Abstract
The hydrophobic effect—the free-energetically favorable association of non-polar solutes in water—makes a dominant contribution to binding of many systems of ligands and proteins. The objective of this study was to examine the hydrophobic effect in biomolecular recognition using two chemically different, but structurally similar hydrophobic groups—aliphatic hydrocarbons and aliphatic fluorocarbons—and to determine whether the hydrophobicity of the two groups could be distinguished by thermodynamic and biostructural analysis. This paper uses isothermal titration calorimetry (ITC) to examine the thermodynamics of binding of benzenesulfonamides substituted in the para position with alkyl and fluoroalkyl chains (H2NSO2C6H4-CONHCH2(CX2)nCX3, n = 0–4, X = H, F) to human carbonic anhydrase II (HCA II). Both alkyl and fluoroalkyl substituents contribute favorably to the enthalpy and the entropy of binding; these contributions increase as the length of chain of the hydrophobic substituent increases. Crystallography of the protein-ligand complexes indicates that the benzenesulfonamide groups of all ligands examined bind with similar geometry, that the tail groups associate with the hydrophobic wall of HCA II (which is made up of the side chains of residues Phe131, Val135, Pro202, and Leu204), and that the structure of the protein is indistinguishable for all but one of the complexes (the longest member of the fluoroalkyl series). Analysis of the thermodynamics of binding as a function of structure is compatible with the hypothesis that hydrophobic binding of both alkyl and fluoroalkyl chains to hydrophobic surface of carbonic anhydrase is due primarily to the release of non-optimally hydrogen-bonded water molecules that hydrate the binding cavity (including the hydrophobic wall) of HCA II and to the release of water molecules that surround the hydrophobic chain of the ligands. This study defines the balance of enthalpic and entropic contributions to the hydrophobic effect in this representative system of protein and ligand: hydrophobic interactions, here, seem to comprise approximately equal contributions from enthalpy (plausibly from strengthening networks among molecules of water hydrogen bonds) and entropy (from release of water from configurationally restricted positions).
Introduction
Hydrophobic Interactions in Protein-Ligand Binding
Hydrophobic interactions—the free energetically favorable aggregation of non-polar molecules in aqueous media—are centrally important in biology because they dominate the folding of proteins, the formation of lipid bilayers, and the association of proteins and ligands.1–3 The classical concept of hydrophobic interactions—which we attribute to Kauzmann and Tanford (KT)—predicts that i) water near the surface of hydrophobic groups is more (or, perhaps, just differently) structured than bulk water, and that ii) entropy dominates the favorable free energy of hydrophobic interactions because association of two non-polar surfaces causes the release of structured molecules of water near non-polar surfaces.1,2 The molecular basis of hydrophobic interactions in protein-ligand association is more complicated than this classical description, and still incompletely understood.4–6 The distinction between hydrophobic effects when different types of groups—aliphatic and aromatic hydrocarbons, or fluorocarbons—interact within a protein-ligand complex is also not clear. This lack of understanding (probably) contributes to the present difficulty in designing ligands that associate tightly with proteins.
Aliphatic Hydrocarbons and Fluorocarbons in Biomolecular Recognition
Both aliphatic hydrocarbons (RH) and aliphatic fluorocarbons (RF) are hydrophobic, in that they are poorly soluble in water,7 but the thermodynamic basis of this hydrophobicity—at least in the context of protein-ligand interactions—is poorly characterized. In drug discovery, replacement of hydrocarbon groups by fluorocarbon groups has been used to modify solubility and basicity, to test for hydrogen bonding interactions, and to improve the metabolic stability, binding affinity, and bioavailability of several compounds.8 Incorporation of fluorocarbons into proteins and peptides results in the stabilization of folded proteins and promotion of self-assembly of alpha-helical peptides into coiled coils.9,10 Resistance of these structures to thermal denaturation suggests that fluorinated analogues are more stable than hydrocarbons, although it remains unclear whether this effect is due to increased hydrophobic surface area alone or to a difference in the character of hydrophobicity.
The results of our own studies involving i) binding of ligands modified with RH and RF tails to bovine carbonic anhydrase (BCA)11 and ii) denaturation of BCA modified with a series of RH and CF3 substituents in the presence of sodium dodecyl sulfate12 suggest, however, that the free energy of interaction of RH and RF with hydrophobic surfaces can be rationalized entirely or predominantly based on the amount of solvent-accessible surface area (CF3CONH groups are 0.05–0.07 kcal mol−1 more hydrophobic than RHCONH groups with the same surface area). (A previous paper and relevant reviews summarize the background on the hydrophobic effect due to RF).12,13
The Thermodynamics of Association of Series of Ligands Presenting RH and RF Groups with Human Carbonic Anhydrase
The current study uses isothermal titration calorimetry to measure the values of the free energy (ΔG°b), enthalpy (ΔH°b), and entropy (−T S°b) for the binding to human carbonic anhydrase II (HCA II, EC 4.2.1.1) of two series of benzenesulfonamide ligands (H2NSO2C6H4CONHCH2RH/F): in one series, the substituents in the 4-position of benzenesulfonamide are RH groups of increasing length, and in the second series, the substituents are RF groups of increasing length. To compare the two series, we estimate the incremental values of the thermodynamic parameters of binding (ΔΔG°b, Δ ΔH°b, and −TΔΔS°b; defined as a difference of thermodynamics of binding for two ligands in the same series and obtained as a slope of ΔJ°b (where J = G, H, S) vs. chain length or surface area) based on measurements of i) the solvent-accessible surface area, and ii) the molecular volume of the RH and RF groups in the crystal structures of the protein-ligand complexes. We determined that values of ΔΔG°b are indistinguishable within statistical uncertainty on the basis of ligand solvent-accessible surface area (for RH ΔΔG°b = −12 ± 1 cal mol−1 Å−2; for RF ΔΔG°b = −14 ± 1 cal mol−1 Å−2), and on the basis of the volume of the ligand (for RH ΔΔG°b = −20 ± 2 cal mol−1 Å−3; for RF ΔΔG°b = −20 ± 2 cal mol−1 Å−3) (Table 1). Both alkyl and fluoroalkyl groups contribute to ΔΔG°b through favorable values of both ΔΔH°b and −TΔΔS°b. The magnitude of ΔΔH°b is indistinguishable (within experimental error) for alkyl and fluoroalkyl groups (for alkyls ΔΔH°b = −5 ± 1 cal mol−1 Å−2 and for fluoroalkyls ΔΔH°b = −7 ± 1 cal mol−1Å−2). Alkyls and fluoroalkyls also have indistinguishable values of −TΔΔS°b (for alkyls −TΔΔS°b = −7 ± 1 cal mol−1 Å−2, for fluoroalkyls −TΔΔS°b = −7 ± 1 cal mol−1Å−2). Table 1 summarizes the results of this study as a guide to subsequent details.
Table 1.
Comparison of the dependence of the ΔG°b, ΔH°b and −TΔS°b on the chain length (n), ligand surface area (A), ligand solvent-accessible surface area (SASA), and volume of the ligand (V) for alkyl (X = H) and fluoroalkyl (X = F) tails of H2NSO2C6H4CONHCH2(CX2)nCX3 (n = 0–4) to HCA II.
| Hydrocarbon, RH | Fluorocarbon, RF | |
|---|---|---|
| ΔΔG°b/Δna (cal mol−1) | −366 ± 30 | −479 ± 35 |
| ΔΔG°b/ΔAb (cal mol−1Å−2) | −18 ± 1 | −18 ± 1 |
| ΔΔG°b/ΔSASAc (cal mol−1Å−2) | −12 ± 1 | −14 ± 1 |
| ΔΔG°b/ΔVd (cal mol−1Å−3) | −20 ± 2 | −20 ± 2 |
|
| ||
| ΔΔH°b/Δna (cal mol−1) | −150 ± 30 | −247 ± 37 |
| ΔΔH°b/ΔAb (cal mol−1Å−2) | −7 ± 1 | −9 ± 1 |
| ΔΔH°b/ΔSASAc (cal mol−1Å−2) | −5 ± 1 | −7 ± 1 |
| ΔΔH°b/ΔVd (cal mol−1Å−3) | −8 ± 2 | −10 ± 2 |
|
| ||
| −TΔΔS°b/Δna (cal mol-1) | −216 ± 23 | −232 ± 48 |
| −TΔΔS°b/ΔAb (cal mol−1Å−2) | −11 ± 1 | −9 ± 2 |
| −TΔ Δ S°b/ΔSASAc (cal mol−1Å−2) | −7 ± 1 | −7 ± 1 |
| −TΔ Δ S°b/ΔVd (cal mol−1Å−3) | −12 ± 1 | −10 ± 2 |
Obtained from the slope of ΔG°b, ΔH°b or −TΔS°b vs. the number of carbon atoms of the tail (Figures 2–4).
Obtained from the slope of ΔG°b, ΔH°b or −TΔS°b vs. the surface area (A) of the tail in the fully extended conformation (Figures S1–S3 in Supporting Information).
Obtained from the slope of ΔG°b, ΔH°b or −TΔS°b vs. the solvent-accessible surface area (SASA) of the tail in the fully extended conformation (Figures 2–4).
Obtained from the slope of ΔG°b, ΔH°b or −TΔS°b vs. the volume (V) of the tail in the fully extended conformation (Figures S1–S3).
We used para-substituted benzenesulfonamides connected to hydrophobic side-chains— “greasy tails”11—via an amide linkage (H2NSO2C6H4-CONHCH2(CX2)nCX3, where n = 0–4, X = H, F) as ligands for HCA II.

In the system of HCA II and derivatives of benzenesulfonamide, association of the benzenesulfonamide moiety (−HNSO2C6H4-) is essentially invariant to most changes in the structure of the R group in the H2NSO2C6H4R group.14 Binding is determined by association of this −HNSO2C6H4- group to the active site Zn2+ ion, and many biostructural data establish that the geometry of the phenyl group in the active site is highly conserved.15 We have used the extreme simplicity of the system of HCA (and structurally very similar BCA) as the basis for detailed physical-organic studies of binding of ligands to HCA II.14
This paper reports the values of free energy of binding (ΔG°b), enthalpy of binding (ΔH°b), and entropy of binding (−TΔΔS°b) for the two series of ligands (R = RH, RF; H2NSO2C6H4CONHR) measured using isothermal titration calorimetry (ITC). We calculated the incremental changes in free energy, enthalpy, and entropy (ΔΔG°b, ΔΔH°b and −TΔΔS°b) of binding by correlating the thermodynamic parameters for binding with i) the change in solvent-accessible surface area of binding, and ii) the molecular volume of the ligand. These incremental values represent the contribution to binding per unit area of hydrocarbon-hydrocarbon and hydrocarbon-fluorocarbon interaction, and per unit volume of hydrocarbon or fluorocarbon for each series.
Residues Phe131, Val135, Pro202, and Leu204 comprise the so-called “hydrophobic wall” of HCA II.14 We guessed, based on the crystallography of structurally similar ligands, and validated by our own structural studies, that the hydrophobic tails of para-substituted benzenesulfonamides would form van der Waals contacts with the hydrophobic wall. Examination of many ligands for HCA II (and BCA II) has demonstrated that hydrophobic groups—and specifically groups of type H2NSO2C6H4-CONHCH2R, with R = n-alkyl, n-fluoroalkyl—increase the strength of binding as the putative area of contact between the ligand and the protein increases. The system that examines binding of benzenesulfonamide ligands (H2NSO2C6H4-R, where R represents various organic moieties) to carbonic anhydrase is thus an excellent one (the best, we believe, so far developed) for physical-organic studies of the hydrophobic effect in a biologically relevant system comprising protein and ligand.14 It is particularly interpretable since the rigidity of the tertiary structure of CA II makes contributions to binding from protein plasticity negligible.14
The first objective of this study was to explore the relationship between the hydrophobic effect and ligand structure, using two chemically different, but structurally related classes of hydrophobic groups: alkyls (RH) and fluoroalkyls (RF). Our hypothesis was that either: i) The hydrophobic effect is due primarily to exclusion of water from the hydrophobic surfaces of the active site and of the ligand; in which case, the magnitude of the effect for homologous RH and RF tails interacting with the hydrophobic wall of HCA II would be the same when adjusted for differences in the solvent-accessible surface areas of the tails. ii) The hydrophobic effect results from the physical properties of RH and RF (i.e., polarizability, van der Waals interactions, etc.); in which case, the magnitude of the effect might be quite different for the two types of tails, since these properties are different for RH and RF.
Our second objective in comparing RH and RF was to define their relative hydrophobicity in the context of protein-ligand interactions. Incorporation of fluorine into small molecules is an important tactic in designing inhibitors of proteins.16 This strategy is often used to increase binding affinity, to improve membrane permeability, and to augment metabolic stability of pharmaceuticals. There is a widespread belief–based primarily and qualitatively on the hydrophobicity and oleophobicity of Teflon–that RH and RF are fundamentally different in their hydrophobicity.17 In many (in fact, most) of the systems studied, the hydrophobicities for RH and RF are different, but there is no thermodynamic evidence to support this argument.
Improved understanding of hydrophobic interactions involving RH and RF in the context of protein-ligand binding will clarify the nature of the hydrophobic effect in biomolecular recognition, assist advances in the rational design of inhibitors for enzymes, and help to understand the basis of interactions of both fluorocarbons and hydrocarbons with proteins.
Additional background information on the hydrophobic effect is summarized in the Supporting Information (available online at pubs.acs.org).
Experimental Design
Choice of Protein-Ligand System
We choose HCA II as a model system for our physical-organic study for five reasons: i) HCA II is an exceptionally stable and rigid protein. It can be obtained readily by expression in E. Coli (~100 mg L−1 of growth medium) using techniques with which we are familiar, and obtained in the quantities necessary for ITC (~ 0.5 mg per experiment)18 and X-ray crystallography; ii) numerous benzenesulfonamide-containing ligands (H2NSO2C6H4-R, with some constraints on R), bind in the active site of HCA II with a conserved geometry;14 iii) previous X-ray and neutron crystallographic studies have detailed the structure of sulfonamide ligands bound in the active site of HCA;14,19 iv) a single protocol for growing crystals of the protein with different ligands can be used; v) one face of the active site of HCA II is a hydrophobic “shelf” or “wall”, comprising residues Phe131, Val135, Pro202, and Leu204.14 This wall has ~250 Å2 of solvent-accessible hydrophobic surface, and substituents in the para-position of benzenesulfonamide are positioned over that part of the active site (Figure 1).
Figure 1.
Our approach to increasing the binding affinity of para-substituted benzensulfonamide ligands to HCA II employs hydrophobic interactions between hydrophobic tails of ligands and the hydrophobic wall of the protein.
Perturbational Approach for Probing Binding
To probe the interactions of “greasy tails” with the hydrophobic region adjacent to the active site of HCA II, we have followed a perturbational approach: we used the para-carboxamido benzenesulfonamide group to anchor the ligand in the active site of the protein in a well-defined, conserved geometry, and we systematically varied the length of RH and RF chains ((CX2)n, where X = H, F and n = 0–4) in the para-position. Previous structural analyses—and data we present here—indicated that this anchor would preserve the geometry of the arylsulfonamide group, which makes the dominant contribution to thermodynamics of binding in this system (~ 8 kcal mol−1), regardless of the nature of the greasy tail.15 This structural rigidity is essential to the perturbational approach that we use because, as we show below, the difference in the contribution to the thermodynamics of the interaction between methylene or fluoromethylene groups and the hydrophobic wall of HCA is less than 0.5 kcal mol−1.
Since this value is roughly the same as the uncertainty in the measurement of ΔH°b (or −TΔS°b) by ITC for any single ligand, the comparison of any pair of ligands would not be statistically meaningful. The perturbational approach, which in this work includes analyses of five ligands of each series, thus allows us to evaluate the similarities (or differences) between RH and RF tails with greater precision than would be possible for pairs of structurally homologous compounds.
One potential limitation of using the p-carboxamido benzenesulfonamide anchor—rather than the N-methylcarboxamides, for example—could be differences in the values of pKa of the carboxamide group for RH and RF series. Involvement of the amide NH group may contribute favorably to the enthalpy of binding via hydrogen-bonding (NH···H2O···Thr200, Pro201), a hydrogen bond that we observe by X-ray crystallography (Figure S4 in Supporting Information). In our previous studies, indeed, we demonstrated that the pKa of the carboxamide group for the RF series is lower than that of the RH series, and the value of ΔG°b of the RF tails were more favorable than that of the RH tails.11 In that work, however, we also measured the values of ΔΔG°b for both series of N-methylcarboxamides and determined that the difference in the pKa of the carboxamide group did not influence the values of ΔΔG°b for either the RH or the RF series. We also show here that, although the NMR shifts of the carboxamide protons of RH and RF are different, they are the same across each series, and we infer that the values of ΔΔH°b and −TΔΔS°b reflect contributions from the hydrophobic interactions between RH/F and the hydrophobic wall.
From our previous work with these groups, we anticipated negative values of ΔΔG°b for both RH and RF tails.11 Our objective was to analyze the enthalpic and entropic contributions to this free energy of binding by using ITC, and to correlate these contributions with the structures and physical properties of the molecules.
Results
Synthesis of the Ligands
We prepared benzenesulfonamides with alkyl and fluoroalkyl tails following the previously reported procedures.11
Purification of the Protein
To purify HCA II (E.C. 4.2.1.1, > 95% pure), we followed the procedures reported by Fierke et al., who also kindly provided the plasmids containing the gene for the protein.20–22 Details of this procedure appear in the Supporting Information.
Collection of Data by ITC
Because of the low solubility (< 50 μM) in aqueous buffer of the ligands that had long (n > 2) tails, we expected it to be challenging to conduct ITC experiments, which require that the concentration of molecule in the cell of the calorimeter to be no higher than 103 × Kd, and that the concentration of molecule in the syringe be ~10 times the concentration of the molecule in the cell.18 Placing solutions of ligand in the cell not only set a lower limit on the concentration of ligand needed, but also allowed us to minimize the contribution to the uncertainty in ΔΔH°b from the uncertainty in the concentration of ligand.23 We titrated aliquots of HCA II (20 μM), taken from a single batch, into solutions of each of the 10 ligands (~ 2.0 μM). The concentration of ligands was measured in the presence of maleic acid as an internal standard in DMSO-d6 by 1H NMR. The observed protein:ligand stoichiometry was typically around 0.9 ± 0.02, possibly, due to the adsorption of greasy tail ligands to the plastic tray. Based on these, and other ITC experiments in our group using carbonic anhydrase, we exclude a possibility that HCA II adsorbs to the surface of the glass syringe; if HCA II were to adsorb to the syringe, the protein:ligand stoichiometry would be greater than 1. By assuming that the concentration of active protein was the same in each experiment, we were able to adjust the stoichiometry of protein-ligand binding to n = 1 during analysis of the data. ITC experiments with each ligand were repeated 7–9 times. We report the average values of ΔG°b, ΔH°b and −TΔS°b and estimate their uncertainties as standard deviations (for number of repeated experiments N ≥ 7).
ΔG°b is proportional to the solvent-accessible surface area of the ligand
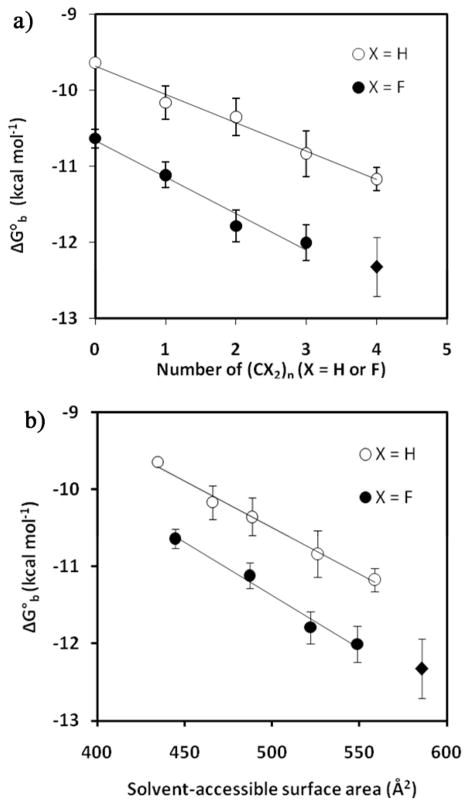
ITC experiments confirmed our previous observation that extending the length of the “greasy tail” resulted in more negative values of ΔG°b and lower values of Kd for dissociation from HCA II (Figure 2). Fluoroalkyls, in general, display higher affinity (~ 1 kcal mol−1) for HCA II than alkyls with the same number of carbon atoms in the “greasy tail”. We rationalize this effect, at least in part, by the fact that CF2 groups have larger hydrophobic surface area than CH2 groups (see below). The electron-withdrawing properties of fluoroalkyl chains, and their absence in alkyl chains, moreover, result in more acidic amidic NH in fluoroalkyl amides (Ar-CONHCH2RF) than in alkyl amides (Ar-CONHCH2RH). This inference of a difference in acidity is supported by chemical shifts in 1H NMR spectra (Figure S5 in Supporting Information). Table 2 summarizes the thermodynamic values for binding of para-substituted benzenesulfonamides to HCA II. Our data are consistent with those reported by Gao et al.11 ΔΔG°b values (defined as ΔG°b/Δn) are −366 ± 30 cal mol−1 per CH2 group and −479 ± 35 cal mol−1 per CF2 group (Gao et al. used fluorescence-based assay in their studies of binding of greasy tail ligands to BCA II and reported values of −560 cal mol−1 per CH2 and −860 cal mol−1 per CF2).
Figure 2.
a) Dependence of ΔG°b for binding of benzenesulfonamide ligands (H2NSO2C6H4-CONHCH2(CX2)nCX3, X = H, F) with alkyl (○) and fluoroalkyl (●) tails on their chain length. The slope of the regression line through the alkyl data is ΔΔG°b = −366 ± 30 cal mol−1 -CH2-−1 (R2 = 0.99), and that for fluoroalkyl data is ΔΔG°b = −479 ± 35 cal mol−1 -CF2-−1 (R2 = 0.97) (N = 7). b) Dependence of ΔG°b for binding of benzenesulfonamide ligands with alkyl (○) and fluoroalkyl (●) tails on their solvent-accessible surface area in the fully extended conformation. The slope of the regression line for the alkyl data is ΔΔG°b = −12 ± 1 cal mol−1 Å−2 (R2 = 0.99), and that for the fluoroalkyl data is ΔΔG°b = −14 ± 1 cal mol−1 Å−2 (R2 = 0.98) (N = 7). The longest fluoroalkyl ligand (◆) is not included in the linear regression, because, in contrast to other fluoroalkyl ligands, it causes a flip in the orientation of Gln136 of HCA II (see the text). The analysis of data with all fluoroalkyl ligands (n = 0–4) gives ΔΔG°b = −418 cal mol−1 -CF2-−1 (R2 = 0.96) and G°b = −12 cal mol−1 A−2 (R2 = 0.98).
Table 2.
Thermodynamic parameters ΔG°b, ΔH°b, −TΔS°b and ΔCp for binding of ligands (H2NSO2C6H4-CONHCH2(CX2)nCX3, n = 0–4, X = H, F) to HCA II. The solvent-accessible surface area (SASA) of ligands was calculated using the Molecular Operating Environment (MOE) suite.
| n | X | SASA (Å2) | Kd (nM) | ΔG°b(kcal mol−1) | ΔH°b (kcal mol−1) | −TΔS°b (kcal mol−1) | ΔCp (cal mol−1 K−1) | PDB ID |
|---|---|---|---|---|---|---|---|---|
| 0 | H | 435 | 86 | −9.6 | −9.2 | −0.4 | −70 ± 8 | 3RYV |
| 1 | H | 466 | 38 | −10.2 | −9.4 | −0.8 | −82 ± 4 | 3RYY |
| 2 | H | 489 | 28 | −10.4 | −9.4 | −1.0 | −88 ± 7 | 3RZ0 |
| 3 | H | 526 | 13 | −10.8 | −9.6 | −1.2 | n.d.a | 3RZ5 |
| 4 | H | 559 | 6.7 | −11.2 | −9.8 | −1.3 | n.d.a | 3RZ8 |
| 0 | F | 445 | 16 | −10.6 | −9.7 | −0.9 | −72 ± 5 | 3RYJ |
| 1 | F | 487 | 7.3 | −11.1 | −10.0 | −1.1 | −80 ± 6 | 3RYX |
| 2 | F | 522 | 2.4 | −11.8 | −10.3 | −1.5 | 91 ± 5 | 3RYZ |
| 3 | F | 549 | 1.8 | −12.0 | −10.5 | −1.6 | n.d.a | 3RZ1 |
| 4 | F | 586 | 1.1 | −12.3 | −10.4 | −1.9 | n.d.a | 3RZ7 |
n.d. not determined
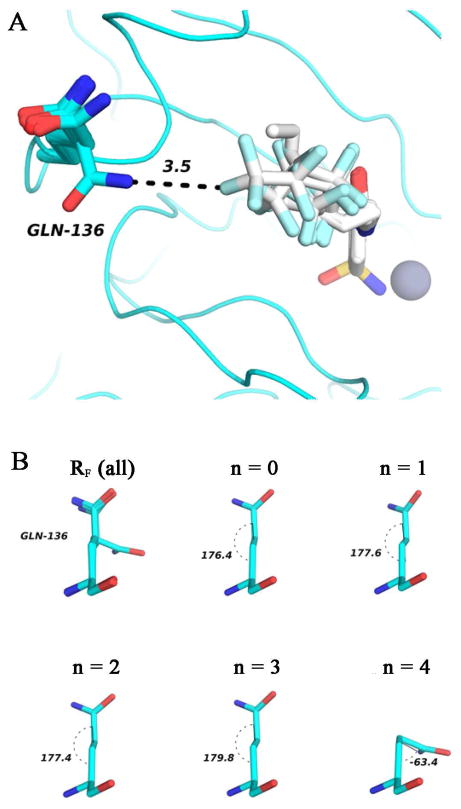
Based on our X-ray crystallographic analysis of HCA II-ligand complexes (see later in this paper), we excluded the data for the longest fluoroalkyl ligand (X = F, n = 4) from the analysis of thermodynamics of interactions for RF (for free energy, enthalpy and entropy of binding; see below). The crystal structure of HCA II in the complex with the fluoroalkyl ligand that contains the longest side-chain (n = 4), in contrast to structures with all other fluoroalkyl and alkyl ligands, shows that Gln136 flips its orientation (the gauche conformation in the case of X = F, n = 4, and the anti conformation in all other cases).
Both series of ligands display a favorable incremental entropy of binding
Figure 3 shows that the free energies of binding for both series of ligands (RH and RF) have a favorable entropic contribution that increases with the length of the hydrophobic tail (for alkyls −TΔΔS°b = −7 ± 1 cal mol−1 Å−2, for fluoroalkyls −TΔΔS°b = −7 ± 1 cal mol−1 Å−2). This contribution could be i) related to changes in the structure of the network of waters that hydrate the ligand and/or the active site of the protein, ii) the result of a change in the conformational degrees of freedom of the tail on binding, or (in principle) iii) the result of changes in the conformational degrees of freedom of amino acid side chains on binding of ligand.
Figure 3.
a) Plots for −TΔS°b versus chain length for alkyl (○) and fluoroalkyl (●) tails of benzensulfonamide ligands (H2NSO2C6H4-CONHCH2(CX2)nCX3, X = H, F). The slope of the regression line through the alkyl data is −TΔΔS°b = −217 ± 23 cal mol−1 -CH2-−1 (R2 = 0.97), and that for fluoroalkyl data is −TΔΔS°b = −232 ± 48 cal mol−1 -CF2-−1 (R2 = 0.94) (N = 7). b) Plots for −TΔS°b versus solvent-accessible surface area for alkyl (○) and fluoroalkyl (●) tails. The slope of the regression line for the alkyl data is −TΔΔS°b = −7 ± 1 cal mol−1 Å−2 (R2 = 0.98), and that for the fluoroalkyl data is −TΔΔS°b = −7 ± 1 cal mol−1 Å−2 (R2 = 0.95) (N = 7). The longest fluoroalkyl ligand (◆) is not included in the trendline, because it causes the conformational change of Gln136 of HCA II (see the text). The analysis of data with all fluoroalkyl ligands (n = 0–4) gives −TΔΔS°b = −234 cal mol−1 -CF2-−1 (R2 = 0.88), −TΔΔS°b = −7 cal mol−1 A−2 (R2 = 0.90).
Both series of ligands also display a favorable incremental enthalpy of binding
Extending the chain length of alkyl and fluoroalkyl tails also contributed to the free energy of binding through a favorable enthalpic term (Figure 4). The slope of enthalpy as a function of chain length (or ligand solvent-accessible surface area) is indistinguishable for alkyl and fluoroalkyl tails (from the alkyl data, ΔΔH°b = −5 ± 1 cal mol−1 Å−2, and from the fluoroalkyl data, ΔΔH°b = −7 ± 1 cal mol−1 Å−2; the uncertainties in these values overlap).
Figure 4.
a) Dependence of ΔH°b for benzenesulfonamide ligands (H2NSO2C6H4-CONHCH2(CX2)nCX3, X = H, F) containing alkyl (○) and fluoroalkyl (●) tails on the length of the chain. The slope of the regression line through the alkyl data is ΔΔH°b = −150 ± 30 cal mol−1 -CH2-−1 (R2 = 0.96), and that for fluoroalkyl data is ΔΔH°b = −247 ± 37 cal mol−1 -CF2-−1 (R2 = 0.95) (N = 7). b) Dependence of ΔH°b for ligands containing alkyl (○) and fluoroalkyl (●) tails on the solvent-accessible surface area. The slope of the regression line for the alkyl data is ΔΔH°b = −5 ± 1 cal mol−1 Å−2 (R2 = 0.99), and that for the fluoroalkyl data is ΔΔH°b = −7 ± 1 cal mol−1 Å−2 (R2 = 0.98) (N = 7). The longest fluoroalkyl ligand (◆) is not included in the trendline, because it causes the conformational change of Gln136 of HCA II (see the text). The analysis of data with all fluoroalkyl ligands (n = 0–4) gives ΔΔH°b = −184 cal mol−1 -CF2-−1 (R2 = 0.80), ΔΔH°b = −5 cal mol−1 A−2 (R2 = 0.81).
Both series have indistinguishable heat capacities
We measured heat capacities (ΔCp) for binding of the first three (n = 0–2) RH and RF ligands to HCA II in the temperature range between 283 K and 303 K. Heat capacities were obtained from the slope of the enthalpy of binding as a function of temperature. ΔCp values for binding of alkyl and fluoroalkyl ligands are indistinguishable (for alkyls −9 ± 1 cal mol−1 K−1 per CH2, for fluoroalkyls −10 ± 1 cal mol−1 K−1 per CF2).
Crystallization of Protein-Ligand Complexes
We grew crystals of HCA II in conditions reported by McKenna and coworkers (1.15 M sodium citrate, 100 mM Tris hydrochloride, pH = 7.8) because under these conditions, crystals of the native protein diffract X-rays to ~1.0 Å resolution.24 We performed soaking experiments using the ligands with short (n = 0 or 1) tails by transferring crystals from their mother liquor to a fresh drop that contained sodium citrate (1.32 M), Tris (100 mM), and ligand (20 – 450 μM). The ligands with n ≥ 2 were insoluble in sodium citrate, which prohibited its use as the medium for soaking experiments.
We expected that the solubility of the ligands would be higher in solutions containing polyethylene glycol (PEG, 30 – 35%) than in sodium citrate, but were unable to grow crystals of HCA II in solutions of PEG. We chose, thus, a solution condition (PEG 1500, 20 %; HEPES 100 mM) that was slightly higher in concentration of PEG than conditions reported previously to crystallize HCA II in the same polymorph as our crystals, and we transferred crystals of native HCA II, grown in sodium citrate, into drops containing PEG and saturated with ligands with longer (n ≥ 2) tails. The strategy was successful in that the resulting crystals diffracted X-rays to 1.5 – 1.8 Å resolution, and the maps of electron density derived from molecular replacement indicated the presence of ligand (Figure S4 in Supporting Information). We refined the crystal structures of each of the ten HCA II-ligand complexes at high resolution (1.83 – 1.05 Å) data (Table 2, Table S1 in Supporting Information).
Crystallography indicates that the structure of the protein is invariant in most of the protein-ligand complexes
To determine whether the thermodynamic trends in binding were the result of structural changes to the protein, we aligned the ten structures and calculated the root-mean-squared deviation (RMSD) for all atoms of the proteins. The average value for RMSD for these structures was 0.091 Å, a result that indicated that the conformation of the protein did not depend on the identity of ligand bound in the active site.
To verify our assumption that the geometry of the ligands in the active site of HCA II was conserved for each complex, we aligned the atoms of the ten HCA II-ligand complexes, and calculated the RMSDs for the atoms of the ligands and the Zn2+ ion (Figure 5). The 10 ligands had the same geometries of binding: the average value of RMSD for the alignment of the heavy atoms of the ligand, the Zn2+ ion, and the heavy atoms of residues His94, His96, His119, Phe131, Thr200 (chosen arbitrarily to allow the three-dimensional alignment) was 0.064 Å, the data that justified our assumption that the carboxybenzenesulfonamide group, the Zn-N bond, and the interaction between the carboxamide group and the protein-bound water at Thr200 were indistinguishable for the ten complexes.
Figure 5. Alignment of the atoms of the ligands.
A) Aligned structures for ten ligands determined by X-ray crystallography appear as ball and stick representations. The Zn2+ cofactor appears as a silver sphere. Individual images of each ligand appear in Figure S6 in Supporting Information.
The structure of HCA II is invariant in nine of ten crystal structures of ligand complexes that we solved, the exception being the structure of HCA II in complex with the longest fluoroalkyl tail (X = F, n = 4, Figure 6). In this case, the side chain of Gln136, which is at the edge of the hydrophobic shelf, flips to contact the terminal -CF3 group of the tail. This conformational flip induces a gauche conformation of the Gln136 side chain (Figure 6B). The number of crystallographically defined water molecules within 4 Å of the –CONH2 group of Gln136, however, did not change: each of the two possible conformations of Gln136 showed four contacts with ordered molecules of water in the crystal structures (Figure S7 in Supporting Information). Crystallography of the protein-ligand complexes verified that the ligands bind to the active site in the same geometry, and that extension of the tails increased the putative surface of contact between the ligand and the protein.
Figure 6. Crystal structures of fluorinated “greasy tails” complexed with HCA II.
A) Superimposition of all ligands and the Gln136 side chain. The distance between Gln136 and one fluorine atom of the ligand (RF, n = 4) appears as a dashed line with its length labeled in Å. B) Conformational analysis for Gln136. Superimposition of ligands (top left) and individual ligands. Gln136 possesses the anti conformation in cases of n = 0–3, while the gauche conformation is observed in the case of n = 4. Values of dihedral angles are labeled.
For the RH series, all of the contacts between the tail and the hydrophobic wall occurred between methylene and methyl groups of the tails and methyl groups of Val135 and Leu204, methylene groups of Pro202, and methine groups of Phe131. This observation validated our first-order analysis of hydrophobic effects in the linear trends in ΔH°b and −TΔS°b across the series. For the RF series, similarly, crystallography validated our first-order analysis of linear trends in ΔH°b and −TΔS°b for the ligands n = 0 – 3, and indicated potential causes (i.e., polar contacts, steric interactions, changes in the hydration of protein, and change in the conformation of Gln136) for deviations from those trends for the ligand n = 4.
Crystallography provides no direct evidence concerning the hydration of the hydrophobic wall or the structure of the network of hydrogen bonds among molecules of water at the surface. It is interesting, however, to analyze both the regions of the active site in which crystallographic waters appear, and those in which they do not. We analyzed three recent structures of HCA II that were solved by high resolution (~1.0 Å) X-ray diffraction and by neutron diffraction.25 These structures show that more than 90% of the observable (crystallographic) waters are in indistinguishable positions. Interestingly, however, no crystallographically identifiable molecules of water appear within 3 Å of the hydrophobic wall. This observation provides no positive support for the idea of structured water near a hydrophobic surface in HCA II (although it also does not demonstrate the absence of such structure).
Discussion
Negative values of ΔΔG°b (ΔG°b, n+1 − ΔG°b, n = ΔG°CX2, protein − ΔG°CX2, solv; ΔG°b, n+1 and ΔG°b, n are free energies of binding for greasy tails with the chain length of n+1 and n, respectively. ΔG°CX2, protein and ΔG°CX2, solv are the free energy of binding of the CX2 group to the protein and the free energy of hydration of the CX2 group in the unbound state, respectively) could be the result of i) favorable desolvation of the protein and/or the ligand (i.e., −ΔΔG°CX2, solv < 0), ii) favorable interactions (e.g. dispersion interactions) between the alkyl and fluoroalkyl tails of the ligand and the hydrophobic wall (i.e., ΔΔG°CX2, protein < 0), iii) an increase in conformational degrees of freedom of the ligand or protein on binding (i.e., ΔΔG°CX2, protein < 0), or iv) favorable solvation of the protein-ligand complex (i.e., ΔΔG°CX2, protein < 0). The analysis we describe below indicates that desolvation of the greasy tails and of the hydrophobic wall determine the thermodynamics of binding in this system.
Dehydration of the ligand accounts for the favorable entropy of binding
Our ITC data show that the entropy of binding becomes more favorable with larger alkyl and fluoroalkyl tails (for alkyls −TΔΔS°b = −7 ± 1 cal mol−1 Å−2, for fluoroalkyls −TΔΔS°b = −7 ± 1 cal mol−1 Å−2, Table 1). There are at least four ways to explain the favorable incremental entropy of binding for RH and RF:
First, as Kauzmann and Tanford would have predicted, the desolvation of RH and RF could be entropically favorable (i.e., TΔΔS°CX2, solv > 0). We assume—based on the large number of calorimetric data for the transfer from octanol into aqueous phase, and for transfer from aqueous phase to vacuum of homologous alkyl-alcohols, and alkyl–amides26,27—that the incremental entropy of desolvation of the RH tails is favorable (−0.9 kcal mol−1 CH2−1).28 This value—which is ~0.6 kcal mol−1 more favorable than −TΔΔS°b for RH and HCA II—indicates that dehydration of the tail makes the dominant favorable contribution to −TΔΔS°b. Our crystallographic data indicate that the CH2 group of the ligand is only partially dehydrated upon binding to HCA II. Partial dehydration of the CH2 group would give a value of the entropy of dehydration that is less favorable than the entropy of dehydration of the whole CH2 group; thus, dehydration of the alkyl tail may account for the observed values of −TΔΔS°b. It is also plausible that the conformational flexibility of the tail in the unbound state is greater than that of the tail in the bound state: loss in conformational flexibility of the tail on binding to HCA would rationalize this difference between −TΔΔS°CH2, solv and −TΔΔS°b. We are not aware of a calorimetric study of the solvation of aliphatic fluorocarbon compounds, presumably because their low solubility in water makes their solvation inherently difficult to study by calorimetry.
Second, Homans et al. showed in studies with major urinary protein (MUP) that increasing the number of methylene groups in ligands made unfavorable contributions to −TΔΔS°b:29 for the series of n-alkyl alcohols, they report a value for –TΔΔS°b = +412 cal mol−1 CH2−1. Values of −TΔS°b, however, include the difference in conformational mobility of the ligand between the unbound and bound states. Although the conformational mobility of alkyl chains on n-alkyl alcohols in the unbound state is likely to be similar to that of our alkyl tails in the unbound state, the conformational mobility of these groups in the bound state is determined by the structure of the binding site of the protein. The structures of the binding sites of HCA II and MUP are very different: the binding site of MUP is a narrow groove lined with Leu, Tyr, and Phe residues that completely surround its ligands, while that of HCA II is an open, conical cleft in which bound ligands retain 30 – 50% of their solvent-accessible surface area. It is difficult to estimate the conformational mobility of ligands in the bound state. It is plausible, however, that alkyl tails in the active site of HCA II retain more conformational mobility than do alkyl alcohols bound in the active site of MUP.30 Such a difference in conformational mobility would rationalize, at least in part, the difference in values of −TΔΔS°b between alkyl tails binding to HCA II and to MUP.
The third contribution to the overall −TΔΔS°b could derive from changes from conformational degrees of freedom for amino acids in the active site upon binding. Our crystallographic data indicate that this contribution is unlikely to be important, because all ligands except the longest fluorinated one (X = F, n = 4) do not change the conformation of side-chains of amino acids in the proximity of ligands (the conformation of all amino acids of HCA II remain the same in complexes with RH and RF ligands, the only exception being Gln136 in the case of RF, n = 4).
Fourth, there is no indication from high-resolution X-ray and neutron diffraction studies of native HCA II that molecules of water are localized near the hydrophobic wall, although configurational restriction of molecules of water that are not observable by crystallography could, at least in principle, make favorable contributions TΔΔS°CX2, protein.
Our results—an increasing favorable entropy with increasing area of alkyl and fluoroalkyl tails—are compatible with Kauzmann-Tanford’s hypothesis for the origin of the hydrophobic effect: the burial of hydrophobic surface area, in both cases, is entropically favorable. The entropic contribution to ΔΔG°b appears to be dominated by the entropically favorable dehydration of the greasy tail. Although it is plausible that association with HCA II restricts the conformational flexibility of the alkyl tails, this restriction could be less unfavorable for the association of greasy tails with HCA II than it is for that of n-alkyl alcohols with MUP because of the differences in the structures of the active sites of the two proteins. The favorable contribution of dehydration of the hydrophobic surface of ligands to the overall free energy of binding is also illustrated by negative values of ΔCp. The heat capacity of desolvation of a CH2 group is around −13 cal mol−1 K−1.28,31 This value is slightly larger than what we have observed for binding of RH ligands to HCA II (−9 cal mol−1 K−1 per CH2). The observed difference presumably results from a partial dehydration of RH ligands in the bound state; this proposal is well supported by X-ray crystallographic data for HCA II-RH complexes.
Is the favorable enthalpy of binding a result of dispersion interactions or dehydration of the hydrophobic wall of HCA II?
Our ITC results show that the enthalpy of binding to HCA II becomes more favorable with larger alkyl and fluoroalkyl tails (for alkyls ΔΔH°b = −5 ± 1 cal mol−1 Å−2, for fluoroalkyls ΔΔH°b = −7 ± 1 cal mol−1 Å−2, Table 1). The values we measure for ΔΔH°b for the alkyl tails are quite different from those reported by Homans for the aliphatic alcohols binding to MUP (ΔΔH°b = −150 ± 30 cal mol−1 for HCA II with alkyl tails; ΔΔH°b = ~ −1350 cal mol−1 for MUP with aliphatic alcohols). Homans et al., however, did not compare thermodynamics of binding for aliphatic alcohols and fluorinated aliphatic alcohols, and they did not correlate the thermodynamic parameters of binding with solvent-accessible surface area of ligands.
Binding of primary alcohols (CH3(CH2)nOH, n = 4–9) to MUP appears to conflict with Kauzmann-Tanford’s view on the hydrophobic effect: Homans et al. found that enthalpy of binding becomes more favorable and the entropy of binding becomes less favorable with the increasing chain length of the ligands. They rationalized that trend by invoking dispersion interactions—which contribute favorably to ΔΔH°b—between the alkyl groups of the alcohols and the active site of the MUP.29
Previous studies of the dehydration of aliphatic compounds (and of other model compounds) suggest that the dehydration of aliphatic surface area is enthalpically unfavorable (−ΔΔH°CH2, solv = ~ 0.7 kcal mol−1).28,29 An unfavorable value for partial dehydration of CH2 group – H°CH2, solv paired with an overall favorable value for H°b requires the sum of the remaining contribution to be favorable (for RH ΔΔH°CH2, protein = ~ −0.6 kcal mol−1). This requirement, in turn, suggests at least three possibilities to obtain the overall favorable value of ΔΔH°b that we observe experimentally: i) noncovalent interactions between the tail moieties and the hydrophobic wall make ΔΔH°CX2, protein < 0, ii) dehydration of the hydrophobic wall of HCA II is enthalpically favorable (ΔΔH°CX2, protein < 0), or iii) hydration of protein-ligand complex is enthalpically favorable (ΔΔH°CX2, protein < 0).
We propose, from the comparison of thermodynamics of binding for RH and RF, and from crystallography, that partial dehydration of the hydrophobic wall of HCA II upon ligand binding releases loosely bound molecules of water that are involved in hydrogen bonding interactions that are weaker than those in bulk water. This release results in the formation of stronger hydrogen-bonds among waters in the bulk— an enthalpically favorable process.
Dispersion interactions presumably do not account for the enthalpic contributions to binding for alkyl and fluoroalkyl tails
Dispersion interactions are considerably weaker in fluorocarbon liquids than they are in hydrocarbon liquids; these interactions are determined by a low polarizability of fluorocarbons inferred from correlating the index of refraction with electronic polarizability. The difference in polarizabilities would predict that the dispersion interactions between the alkyl tails (RH) and the hydrophobic wall would be more enthalpically favorable than the analogous interactions of fluoroalkyl tails (RF)—a difference that is not observed in our measurements (for alkyls ΔΔH°b = −5 ± 1 cal mol−1 Å−2, for fluoroalkyls ΔΔH°b = −7 ± 1 cal mol−1 Å−2). We conclude, therefore, that the difference in dispersion interactions between fluorocarbons and hydrocarbons does not account for the value of ΔΔH°b for the two series of ligands.
Favorable enthalpic contribution results from non-optimal hydration of the hydrophobic wall of HCA II
It is plausible that the hydrophobic wall of HCA II is solvated with molecules of water that, because of the structure of the active site, are connected by weaker hydrogen bonds than are water molecules of the bulk. The displacement of such near-surface molecules of water (or, alternatively, of molecules of water disordered in the conical active site) by the ligand would be enthalpically favorable regardless of the structure and chemical composition of the ligand that is doing the displacement. In addition, larger ligands should displace more of these water molecules from the hydrophobic wall, and the favorable enthalpic contribution to binding would be proportional to the surface (and also volume) of the ligand.
The absence of observable, crystallographically-bound water molecules could indicate, but does not prove, that hydration of the hydrophobic wall does not occur with the ordering of water molecules that Kauzmann and Tanford suggested as being the origin of an entropy-dominated hydrophobic effect.
Crystal structures support thermodynamic data in suggesting that the molecular driving forces for binding of RH and RF to HCA II are indistinguishable. The change in conformation for Gln136 on binding correlates with a deviation in the trends of enthalpy and entropy of binding for the ligand with the longest fluoroalkyl tail compared to the other members of the series. This result suggests that the binding of alkyl and fluoroalkyl tails (except for X = F, n = 4) to HCA II is determined by similar molecular interactions (on an area- and volume-corrected basis) at the hydrophobic wall of HCA II.
The differences in polarizability of RF and RH could plausibly make the enthalpy of binding of RH slightly more favorable than RF. In contrast to other alkyls (n = 0 – 4) and fluoroalkyls (n = 0 – 3), crystallographic analysis of the longest fluorinated sulfonamide (n = 4) shows a major structural difference compared to other tails. Gln136 possesses the gauche conformation in the case of this fluoroalkyl (n = 4), while in the presence of all other ligands, the anti conformation of Gln136 is observed (Figure 6). Overall, we believe that this difference contributes to the lower value of ΔH°b for the longest fluorinated ligand (n = 4), and explains the inconsistency (~0.4 kcal mol−1 less than what would be predicted using the least squares linear regression fit obtained from n = 0–3) in its value of ΔH°b with those of the other fluoroalkyl ligands (Figure 4).
Conclusions
RF and RH have indistinguishable hydrophobicities
ITC demonstrates that the increasingly favorable binding of hydrophobic tails (RH and RF) to HCA II with increasing length of RH or RF chain results from favorable contributions from both enthalpy and entropy. These thermodynamics also show that alkyl and fluoroalkyl tails have indistinguishable thermodynamic signatures after correction for the differences in solvent-accessible surface area. This similarity indicates that the molecular basis for increasing affinity with increasing surface area of the tail group is similar for both. Our data are consistent with the hypothesis that the hydrophobic effect, in this case, results from the exclusion of water molecules from the contact region between the hydrophobic surface of the ligand, the hydrophobic wall of HCA II, and from the active site cavity, and not from different physical properties of RH and RF. Based on the thermodynamics of binding, we conclude that hydrocarbons and fluorocarbons are virtually indistinguishable when interacting with the hydrophobic surface of HCA II. Apparent differences between fluorocarbons and hydrocarbons in this study result primarily from differences in their hydrophobic surface area, and not from differences in dispersion interactions.
The origin of the hydrophobic effect is release of water molecules from the protein binding pocket and from the surface of the ligand
Most of the favorable free energy of binding is gained from interactions of water with non-polar surfaces. In this particular case, dehydration of the ligand (which presents a convex hydrophobic surface area) results in a favorable change in entropy of binding, and dehydration of the hydrophobic wall of HCA II (which is a concave hydrophobic surface area) results in a favorable change in enthalpy of binding. Theoretical studies by Rossky, Berne, Abel, Friesner and others have, time and again, suggested that the free energy of water molecules that solvate hydrophobic surfaces depend on the shape of the surface.24,26,27,32–36 Our experimental observations are compatible with this view, and indicate that favorable contributions to ΔG°b can arise simultaneously from the entropy of dehydration of the greasy tail of the ligand and from the enthalpy of dehydration of the hydrophobic wall of the protein. Whether or not there are general relationships between the curvature of hydrophobic surfaces and the thermodynamic parameters for the dehydration of them remains to be determined, although understanding these relationships may be centrally important for designing ligands that bind tightly to proteins.
Rational ligand design may require the explicit consideration of water
A better understanding of the thermodynamics of water interacting with non-polar surfaces in various biological systems is required for generating predictive algorithms in rational ligand design. Knowledge of the hydration of the active sites of medicinally-relevant proteins might be useful in designing high affinity ligands. Releasing water molecules of an active site that is partially (non-optimally) hydrated by a ligand would provide an enthalpically favorable component to the free energy of binding. In this respect, ligands with larger solvent-accessible surface area (and also larger volume) would release more water molecules upon binding than ligands with smaller SASA (or smaller volume). Thus, designing new inhibitors would involve an approach where ligands with larger SASA (and perhaps also volume) would be better targets than those with smaller SASA and volume. Incorporation of fluorine instead of hydrogen is one way to achieve larger SASA, but there are other functional groups (e.g., CH3 instead of H) that could provide a similar effect.
Our results demonstrate that water must be considered when designing ligands to bind tightly to proteins. Structural characterization of proteins by crystallography describes only part of the influence of water on molecular recognition. Nuclear magnetic resonance may fill in some of the details,37,38 but its application to proteins much larger than about 25 kDa has not yet provided detailed information about locations of water molecules. Rational ligand designers need theoretical approaches that predict accurately the structure of water in and around the active sites of proteins. Toward that aim, we believe, it is important to provide the theoretical community with the integrated structural and thermodynamic characterization of well-defined model systems of ligands and proteins against which to validate theoretical models. Carbonic anhydrase and arylsulfonamides are particularly well suited for this purpose.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (GM051559, GM030367) and a predoctoral fellowship from Eli Lilly (K.A.M.). Crystallography data for this study were measured at beamlines X25 and X29 of the National Synchrotron Light Source. Financial support comes principally from the Offices of Biological and Environmental Research and of Basic Energy Sciences of the US Department of Energy, and from the National Center for Research Resources of the National Institutes of Health grant number P41RR012408.
Footnotes
SUPPORTING INFORMATION. Background about the hydrophobic effect, additional thermodynamic analysis and crystallographic data. This material is available free of charge at http://pubs.acs.org.
References
- 1.Kauzmann W. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- 2.Tanford C. Science. 1978;200:1012–1018. doi: 10.1126/science.653353. [DOI] [PubMed] [Google Scholar]
- 3.Tanford C. J Mol Biol. 1972;67:59–74. doi: 10.1016/0022-2836(72)90386-5. [DOI] [PubMed] [Google Scholar]
- 4.Ball P. Chem Rev. 2008;108:74–108. doi: 10.1021/cr068037a. [DOI] [PubMed] [Google Scholar]
- 5.Southall NT, Dill KA, Haymet ADJ. J Phys Chem B. 2002;106:521–533. [Google Scholar]
- 6.Blokzijl W, Engberts JBFN. Angew Chem Int Ed. 1993;32:1545–1579. [Google Scholar]
- 7.Dalvi VH, Rossky PJ. Proc Natl Acad Sci USA. 2010;107:13603–13607. doi: 10.1073/pnas.0915169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 9.Montclare JK, Son S, Clark GA, Kumar K, Tirrell DA. ChemBioChem. 2009;10:84–86. doi: 10.1002/cbic.200800164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoder NC, Kumar K. Chem Soc Rev. 2002;31:335–341. doi: 10.1039/b201097f. [DOI] [PubMed] [Google Scholar]
- 11.Gao J, Qiao S, Whitesides GM. J Med Chem. 1995;38:2292–2301. doi: 10.1021/jm00013a005. [DOI] [PubMed] [Google Scholar]
- 12.Lee A, Mirica KA, Whitesides GM. J Phys Chem B. 2011;115:1199–1210. doi: 10.1021/jp107765h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunitz JD. ChemBioChem. 2004;5:614–621. doi: 10.1002/cbic.200300801. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamurthy VM, Kaufman GK, Urbach AR, Gitlin I, Gudiksen KL, Weibel DB, Whitesides GM. Chem Rev. 2008;108:946–1051. doi: 10.1021/cr050262p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamurthy V, Bohall B, Kim CY, Moustakas D, Christianson D, Whitesides G. Chem Asian J. 2007;2:94–105. doi: 10.1002/asia.200600360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Böhm H-J, Banner D, Bendels S, Kansy M, Kuhn B, Müller K, Obst-Sander U, Stahl M. ChemBioChem. 2004;5:637–643. doi: 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- 17.Kwon O-H, Yoo TH, Othon CM, Van Deventer JA, Tirrell DA, Zewail AH. Proc Natl Acad Sci USA. 2010;107:17101–17106. doi: 10.1073/pnas.1011569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiseman T, Williston S, Brandts JF, Lin L-N. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- 19.Boriack-Sjodin PA, Zeitlin S, Christianson DW, Chen H-H, Crenshaw L, Gross S, Dantanarayana A, Delgado P, May JA, Dean T. Prot Sci. 1998;7:2483–2489. doi: 10.1002/pro.5560071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair SK, Calderone TL, Christianson DW, Fierke CA. J Biol Chem. 1991;266:17320–17325. [PubMed] [Google Scholar]
- 21.Burton RE, Oas TG, Fierke CA, Hunt JA. Prot Sci. 2000;9:776–785. doi: 10.1110/ps.9.4.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalifah RG, Strader DJ, Bryant SH, Gibson SM. Biochemistry. 1977;16:2241–2247. doi: 10.1021/bi00629a031. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull WB, Daranas AH. J Am Chem Soc. 2003;125:14859–14866. doi: 10.1021/ja036166s. [DOI] [PubMed] [Google Scholar]
- 24.Berne BJ, Weeks JD, Zhou R. Ann Rev Phys Chem. 2009;60:85–103. doi: 10.1146/annurev.physchem.58.032806.104445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pratt LR, Pohorille A. Chem Rev. 2002;102:2671–2692. doi: 10.1021/cr000692+. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Berne BJ, Friesner RA. Proc Natl Acad Sci USA. 2011;108:1326–1330. doi: 10.1073/pnas.1016793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young T, Abel R, Kim B, Berne BJ, Friesner RA. Proc Natl Acad Sci USA. 2007;104:808–813. doi: 10.1073/pnas.0610202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plyasunov AV, Shock EL. Geochim Cosmochim Acta. 2000;64:439–468. [Google Scholar]
- 29.Malham R, Johnstone S, Bingham RJ, Barratt E, Phillips SEV, Laughton CA, Homans SW. J Am Chem Soc. 2005;127:17061–17067. doi: 10.1021/ja055454g. [DOI] [PubMed] [Google Scholar]
- 30.Krishnamurthy VM, Bohall BR, Semetey V, Whitesides GM. J Am Chem Soc. 2006;128:5802–5812. doi: 10.1021/ja060070r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syme NR, Dennis C, Phillips SEV, Homans SW. ChemBioChem. 2007;8:1509–1511. doi: 10.1002/cbic.200700281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng Y-K, Rossky PJ. Nature. 1998;392:696–699. doi: 10.1038/33653. [DOI] [PubMed] [Google Scholar]
- 33.Giovambattista N, Lopez CF, Rossky PJ, Debenedetti PG. Proc Natl Acad Sci USA. 2008;105:2274–2279. doi: 10.1073/pnas.0708088105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu P, Huang X, Zhou R, Berne BJ. Nature. 2005;437:159–162. doi: 10.1038/nature03926. [DOI] [PubMed] [Google Scholar]
- 35.Zhou R, Huang X, Margulis CJ, Berne BJ. Science. 2004;305:1605–1609. doi: 10.1126/science.1101176. [DOI] [PubMed] [Google Scholar]
- 36.Abel R, Salam NK, Shelley J, Farid R, Friesner RA, Sherman W. ChemMedChem. 2011;6:1049–1066. doi: 10.1002/cmdc.201000533. [DOI] [PubMed] [Google Scholar]
- 37.Wider G, Wuthrich K. Curr Opin Struct Biol. 1999;9:594–601. doi: 10.1016/s0959-440x(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 38.Wuthrich K, Otting G, Liepinsh E. Faraday Discuss. 1992;93:35–45. doi: 10.1039/fd9929300035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.