Abstract
Purpose
To compare intraocular pressure (IOP) measurements with Goldmann applanation tonometry (GAT) and iCare tonometry in normal and post-keratoplasty corneas and to assess the influence of central corneal thickness (CCT), corneal curvature (CC), and corneal astigmatism (CA) on IOP.
Methods
This prospective cross-sectional study included one eye of 101 subjects with normal corneas (58 healthy subjects, 43 glaucoma); and 90 post-keratoplasty patients: 34 penetrating keratoplasties (PK); 20 automated-lamellar-therapeutic keratoplasties (ALTK); 19 Descemet-stripping-automated-endothelial keratoplasties (DSAEK); 17 edematous grafts. All subjects underwent GAT and iCare IOP measurements in random order, and CCT, CC, and CA evaluation. The Bland–Altman method and multivariate regression analysis were used to assess inter-tonometer agreement and the influence of CCT, CC, and CA on IOP.
Results
iCare significantly underestimated IOP in all groups compared with GAT (GAT minus iCare of 3.5±3.5 mm Hg, P<0.001), but overestimated IOP in the edematous grafts (GAT minus iCare of −6.5±1.9 mm Hg, P<0.001). In normal corneas, both tonometer measurements were directly related to CCT values; iCare readings appeared inversely related to CC. There was no significant relationship between IOP and CCT, CC and CA in post-keratoplasty eyes, except between CC and iCare measurements for PK eyes.
Conclusions
The agreement between GAT and iCare was clinically acceptable in control, ALTK and DSAEK groups, and poor in PK and edematous grafts eyes. In normal corneas, GAT was significantly affected by CCT; iCare was influenced by CCT and CC. The iCare appeared less influenced by corneal edema when compared with GAT. High IOP readings taken with both tonometers in grafts should raise suspicion of true elevated IOP.
Keywords: intraocular pressure, Goldmann applanation tonometer, iCare tonometer, corneal thickness, corneal curvature, keratoplasty
Introduction
The accuracy of intraocular pressure (IOP) measurement is crucial in the screening, diagnosis, and management of glaucoma and for all types of intraocular surgical procedures. Goldmann applanation tonometry (GAT) is considered the gold standard for IOP measurement;1 its accuracy is, however, influenced by corneal thickness, curvature, and biomechanical properties, such as rigidity, viscosity, elasticity, hydration,2, 3, 4, 5, 6 which have shown to have high interindividual variability and to be affected by corneal pathology and surgery.3, 4, 7, 8, 9
Studies have shown erroneous postoperative IOP readings with GAT after keratoplasty.8, 9, 10, 11 Although not fully understood and predictable, GAT IOP errors in corneal grafts may be due to several factors including the following: surgically induced thinning or thickening of the cornea; high and/or irregular postoperative astigmatism; disruption of the mechanical integrity and remodeling of corneal tissue, which include the compliance forces present in the host eye, variable graft-host interface mechanics, changes in corneal biomechanical properties, and difficulties in aligning the tonometer head properly on the corneal surface.8, 9, 10, 11
Several alternative tonometers have been developed that claim to provide measurements that are not influenced by corneal properties, unlike GAT. The iCare (Tiolat Oy, Helsinki, Finland) is a new handheld tonometer, which is based on the impact-induction principle also known as rebound tonometry.12 The main advantages of this device include its quick and simple use, and that local anesthesia and slitlamp are not needed. The iCare tonometer has shown good reproducibility13 and correlation with GAT and other tonometers in healthy and glaucomatous eyes.13, 14, 15, 16 Although iCare was designed not to be influenced by corneal properties, studies have shown that central corneal thickness (CCT) and other corneal structural characteristics affect iCare IOP readings.13, 14, 15, 16, 17
To the best of our knowledge, studies reporting the influence of corneal curvature (CC) on iCare and comparative IOP measurements in post-keratoplasty eyes are limited.
The purpose of our study was to compare IOP readings taken with GAT and iCare in subjects with normal corneas and in patients that underwent lamellar or penetrating keratoplasty and to assess the influence of CCT, corneal curvature (CC) and corneal astigmatism (CA) on IOP measurements.
Materials and methods
This observational, prospective, cross-sectional study included the following groups of consecutive subjects: 101 eyes with normal cornea (control group: 58 healthy subjects and 43 with primary open-angle glaucoma or POAG subjects); and 90 eyes that underwent keratoplasty (34 penetrating keratoplasties or PK; 20 automated lamellar therapeutic keratoplasties or ALTK; 19 Descemet stripping automated endothelial keratoplasties or DSAEK; 17 edematous corneal grafts). Only one eye per subject was randomly selected using a computer-generated randomized number assignment. The study was in compliance with the tenets of the Helsinki's Declaration, and informed consent was obtained from all participants before testing. Each participant underwent the following examinations on the same day: complete ophthalmologic examination, including best-corrected visual acuity evaluation, slit-lamp examination by our corneal specialists (to determine signs of clinical corneal edema), and fundus biomicroscopy with a 90D lens; IOP measurement with GAT and iCare tonometer taken in random order, followed by CCT, CC, and CA measurements. Normal subjects were recruited from staff members and volunteers. POAG and post-keratoplasty patients were recruited from the Glaucoma and Cornea Clinics of the Department of Ophthalmology of the S. Maria della Misericordia Hospital, Udine, Italy. The study was in compliance with Institutional Review Boards (IRBs) and HIPAA requirements for the institute involved in the study. The study was approved by the IRB of the Azienda Ospedaliero-Universitaria ‘S. Maria della Misericordia', Udine, Italy.
Inclusion criteria were as follows: open anterior chamber angle; absence of ocular pathology other than glaucoma, ocular hypertension, mild nuclear sclerosis and rare drusen; at least 3 months after surgery in grafted subjects; reliable IOP and iCare measurements; and willingness to provide informed written consent.
Exclusion criteria included: presence of secondary causes of glaucoma; microphthalmus; ocular inflammation; history of intraocular surgery other than keratoplasty; and, contact lens wear.
Normal eyes were defined as GAT IOP≤21 mm Hg; normal optic nerve head (ONH) and retinal nerve fiber layer (RNFL) appearance, normal standard automated perimetry (SAP) visual field (VF) results; no family history of glaucoma, and other ocular pathologies. POAG was defined as IOP>21 mm Hg before medication, ONH or RNFL thickness with typical glaucomatous changes, and/or reproducible glaucomatous SAP VF defects.
The corneal diseases requiring keratoplasty were as follows: keratoconus with apex opacity or contact lens intolerance, stromal dystrophies, corneal leucoma, stromal opacity occurring after herpes keratitis, penetrating injuries and opacity of the donor button after previous PK for PK or ATLK; Fuchs endothelial dystrophy and bullous keratopathy for DSAEK. The clinical corneal edema was caused by graft failure or reject.
All keratoplasty surgeries were performed by a single surgeon (PB) from March 2006 to January 2009. PK,18 ALTK,19 and DSAEK20 techniques have been extensively described elsewhere. Four interrupted sutures and a single 16-bite 10-0 nylon suture (Ethicon Inc., Somerville, NJ, USA) were placed in all PK and ALTK cases. The postoperative medication included 0.1% dexamethasone sodium phosphate and ofloxacin eye drops four times daily for 1 week. Dexamethasone eye drops were tapered for 12 months in all groups. In PK and ALTK eyes, the running suture was removed 6 and 12 months after surgery, respectively. The presence or absence of the running suture at the time of examination was recorded for each eye.
All CCT, CC, and CA measurements were performed by the same experienced examiner (LP). CCT was measured with an ultrasonic pachymeter (Altair pachymeter, Optikon 2000, Rome, Italy) after topical anesthesia with 0.4% benoxinate hydrochloride eye drops. The mean of three readings within a range of ±5 microns was considered.
CC measurements were taken with the Automatic Refractor/Keratometer 599 (Zeiss Humphrey, Dublin, CA, USA). Simulated keratometric values include diopter power and axis of the steepest meridian and at 90° (K1 and K2). K1 and K2 were averaged to obtain a single CC value. The CA value was defined as the absolute value for K2 minus K1.
All IOP measurements were taken by the same experienced examiner (MLS) who was masked to previous readings. A different observer (LP) read and recorded the IOP readings to minimize biases. GAT and iCare tonometry methods were performed according to manufacturer's guidelines.1, 12 All subjects underwent IOP measurements with GAT and iCare tonometer in random order, with a minimum 5-min time interval between readings to minimize the tonographic effect of applanation tonometry. To minimize the potential confounding effect of diurnal variation in IOP, all measurements were taken from 1000 to 1130 hours.
GAT (Haag Streit International, Koeniz, Switzerland) was performed with a BQ 900 slitlamp (Haag Streit, Bern, Switzerland) using local anesthetic (0.4% benoxinate hydrochloride) and fluoresceine sodium 2% strips. GAT was calibrated weekly and performed in the manner originally described by Goldmann and Schimdt.1 Before each reading, the measurement drum was reset to ∼6 mm Hg. If IOP fluctuated during the cardiac pulse cycle, GAT measurements were taken in the middle of the pulsation amplitude. The mean of three consecutive IOP readings was considered for analysis. In eyes with irregular astigmatism or regular astigmatism >3 diopters (D), three readings on the steepest and three on the flattest axis were averaged for each eye.21 A minimum 3-min time gap between GAT readings was used to minimize the tonographic effect of applanation tonometry.
The iCare tonometry has been thoroughly described elsewhere.12 In brief, the tonometer is a small handheld device composed of a probe and a solenoid. The probe is positioned at a distance of ∼4–8 mm from the cornea, utilizing the forehead as a base support. In pressing the button, an electrical pulse generates a magnetic field in the solenoid, which repels the magnet and the probe. The probe moves towards, then rebounds off the cornea, which is used in the calculation of IOP.12 The software is programmed for six measurements: the average IOP value is then automatically calculated excluding the highest and the lowest readings. After the sixth measurement, the letter P appears on the display, followed by the mean IOP reading. The mean of three consecutive IOP readings was recorded. The iCare tonometer utilizes different symbols after the letter ‘P' to indicate the quality of measurement taken. As suggested by other authors,17 we discarded any iCare reading with an error bar.
The differences between GAT and iCare tonometer results were calculated with the paired t-test; the comparisons among groups of subjects were assessed using the analysis of variance; the Duncan's multiple range test and the Bonferroni correction were used for multiple comparisons. Percentages were compared using the χ2-test. The correlation between tonometers was calculated using the Pearson correlation coefficient. Agreement between tonometers was assessed using the Bland–Altman method.22 The percentage of eyes with a IOP difference (ΔIOP) between tonometers within ±1, 2, 3, 4, 5, and >5 mm Hg was calculated. A ΔIOP between tonometers of >±3 mm Hg was considered as clinically significant in our study. Multiple linear regression analysis (MLRA) was used to evaluate the influence of CCT, CC, CA, and the mean IOP value of the tonometers ((GAT+iCare)/2) on the IOP measurements and the ΔIOP (GAT minus iCare). Significance levels were assessed by the paired two-tailed t-test. The statistical analysis was performed using SPSS 11.0 (SPSS, Chicago, IL, USA). Statistical significance was defined as P<0.05. For multiple comparisons a P-value of <0.01 was considered as statistically significant.
Considering that our study defined a ΔIOP of greater than ±3 mm Hg as clinically relevant and that the SD of IOP differences between tonometers was ∼3.5 mm Hg (Table 2), the power of our study in detecting a 3 mm Hg difference (at the 5% significance level, with an error type II of 20%) was 100% in a group of 100 subjects (control eyes) and 75.2% in a group of 20 subjects (post-keratoplasty eyes).
Results
Demographics and corneal structural characteristics are listed in Table 1. The mean time between grafting and last considered follow-up was 12.7±15 (range 4–38) months. Three PK eyes and two ALTK eyes had a running suture in situ.
Table 1. Demographics and corneal characteristics.
| Age (years) | CCT (μm) | CC (diopters) | CA (diopters) | |
|---|---|---|---|---|
| Control group (n=101) | 63.2±11.1 (34–83) | 557.6±34.9 (472.7–624) | 43.5±1.6 (40.0–46.3) | 0.9±0.8 (0–3.7) |
| PK group (n=34) | 64.0±14.2 (32–88) | 569.2±50.4 (478–698) | 45.4±2.7 (39.7–51.1) | 5.7±3.6 (0–12.2) |
| ALTK group (n=20) | 66.3±11.7 (43–87) | 562.5±43.5 (475–635) | 43.6±2.3 (38.4–46.7) | 3.9±2.6 (0.7–12.0) |
| DSAEK group (n=19) | 75.5±11.9 (53–88) | 650.2±61.6 (545–760) | 43.9±2.2 (38.0–47.6) | 1.7±1.1 (0.2–5.0) |
| Edematous grafts group (n=17) | 71.3±14.1 (44–88) | 784.9±61.4 (696–903) | 43.1±2.7 (39.4–44.5) | 2.5±2.1 (0–8.5) |
| P^ | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Abbreviations: ALTK, automated-lamellar-therapeutic keratoplasty; CA, corneal astigmatism; CC, corneal curvature; CCT, central corneal thickness; DSAEK, Descemet-stripping-automated-endothelial keratoplasty; PK, penetrating keratoplasty; ^, analysis of variance.
Results are given as mean±SD (95% confidence interval).
Table 2 shows the IOP measurement results. Mean IOP measurements were significantly higher with GAT than with iCare in controls, PK, ALTK, and DSAEK groups, and significantly lower in the edematous grafts group. GAT and iCare reading appeared highly correlated in all groups. The mean ΔIOP in absolute value were highest in the edematous grafts and PK groups, lower in the DSAEK group, and lowest in the ALTK and controls groups.
Table 2. Intraocular pressure measurements results.
| GAT IOP | iCare IOP | P^ | r* | GAT-iCare | |
|---|---|---|---|---|---|
| (mm Hg) | (mm Hg) | P | (mm Hg) | ||
| Control group (n=101) | 17.6±4.9 (9.5–28) | 14.9±4.5 (8–24.4) | <0.0001 | 0.83 | 2.7±3.4 (−5/9) |
| <0.0001 | |||||
| Healthy subjects (n=58) | 15.3±3.8 (9.5–21) | 13.9±4.1 (8–20) | <0.0001 | 0.86 | 1.4±3.5 (−5/9) |
| <0.0001 | |||||
| POAG patients (n=43) | 20.6±4.5 (12–28) | 16.2±4.7 (10–24.4) | <0.0001 | 0.82 | 4.4±2.4 (0/9) |
| <0.0001 | |||||
| PK group (n=34) | 20.3±5.0 (12–34) | 14.7±5.4 (8–31) | <0.0001 | 0.76 | 5.5±3.6 (−3/12) |
| <0.0001 | |||||
| ALTK group (n=20) | 14.1±4.6 (6–24) | 11.8±3.7 (5–18) | <0.0001 | 0.78 | 2.3±2.9 (−4/7) |
| <0.0001 | |||||
| DSAEK group (n=19) | 17.7±3.8 (11–25) | 13.6±4.6 (8–26) | <0.0001 | 0.70 | 4.0±3.3 (−2/10) |
| <0.001 | |||||
| Edematous grafts group (n=17) | 9.1±3.1 (5–14) | 13.9±3.8 (10–22) | <0.0001 | 0.68 | −6.5±1.9 (−11/−4) |
| <0.002 | |||||
| P** | <0.0001 | <0.01 | <0.0001 |
Abbreviations: ALTK, automated-lamellar-therapeutic keratoplasty; DSAEK, Descemet-stripping-automated-endothelial keratoplasty; PK, penetrating keratoplasty; POAG, primary open-angle glaucoma; ^, paired t-test
Results are given as mean±SD (95% confidence interval). *Pearson's correlation coefficient; **analysis of variance.
When the measurements were taken in two different sequences in all groups (GAT first vs GAT second), the ΔIOP were not significantly different.
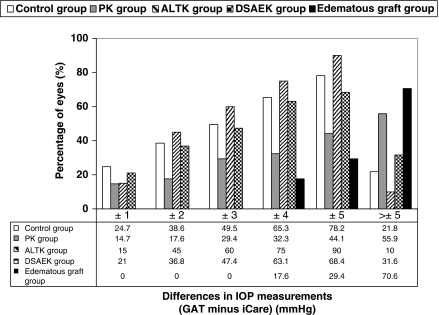
Figure 1 shows the percentage of eyes following within different intervals of ΔIOP between tonometers (GAT minus iCare) for all groups. The percentage of eyes with a ΔIOP within ±3 mm Hg were highest in healthy eyes and ALTK group, higher in the DSAEK group, lower in POAG eyes and PK group, and lowest in the edematous grafts. The ΔIOP were within ±3 mm Hg in 42.4% of all eyes.
Figure 1.
Frequency histogram of the differences in IOP measurements between GAT and iCare. The histogram shows the percentage of eyes falling within different intervals of differences in IOP measurement between GAT and iCare (GAT minus iCare) in subjects with normal corneas (control group), patients after penetrating keratoplasty (PK group); patients after automated-lamellar-therapeutic keratoplasty (ALTK group), patients after Descemet-stripping-automated-endothelial keratoplasty (DSAEK group), and patients after keratoplasty with corneal edema (edematous grafts group).
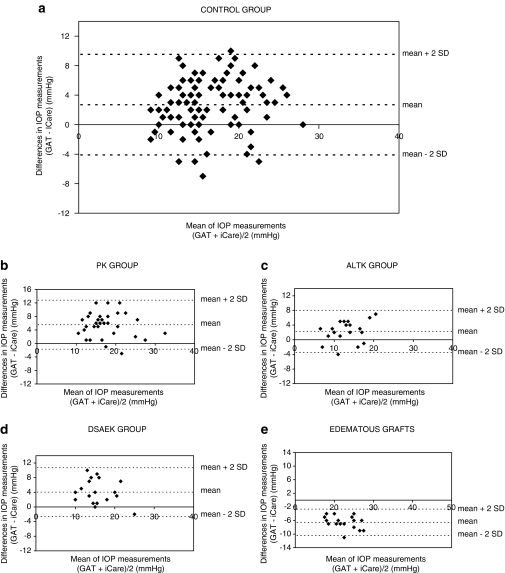
The Bland–Altman plots of the agreement between GAT and iCare IOP measurements are shown in Figure 2. The plots show the distribution of ΔIOP (GAT minus iCare) on the y axis and the mean IOP value of the tonometers on the x axis for each measured eye. The mean of the ΔIOP (GAT minus iCare) was positive in the control, PK, ALTK, and DSAEK groups; however, was negative in the edematous graft group.
Figure 2.
Bland–Altman plots of the agreement between GAT and iCare IOP measurements. The plot shows the distribution of ΔIOP (GAT-iCare) (y axis) against the mean IOP value of the tonometers (x axis) for each measured eye in subjects with normal corneas ((a) control group), patients after penetrating keratoplasty ((b) PK group), patients after automated-lamellar-therapeutic keratoplasty ((c) ALTK group), patients after Descemet-stripping-automated-endothelial keratoplasty ((d) DSAEK group), and patients after keratoplasty with corneal edema ((e) edematous grafts group).
The MLRA results are listed in Table 3. The dependent variables considered in our study (CCT, CC, CA, and mean IOP value) were able to explain the variation of the independent variables (GAT and iCare IOP readings) in eyes with normal cornea (P<0.05) but not in post-keratoplasty eyes. Healthy subjects and POAG eyes showed comparable MLRA results (data not shown). With regards to the control group, both tonometer IOP readings were significantly directly related to CCT, which appeared slightly higher for iCare. IOP measurements with iCare appeared significantly inversely related to CC. The ΔIOP (GAT minus iCare) appeared significantly directly related to CC and mean IOP. The relationship between IOP readings and CCT, CC and CA in post-keratoplasty eyes were not significant, except for CC on the iCare IOP measurements in the PK group that showed a significant inverse effect. IOP values of both tonometers were not significantly influenced by patient age or the presence of a running suture (P>0.05).
Table 3. Regression coefficients (P-value) of the multiple linear regression analysis.
|
Independent variable |
P* | ||||||
|---|---|---|---|---|---|---|---|
| Dependent | Intercept | CCT | CC | CA | (GAT+iCare)/2 | ||
| variable | (μm) | (diopters) | (diopters) | (mm Hg) | |||
| Control group | GAT IOP | −2.2 | 0.041 (0.01) | 0.06 (0.85) | −0.19 (0.76) | n.a. | 0.047 |
| (n=101) | iCare IOP | 20.6 | 0.050 (0.0001) | −0.76 (0.008) | −0.23 (0.68) | n.a. | 0.0001 |
| GAT–iCare | −24.3 | −0.016 (0.10) | 0.76 (0.0001) | 0.072 (0.85) | 0.17 (0.015) | 0.001 | |
| PK group | GAT IOP | 22.2 | 0.012 (0.50) | 0.20 (0.56) | 0.03 (0.92) | n.a. | 0.82 |
| (n=34) | iCare IOP | 44.9 | 0.009 (0.62) | −0.77 (0.031) | −0.06 (0.81) | n.a. | 0.15 |
| GAT–iCare | −22.1 | 0.003 (0.80) | 0.56 (0.023) | 0.085 (0.61) | −0.02 (0.88) | 0.17 | |
| ALTK group | GAT IOP | −28.5 | −0.003 (0.87) | 0.22 (0.57) | −0.6 (0.10) | n.a. | 0.55 |
| (n=20) | iCare IOP | 7.8 | −0.030 (0.11) | 0.12 (0.72) | −0.50 (0.11) | n.a. | 0.62 |
| GAT–iCare | −34.1 | 0.030 (0.06) | 0.10 (0.62) | −0.02 (0.94) | 0.20 (0.29) | 0.11 | |
| DSAEK group | GAT IOP | 10.7 | −0.009 (0.55) | 0.26 (0.59) | 0.34 (0.35) | n.a. | 0.37 |
| (n=19) | iCare IOP | −6.0 | -0.018 (0.38) | 0.19 (0.76) | −0.20 (0.86) | n.a. | 0.85 |
| GAT–iCare | 17.4 | 0.010 (0.11) | 0.13 (0.68) | 0.13 (0.37) | −0.29 (0.10) | 0.19 | |
| Edematous grafts | GAT IOP | 3.7 | −0.017 (0.16) | 0.41 (0.60) | 0.56 (0.44) | n.a. | 0.66 |
| Group (n=17) | iCare IOP | 16.0 | −0.035 (0.16) | 0.26 (0.57) | −0.13 (0.87) | n.a. | 0.52 |
| GAT–iCare | −10.6 | 0.013 (0.34) | 0.15 (0.73) | 0.73 (0.13) | −0.16 (0.49) | 0.37 | |
Abbreviations: ALTK, automated-lamellar-therapeutic keratoplasty; CA, corneal astigmatism; CC, corneal curvature; CCT, central corneal thickness; DSAEK, Descemet-stripping-automated-endothelial keratoplasty; PK, penetrating keratoplasty; n.a., not applicable. *significance value of the model in explaining the variation of the dependent variable. Statistically significant values are indicated in bold.
Discussion
In accordance with previous reports,13, 14, 15, 16, 23 GAT and iCare IOP measurements were highly correlated (Table 2). The iCare tonometer, however, significantly underestimated GAT IOP in all groups (Table 2; Figure 2a–d), with exception of edematous graft corneas, in which IOP tended to be overestimated compared with GAT (Table 2; Figure 2e). The relationship between GAT and iCare readings is a still debatable issue. Although iCare has not been validated with manometric studies in human eyes, studies have shown that rebound tonometry provides accurate IOP measurements compared with manometric studies on animal models,24, 25 and tend to be preferable to TonPen IOP readings.24 Our results for healthy and glaucomatous eyes are in accordance with some studies,12, 16 yet in disagreement with others,13, 15, 17 which may be due to the differences in the cohort of subjects.
The mean IOP difference between GAT and iCare readings was higher in glaucomatous patients than in healthy eyes (Table 2). This finding could be due to the difference in age, mean IOP (MLRA results, Table 3), or corneal biomechanical properties. All POAG patients were receiving anti-glaucomatous eye drops, which have shown to influence corneal extracellular matrix;26 thus, corneal rigidity or elasticity may have been altered in the POAG eyes.
The iCare tonometer tended to significantly underestimate GAT readings in post-keratoplasty eyes, with exception to eyes with corneal edema (Table 2; Figure 2a–e). Unlike GAT, which has shown to significantly underestimate IOP in edematous corneas,2, 3 iCare tended to be less influenced by corneal hydration.
The reproducibility of GAT has shown to be quite good, ranging from about 2.5 mm Hg in normals and 3.5 mm Hg in post-KP eyes.27 In accordance with previous studies,28, 29 differences that exceed these limits tend to be clinically relevant for diagnosis, follow-up, and treatment.
GAT and iCare measurements were not interchangeable in the majority of cases in both normal and post-surgical corneas, considering that 57.6% of eyes showed an IOP difference between tonometers of more than ±3 mm Hg (Figure 1). When considering a ΔIOP between tonometers of >±3 mm Hg as clinically relevant, the agreement between tonometers appeared clinically acceptable in the control, ALTK, and DSAEK groups (Figure 1). The agreement, however, was poor in the PK group, considering that 56% of these eyes showed a ΔIOP between tonometers of >±5 mm Hg. It was clinically unacceptable in eyes with corneal edema, considering that 71% of these eyes showed a ΔIOP between tonometers of >±5 mm Hg.
CCT significantly affected both GAT and iCare IOP measurements in healthy corneas, however, did not in post-keratoplasty eyes (Table 3). In accordance with other studies regarding GAT5, 30, 31, 32, 33 and iCare,14, 15, 17 our data showed that the IOP was significantly directly related to CCT in subjects with normal cornea (Table 3), with an increase IOP of 0.41 mm Hg for GAT and 0.50 mm Hg for iCare per 10 μm increase in CCT (Table 3).
Our post-keratoplasty eyes did not show any statistically significant relationship between CCT and both tonometers IOP readings, which is in accordance to previous studies regarding GAT in post-PK,8, 11 post-deep anterior LK,8 and post-DSEK.9 It is likely that keratoplasty may alter corneal biomechanics,9 and corneal rigidity may not necessarily increase in thicker post-keratoplasty corneas. Elevated IOP readings with both tonometers after keratoplasty should thus raise suspicion of true high IOP.
CC appeared to significantly affect iCare IOP in normal and post-PK corneas, however, it did not influence GAT IOP (Table 3). Studies have shown that the influence of CC on GAT IOP readings is debatable, yet tends to be marginal in normal eyes.4, 5 In accordance to our results, previous authors have reported that there are no correlations between CC and GAT IOP;5, 28, 34 however, other studies have shown some correlation.35 Moreover, CC has shown to have no significant effect on GAT IOP in post-PK and post-deep LK eyes,8 which is in agreement with our data.
In disagreement with previous studies,36 our results show that iCare tended to underestimate IOP in healthy steep corneas and overestimate IOP in normal flat corneas, with an IOP decrease of 0.76 mm Hg per 1 D in CC (Table 3). Steeper corneas may hypothetically decrease the iCare probe velocity, thus artificially underestimate IOP.
The relationship between CA and IOP with both instruments in controls and post-keratoplasty eyes was not significant (Table 3). Studies have shown that CA greatly influences GAT.2, 35, 37 The method used to measure IOP with GAT in eyes with high astigmatism (the mean of two readings taken at the flattest and steepest meridian)21 could have influenced the results in our cohort of patients. Moreover, studies have reported that CA has no significant effect on GAT in post-PK and post-deep LK eyes,8 which is in accordance to our results. Unlike GAT, the iCare probe is in contact with a very small area on the corneal surface, thus theoretically less influenced by CA.
The variables CC, CA, CCT, and mean IOP value considered in our study were significantly related to the IOP measurements taken with GAT and iCare in normal corneas, but not in post-keratoplasty eyes (Table 3); this may be indicative of other confounding factors not considered (especially in grafted corneas), such as corneal altered biomechanics, which are known to induce errors on GAT 4, 6 and rebound tonometry 17 measurements.
From a clinical perspective, the iCare tonometer appeared easier and quicker to use than GAT in post-keratoplasty eyes in our study, especially in eyes with elevated astigmatism that required several repeat GAT measurements. It is important, however, to note some limits of our study. Our cohort of subjects, especially the post-keratoplasty groups, was relatively small. Furthermore, the eyes considered did not have a large range in CCT in both groups, thus the effect of thin or thick corneas was not assessed. Our study is also limiting in that the patients considered are quite variable, and ocular intrinsic differences (ie, axial length, corneal biomechanical properties, postoperative induced changes, etc.) were not assessed in our analysis. Moreover, subjects on topical medication with anti-glaucomatous or steroid drops were not excluded, which may have influenced results due to their effect on corneal biomechanics and CCT.26
In conclusion, GAT and iCare IOP readings were highly correlated but not interchangeable. The inter-tonometer agreement was clinically acceptable in control, ALTK, and DSAEK groups; it appeared, however, poor in the PK and edematous graft groups. In eyes with normal cornea, GAT appeared to be significantly influenced by CCT, whereas iCare tended to be influenced by both CCT and CC. The iCare tonometer appeared less influenced by corneal edema when compared with GAT. High IOP readings with both tonometers in grafts should raise suspicion of true elevated IOP. Further studies are needed, including intraocular manometric measurements, to determine the most accurate tonometry method in post-keratoplasty eyes.
The authors declare no conflict of interest.
References
- Goldmann H, Schmidt T. Ueber Applanationstonometrie. Ophthalmologica. 1957;134:221–242. doi: 10.1159/000303213. [DOI] [PubMed] [Google Scholar]
- Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38:1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
- Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurements: quantitative analysis. J Cataract Refract Surg. 2005;31:146–155. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Kohlhaas M, Boehm AG, Spoerl E, Pursten A, Grein HJ, Pillunat LE. Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch Ophthalmol. 2006;124:471–476. doi: 10.1001/archopht.124.4.471. [DOI] [PubMed] [Google Scholar]
- Broman AT, Congdon NG, Bandee-Roche K, Quigley HA. Influence of corneal structure, corneal responsiveness, and other ocular parameters on tonometric measurement of intraocular pressure. J Glaucoma. 2007;16:581–588. doi: 10.1097/IJG.0b013e3180640f40. [DOI] [PubMed] [Google Scholar]
- Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Ceruti P, Morbio R, Marraffa M, Marchini G. Comparison of dynamic contour tonometry and Goldmann applanation tonometry in deep lamellar and penetrating keratoplasty. Am J Ophthalmol. 2008;145:215–221. doi: 10.1016/j.ajo.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Vajaranant TS, Price MO, Price FW, Wilensky JT, Edward DP. Intraocular pressure measurements following Descemet stripping endothelial keratoplasty. Am J Ophthalmol. 2008;145:780–786. doi: 10.1016/j.ajo.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Patel S, McLaughlin MJ. Effects of central corneal thickness on measurement of intra-ocular pressure in keratoconus and post-keratoplasty. Ophthal Physiol Opt. 1999;3:236–241. doi: 10.1046/j.1475-1313.1999.00420.x. [DOI] [PubMed] [Google Scholar]
- Ismail AR, Lamont M, Perera S, Khan-Lim D, Mehta R, Macleod JDA, et al. Comparison of IOP measurement using GAT and DCT in patients with penetrating keratoplasties. Br J Ophthalmol. 2007;91:980–981. doi: 10.1136/bjo.2006.099564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontiola AI. A new induction-based impact method for measuring intraocular pressure. Acta Ophthalmol Scand. 2000;78:142–145. doi: 10.1034/j.1600-0420.2000.078002142.x. [DOI] [PubMed] [Google Scholar]
- Martinez-de-la-Casa JM, Garcia-Feijoo J, Castillo A, Garcia-Sanchez J. Reproducibility and clinical evaluation of rebound tonometry. Invest Ophthalmol Vis Sci. 2005;46:4578–4580. doi: 10.1167/iovs.05-0586. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Darhad U, Tatsumi Y, Fujioka M, Kusuhara A, Maeda H, et al. Agreement of rebound tonometer in measuring intraocular pressure with three types of applanation tonometers. Am J Ophthalmol. 2006;142:332–334. doi: 10.1016/j.ajo.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Martinez-de-la-Casa JM, Garcia-Feijoo J, Vico E, Fernandez-Vidal A, Benitez del Castillo JM, Wasfi M, et al. Effect of corneal thickness on dynamic contour, rebound, and Goldmann tonometry. Ophthalmology. 2006;113:2156–2162. doi: 10.1016/j.ophtha.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L. Comparison of ICare tonometer with Goldmann applanation tonometer in glaucoma patients. J Glaucoma. 2006;15:213–217. doi: 10.1097/01.ijg.0000212208.87523.66. [DOI] [PubMed] [Google Scholar]
- Chui W, Lam A, Chen D, Chiu R. The influence of corneal properties on rebound tonometry. Ophthalmology. 2008;115:80–84. doi: 10.1016/j.ophtha.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Bahar I, Kaiserman I, McAllum P, Slomovis A, Rootman D. Comparison of posterior lamellar keratoplasty techniques to penetrating keratoplasty. Ophthalmology. 2008;115:1525–1533. doi: 10.1016/j.ophtha.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Busin M, Zambianchi L, Arffa RC. Microkeratome-assisted lamellar keratoplasty for the surgical treatment of keratoconus. Ophthalmology. 2005;112:987–997. doi: 10.1016/j.ophtha.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Price FW, Jr, Price MO. Descemet's stripping with endothelial keratoplasty in 200 eyes: early challenger and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32:411–418. doi: 10.1016/j.jcrs.2005.12.078. [DOI] [PubMed] [Google Scholar]
- Holladay JT, Allison ME, Prager TC. Goldmann applanation tonometry in patients with regular astigmatism. Am J Ophthalmol. 1983;96:90–93. doi: 10.1016/0002-9394(83)90459-2. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Kontiola A, Puska P. Measuring intraocular pressure with the Pulsair 3000 and Rebound tonometers in elderly patients without an anesthetic. Graefe's Arch Clin Exp Ophthalmol. 2004;242:3–7. doi: 10.1007/s00417-003-0671-3. [DOI] [PubMed] [Google Scholar]
- Pease ME, Hammond JC, Quigley HA. Manometric calibration and comparison of TonLab and TonoPen tonometers in rat with experimental glaucoma and in normal mice. J Glaucoma. 2006;15:512–519. doi: 10.1097/01.ijg.0000212276.57853.19. [DOI] [PubMed] [Google Scholar]
- Pease ME, Cone FE, Gelman S, Son JL, Quigley HA. Calibration of the TonLab tonometer in mice with spontaneous or experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;52:858–864. doi: 10.1167/iovs.10-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Ohguro H, Mamiya K, Ohguro I, Nakazawa M. Effect of antiglaucoma drops on MMP and TIMP balance in conjunctival and subconjunctival tissue. Invest Ophthalmol Vis Sci. 2006;47:823–830. doi: 10.1167/iovs.05-0902. [DOI] [PubMed] [Google Scholar]
- Pandav SS, Sharma A, Gupta A, Sharma SK, Gupta A, Patnaik B. Reliability of ProTon and Goldmann applanation tonometers in normal and postkeratoplasty eyes. Ophthalmology. 2002;109:979–984. doi: 10.1016/s0161-6420(02)00974-0. [DOI] [PubMed] [Google Scholar]
- Francis BA, Hsieh A, Lai M-Y, Chopra V, Pena F, Azen S, et al. Los Angeles Latino Eye Study Group. Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and Dynamic contour tonometry. Ophthalmology. 2007;114:20–26. doi: 10.1016/j.ophtha.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Rao VJ, Gnanaraj L, Mitchell KW, Figueiredo FC. Clinical comparison of ocular blood flow tonometer, tonopen and Goldmann applanation tonometer for measuring intraocular pressure in postkeratoplasty eyes. Cornea. 2001;20:834–838. doi: 10.1097/00003226-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am J Ophthalmol. 1993;115:592–596. doi: 10.1016/s0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- Wolfs RC, Klaver CC, Vingerling JR, Grobbee DE, Hofman A, de Jong PT. Distribution of corneal central thickness and its association with intraocular pressure: The Rotterdam Study. Am J Ophthalmol. 1997;123:767–772. doi: 10.1016/s0002-9394(14)71125-0. [DOI] [PubMed] [Google Scholar]
- Orssengo G, Pye DC. Determination of the true intraocular pressure and modulus of elasticity of the human cornea in vivo. Bull Math Biol. 1999;61:551–572. doi: 10.1006/bulm.1999.0102. [DOI] [PubMed] [Google Scholar]
- Shimmyo M, Ross AJ, Moy A, Mostafavi R. Intraocular pressure, Goldmann applanation tension, corneal thickness, and corneal curvature in Caucasians, Asians, Hispanics and African Americans. Am J Ophthalmol. 2003;136:603–613. doi: 10.1016/s0002-9394(03)00424-0. [DOI] [PubMed] [Google Scholar]
- Saleh TA, Adams M, McDermott B, Claridge KG, Ewings P. Effects of central corneal thickness and corneal curvature on the intraocular pressure measurements by Goldmann applanation tonometry and ocular blood flow pneumatonometer. Clin Exp Ophthalmol. 2006;34:516–520. doi: 10.1111/j.1442-9071.2006.01266.x. [DOI] [PubMed] [Google Scholar]
- Mark HN. Corneal curvature in applanation tonometry. Am J Ophthalmol. 1973;76:223–224. doi: 10.1016/0002-9394(73)90164-5. [DOI] [PubMed] [Google Scholar]
- Johannesson G, Hallberg P, Eklund A, Linden C. Pascal, ICare and Goldmann applanation tonometry- a comparative study. Acta Ophthalmol. 2008;86:614–621. doi: 10.1111/j.1600-0420.2007.01112.x. [DOI] [PubMed] [Google Scholar]
- Rask G, Behndig A. Effects of corneal thickness, curvature, astigmatism and direction of gaze on Goldmann applanation tonometry readings. Ophthalmic Res. 2006;38:49–55. doi: 10.1159/000089762. [DOI] [PubMed] [Google Scholar]