Abstract
Testosterone influences the hypothalamic-pituitary-adrenal axis, anxiety-related behavior, and sensorimotor gating in rodents, but little is known about the role of the androgen receptor (AR) in mediating these influences. We compared levels of the stress hormone corticosterone at baseline and following exposure to a novel object in an open field in wild type (wt) male and female rats, and male rats with the testicular feminization mutation (Tfm) of the AR, which disables its function. Basal corticosterone was equivalent in all groups, but exposure to a novel object in an open field elicited a greater increase in corticosterone in Tfm males and wt females than in wt males. Tfm males also showed increased behavioral indices of anxiety compared to wt males and females in the test. Analysis of the immediate early gene c-Fos expression after exposure to a novel object revealed greater activation in Tfm males than wt males in some regions (medial preoptic area) and lesser activation in others (dentate gyrus, posterodorsal medial amygdala). No differences were found in a measure of sensorimotor gating (prepulse inhibition of the acoustic startle response), although Tfm males had an increased acoustic startle response compared to wt males and females. These findings demonstrate that ARs play a role in regulating anxiety-related behaviors, as well as corticosterone responses and neural activation following exposure to a mild stressor in rats.
Introduction
In humans, gonadal hormones influence mood disorders including anxiety and depression. Women are diagnosed with anxiety disorders and depression more often than are men, and these disorders often coincide with a decline in levels of estrogen during menopause (Arpels, 1996). Furthermore, estrogen replacement therapy has been reported to decrease anxiety in postmenopausal women (Yazici et al., 2003). In men, a similar but less abrupt decline in androgen levels with age is also often accompanied by symptoms of anxiety and depression (Kaminetsky, 2005; Lund et al., 1999; Eskelinen et al., 2007; Cooper and Richie, 2000). Androgen treatment of aging men, or of younger men with decreased testicular production of testosterone (T), ameliorates some of these symptoms (Amore et al., 2009; Genazzani et al., 2004; Seidman et al., 2009; Kaminetsky, 2005; Cooper and Richie, 2000; Kumano, 2007). Similarly, boys and girls with low T levels show greater indices of depression and anxiety than those with high T (Granger et al., 2003).
Gonadal hormones also appear to influence anxiety and depression-related behaviors in rodents. In rats, females often show fewer anxiety-related behaviors than males (Archer, 1975; Masur et al., 1980; Slob et al., 1981; Seliger, 1977; Lucion et al., 1996). Furthermore, administration of either estrogens or androgens generally results in decreased indices of anxiety and depression-related behaviors in rodents (Frye and Lacey, 2001; Walf and Frye, 2005; Lund et al., 2005; Frye et al., 2008; Bing et al., 1998; Bitran et al., 1993). Evidence suggests that anxiolytic actions of estrogens are largely mediated through activation of the estrogen receptor (ER) isoform ERβ (Lund et al., 2005; Imwalle et al., 2005). On the other hand, androgen action can be mediated through several mechanisms including androgen receptors (ARs), GABA-A receptors (Edinger and Frye, 2006; Lund et al., 2005; Edinger and Frye, 2005), and ERs.
Sex hormones also modulate hypothalamic-pituitary-adrenal (HPA) axis activity (Handa et al., 1994a; Lund et al., 2005). These hormones may in turn affect behavior in rodents exposed to an aversive situation, since hyperactivity of the HPA axis is a symptom of rats that display trait anxiety (Jankoveski et al., 2008; Landgraph et al., 1999). In particular, T treatment decreases, while estrogen treatment increases, the release of stress hormones adrenocorticotropic hormone (ACTH) from the pituitary gland, and corticosterone from the adrenal cortex. Specific activation of sex hormone receptors ERα and ERβ increase and decrease HPA axis activity respectively, while AR activation also appears to decrease HPA activity, although its role is less clear (Handa et al., 1994b; Lund et al., 2004; Lund et al., 2005; Lund et al., 2006).
There are also sex differences in sensorimotor gating (men > women; Swerdlow et al., 1993) as assessed by an experimental model of sensorimotor gating, prepulse inhibition of the acoustic startle response (PPI; Swerdlow et al., 1996). Furthermore, PPI varies in women according to the stage of the menstrual cycle, suggesting that variations in sex hormones can influence PPI (Swerdlow et al., 1997; Jovanovic et al., 2004). Sex differences in PPI have also been reported in Wistar rats and in mice (males > females; Lehmann et al., 1999; Ralph et al., 2001; Ison and Allen, 2007), and PPI varies across the estrus cycle in rats (Koch, 1998). Administration of estrogens and androgens can facilitate PPI (van den Buuse and Eikelis, 2001; Gogos and Van den Buuse, 2003), although little is known about the role of specific hormone receptors, including the AR, that may mediate these changes.
Use of pharmacological agents to probe which steroid receptors mediate a response to T are useful, but interpretation of these experiments can be complicated. DHT, a non-aromatizable androgen, can also be metabolized to 3α-androstanediol (3α-diol) which has a high affinity for GABA-A receptors. Moreover, the 3β-diol metabolite of DHT is an estrogenic compound, with greater affinity for ERβ than ERα (Lund et al. 2004). Another approach to ascertain the receptor(s) mediating T effects on stress hormone responses and behavior is to examine animals with a dysfunctional AR allele, the testicular feminization mutation (Tfm; Allison, 1965; Yarbrough et al., 1990; Zuloaga et al., 2008a). If XY males carrying this allele behave identically to wildtype (wt) males, then AR is not necessary for the behavior. Alternatively, if Tfm males differ from wt males for any given behavior, then AR normally influences that behavior.
We previously investigated corticosterone responses and anxiety-related behaviors in response to a mild stressor in wt male and Tfm male mice and found that a dysfunctional AR led to increases in both (Zuloaga et al., 2008b). We chose to examine these same responses to stress in the Tfm rat model to determine whether the apparent role of AR in the HPA axis is idiosyncratic to mice or more broadly relevant to other mammals. Another reason to examine Tfm male rats stems from a drawback of the Tfm mutation in mice; namely, Tfm male mice secrete virtually no T in adulthood, so behavioral differences between wild-type and Tfm male mice could be due either to differences in AR function or to differences in circulating T, either during or before the time of testing. While androgen can be pharmacologically replaced in Tfm mice, such paradigms are necessarily imperfect. Furthermore, because both Tfm rats and humans with complete androgen insensitivity secrete levels of T that are higher than in normal males (Rosseli et al., 1987; Vague, 1983), Tfm rats may be a better model for the human condition than Tfm mice. In Tfm rats, as in CAIS humans, reduced AR-mediated negative feedback causes increased gonadotropin release and therefore greater androgen secretion (Yarbrough et al., 1990). While the Tfm mutation in rats does not completely render the AR nonfunctional, as it does in the mouse, the decreases in function by 85–90% (Yarbrough et al., 1990) is sufficient to produce an entirely feminine external phenotype (Allison, 1965). As a result, the Tfm rat may also be a better model for the human partial androgen insensitivity syndrome. In the present study, we investigated the role of ARs in the regulation of the HPA axis, anxiety-related behavior, and sensorimotor gating in the Tfm rat model. Our results suggest that in rats, as in mice, AR normally dampens HPA axis activation and anxiety-related behaviors. Furthermore, we present evidence of three brain regions that may mediate these effects of AR.
Materials and Methods
Animals
Long Evans rats carrying the Tfm allele, which were bred with commercially purchased Long Evans (Charles River) sires for over 10 generations, were group housed in our colony at Michigan State University with a 12/12 L/D cycle, lights on at 0600. These rats are descendents of the original King-Holtzman strain of Tfm rats (Allison, 1965). Upon weaning at 21 days of age, ear punches were obtained to determine genotype using a modified polymerase chain reaction (PCR) to detect Tfm versus wt alleles for AR, and the presence or absence of the Sry gene found only on the Y chromosome (Fernandez et al, 2003). Wt males, wt females that were not carrying the Tfm allele, and Tfm males were used. All animals received care that meets standards of the National Institutes of Health and all experiments were approved by the Michigan State University IACUC.
Experiment 1: The role of ARs in HPA response to mild stress
Plasma corticosterone was collected from 120–180 day old rats at baseline (wt male: N=10; wt female: N=10; Tfm male: N=10) or 20 minutes after initial exposure to an open field with a novel object (wt male: N=10; wt female: N=10; Tfm male: N=10).
Experiment 2: The role of ARs in HPA axis recovery
To assess whether the time course of HPA axis recovery differed in wt and Tfm males following exposure to a mild stressor, 120–180 day old rats were assayed for corticosterone at baseline (wt male: N=7; Tfm male: N=6), or at 20 (wt male: N=7; Tfm male: N=7), 40 (wt male: N=6; Tfm male: N=6), 60 (wt male: N=6; Tfm male: N=6), or 120 (wt male: N=6; Tfm male: N=6) minutes after exposure to an open field with a novel object. This experiment focused only on wt and Tfm males to further probe the role of ARs in the male stress response, as sex differences in rodent corticosterone recovery from stress are already well documented (Handa et al., 1994a; Kudielka and Kirschbaum, 2005).
Experiment 3: The role of ARs in anxiety-related behavior
One hundred twenty-150 day old wt male (N=8), wt female (N=5), and Tfm male rats (N=11) were tested for anxiety-related behaviors in the open field/novel object test as described below.
Experiment 4: The role of ARs in immediate early gene activation
To investigate how specific brain areas may be activated differently due to the presence or absence of functional ARs in males, we immunostained for the immediate early gene c-Fos in 120–150 day old Tfm and wt male rats at baseline (wt male: N=7; Tfm male: N=7) and after exposure to an open field with a novel object (wt male: N=7; Tfm male: N=7).
Experiment 5: The role of ARs in sensorimotor gating
One hundred twenty-150 day old wt male (N=9), wt female (N=11), and Tfm male rats (N=10) were tested for PPI and acoustic startle response (ASR) as described below.
Open Field/Novel object test
Open field/novel object testing was conducted between 1000 and 1400 in a 122cm × 122cm white plastic box illuminated from directly above by a 60 watt light, a meter above the floor of the box. A grid was drawn in the box (individual grids were 20.3 cm × 20.3 cm) to demarcate entries into the center area and activity (grid crossings). For corticosterone and immediate early gene analysis, rats were placed into the open field containing a novel object (a 4” diameter × 8” high cylindrical metal oxygen tank cap) for 10 minutes. For behavioral analysis, rats were first placed into a corner of the empty open field and behavior was recorded via an overhead video camera for 5 minutes. After 5 minutes rats were removed, the box was cleaned with 70% ethanol, and a novel object was placed in the center of the chamber. Three minutes after removal from the open field, rats were replaced into the chamber now containing the novel object and behavior was recorded for another 5 minutes. We recorded the amount of time spent grooming, number of entries into the center area, time spent in the center area, visits to the novel object, and time spent visiting the novel object, which have previously been reported to reflect anxiety in rodents (Delini-Stula and Hunn, 1988). The number of grid crossings and rearings were assessed as measures of activity and all behaviors were coded at a later time by a “blind” observer. The box was again cleaned with 70% ethanol following the novel object test.
Plasma Collection and Hormone Assays
In Experiment 1, blood was collected from adult rats between 0900–1100 at baseline or 20 minutes after initial exposure to the open field with a novel object, a test in which we previously found an increased corticosterone response in Tfm mice compared to wt males (Zuloaga et al., 2008b). In Experiment 2, blood was collected from different cohorts of adult rats between 0900–1100 at 20, 40, 60, and 120 minutes after exposure to an open field with a novel object or at baseline. Novel object exposed rats were placed in an open field with a novel object for 10 minutes after which they were returned to their home cage where they remained until sacrifice. Baseline rats remained unhandled in their home cage until sacrifice. Rats were deeply anaesthetized with isoflurane and decapitated, with trunk blood collected within 2 minutes of cage disturbance. All blood was collected in 1.5 ml tubes containing 250µl of heparin and held on crushed ice until centrifugation. After centrifugation, plasma was collected and frozen at −20° C until the assay was performed. Plasma was assayed for corticosterone and T at the Diagnostic Center for Population and Animal Health at Michigan State University using Coat-A-Count Corticosterone and Coat-A-Count Total Testosterone kits according to the manufacturer’s instructions (Diagnostics Products Corporation, Los Angeles, CA, USA). All plasma samples were run in duplicate and results were averaged. Intraassay and interassay coefficients of variation were < 5 and < 7%, respectively for corticosterone and < 12 and < 7%, respectively for testosterone.
Tissue Processing and c-Fos Immunocytochemistry
One hour after a 10 minute exposure to an open field with a novel object or at baseline, rats were administered an intraperitoneal overdose of pentobarbital, and perfused through the heart with 100 ml of 0.9% saline followed by 200 ml of phosphate-buffered 4% paraformaldehyde. Brains were removed, placed in 4% paraformaldehyde, and refrigerated overnight. The following morning brains were transferred into a 20% sucrose solution where they remained at 4° C until sectioning. No later than one week after collection, brains were sectioned at 40 µm on a freezing microtome into three alternative series and stored at −20° C in cryoprotectant (de Olmos et al., 1978). For c-Fos labeling, one series of sections was rinsed in several changes of 0.05 M tris buffered saline (TBS) to clear cryoprotectant and then incubated as free-floating sections in 0.1% sodium borohydride in TBS for 15 minutes to clear residual fixative. Sections were then rinsed in TBS, incubated in 1% hydrogen peroxide and 0.3% Triton-X in TBS (TBS-TX) for 10 minutes, again rinsed in TBS, then incubated in 20% normal goat serum (NGS) in TBS-TX for 25 minutes. After rinsing in TBS, tissue was incubated in primary antisera (c-Fos rabbit polyclonal: 1:10,000, Santa Cruz Biotechnology, sc52) in 2% normal goat serum and TBS-TX for 48 hours at room temperature. Tissue was then rinsed in TBS and incubated for 1 hour in biotinylated goat-anti rabbit antibody in TBS-TX (1:500, Vector Laboratories, Burlingame, CA) followed by rinses in TBS and a 1 hour incubation in avidin-biotin peroxidase complex (ABC Elite kit, Vector Laboratories, Burlingame, CA). Following rinses in TBS, tissue was developed for visualization of c-Fos positive cells in a hydrogen peroxide, diaminobenzidine, and nickel solution for 5 minutes, after which sections were rinsed in TBS and immediately mounted on slides. The following day, sections were counterstained with neutral red, dehydrated in ethanol, defatted in xylene, and coverslipped with Permount.
Microscope analysis
Analysis of c-Fos positive cells was conducted on a Nikon light microscope equipped with a video camera and Bioquant stereological software (Bioquant, Nashville, TN, USA). Briefly, anatomical position was determined using a stereotaxic atlas (Paxinos and Watson, 1998). Discrete brain regions were outlined to obtain a measure of regional area using a 20× objective and cells considered c-Fos positive (containing black nuclear label) within this region were counted. C-Fos positive cells per mm2 were quantified for each region by dividing the number of cells counted within a region by the area of that region. C-Fos immunoreactivity was quantified in brain regions that express AR and/or have been implicated in stress response, including the basolateral amygdala (BLA), medial amygdala (MEA), posterodorsal medial amygdala (MePD), ventral lateral septum (VLS), posteromedial nucleus of the bed nucleus of the stria terminalis (BSTMPM), paraventricular nucleus of the hypothalamus (PVN), ventromedial nucleus of the hypothalamus (VMH), medial preoptic area (MPOA), dentate gyrus (DG), and hippocampal CA1. The observer was blind to the experimental condition of the tissue.
Prepulse Inhibition
Rats were tested for PPI 1 hour after lights off in a room illuminated by dim red light. PPI was measured in acoustic startle response chambers (SR Lab startle response system, San Diego Instruments, San Diego, CA). Animals were placed into the chamber for 18 minutes, the first 5 minutes of which is an acclimation period. For the remaining 13 minutes the fast muscle twitch startle responses of animals were recorded to a 100 decibel tone alone (acoustic startle response; ASR), or to that same tone preceded by a prepulse or “warning” tone of 3, 8, 10, and 15 decibels via SR Lab software (San Diego Instruments). The prepulse should permit subjects to anticipate the loud pulse, and consequently startle less severely. Each of these trials was repeated 6 times at pseudo-random intervals. After the test, animals were removed and the chamber was cleaned with 70% ethanol.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5.0 and SPSS 15.0. For PPI, a mixed design repeated measures analysis of variance (ANOVA) was used to analyze data with genotype as a between groups factor and prepulse intensity as within (or repeated) groups factor. ASR and anxiety-related behaviors were analyzed using one-way ANOVAs. Acoustic startle response by trial was analyzed using a repeated measures 2-way ANOVA with ASR trial order (1–6) and genotype as factors. T and corticosterone concentrations were analyzed using a 2-way ANOVA with testing condition (novel object exposure, no manipulation) and genotype (wt male, wt female, and Tfm male) as factors. For analysis of corticosterone recovery, differences in corticosterone were analyzed using a two-way ANOVA (genotype × time). Open field and novel object behaviors were analyzed using one-way ANOVAs. Separate 2-way ANOVAs were run for each brain region analyzed for c-Fos expression with testing condition and genotype as factors. All significant main effects or interactions were further analyzed using Tukey’s post hoc tests. Differences were considered significant when p<0.05 and all data are reported as means ± standard error of the mean (SEM) with N= the number of animals.
Results
Experiment 1: The role of ARs in HPA axis regulation
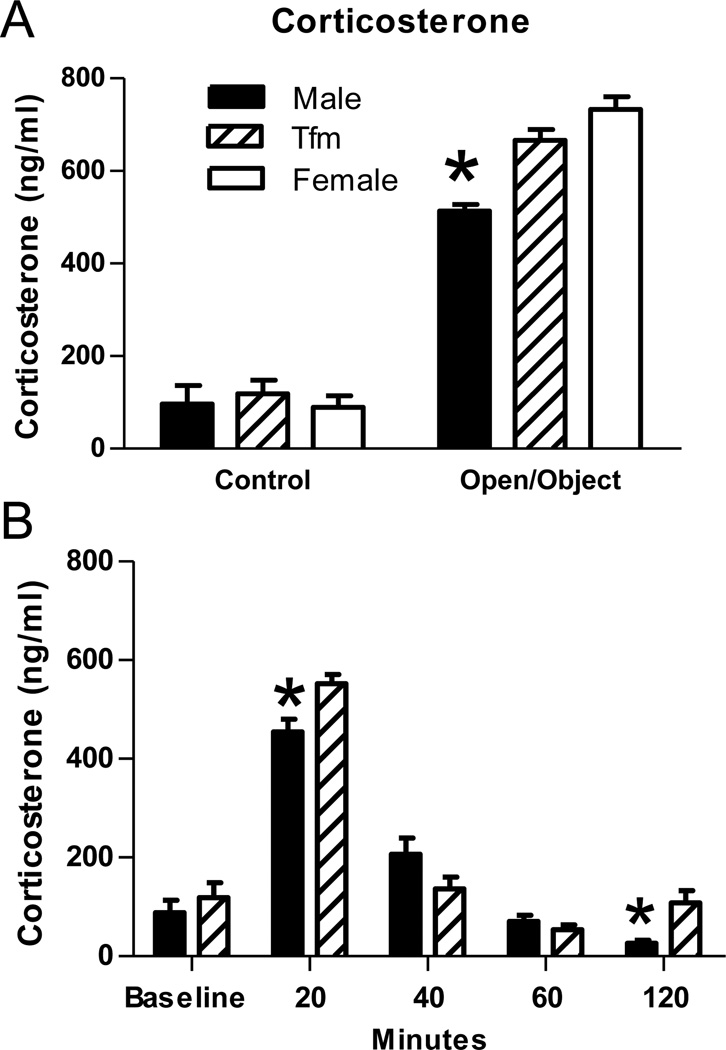
Two-way ANOVA revealed a significant main effect of exposure (F(1,54)= 585.8, p<0.001) in which rats exposed to an open field with a novel object showed an increased corticosterone response 20 minutes after exposure compared to rats in the basal condition. There was also a significant main effect of genotype (F(2,54)= 8.484, p<0.001) and a significant interaction between exposure and genotype on corticosterone concentrations (F(2,54)= 8.50, p<0.001). Post hoc comparisons revealed that all groups had similar blood corticosterone levels at baseline, but after exposure to an open field with a novel object, Tfm males and wt females showed a greater corticosterone response than wt males (ps<0.001; Fig 1a). Two-way ANOVA revealed the expected significant main effect of genotype on T levels (F(2,54)= 11.40, p<0.001), with no main effect of exposure or genotype × exposure interaction. Specifically, T levels (collapsed across stress and baseline conditions) were greater in Tfm males (16.3 ± 4.0 nmol/L) than in wt males (5.9 ± 1.3 nmol/L; p<0.05), and both groups had higher T levels than wt females (0.1 ± 0.03; ps<0.01).
Figure 1.
HPA axis response in Tfm male, wt male and wt female rats. (A) Plasma corticosterone levels at baseline did not differ between the three groups. However, 20 minutes after initial exposure to an open field with a novel object, corticosterone levels were elevated in Tfm males and wt females compared to wt males (* indicates p< 0.001). (B) Plasma corticosterone levels from a second cohort of Tfm and wt male rats were again equivalent at baseline and elevated at 20 minutes after exposure to an open field with a novel object, with levels greater in Tfm males than wt males. Thereafter the groups differed only at 120 minutes after initial exposure suggesting that recovery levels were normal in Tfm males. * indicates p< 0.05 compared to Tfm males at the same time point.
Experiment 2: The role of ARs in HPA axis recovery
Two-way ANOVA revealed the expected effect of time following test exposure on corticosterone levels (F(4,50)= 95.22, p<0.001). Compared to basal corticosterone levels, corticosterone levels were greater in wt and Tfm males at 20 and 40 minutes after the onset of novel object exposure (ps< 0.01), returning to baseline by 60 minutes. A significant genotype × time interaction was also revealed (F(4,50)= 3.874, p<0.01). Tfm males showed increased corticosterone levels compared to wt males at 20 and 120 (ps<0.05) minutes after novel object exposure, but there were no significant differences at baseline, 40, and 60 minutes (Figure 1b) indicating normal basal and recovery hormone levels in Tfm males. A significant main effect of genotype was not found in this test.
Experiment 3: The role of ARs in anxiety-related behavior
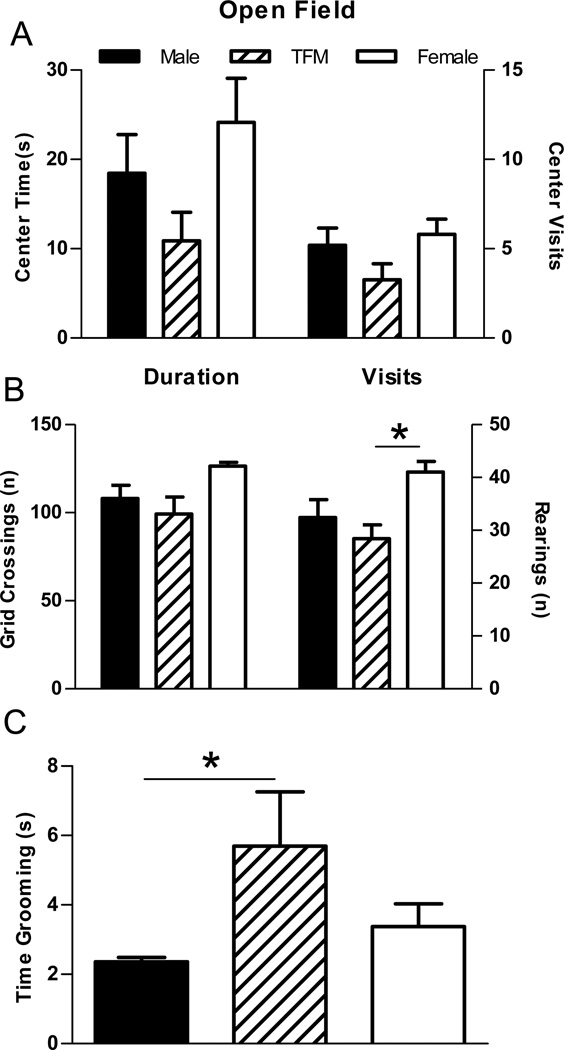
In the open field before introduction of a novel object, there were no significant group differences for time spent in the center area of the open field, number of visits to the center area, or grid crossings. However, Tfm males tended to show fewer visits and decreased time in the center area of the open field compared to wt males and females (Figure 2a). There was a significant effect of genotype for the number of rearings (F(2,21)= 3.458, p<0.05), with wt females rearing more than did Tfm males (p<0.05; Figure 2b). A significant main effect was also found for time spent grooming in the open field (F(2,21)= 3.747, p<0.05) with Tfm males grooming more than wt males (p<0.05; Figure 2c).
Figure 2.
Open field behavior in wt male, Tfm male, and wt female rats. (A) The amount of time spent in and number of visits to the center area of the open field. (B) The number of grid crossing/rearings and (C) time spent grooming in the open field test. Tfm males showed some indices of elevated anxiety as evidenced by increased time grooming (C), although no significant differences were found for time spent in or visits to the center area (A). No significant differences were found for the number of grid crossings, although rearings were decreased for Tfm males compared to wt females, suggesting a moderate activity decrease in Tfm males. Test duration was 300 seconds. * indicates p<0.05 Tfm males compared to wt females (B) and compared to wt males (C).
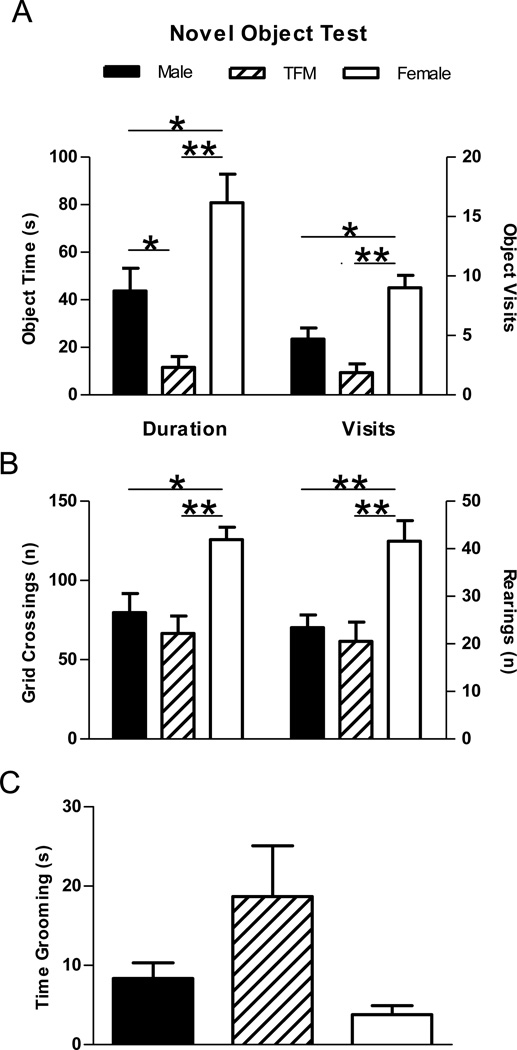
In the novel object test there was a significant effect for time spent visiting the novel object (F(2,21)= 17.55, p<0.001; Figure 3a) and the number of novel object visits (F(2,21)= 12.12, p<0.001; Figure 3a). Post hoc comparisons revealed that Tfm males spent less time visiting the novel object than either wt males (p<0.05) or females (p<0.01) and visited the object less frequently than did wt females (p<0.01). Wt males also spent less time visiting the novel object and had a fewer number of visits to the object than did wt females (ps<0.05). There was a significant effect of genotype on the number of grid crossings (F(2,21)= 5.833, p<0.01; Figure 3b) with females showing a greater number of grid crossings than wt males (p<0.05) and Tfm males (p<0.01), and a significant difference in the number of rearings F(2,21)= 8.420, p<0.01), with females showing a greater number of rearings than wt and Tfm males (ps<0.01). No significant differences were found for time spent grooming in the novel object test (Figure 3c).
Figure 3.
Novel object test for anxiety-related behavior in wt male, Tfm male, and wt female rats. (A) The amount of time spent with and number of visits to the novel object. (B) The number of grid crossing/rearings and (C) time spent grooming in the open field test. Tfm males showed increased indices of anxiety, exhibiting decreased time spent with the novel object compared to both wt males and females, and fewer visits to the object compared to wt females (A). The number of grid crossings and rearings both suggest that motor activity was comparable between wt and Tfm males, although reduced compared to wt females. No significant differences were found for time spent grooming. Test duration was 300 seconds. * indicates p<0.05, ** p<0.01.
Experiment 4: The role of ARs in immediate early gene activation
Replicating our earlier findings (Zuloaga et al., 2008a), body weight was significantly greater for wt males (526.4 ± 24.4g) than comparatively aged Tfm males (379.5 ± 18.6g), while brain weight did not significantly differ between them (wt male: 1.68 ± 0.03g; Tfm male: 1.62 ± 0.04g) indicating that the Tfm mutation does not affect overall brain size, despite effects on the morphology of several specific brain regions (Morris et al., 2005). A significant main effect of exposure to the novel object in an open field was found on the number of c-Fos immunopositive cells per unit area in every brain region examined, with an overall increase in the number of c-Fos immunoreactive (ir) neurons after exposure to an open field with a novel object independent of genotype (ps<0.05). However, for most brain regions, there was no significant difference between wt and Tfm males in the number of c-Fos-ir neurons, either at baseline or in response to the novel object test (Table 1).
Table 1.
The number of c-Fos positive cells per mm2 in wt and Tfm male rats at baseline or after exposure to an open field with a novel object.
| Baseline | Open field w/Novel Object | ||||
|---|---|---|---|---|---|
| Brain Region | Bregma | wt male | Tfm male | wt male | Tfm male |
| Forebrain | |||||
| LSV | 0.7 mm | 74.88 ± 17.28 | 58.45 ± 7.72 | 461.50 ± 32.23* | 429.45 ± 35.78* |
| BSTMPM | −0.8 mm | 24.78 ± 4.46 | 25.03 ± 2.69 | 148.99 ± 15.01* | 127.07 ± 10.17* |
| Hypothalamus | |||||
| PVN | −1.8 mm | 94.45 ± 15.78 | 102.51 ± 11.94 | 396.27 ± 45.89* | 400.32 ± 59.40* |
| VMH | −3.14 mm | 12.23 ± 2.60 | 14.78 ± 2.214 | 93.52 ± 18.09* | 83.12 ± 7.45* |
| Amygdala | |||||
| BLA | −3.14 mm | 26.54 ± 4.61 | 34.58 ± 5.62 | 72.11 ± 9.20* | 60.83 ± 17.28# |
| MEA | −2.56 mm | 13.99 ± 2.69 | 17.356 ± 4.46 | 154.26 ± 8.41* | 151.22 ± 24.80* |
| Hippocampus | |||||
| CA1 | −3.6 mm | 51.22 ± 7.73 | 63.57 ± 6.96 | 111.39 ± 9.43* | 105.29 ± 11.42* |
indicates p<0.01 compared to same group at baseline,
indicates p<0.05 compared to same group at baseline.
No significant differences were found between Tfm and wt males in the number of c-Fos positive cells within these brain regions.
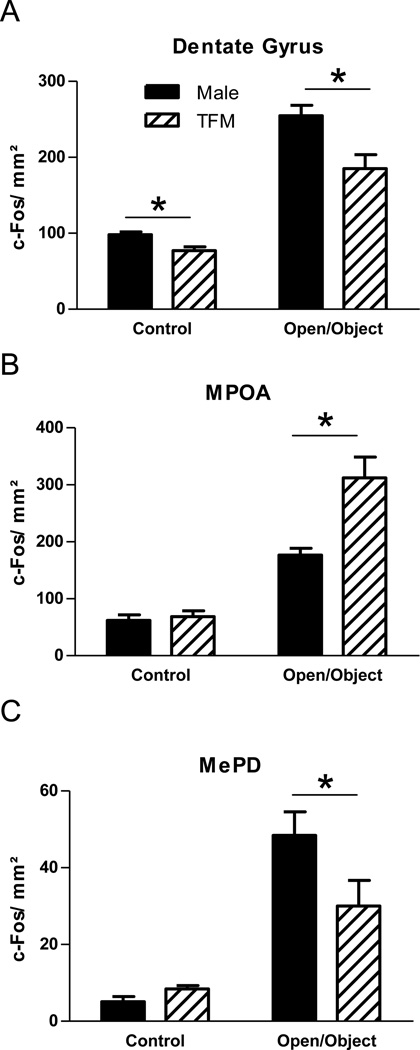
The number of c-Fos-ir cells differed in Tfm and wt males in three brain regions—DG, MPOA and MePD. In the DG, a main effect of genotype was revealed (F(1,24)= 14.85, p<0.001), reflecting the fact that wt males had more c-Fos-ir neurons overall compared to Tfm males (p<0.001; Figure 4a). There was also a main effect of genotype in the MPOA (F(1,24)= 12.35, p<0.01). Post hoc comparisons revealed that exposure to a novel object induced a significant increase in the number of cFos-ir neurons in both Tfm and wt males (p<0.001; Figure 4b). However, a significant interaction was also found in the MPOA (F(1,24)= 10.08, p<0.01), because exposure to a novel object induced a greater cFos response in Tfm males than wt males. In the MePD, in addition to the overall increase in the number of c-Fos-ir neurons in response to a novel object, a significant interaction was also found (1,24)= 5.630, p<0.05), due to a greater increase in c-Fos expression after exposure to a novel object in wt males than Tfm males (Figure 4c). No other significant main effects of genotype or interactions were found for the brain regions examined.
Figure 4.
The number of c-Fos positive cells per mm2 in wt and Tfm male rats at baseline or after exposure to an open field with a novel object. Exposure to a novel object induced a significant increase in the number of cFos-ir neurons in DG, MPOA and MePD but the magnitude of the cFos response in these brain regions varied depending on genotype. Tfm males displayed fewer c-Fos positive cells in the DG and MePD, and more c-Fos positive cells in the MPOA, than did wt males. Tfm males also showed fewer c-fos immunoreactive cells than wt males in the dentate gyrus at baseline. * indicates p< 0.05.
Experiment 5: The role of ARs in PPI and ASR
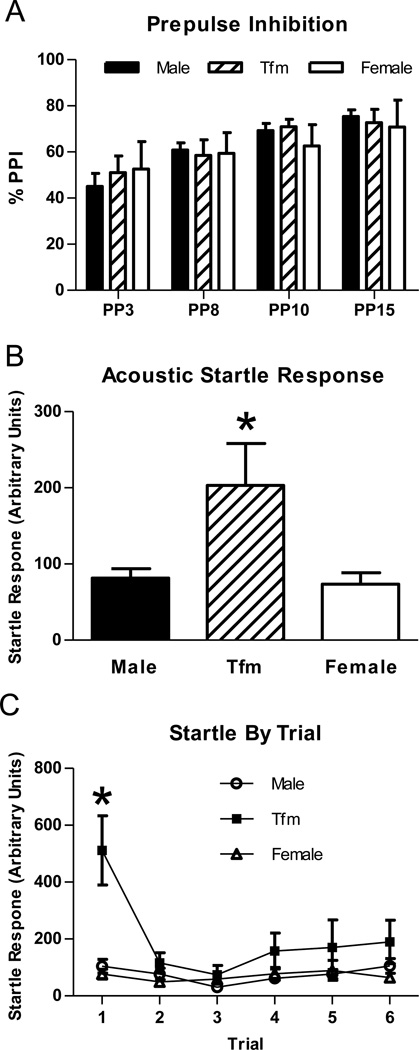
A mixed design repeated measures ANOVA for PPI revealed the expected effect of prepulse intensity (F(3,108)= 3.793, p<0.01; Figure 5a) in which PPI increased along with an increase in intensity of the prepulse, with no main effect of genotype or interaction between genotype and prepulse intensity. Analysis of ASR (response to the pulse in the absence of a prepulse) revealed a significant main effect of genotype (F(2,27)= 4.668, p<0.01). Post hoc comparisons revealed that Tfm males showed an increased ASR compared to both wt males and females (ps<0.05; Figure 5b), which was almost entirely due to a much greater startle in response to the first presentation of the auditory signal (ps<0.001; Figure 5c).
Figure 5.
Prepulse inhibition (PPI; A), acoustic startle response (B), and acoustic startle response by trial in gonadally intact wt male, Tfm male, and wt female rats. There were no group differences in PPI, but ASR was greater in Tfm males than either wt males or females, primarily due to differences in startle response to the first pulse. * indicates p<0.05 (A) or p<0.001 (B) compared to wt males and females.
Discussion
Although it would appear to be a very mild stressor, exposure to an open field with a novel object significantly increased corticosterone levels to within the range of levels previously reported in rats exposed to tests of anxiety (Rees et al., 2006; Green et al., 2007). Consistent with other studies, we also report sex differences in the corticosterone response to a stressful situation (Handa et al., 1994a; Seale at al., 2005), with wt female rats showing a greater corticosterone response than wt males after exposure to the stressor. A novel finding in our study is that Tfm male rats, like wt females, showed a greater peak corticosterone response (20 min) than wt males, indicating that they are feminine in this respect. This difference between Tfm and wt males in circulating corticosterone 20 minutes after exposure to the novel object test observed in experiment 1 was replicated in measures at the 20 minute mark in experiment 2, indicating that the difference in peak corticiosterone levels induced by exposure to a novel object in wt and Tfm males reflects a reliable difference in response to a mild stressor. Since Tfm males had elevated T levels compared to wt males, and T generally has an inhibitory effect on corticosterone release (Handa et al., 1994b; Seale et al., 2004; Mitsushima et al., 2008), the difference between Tfm and wt males in corticosterone response cannot be readily attributed to differences in circulating T. Rather, these findings suggest that AR normally plays a role in dampening the corticosterone response to stress in male rats.
While there was also a difference in corticosterone levels between wt and Tfm males at 120 minutes after exposure to a novel object, the similar levels at 40 and 60 minutes after exposure indicates that any differences between the two types of males in corticosterone recovery are either subtle or more complicated than simply faster versus slower recovery. We previously found that Tfm male mice also show a heightened corticosterone response to a novel object compared to wt males (Zuloaga et al., 2008b), supporting the notion that AR may play a role in adrenal steroid regulation across rodents, and therefore possibly in other mammals as well. Taken together, these results suggest that humans with complete androgen insensitivity might display an exaggerated adrenal response to stress compared to control males.
In the novel object test, Tfm male rats showed increased indices of anxiety with decreased exploration of the novel object compared to both wt males and females. In the open field test, Tfm males also demonstrated a slight increase in anxiety-related behavior, showing a greater amount of time spent grooming compared to wt males, although exploration of the center area of the open field did not significantly differ between groups. These results support previous research indicating involvement of ARs in the regulation of anxiety-related behavior in rodents (Edinger and Frye, 2006; Fernandez-Guasti and Martinez-Mota, 2005), including our previous findings in Tfm mice (Zuloaga et al., 2008b). As AR is dysfunctional in Tfm rats throughout life, the present results cannot indicate whether these effects are due to differences in AR activation in adulthood or development. Other evidence indicates that neonatal gonadectomy results in an equivalent decrease in anxiety-related behaviors in wt and Tfm males, suggesting that differences reported here may involve adult activation of AR (Zuloaga et al., 2011).
We previously found that Tfm rats that were sham-gonadectomized at birth, then gonadectomized and provided T replacement in adulthood showed comparable indices of anxiety in the open field and novel object tests compared to similarly treated wt male rats (Zuloaga et al., 2011). It is possible that exposure to a surgical procedure (gonadectomy) two weeks prior to behavior testing and/or neonatal anesthesia may have affected anxiety-related behaviors in such a way as to eliminate differences between these groups. Neonatal anesthesia has previously been demonstrated to affect rat hippocampal morphology and cognitive behavior (Rothstein et al., 2008; Nunez et al., 2000).
Tfm males, like wt males, were also consistently less active than wt females as assessed by the number of rearings and grid crossings in the open field and novel object tests, consistent with reports of sex differences in motor activity (e.g., ambulation and rearing), with females more active (Archer, 1975; Masur et al., 1980; Slob et al., 1981; Seliger, 1977; Lucion et al., 1996). Activity measures did not differ between Tfm and wt males, suggesting that sex differences in activity in rats do not depend on AR and may be mediated via differential activation of ERs. Since ambulation was generally greater in wt females than Tfm males, this difference in activity may have contributed to differences between those two groups in anxiety-related behaviors, particularly the number of entries into the center of the open field and visits to the novel object. Therefore, it is possible that the difference between females and Tfm males reflects, in part, a difference in activity, a confound inherent to activity based tests of rodent anxiety. However, since ambulation was similar in wt and Tfm males, activity differences are not likely to have contributed to their differences in anxiety-related behavior in the novel object test.
Although an increased corticosterone response to stress in females compared to males is not associated with increased indices of anxiety, increased corticosterone responses within a gender have been associated with elevated anxiety. In both male and female rats, pharmacological stimulation of ERβ reduces anxiety as well as corticosterone levels in the EPM (Lund et al., 2005; Lund et al., 2004; Lund et al., 2006). Furthermore, male rats bred for high anxiety also show increases in corticosterone compared to rats showing normal anxiety behaviors (Landgraf et al., 1999). Therefore, elevated anxiety-related behaviors and corticosterone responses in Tfm males compared to wt males are likely related.
As expected, there was greater c-Fos activation in all brain regions examined following exposure to the novel object compared to baseline. However, c-Fos expression by such exposure differed between wt and Tfm males in some brain areas (DG, MPOA, MePD) but not others (LSV, PVN, BSTMPM, VMH, BLA, MEA, CA1). In brain regions in which c-Fos activation differed between types of males, there were examples of both increased (MPOA) and decreased (DG, MePD) immunoreactivity in Tfm males, suggesting that AR modulates neural activity differently in discrete brain regions, which may regulate anxiety-related behaviors and stress hormone responses.
Fewer cells displayed activation of c-Fos in the DG after novel object exposure in Tfm males than in wt males. The valence of this response matches that in another study, in which rats bred for high anxiety showed decreased c-Fos activation in the DG compared to those bred for low anxiety (Salome et al., 2004). The hippocampal formation is an androgen responsive brain region, with abundant ARs particularly in CA1 (Xiao and Jordan, 2002) and lower levels in the DG (Brannvall et al., 2005; Tabori et al., 2005), which plays a role in anxiety-related behavior (Edinger and Frye, 2006, Eren-Koçak et al., 2011). The hippocampus also regulates HPA axis feedback via inhibitory projections to the PVN, where increased c-Fos expression correlates with increased HPA axis activity and anxiety (Sink et al, 2011; Salome et al., 2004). While this could be a pathway through which androgens inhibit anxiety and HPA axis activity, we found no differences in c-Fos activation of the PVN.
Exposure to a novel object induced a greater c-Fos response in neurons of the MPOA of Tfm males than wt males. This increase is consistent with previous findings demonstrating a positive correlation between c-Fos activation of the MPOA and anxiety-related behavior in an open field and the elevated plus maze (Salome et al., 2004). The MPOA contains abundant ARs (Handa et al., 1996; Simerly et al., 1990) and has been identified as an androgen responsive brain region involved in sexual behaviors as well as the regulation of the HPA axis (McCormick et al., 2002; Viau and Meaney, 1996). Therefore, differences in AR function within the MPOA may underlie both the difference in c-Fos activation in this brain region and the difference in HPA axis activity between wt and Tfm male rats.
Fewer c-Fos-ir cells were seen in Tfm males than wt males in the MePD, the volume of which is less in Tfm male rats compared to wt males (Morris et al., 2005). Given that our counts of c-Fos positive neurons are per mm2, and thus independent of changes in volume, it is likely that the decreased number of c-Fos positive neurons in the MePD of Tfm male rats after exposure to a novel object reflects a true decrement in c-Fos activation in response to a mild stressor. The MePD receives olfactory and pheromonal information and is important for some aspects of male sexual behavior (Lehman et al., 1983; Swann et al., 2001). However, the MePD projects to two other hormone and stress responsive regions (BNST, MPOA) and through these projections may regulate anxiety-related behaviors (Coolen and Wood, 1998; Gomez and Newman, 1992). In areas where we detected no change in c-Fos activation between Tfm and wt males, it remains possible that there were actual differences in the activation of specific cell types within these regions yet net cell activation was the same. Differences between Tfm and wt males, particularly in HPA axis activation, could also result from AR-mediated changes in pituitary activity. Cre-lox generated AR knockout male mice show elevations in ACTH and corticosterone that appear to be caused by impaired negative feedback caused by decreased pituitary glucocorticoid receptors (Miyamoto et al., 2007).
PPI did not differ between groups, indicating that the AR plays a minimal role in this measure of sensorimotor gating in rats. These findings are in line with other studies showing that administration of DHT does not affect PPI in male and female rats gonadectomized in adulthood (Turvin et al., 2007; van den Buuse and Eikelis, 2001). Since AR deficiency is present from ontogeny in Tfm male rats, these findings further suggest that activation of ARs during development has negligible effect on PPI, which is in agreement with our finding that neonatal gonadectomy had similar effects on PPI in wt and Tfm male rats (Zuloaga et al., 2011). Thus ERs likely mediate T effects on PPI (van den Buuse and Eikelis, 2001).
Although PPI did not differ across groups, ASR was enhanced in Tfm males compared to wt males and females in this test, suggesting a role for ARs in this behavior. Elevated ASR in Tfm males is in line with studies showing administration of T (Turvin et al., 2007; Toufexis et al., 2005) and DHT can attenuate ASR (Turvin et al., 2007). Elevated ASR in Tfm rats likely results from decreased activation of ARs in adulthood rather than during development (Zuloaga et al., 2011). Increased ASR in Tfm male rats is consistent with our findings of increased anxiety-related behaviors in Tfm males, since elevations in ASR have been suggested to reflect anxiety in mice and rats (Walker and Davis, 1997; Crawley et al., 1997; Belzung et al., 2000; Hode et al., 2000). Therefore, increased ASR in Tfm male rats likely also reflects increased anxiety-related behavior. Importantly, differences in this test cannot be attributed to putative differences in activity. The difference in ASR is largely due to an elevated response in Tfm males following the first acoustic pulse, indicating that Tfm males can acclimate to this response.
Aromatase activity is also decreased in several areas of the brain in adult Tfm rats compared to wt males (Rosseli et al., 1987), so we cannot discount the possibility that differences between these groups result from decreased activation of ERs in Tfm males. Thus our findings that AR plays a role in regulating anxiety-related behavior and corticosterone response should not be taken as evidence that ERs play no role. It is likely that both classes of receptors are involved normally.
In conclusion, compared to wt males, Tfm males showed increased peak corticosterone responses and differences in immediate-early gene activation in select brain regions (DG, MPOA, and MePD) following exposure to an open field with a novel object, which is consistent with greater anxiety in Tfm males than wt males. These outcomes largely replicate findings in the Tfm mouse (Zuloaga et al, 2008b), suggesting that AR may play this role in mammals generally. Furthermore, Tfm and wt males differed in the extent of c-Fos activation in several brain regions known both to respond to androgenic hormones and to be involved in anxiety-related behaviors and the HPA axis. Together, these data indicate a role of brain AR in the regulation of the HPA axis, anxiety-related behavior, and activation of discrete brain regions that may underlie these functions in rats.
Highlights.
> Tfm rats show HPA axis hyper-activity. > Also show elevated indices of anxiety. > Sensorimotor gating is normal in Tfm rats.
Acknowledgements
Special thanks to Diane Redenius, Heather Malinowski, Sandra Troxell, Peer Karmaus, Cindy Knaff, Ernestine Mitchell, Kentaro Oki, and Susan Beyerlein for their expert technical assistance. This work was supported by NIH NS28421, NIH NS045195 and NIH predoctoral NRSA Grant F31-MH78273.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison JE. Testicular Feminization. J Okla State Med Assoc. 1965;58:378–380. [PubMed] [Google Scholar]
- Amore M, Scarlatti F, Quarta AL, Tagariello P. Partial androgen deficiency, depression and testosterone treatment in aging men. Aging Clin Exp Res. 2009;21(1):1–8. doi: 10.1007/BF03324891. [DOI] [PubMed] [Google Scholar]
- Archer J. Rodent sex differences in emotional and related behavior. Behavioral Biology. 1975;14:451–479. doi: 10.1016/s0091-6773(75)90636-7. [DOI] [PubMed] [Google Scholar]
- Arpels JC. The female brain hypoestrogenic continuum from the premenstrual syndrome to menopause. A hypothesis and review of supporting data. J Reprod Med. 1996;41(9):633–639. [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Crestani F. Flumazenil induces benzodiazepine partial agonist-like effects in BALB/c but not C57BL/6 mice. Psychopharmacology. 2000;148:24–32. doi: 10.1007/s002130050021. [DOI] [PubMed] [Google Scholar]
- Bing O, Heilig M, Kakoulidis P, Sundblad C, Wiklund L, Eriksson E. High doses of testosterone increase anticonflict behaviour in rat. European Neuropsychopharmacology. 1998;8:321–323. doi: 10.1016/s0924-977x(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm. Behav. 1993;27(4):568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Brännvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. European Journal of Neuroscience. 2005;21(4):871–878. doi: 10.1111/j.1460-9568.2005.03942.x. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. Journal of Comparative Neurology. 1998;399(2):189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Ritchie EC. Testosterone replacement therapy for anxiety. American Journal of Psychiatry. 2000;157(11):1884. doi: 10.1176/appi.ajp.157.11.1884. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology Berlin. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Delini-Stula A, Hunn C. Differential effects of anxiolytics and beta-receptor blockingdrugs on novelty-oriented ("neophobic") behavior in the rat. Pharmacopsychiatry. 1988;21(4):186–191. doi: 10.1055/s-2007-1014673. [DOI] [PubMed] [Google Scholar]
- de Olmos J, Hardy H, Heimer L. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. Journal of Comparative Neurology. 1978;181:213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Differential projections of the anterior and posterior regions of the medial amygdaloid nucleus in the Syrian hamster. Journal of Comparative Neurology. 1992;317(2):195–218. doi: 10.1002/cne.903170208. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone's analgesic, anxiolytic, and cognitiveenhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behavioral Neuroscience. 2004;118(6):1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30(5):418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Hormones and Behavior. 2006;50(2):216–222. doi: 10.1016/j.yhbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Eren-Koçak E, Turner CA, Watson SJ, Akil H. Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biological Psychiatry. 2011;69(6):534–540. doi: 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen SI, Vahlberg TJ, Isoaho RE, Kivelä SL, Irjala KM. Associations of sex hormone concentrations with health and life satisfaction in elderly men. Endocrine Practice. 2007;13(7):743–749. doi: 10.4158/EP.13.7.743. [DOI] [PubMed] [Google Scholar]
- Fernandez R, Collado P, Garcia Doval S, Garcia-Falgueras A, Guillamon A, Pasaro E. A molecular method for classifying the genotypes obtained in a breeding colony from testicular feminized (Tfm) rats. Hormone and Metabolic Research. 2003;35(3):197–200. doi: 10.1055/s-2003-39081. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Martínez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30(8):762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen Administration to Aged Male Mice Increases Anti-Anxiety Behavior and Enhances Cognitive Performance. Neuropsychopharmacology. 2008;33(5):1049–1061. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Posttraining androgens' enhancement of cognitive performance is temporally distinct from androgens' increases in affective behavior. Cognitive, Affective, and Behavioral Neuroscience. 2001;1(2):172–182. doi: 10.3758/cabn.1.2.172. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Inglese S, Lombardi I, Pieri M, Bernardi F, Genazzani AD, Rovati L, Luisi M. Long-term low-dose dehydroepiandrosterone replacement therapy in aging males with partial androgen deficiency. Aging Male. 2004;7(2):133–143. doi: 10.1080/13685530412331284669. [DOI] [PubMed] [Google Scholar]
- Gogos A, van den Buuse M. Castration reduces the effect of serotonin-1A receptor stimulation on prepulse inhibition in rats. Behavioral Neuroscience. 2003;117(6):1407–1415. doi: 10.1037/0735-7044.117.6.1407. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Developmental Psychopathology. 2003;15(2):431–449. [PubMed] [Google Scholar]
- Green MK, Barbieri EV, Brown BD, Chen KW, Devine DP. Roles of the bed nucleus of stria terminalis and of the amygdala in N/OFQ-mediated anxiety and HPA axis activation. Neuropeptides. 2007;41(6):399–410. doi: 10.1016/j.npep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior. 1994a;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiology and Behavior. 1994b;55(1):117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Kerr JE, DonCarlos LL, McGivern RF, Hejna G. Hormonal regulation of androgen receptor messenger RNA in the medial preoptic area of the male rat. Brain Research: Molecular Brain Research. 1996;39(1–2):57–67. doi: 10.1016/0169-328x(95)00353-t. [DOI] [PubMed] [Google Scholar]
- Hode Y, Ratomponirina C, Gobaille S, Maitre M, Kopp C, Misslin R. Hypoexpression of benzodiazepine receptors in the amygdala of neophobic BALB/c mice compared to C57BL/6 mice. Pharmacology, Biochemistry, and Behavior. 2000;65:35–38. doi: 10.1016/s0091-3057(99)00131-8. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiology and Behavior. 2005;84(1):157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD. Pre- but not post-menopausal female CBA/CaJ mice show less prepulse inhibition than male mice of the same age. Behavioural Brain Research. 2007;185(2):76–81. doi: 10.1016/j.bbr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, Schwartz MP, Gonzenbach S, Rotrosen JP, Duncan EJ. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41(3):401–406. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Kaminetsky JC. Benefits of a new testosterone gel formulation for hypogonadal men. Clinical Cornerstone. 2005;7 Suppl 4:S8–S12. doi: 10.1016/s1098-3597(05)80091-2. [DOI] [PubMed] [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiology and Behavior. 1998;64(5):625–628. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kumano H. Hormone replacement Up-to-date. The effects of androgen replacement therapy on brain function. Clinical Calcium. 2007;17(9):1378–1383. [PubMed] [Google Scholar]
- Landgraf R, Wigger A, Holsboer F, Neumann ID. Hyper-reactive hypothalamo-pituitary adrenocortical axis in rats bred for high anxiety-related behaviour. Journal of Neuroendocrinology. 1999;11(6):405–407. doi: 10.1046/j.1365-2826.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Feldon J. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behavioural Brain Research. 1999;104(1–2):113–117. doi: 10.1016/s0166-4328(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Powers JB, Winans SS. Stria terminalis lesions alter the temporal pattern of copulatory behavior in the male golden hamster. Behavioural Brain Research. 1983;8(1):109–128. doi: 10.1016/0166-4328(83)90174-2. [DOI] [PubMed] [Google Scholar]
- Lucion AB, Charchat H, Pereira GA, Rasia-Filho AA. Influence of early postnatal gonadal hormones on anxiety in adult male rats. Physiology and Behavior. 1996;60(6):1419–1423. doi: 10.1016/s0031-9384(96)00246-6. [DOI] [PubMed] [Google Scholar]
- Lund BC, Bever-Stille KA, Perry PJ. Testosterone and andropause: the feasibility of testosterone replacement therapy in elderly men. Pharmacotherapy. 1999;19(8):951–956. doi: 10.1592/phco.19.11.951.31574. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. Journal of Neuroscience. 2006;26(5):1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neuroscience Letters. 2004;365(1):43–47. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Masur J, Schutz MT, Boerngen R. Gender differences in open-field behavior as a function of age. Developmental Psychobiology. 1980;13:107–110. doi: 10.1002/dev.420130202. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5(4):235–247. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Takase K, Funabashi T, Kimura F. Gonadal steroid hormones maintain the stress-induced acetylcholine release in the hippocampus: simultaneous measurements of the extracellular acetylcholine and serum corticosterone levels in the same subjects. Endocrinology. 2008;149(2):802–811. doi: 10.1210/en.2007-0827. [DOI] [PubMed] [Google Scholar]
- Miyamoto J, Matsumoto T, Shiina H, Inoue K, Takada I, Ito S, Itoh J, Minematsu T, Sato T, Yanase T, Nawata H, Osamura YR, Kato S. The pituitary function of androgen receptor constitutes a glucocorticoid production circuit. Molecular and Cellular Biology. 2007;27(13):4807–4814. doi: 10.1128/MCB.02039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. Journal of Comparative Neurology. 2005;487(2):217–226. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Koss WA, Juraska JM. Hippocampal anatomy and water maze performance are affected by neonatal cryoanesthesia in rats of both sexes. Horm Behav. 2000;37(3):169–178. doi: 10.1006/hbeh.2000.1572. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. Journal of Neuroscience. 2001;21(1):305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees SL, Steiner M, Fleming AS. Early deprivation, but not maternal separation, attenuates rise in corticosterone levels after exposure to a novel environment in both juvenile and adult female rats. Behav Brain Res. 2006;175(2):383–391. doi: 10.1016/j.bbr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Salisbury RL, Resko JA. Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology. 1987;121:2205–2210. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- Rothstein S, Simkins T, Nuñez JL. Response to neonatal anesthesia: Effect of sex on anatomical and behavioral outcome. Neuroscience. 2008;152(4):959–969. doi: 10.1016/j.neuroscience.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé N, Salchner P, Viltart O, Sequeira H, Wigger A, Landgraf R, Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential Fos expression in HAB and LAB rats. Biological Psychiatry. 2004;55(7):715–723. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. Journal of Neuroendocrinology. 2004;16(12):989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- Seidman SN, Orr G, Raviv G, Levi R, Roose SP, Kravitz E, Amiaz R, Weiser M. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216–221. doi: 10.1097/JCP.0b013e3181a39137. [DOI] [PubMed] [Google Scholar]
- Seliger DL. Effects of age, sex, and brightness of field on open-field behaviors of rats. Perceptual and Motor Skills. 1977;45(3 Pt 2):1059–1067. doi: 10.2466/pms.1977.45.3f.1059. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. Journal of Comparative Neurology. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. Journal of Neuroscience. 2011;31(5):1802–1810. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slob AK, Bogers H, van Stolk MA. Effects of gonadectomy and exogenous gonadal steroids on sex differences in open field behaviour of adult rats. Behavioural Brain Research. 1981;2(3):347–362. doi: 10.1016/0166-4328(81)90017-6. [DOI] [PubMed] [Google Scholar]
- Swann J, Rahaman F, Bijak T, Fiber J. The main olfactory system mediatespheromone-induced fos expression in the extended amygdala and preoptic area of the male Syrian hamster. Neuroscience. 2001;105(3):695–706. doi: 10.1016/s0306-4522(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Auerbach P, Monroe SM, Hartston H, Geyer MA, Braff DL. Men are more inhibited than women by weak prepulses. Biological Psychiatry. 1993;34(4):253–260. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Bakshi VP, Geyer MA. An animal model of sensorimotor gating deficits in schizophrenia predicts antipsychotic drug action. In: Csernansky JG, editor. Antipsychotics. Berlin: Springer; 1996. pp. 289–312. [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biological Psychiatry. 1997;41(4):452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130(1):151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Toufexis D, Davis C, Hammond A, Davis M. Sex differences in hormonal modulation of anxiety measured with light-enhanced startle: possible role for arginine vasopressin in the male. Journal of Neuroscience. 2005;25(39):9010–9016. doi: 10.1523/JNEUROSCI.0127-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvin JC, Messer WS, Jr, Kritzer MF. On again, off again effects of gonadectomy on the acoustic startle reflex in adult male rats. Physiology and Behavior. 2007;90(2–3):473–482. doi: 10.1016/j.physbeh.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vague J. Testicular feminization syndrome. An experimental model for the study of hormone action on sexual behavior. Horm. Res. 1983;18:62–68. doi: 10.1159/000179779. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Eikelis N. Estrogen increases prepulse inhibition of acoustic startle in rats. European Journal of Pharmacology. 2001;425(1):33–41. doi: 10.1016/s0014-2999(01)01139-6. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. Journal of Neuroscience. 1996;16(5):1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30(7):1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biological Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jordan CL. Sex differences, laterality, and hormonal regulation of androgen receptor immunoreactivity in rat hippocampus. Hormones and Behavior. 2002;42(3):327–336. doi: 10.1006/hbeh.2002.1822. [DOI] [PubMed] [Google Scholar]
- Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. Journal of Biol Chem. 1990;265(15):8893–8900. [PubMed] [Google Scholar]
- Yazici K, Pata O, Yazici A, Aktas A, Tot S, Kanik A. The effects of hormone replacement therapy in menopause on symptoms of anxiety and depression. Turkish Journal of Psychiatry. 2003;14:101–105. [PubMed] [Google Scholar]
- Zuloaga DG, Jordan CL, Breedlove SM. The organizational role of testicular hormones and the androgen receptor in anxiety-related behaviors and sensorimotor gating in rats. Endocrinology. 2011;152(4):1572–1581. doi: 10.1210/en.2010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm. Behav. 2008b;54(5):758–766. doi: 10.1016/j.yhbeh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: What we've learned from the testicular feminization mutation. Hormones and Behavior. 2008a;53(5):613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]