefore, it is most likely that spinous synaptogenesis occurs by a fundamentally different mechanism from shaft synaptogenesis, a possibility that has not been directly addressed experimentally. Possibly, spinous synaptogenesis is somewhat dependent on previously established shaft or spinous synapses as templates for directing sites of spine emergence.
Various models describing possible mechanisms of spinogenesis have been under consideration (Yuste and Bonhoeffer, 2004). In the Miller/Peters model, shaft synapses transform into spines while maintaining synaptic contact throughout the transformation process (Miller and Peters, 1981). Another model specifies that filopodia transform into spines (De Roo et al., 2008; Ziv and Smith, 1996). There are also some hybrid models in which filopodia promote formation of stubby or shaft synapses which subsequently transform into bona fide spines (Dailey and Smith, 1996; Fiala et al., 1998; Harris, 1999). Finally, the Sotelo model, based on studies of Purkinje cells in the cerebellum, specifies that dendritic spines first emerge then axons grow out to meet them (Sotelo, 1978). None of these models have been conclusively supported or ruled out.
To collect direct evidence for the cellular mechanism responsible for the shaft-to-spinous synapse transition in living neurons, we employed live imaging GFP-filled dendrites in contact with VAMP2-DsRed-labeled boutons in dissociated culture. Prior to conducting the live imaging experiments, electron microscopy revealed that shaft synapses were abundant in the cultures, many adjacent to either synaptic spines or other protrusions without synapses. This configuration is similar to previous in vivo observations made by others of synapses at the bases of filopodia (Fiala et al., 1998; Ulfhake and Cullheim, 1988). Pre- and postsynaptic immunofluorescent labeling of GFP-filled cultured neurons fixed between 1 and 3 weeks in vitro further revealed a developmental shift in predominance from shaft to spinous synapses indicating that the shaft-to-spinous transition occurs in the cultures, mirroring that which occurs in vivo. Finally, live imaging of developing dendrites and their contacting presynaptic boutons showed that shaft synapses can co-exist adjacent to both newly emerged and pre-existing dendritic spines. Alternatively, a shaft synapse bouton can redistribute to contact an adjacent newly emerged dendritic spine. These findings support the conclusion that, rather than being directly converted into spinous synapses, shaft synapses serve as templates defining sites of spine emergence along the dendrite.
2. Material and Methods
2.1 Animals used for neuron cultures
Pregnant female Sprague-Dawley rats were purchased from Charles River Laboratories and housed in the Center for Comparative Medicine and Surgery at the Mount Sinai School of Medicine which holds an Animal Welfare Assurance Number (A3111-01) signifying that it adheres to all National Institutes of Health guidelines for the care and treatment of laboratory animals. To obtain embryos for neuron culture preparation, pregnant rats were euthanized at E18.5 by CO2 asphyxiation and the embryos removed by caesarean section. All procedures were approved by the Mount Sinai School of Medicine Institutional Animal Care and Use Committee (IACUC).
2.2 Dissociated neuron culture preparation
To obtain neuron cultures for immunocytochemistry, hippocampi were harvested from E18.5 rat embryos and dissociated. 4.5 × 106 neurons were electroporated with 3 μg pEGFP-N1 plasmid DNA using the Rat Neuron Nucleofector Kit (Lonza Group Ltd.) to express cell-filling EGFP and plated at a density of approximately 4 × 105 cells/dish in 60 mm Petri dishes on 18 mm coverglasses (Fisherbrand coverglass for growth, Thermo Fisher Scientific) in MEM (Invitrogen) supplemented with l-glutamine. Prior to plating, the coverglasses were treated with > 65% pure nitric acid for 72 hours, washed 3 × 20 minutes with water, then sterilized and dried by heating in an oven. After being left to cool, the coverglasses were treated for 8 hours with 1 mg/ml poly-l-lysine (Sigma-Aldrich) in borate buffer, washed 3 × 10 minutes with water, then left to dry completely. 4 hours after plating, the MEM was replaced with Neurobasal (Invitrogen) supplemented with B27 (Invitrogen) and L-glutamine. Neurons remained in this media at 37°C and 5% CO2 until fixation with 4% paraformaldehyde.
To obtain neuron cultures for electron microscopy, neurons were obtained, electroporated with EGFP, and cultured under the same conditions as above. However, the neurons were plated in 35 mm glass bottom dishes (MatTek Corp.) at a density of approximately 1.7 × 105 cells/dish. Prior to plating, the glass bottoms of the dishes were treated for 12 hours with 1 M hydrochloric acid, washed 3 × 20 minutes with water, left to dry completely, treated for 8 hours with 1 mg/ml poly-L-lysine (Sigma-Aldrich) in borate buffer, washed 3 × 10 minutes with water, then again left to dry completely.
To obtain neuron cultures for live LSCM, neurons were again obtained and cultured under the same conditions as above but divided between two tubes, each containing 4.5 × 106 cells. The cells in one tube were electroporated with EGFP as described above while the cells in the other tube were electroporated with with 3 μg VAMP2-DsRed plasmid DNA (obtained from Dr. Kimberley McAllister, UC Davis) (Sabo et al., 2006) then plated in 35 mm glass bottom dishes at a ratio of 1.5 VAMP2-DsRed to 1 GFP transfect at a total density of approximately 3 × 105 cells/dish. This density and plating ratio maximized the number of axodendritic contacts between GFP-filled spines and VAMP2-DsRed-labeled boutons.
2.3 Neuron processing for EM
Neurons cultured in glass bottom dishes as described above were processed for EM as previously described (Hanson et al., 2010). Briefly, neurons were fixed with 4% glutaraldehyde in 0.1 M cacodylate buffer, treated with 1% osmium tetroxide plus 1.5% potassium ferricyanide in 0.1 M cacodylate buffer, dehydrated in an ascending ethanol series (50%, 60%, 70%), left in 3% uranyl acetate in 70% ethanol for 12 hours at 4°C, washed in 70% ethanol, then further dehydrated in an ascending ethanol series (80%, 90%, 100%). The dehydrated neurons were infiltrated with a 1:1 solution of resin (Embed 812 kit, Electron Microscopy Sciences) and 100% ethanol for 24 hours at room temperature. The resin-ethanol solution was then replaced with a thin layer of pure resin and neurons of interest were embedded and sectioned through at 70 nm. Serial sections were collected on Formvar-coated slot grids. Sections were contrasted with lead citrate and uranyl acetate and serial sections of the cell of interest were documented on an Hitachi H-7000 (Hitachi, Ltd.) transmission electron microscope at 12-30k magnification and 75 kV voltage.
2.4 Immunocytochemistry
Fluorescent immunolabeling was carried out as described (Fernandez-Monreal et al., 2009; Fernandez-Monreal et al., 2010) using primary antibodies against vesicular glutamate transporter 1 (vGlut1; Millipore) and PSD95 (Thermo Fisher Scientific).
2.5 LSCM
LSCM of immunofluorescently labeled neurons was performed using a Zeiss LSM 710 (Carl Zeiss, Inc.) with a 63x oil objective (plan apochromat, numerical aperture 1.4). The cell-filling EGFP was excited by 488 nm wavelength laser light. The immunofluorescently labeled vGlut1 was excited by 633 nm laser light and the PSD95 with 561 nm laser light. All emissions were detected in separate channels through a beamsplitter and a pinhole set to 1 Airy unit. Z-stacks of approximately 75 images each were taken with an x-y pixel size of 0.13 μm2, an optical slice thickness of, and a z-step of 0.14 μm. The exact number of images in each z-stack depended on the thickness of the neuron in the stack. The scan speed was 3.15 with μs/pixel with 2 line averaging.
Long-term live LSCM was performed using a Zeiss LSM 510 with a 63x oil objective (plan apochromat, numerical aperture 1.4). The microscope was enclosed in an incubation chamber to maintain temperature, CO2, and humidity. The cell-filling EGFP was excited by 488 nm wavelength laserlight and its emission detected through a long pass 505 nm filter. The DsRed tagging VAMP2 was excited by 543 nm wavelength helium neon laser light and its emission detected through a band pass 585-615 nm filter. The EGFP and DsRed emissions were detected separately in different channels. Z-stacks were acquired every hour for 13 to 31 hours with a scan time of approximately 5 minutes for each image depending on the size of the region in the z-stack. Prior to placing the neuron culture dish on the microscope, the incubation chamber was equilibrated to 37°C and 5% CO2 for at least 1 hour. After focusing the objective on the dendritic segment to be imaged, the system was left to equilibrate for at least 1 more hour to prevent vertical drift during the long-term imaging session. Dishes were covered with a PTFE (polytetrafluoroethylene) membrane (DuPont) sealed around the dish using a rubber band to prevent media evaporation while allowing gas exchange. This covering has been used previously to ensure long-term survival during live microscopy (Chen et al., 2007).
2.6 Quantification and statistics
To evaluate the shaft-to-spinous synapse transition in dissociated culture, dendritic spines and fluorescently immunolabeled vGlut1 and PSD95 puncta were identified manually by visual inspection in LSCM z-stack projections of GFP-filled dendritic segments at 7, 15, and 22 DIV. Dendritic spines were categorized as having either vGlut1, PSD95, both, or neither puncta coincident with their heads. vGlut1 or PSD95 puncta coincident with dendritic shafts were categorized as having either only either vGlut1, PSD95, or both puncta present. Only synapses having both vGlut1 and PSD95 puncta were used in the synapse density calculations. All measurements were made in ImageJ (NIH). All numeric analyses and statistical tests were performed using Microsoft Excel or SPSS (IBM). Significance was set at p < 0.05 for all tests.
3. Results
3.1 Dendritic spines protrude adjacent to shaft synapses
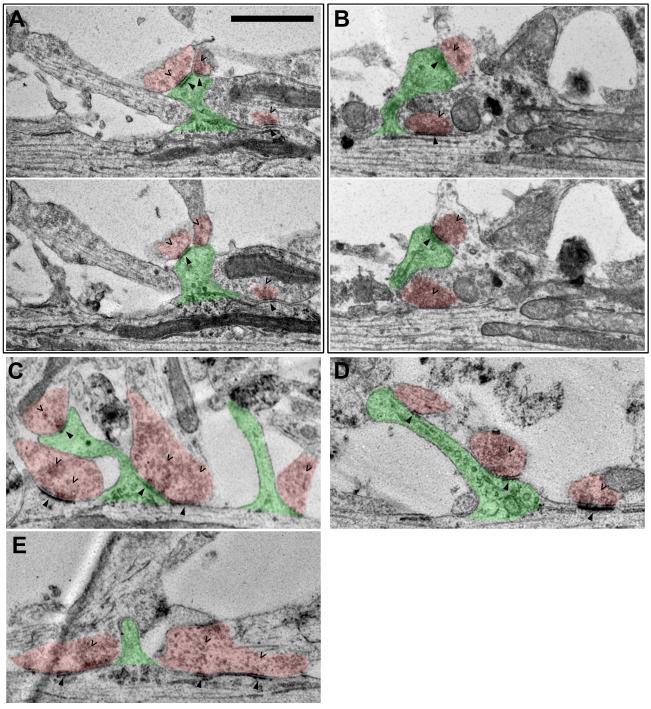
To begin this study of the shaft-to-spinous synapse transition, we used EM to determine the prevalence of shaft synapses in dissociated cultured neurons and to evaluate their spatial relationship to dendritic protrusions and spines. Not only did we find many shaft synapses clearly identifiable by both having postsynaptic densities and contacting presynaptic vesicle boutons, but we also frequently observed synaptic spines or other dendritic protrusions emerging adjacent to the shaft synapses (Fig. 1). Some of the protrusions adjacent to the shaft synapses had postsynaptic densities and contacted boutons containing presynaptic vesicles thus identifying them as synaptic spines (Fig. 1A-D, indicated by arrowheads and carets, respectively). Protrusions that lacked either of these components were considered to be non-synaptic protrusions or protospines (Fig. 1C, E; green shaded spines without arrowheads and carets). In addition to the representative examples shown in Figure 1, we encountered many more examples of shaft synapses both with and without accompanying dendritic spines or other protrusions. These results provided a basis for our live imaging experiments by showing both that shaft synapses are abundant in our dissociated cultured neurons and that they are frequently adjacent to dendtric spines or non-synaptic protrusions.
Figure 1. Serial section EM reveals dendritic spines protruding adjacent to shaft synapses.
Spines are shaded in green. Axo-shaft and axo-dendritic boutons are both shaded in red. Carets point to presynaptic vesicles in the boutons. Arrowheads point to postsynaptic densities in both dendritic shafts and spines. All neurons shown are at 3-4 weeks in vitro. Scale bar is 1 μm. (A, B) show two consecutive slices in the serial sections in which both pre- and postsynaptic features are visible. (C-E) show single slices of the serial sections in which both pre- and postsynaptic features are visible.
3.2 The shaft-to-spinous synapse transition occurs in culture
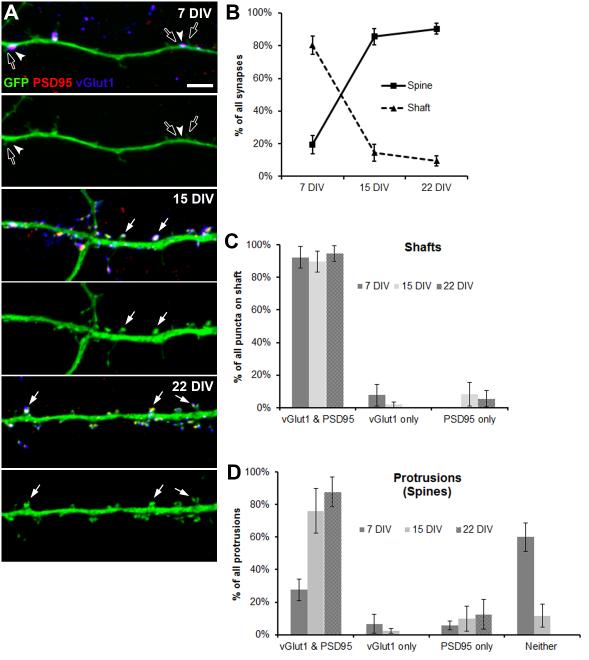
To continue to characterize the shaft-to-spinous transition, we conducted pre- (vGlut1) and postsynaptic (PSD95) fluorescent immunolabeling of GFP-filled neurons in dissociated culture to evaluate shaft and spinous synapses at three developmental stages: 7, 15, and 22 DIV (Fig. 2A). The vast majority of synapses examined were on either primary or secondary dendrites with a small minority on the soma. LSCM imaging and quantitation of the synapses revealed that a shift from mostly shaft to mostly spinous synapses occurs between 7 and 15 DIV (Fig. 2B). At 7 DIV, approximately 80% of synapses are on the shaft while the remaining 20% are spinous. However, by 15 DIV and persisting until at least 22 DIV, over 90% of synapses are spinous while only the remaining 10% are on the shaft (Fig. 2B). Both the decrease in shaft synapses and the increase in spinous synapses from 7 to 15 DIV are statistically significant by multiple analysis of variance tests. Shaft and spinous synapse densities at each time point are shown in Table 1. Of these, the only statistically significant change was the increase in spinous synapse density from 7 to 15 DIV by a one-tailed t test. These findings in dissociated culture, consistent with those of previous in vivo studies (Boyer et al., 1998; Fiala et al., 1998), prompted us to investigate the transition with long-term live LSCM.
Figure 2. Shaft-to-spinous transition in dissociated culture.
(A) LSCM z-stack projections showing shaft and spinous synapses across a developmental time course of 7 to 22 DIV on GFP-filled dendritic segments (green) with immunolabeled pre- (vGlut1, blue) and postsynaptic (PSD95, red) markers. Arrowheads point to pre- and postsynaptically labeled shaft synapses. Solid arrows point to pre- and postsynaptically labeled spinous synapses. Open arrows point to protrusions adjacent to shaft synapses. Bottom panels at each time point show only the GFP (green) to clearly show the presence or absence of spines at synapse locations. Scale bar is 5 μm. (B) Line graph showing percent of shaft versus spinous synapses over the developmental time course of 7 to 22 DIV. Error bars represent standard deviation. (C) Bar graph showing percent of all pre- (vGlut1) or postsynaptic (PSD95) puncta on shaft with both pre- and postsynaptic markers (vGlut1 and PSD95), only presynaptic (vGlut1), and only postsynaptic (PSD95). Error bars represent standard deviation. (D) Bar graph showing percent of all spines with both pre- and postsynaptic markers (vGlut1 and PSD95), only presynaptic (vGlut1), only postsynaptic (PSD95), and neither pre- nor postsynaptic (no vGlut1 or PSD95). Error bars represent standard deviation.
Table 1. Shaft and spinous synapses per unit length of dendrite.
Error is shown as standard deviation.
| DIV | Shaft synapses per 10 μm of dendrite |
Spinous synapses per 10 μm of dendrite |
|---|---|---|
| 7 | 0.48 ± 0.05 | 0.11 ± 0.03 |
| 15 | 0.23 ± 0.13 | 1.14 ± 0.24 |
| 22 | 0.30 ± 0.18 | 2.37 ± 0.66 |
Our fluorescent labeling of GFP-filled neurons also outlined the developmental sequence from 7 to 22 DIV by which pre- and postsynaptic components appear at shaft and spinous synapses. For shaft synapses, both pre- and postsynaptic puncta are present from 7 to 22 DIV with very few examples of one absent of the other (Fig. 2C). Of the dendritic protrusions present at 7 DIV most are devoid of both pre- and postsynaptic immunolabel (Fig. 2D). This is in line with the idea that spines grow first then form synapses (Knott et al., 2006). At 15 and 22 DIV, however, over 75% of dendritic protrusions have both pre- and postsynaptic puncta at their tips suggesting that they are functional synaptic spines. Each of these majorities were significant by one-tailed t tests.
Although there are many more shaft than spinous synapses on 7 DIV dendrites, non-synaptic protrusions were present at a high density. Consistent with our EM observation of dendritic protrusions and spines adjacent to shaft synapses (Fig. 1), we also frequently observed protrusions adjacent to shaft synapses in our fluorescent synaptic labeling of GFP-filled neurons (Fig. 2A, open arrows). The nearness of shaft synapses to dendritic protrusions suggests a possible interaction between the two, the dynamics of which might be involved in the shaft-to-spinous synapse transition.
3.3 Live microscopy reveals spines emerging adjacent to existing shaft synapses
To observe the fate of shaft synapses, we employed long term live LSCM of neurons between 14 and 22 DIV transfected with cell-filling GFP in dissociated co-culture with neurons transfected with VAMP2-DsRed (Fig. 3). Images were taken hourly for up to 31 continuous hours. Instances of VAMP2-DsRed puncta in persistent contact with GFP-filled dendritic shafts were identified as shaft synapses (Fig. 4, arrowheads). It is quite unlikely that these shaft contacts were nonsynaptic as our fluorescent labeling of GFP-filled neurons showed that only 2% of vGlut1 presynaptic puncta at the dendritic shaft were without postsynaptic partners at 15 DIV and none were without partners at 22 DIV (Fig. 2C). All synapses examined were on either primary or secondary dendrites.
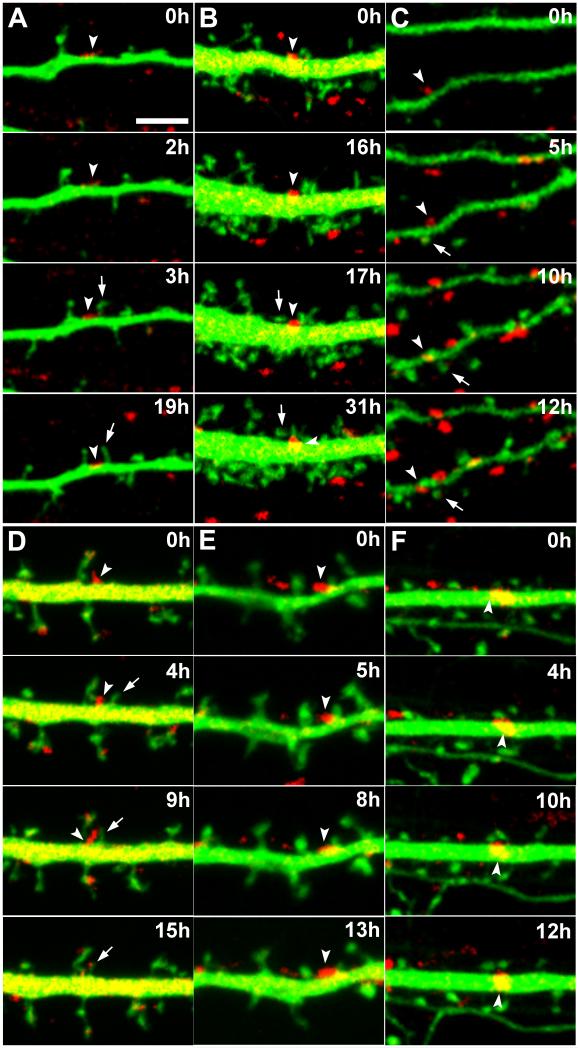
Figure 3. Long-term live imaging of synaptic contacts.
Representative time lapse live LSCM z-stack projections of GFP-filled dendrites (green) with VAMP2-DsRed labeled boutons (red) from which the recordings shown in Figure 4 were obtained. Scale bar is 10 μm. The boxed region is shown in Figure 4B.
Figure 4. Spine outgrowth and persistence adjacent to pre-existing shaft synapses.
Live LSCM z-stack projections of GFP-filled dendrites (green) with VAMP2-DsRed labeled boutons (red) between 14 and 22 DIV. Scale bar is 5 μm. Arrowheads point to shaft synapses. Arrows point to new dendritic protrusions or spines. (A-C) Dendritic protrusions emerging then persisting adjacent to pre-existing shaft synapses for up to at least 16 hours (A). (B) is boxed region in Figure 3. (D) A newly emerged dendritic protrusion acquiring the bouton of a pre-existing shaft synapse. (E,F) Additional examples of dendritic protrusions persisting adjacent to shaft synapses for up to at least 13 hours (E).
Close inspection of the time lapse imaging series revealed dendritic protrusions emerging adjacent to existing shaft synapses (Fig. 4A, B, arrows). After emergence of the protrusions, we observed shaft synapses continuing to persist for at least as long as the remaining 14 to 16 hours of the imaging sessions allowed us to observe. Furthermore, we also observed dendritic protrusions with a stable morphology characteristic of mature established spines adjacent to persistent shaft synapses (Fig. 4C). However, not all shaft synapses persist upon emergence of an adjacent dendritic protrusion. In one instance, several hours after protrusion emergence, we observed redistribution of the bouton from the existing shaft synapse to contact an adjacent protrusion (Fig. 4D). A close frame-by-frame inspection of the live imaging series shows the bouton clearly at a new location beginning at hour 11 through to hour 15, the last time point examined (Supp. Video 4). The constancy of the bouton at the new location for several hours in contact with the new protrusion suggests that it has been redistributed to this location. It is unlikely that this spinous contact was nonsynaptic as our fluorescent labeling of GFP-filled neurons showed that only 2.4% of vGlut1 presynaptic puncta at spines were without postsynaptic partners at 15 DIV and none were without partners at 22 DIV (Fig. 2D). In no case did we observe any evidence of shaft synapses transforming themselves into spinous synapses.
4. Discussion
4.1 Early synapses form on dendritic shafts; later synapses form on dendritic spines
During the first postnatal week in rats, most excitatory synapses in the hippocampus are on dendritic shafts (Boyer et al., 1998; Fiala et al., 1998). Then, from the second postnatal week onward into adulthood, most of these synapses are on dendritic spines. Since their initial discovery more than 100 years ago (Ramón y Cajal, 1891), dendritic spines have been studied extensively. However, much remains to be determined in terms of their formation and elimination, motility, connectivity, structure, morphology, and spatial arrangement along dendrites and within axodendritic networks.
Early in development, formation of the first shaft synapses may be directed by axodendritic contact leading to vesicle stabilization and synapse formation (Ahmari et al., 2000; Ziv and Smith, 1996). Alternatively, events entirely within axons can initiate this process independently of dendritic or glial contact (Sabo et al., 2006). In these lone axons, synaptic vesicles pause and accumulate at points where boutons will later form. Eventually, when dendritic filopodia emerge and contact axons, they do so preferentially at these pause sites.
4.2 Existing models of spinogenesis
According to the Miller/Peters hypothesis, an early model for spinogenesis based on EM observations, shaft synapses transform into spinous synapses by spine growth out of existing shaft synapses (Miller and Peters, 1981). However, the occurence of such a transformation has not been supported by direct observations, such as with live imaging. On the contrary, it has been suggested that axons (De Paola et al., 2006) and dendrites (Lee et al., 2006; Trachtenberg et al., 2002) are likely too stable and the axodendritic network too dense to accomodate spines growing out of existing shaft synapses (Fiala et al., 2002). Furthermore, live imaging has shown that a spine can grow prior to formation of a synapse at its tip (Knott et al., 2006). These new spines go on to make functional synapses with both pre- and postsynaptic components within a few hours after spine emergence (Zito et al., 2009). Similarly, according to another early model of spinogenesis, the Sotelo model, which was developed based on studies with cerebellar Purkinje cells, spines emerge first then are met by growing axons to establish synaptic contact (Sotelo, 1978). The initial growth of spines without synapses argues against transformation of shaft synapses into spines because, if such a transformation were to occur, the spine would have a synapse on it from the moment it started to grow.
Other models for spinogenesis feature transformation of filopodia into spines. Support for these models comes from the observation that decreases in the number of filopodia coincide with increases in the number of spines (Ziv and Smith, 1996). On the other hand, it has been shown with live imaging that spines can appear by themselves without precursor filopodia or any other precursor protrusions (Engert and Bonhoeffer, 1999; Trachtenberg et al., 2002). In an alternative filopodial model, contact between filopodia and an axon or bouton somehow causes the axon or bouton to be brought into apposition with the dendritic shaft, which in turn leads to formation of a stubby or shaft synapse (Dailey and Smith, 1996; Fiala et al., 1998; Harris, 1999). Then, according to these models, the stubby or shaft synapse could later mature into a mushroom spine.
4.3 Our proposed model for spinogenesis
We propose a model in which spine growth occurs adjacent to pre-existing shaft synapses (Fig. 4). In our model, shaft synapses would constitute a separate population of synapses that is present to a higher degree early in development. Possibly, in our model, sampling by filopodia would determine the locations of these boutons to be contacted. As evidenced by the finding that glutamate (Smith, 1992) and calcium ions (Williams et al., 1995) can cause filopodial growth, synaptic activity at the shaft synapse could be the first signal for this to occur. Moreover, it has been demonstrated that motile filopodia likely serve to contact and possibly stabilize presynaptic regions along the axon (Konur and Yuste, 2004; Lohmann et al., 2002; Ziv and Smith, 1996). Also, it has recently been shown that, within NMDA synapses, NR2A subunits promote stabilization of the synapse while NR2B subunits are required to allow structural changes in the synapse (Gambrill and Barria, 2011). Thus, spinogenesis may occur preferentially near to shaft synapses because, as the shaft synapses are already making synaptic contact, the increased activity at that location could initiate a spinogenic signal, possibily mediated through transient filopodia and NMDA receptor subunit composition.
Supplementary Material
Supplementary Video 1. Dendritic protrusions emerging then persisting adjacent to pre-existing shaft synapses for up to at least 16 hours (Video 1).
Supplementary Video 2. Dendritic protrusions emerging then persisting adjacent to pre-existing shaft synapses for up to at least 16 hours (Video 1).
Supplementary Video 3. Dendritic protrusions emerging then persisting adjacent to pre-existing shaft synapses for up to at least 16 hours (Video 1).
Supplementary Video 4. A newly emerged dendritic protrusion acquiring the bouton of a pre-existing shaft synapse.
Supplementary Video 5. Additional examples of dendritic protrusions persisting adjacent to shaft synapses for up to at least 13 hours (Video 5).
Supplementary Video 6. Additional examples of dendritic protrusions persisting adjacent to shaft synapses for up to at least 13 hours (Video 5).
Figure 5. “Synapse template” model of spinogenesis.
(1) A shaft synapse forms on the dendrite where it comes in contact with a presynaptic bouton. (2) A new spine emerges adjacent to the established shaft synapse. (3) A postsynaptic density forms within the spine and a new presynaptic bouton forms to contact the newly formed spine thus completing the new spinous synapse.
Acknowledgements
This work was supported by NS051238 and an Irma T. Hirschl Award (both to GRP). We thank Dr. Kimberley McAllister for the VAMP2-DsRed plasmid and Dr. Graham Ellis-Davies for advice on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Videos 1-6 correspond to Figure 3A-F, respectively.
References
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Boyer C, Schikorski T, Stevens CF. Comparison of hippocampal dendritic spines in culture and in brain. J Neurosci. 1998;18:5294–5300. doi: 10.1523/JNEUROSCI.18-14-05294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Owens GC, Crossin KL, Edelman DB. Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol Cell Neurosci. 2007;36:472–483. doi: 10.1016/j.mcn.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Mendez P, Poglia L, Muller D. Activity-dependent PSD formation and stabilization of newly formed spines in hippocampal slice cultures. Cereb Cortex. 2008;18:151–161. doi: 10.1093/cercor/bhm041. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fernandez-Monreal M, Kang S, Phillips GR. Gamma-protocadherin homophilic interaction and intracellular trafficking is controlled by the cytoplasmic domain in neurons. Mol Cell Neurosci. 2009;40:344–353. doi: 10.1016/j.mcn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Monreal M, Oung T, Hanson HH, O’Leary R, Janssen WG, Dolios G, Wang R, Phillips GR. gamma-protocadherins are enriched and transported in specialized vesicles associated with the secretory pathway in neurons. Eur J Neurosci. 2010;32:921–931. doi: 10.1111/j.1460-9568.2010.07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Allwardt B, Harris KM. Dendritic spines do not split during hippocampal LTP or maturation. Nat Neurosci. 2002;5:297–298. doi: 10.1038/nn830. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A. 2011;108:5855–5860. doi: 10.1073/pnas.1012676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson HH, Reilly JE, Lee R, Janssen WG, Phillips GR. Streamlined embedding of cell monolayers on gridded glass-bottom imaging dishes for correlative light and electron microscopy. Microsc Microanal. 2010;16:747–754. doi: 10.1017/S1431927610094092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Konur S, Yuste R. Imaging the motility of dendritic protrusions and axon terminals: roles in axon sampling and synaptic competition. Mol Cell Neurosci. 2004;27:427–440. doi: 10.1016/j.mcn.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Lee WC, Huang H, Feng G, Sanes JR, Brown EN, So PT, Nedivi E. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 2006;4:e29. doi: 10.1371/journal.pbio.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- Miller M, Peters A. Maturation of rat visual cortex. II. A combined Golgi-electron microscope study of pyramidal neurons. J Comp Neurol. 1981;203:555–573. doi: 10.1002/cne.902030402. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Sur la structure de l’écorce cérébrale de quelques mammifères. La Cellule. 1891. pp. 125–176.
- Sabo SL, Gomes RA, McAllister AK. Formation of presynaptic terminals at predefined sites along axons. J Neurosci. 2006;26:10813–10825. doi: 10.1523/JNEUROSCI.2052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJC. Rapid induction of filopodial sprouting by application of glutamate lo hippocampal neurons. In: Letourneau PK,S, Macagno E, editors. The nerve growth cone. Raven Press Ltd; New York: 1992. pp. 19–26. [Google Scholar]
- Sotelo C. Purkinje cell ontogeny: formation and maintenance of spines. Prog Brain Res. 1978;48:149–170. doi: 10.1016/S0079-6123(08)61021-3. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Cullheim S. Postnatal development of cat hind limb motoneurons. II: In vivo morphology of dendritic growth cones and the maturation of dendrite morphology. J Comp Neurol. 1988;278:88–102. doi: 10.1002/cne.902780106. [DOI] [PubMed] [Google Scholar]
- Williams CV, Davenport RW, Dou P, Kater SB. Developmental regulation of plasticity along neurite shafts. J Neurobiol. 1995;27:127–140. doi: 10.1002/neu.480270202. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1. Dendritic protrusions emerging then persisting adjacent to pre-existing shaft synapses for up to at least 16 hours (Video 1).
Supplementary Video 2. Dendritic protrusions emerging then persisting adjacent to pre-existing shaft synapses for up to at least 16 hours (Video 1).
Supplementary Video 3. Dendritic protrusions emerging then persisting adjacent to pre-existing shaft synapses for up to at least 16 hours (Video 1).
Supplementary Video 4. A newly emerged dendritic protrusion acquiring the bouton of a pre-existing shaft synapse.
Supplementary Video 5. Additional examples of dendritic protrusions persisting adjacent to shaft synapses for up to at least 13 hours (Video 5).
Supplementary Video 6. Additional examples of dendritic protrusions persisting adjacent to shaft synapses for up to at least 13 hours (Video 5).