Abstract
Objective
Many vascular surgeons construct arteriovenous fistula (AVF) for hemodialysis access as the primary choice access. However, a significant number of AVF fail to mature leading to patient frustration and repeated operations. MMP activity, particularly MMP-2 and MMP-9 may be important for AVF maturation. We therefore sought to identify whether serum MMPs could serve as a biomarker for predicting future successful AVF maturation.
Methods
Patients with chronic renal insufficiency requiring long-term access had blood collected at the time of surgery. Serum was separated from whole blood by the use of an ultracentrifuge at 1000g for 10 minutes. Serum aliquots were then frozen at -80C until used for analysis. MMP-2, MMP-9, TIMP-2, and TIMP-4 were assayed using the ELISA technique. Patients were divided into failed and matured groups depending upon clinical endpoints. Successful maturation was considered in patients who had specific duplex findings or one month of successful two needle cannulation hemodialysis. MMP/TIMP ratios were calculated as an index of the MMP axis activity since MMP activity parallel alterations in their TIMPs.
Results
Twenty patients were enrolled, 13 patients had successful maturation and 7 had failure of AVF maturation. Significantly higher serum levels of MMP-2/TIMP-2 was found in patients who had AVF that matured compared to those that failed (P=.003). Similarly, a trend towards increased serum levels of MMP-9/TIMP-4 were found in patients with successful AVF (P=.06).
Conclusions
MMP-2 and TIMP-2 levels were different among patients who mature their fistulae versus those who did not. Further follow-up studies to determine the predictability of AVF maturation using relative patient serum levels of MMP-2 and TIMP-2 should be performed.
Introduction
A majority of patients with end stage renal failure require hemodialysis and the arteriovenous fistula (AVF) is the preferred method for access. AVF are the most durable, resistant to infection, and thrombosis and are preferred for lower mortality and cost profiles.1 Recognizing the superiority of this access, the National Kidney Foundation recommends an aggressive approach to the creation of AVF.2 However, 20-60% of primary AVF fail to develop into a functioning dialysis access.3-4 Failure can be from impaired vein remodeling, intimal hyperplasia, technical problems, unrecognized stenoses within the outflow vein, inflow problems, or steal syndromes, all leading to failure of achieving a mature AVF.
Vascular access failure is the most important cause for morbidity, repeat surgery, and hospitalization.5 Given the challenges of successful access surgery, the biochemical and pathological changes associated with AVF maturation and intimal hyperplasia should be sought. Recent studies have shown an important role of matrix metalloproteinases (MMP) in the process of AVF maturation.6-7 MMPs belong to a group of zinc-dependent proteases capable of degrading extracellular matrix (ECM) proteins.8-9 In particular, MMP-2 is expressed by a variety of cell types and activated by membrane-bound membrane type-1 MMP (MT1-MMP) and is inhibited by tissue inhibitor metallopreoteinases type 2 (TIMP-2).10 MMP-9 is also expressed by a variety of cell types and is inhibited by tissue inhibitor metalloproteinases type 4 (TIMP-4). Because MMP-2 and MMP-9 have been found to have increased expression in the outflow vein tissue, after AVF construction.7, 11 MMP expression in human patient serum at the time of initial surgery may serve as an important biomarker of AVF maturation. In this study, we sought to identify whether patient serum MMP expression and inhibition are associated with successful AVF maturation.
Methods
Human Study Methods
A prospective evaluation of patients undergoing AVF construction for chronic renal insufficiency was performed under institutional review board approval at the Northern California Veterans Affairs Health Care System. All patients were enrolled and followed at the Sacramento Veterans Affairs Medical Center.
After informed written consent was obtained, a patient history and physical examination was performed and pertinent medical history documented. All patients underwent pre-operative vein mapping (without a tourniquet) using duplex ultrasonography documenting vein diameter, patency as well as arterial patency of both upper extremities. As per the 2006 Dialysis on Quality Initiative (DOQI) vascular access guidelines, the following was used as a guide.12 A radial artery diameter of greater than 0.2 cm and a vein diameter of greater than 0.2 cm at the wrist or greater than 0.3 cm at the antecubital fossa was a requirement for primary AVF. The minimum diameter was also a determinant in the location with which the AVF would be constructed. Both upper extremities were mapped and the most distal vein segment in the non-dominant extremity was used if all diameter criteria were met.
The construction of the AVF was performed under local anesthesia with monitored anesthesia care. The arterial and venous segments were dissected as per routine surgical care. Intraoperatively, 20 ml of blood was obtained from the open artery at the time of surgery via an 18-gauge angio-catheter. Blood samples were immediately placed in heparin containing tubes (green top tubes) and kept at room temperature for immediate transport to the laboratory. Immediate centrifugation and preparation was performed within 2 hours of sample collection. Once a serum pellet was collected, the sample was then kept in -80°C freezer for later ELISA analysis. After surgical construction, the patients were seen in the vascular surgery clinic at 2 weeks and 6 weeks to assess for surgical healing and patency of the AVF. Patients were followed longitudinally until a mature AVF was achieved. If the AVF was abandoned, for another site and a new AVF constructed, the follow up ended for the abandoned access site. Fistula with velocity findings consistent with stenosis was recommended intervention. Patients with deep vein segments underwent secondary superficialization either through a fistula elevation procedure or a basilic vein transposition.
Determination of Fistula maturation
Patients were deemed to have a mature AVF when the first 2 of 3 criteria were met for patients not yet on dialysis or at least the third criteria was met if the patient required dialysis:
Vein diameter of greater than 0.6 mm.
Flow volumes of greater than 600 ml/min.
Complete two-needle cannulation for two-thirds or more of all dialysis runs for 1 month after initiating dialysis.
A duplex assessment of the AVF was performed 6 weeks after AVF creation, but no specific time frame from AVF creation to initial dialysis access was established, because early referral for AVF, may lead to the diagnosis of “failed AVF,” simply due to the non-use of the dialysis access. If the hemodialysis access was unable to be accessed or the vein segments were too small for hemodialysis access based on duplex ultrasonography, the patients may undergo subsequent hemodialysis access procedure. If the AVF was found to be thrombosed upon examination, the access procedure was then considered a failure. The patients were divided into a successful AVF group and a failed AVF group for protein analysis.
Serum Matrix metalloproteinase (MMP) methods
Patient serum levels of MMP-2, MMP-9, TIMP-2, and TIMP-4 were profiled using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D systems, MN). Background activity in the negative control wells were subtracted from the experimental wells in reporting the data. The positive controls were the recombinant protein standards, the negative controls were the calibrator diluents. The sensitivity of the assay for each protein was as follows: MMP-2: 0.0147 ng/mL, MMP-9: less than 0.156 ng/mL, TIMP-2: 0.011 ng/mL, and TIMP-4: 4.91 pg/mL.
After peripheral blood was collected from the patient, serum was obtained by centrifugation. Serum was centrifuged at 400g for 30min and stored at -80°C until tested. Patient sera were divided according the clinical outcome of failed versus matured. Upon testing, serum was thawed and diluted with an appropriate concentration of dilution buffer. Sample and controls were added to 96-well plates which were coated with an antibody specific to the protein of interest. After 2 hours of incubation, plates were washed and then a secondary antibody to the protein of interest which was conjugated to horseradish peroxidase were added. After 2 hours of incubation, plates were washed and incubated with substrate solution for 30min, then an acid stop solution were added and the plate was read on a SpectraMax 190 microplate reader (Molecular Devices, CA) at 450nm with wavelength correction at 540nm. A ratio of MMP/TIMP was used as an indicator of MMP activity: a higher ratio means higher activity.13
Statistics
Results are reported as mean ± standard deviation. Microsoft Excel (Redmond, WA) was utilized to perform a two-tailed t-test was used to compare groups with continuous variables and a Chi-squared analysis for proportions. A difference was considered significant for a P-value <0.05.
Results
Twenty patients were enrolled, 13 patients had successful maturation (Age ± SD: 64.7 ± 14.2) and 7 had failure (Age: 60.8 ± 7 years) of AVF maturation (P=0.41). There were no differences in the pre-operative vein diameters in the matured group 0.37 mm ± .014 versus the failed group 0.36 ± 0.14 (P=0.82). There were 15% wrist fistulae in the mature group and 57% wrist fistula in the failed group (P=0.17). There were no significant differences in the co-morbidities between groups (Table). The average follow up for the matured group was 18 months, and the average time to abandonment for the failed group was 4 months. Thirteen of twenty (65%) of patients required catheter placement during the follow up period.
Table.
Data for all patients within the study. Continuous variables are reported with ± standard deviation. DIAB=diabetes, HTN=hypertension, Tob=tobacco use, CAD=coronary artery disease, CVA=stroke, Statin=statin use. ESRF DX=diagnosis causing renal failure, FSGS=focal sclerosis glomerulosclerosis, Dialysis Access=at the time of index AVF construction whether another access site was achieved or was an initial access site, CATH=catheter dialysis, NM=Nonmaturation, Thromb=Thrombosis, F/U=follow-up, vein diameter pre and post AVF construction= mm, flow volume=ml/min. Bottom row are the P-values comparing the two variables, *Statistically significant.
| Pt | Age ±SD | Procedure | DIAB | HTN | Tob | CAD | CVA | Statin | ESRF DX | Dialysis Access | Time on dialysis | Time to AVF use | F/U | Pre Vein diam | Post Vein diam | Flow volume | 2° proc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | Wrist | Y | Y | N | N | N | N | FSGS | CATH | 5 | NM | 3 | 0.26 | 0.33 | 162 | N |
| 2 | 48 | Wrist | N | Y | N | N | N | Y | FSGS | CATH | 2 | THROMB | 1 | 0.63 | -- | N | |
| 3 | 54 | BB | N | Y | Y | N | N | Y | FSGS | CATH | 13 | NM | 5 | 0.36 | 0.6 | 609 | N |
| 4 | 67 | BB | Y | Y | N | N | N | Y | DIAB | INITIAL | 0 | NM | 10 | 0.22 | 0.38 | 248 | Y |
| 5 | 84 | BB | N | Y | N | N | N | Y | HTN | INITIAL | 3 | THROMB | 3 | 0.38 | -- | -- | N |
| 6 | 67 | Wrist | Y | Y | N | N | N | Y | DIAB | INITIAL | 0 | NM | 2 | 0.23 | 0.37 | 300 | N |
| 7 | 81 | BB | N | N | N | N | N | N | HTN | CATH | 168 | THROMB | 2 | 0.41 | -- | -- | N |
| 65±14 | 57% | 43% | 86% | 14% | 0% | 0% | 71% | 57% | 27 | --- | 3.7 | 0.36 ±.14 | 0.42 ±.12 | 329.8 ±195 | |||
| 8 | 52 | BB | Y | Y | N | N | N | N | FSGS | CATH | 9 | 10 | 17 | 0.48 | 0.74 | 1435 | Y |
| 9 | 69 | BB | Y | Y | Y | N | N | Y | HTN | CATH | 1 | 5 | 18 | 0.29 | 0.8 | 1048 | Y |
| 10 | 68 | BB | Y | Y | N | Y | N | Y | DIAB | INITIAL | 0 | 10 | 16 | 0.37 | 0.62 | 1300 | Y |
| 11 | 61 | Wrist | Y | Y | N | Y | N | Y | DIAB | CATH | 2 | 2 | 2 | 0.24 | -- | -- | N |
| 12 | 62 | BC | Y | Y | N | N | N | Y | DIAB | INITIAL | 0 | 4 | 15 | 0.29 | 0.85 | 972 | Y |
| 13 | 64 | BB | Y | Y | N | Y | N | Y | DIAB | CATH | 48 | 3 | 3 | 0.23 | 0.84 | 1009 | Y |
| 14 | 56 | BB | Y | Y | N | N | N | N | DIAB | CATH | 1 | 8 | 8 | 0.41 | 0.80 | 1000 | Y |
| 15 | 58 | BB | Y | Y | N | N | N | Y | DIAB | INITIAL | 0 | 9 | 22 | 0.56 | 1.3 | 4600 | Y |
| 16 | 46 | BC | Y | Y | N | Y | N | Y | DIAB | CATH | 1 | 3 | 3 | 0.67 | 1.0 | 1070 | Y |
| 17 | 61 | Wrist | Y | Y | Y | N | Y | Y | DIAB | CATH | 4 | 4 | 26 | 0.22 | -- | -- | N |
| 18 | 68 | BC | Y | Y | N | N | N | Y | DIAB | CATH | 5 | 4 | 18 | 0.42 | 0.79 | 1189 | Y |
| 19 | 67 | BC | N | Y | N | N | N | Y | HTN | INITIAL | 0 | 50 | 50 | 0.3 | 0.99 | 1901 | Y |
| 20 | 59 | BC | Y | Y | Y | N | N | N | DIAB | CATH | 13 | 4 | 30 | 0.34 | 0.84 | 800 | Y |
| 61±7 | 15% | 92% | 100% | 23% | 31% | 8% | 77% | 69% | 6.5 | 8.9 | 17.5 | 0.37 ±0.14 | 0.87 ±0.18 | 1484 ±1075 | |||
| 0.41 | 0.17 | 0.33 | 0.82 | 0.7 | 0.94 | 0.47 | 0.92 | 0.80 | 0.25 | *.01 | 0.82 | *.0005 | .06 |
At one month post- index AVF construction, no AVF was deemed mature. The average time from AVF creation to actual dialysis access was 8.9 months. Eleven out of 13 patients who had matured AVF required a secondary fistula elevation or transposition procedure to allow for successful access of the index AVF.
Of the 7 patients that had failed AVF, 4 patients had a patent AVF but non-maturation. One patient in the non-maturation group had a maximum vein diameter of the AVF of 0.38 mm and flow volumes of 248 ml/min. This patient underwent fistulogram and balloon angioplasty, with marginal success in the attempt to accelerate maturation.
Significantly higher serum levels of MMP-2/TIMP-2 were found in patients who had AVF that matured (23.8 ± 0.8) compared to those that failed (20.6 ± 0.9) (P=.003). Similarly, a trend towards increased serum levels of MMP-9/TIMP-4 were found in patients with successful AVF (128.6 ± 34.3) versus serum levels in patient with failed AVF (40.2 ± 10.1) (P=.06). See Figures 1 and 2.
Figure 1.
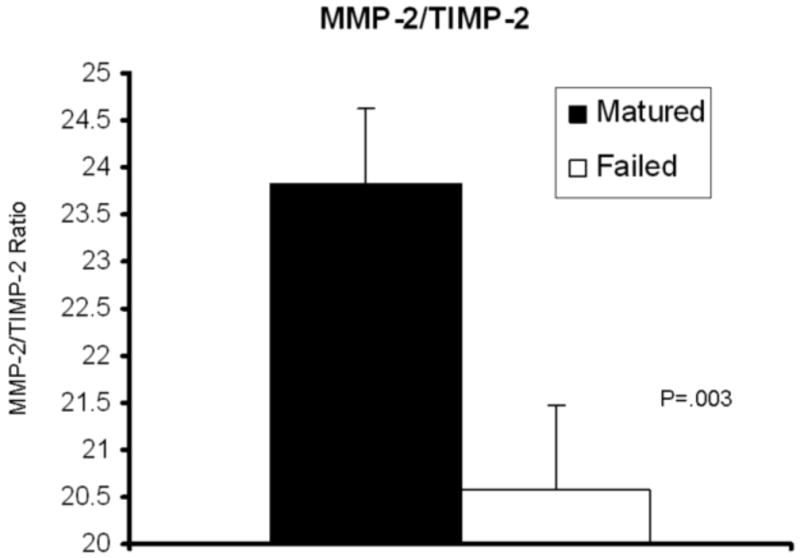
Significantly higher levels of MMP-2 to TIMP-2 ratios are identified in serum of patients who had successful maturation of their arteriovenous fistula when compared to the serum of patients who had failure (P=.003).
Figure 2.

Higher levels of MMP-9 to TIMP-4 ratios are identified in serum of patients who had successful maturation of their arteriovenous fistula when compared to the serum of patients who had failure (P=.06).
Discussion
Since the National Kidney Foundation introduced the Dialysis Outcomes and Quality Initiative, guidelines and access management criteria are being used to judge vascular access programs.2 In a recent review of the natural history of autogenous fistulas for first-time dialysis access, only 11% of primary AVF matured without intervention and a total of 48% of the primary AVF were ultimately utilized for hemodialysis access. The mean time to AVF maturation was 146 days and the mean time for AVF abandonment was 162 days.14 Given these results, many patients undergo repetitive unsuccessful procedures. To improve upon this clinical problem, investigators have focused on vascular remodeling and vascular pathology. In previous studies, the animal model has been used to evaluate MMPs which would be an obvious choice as these proteins have played a well-established role in both intimal hyperplasia15 and vessel enlargement6-7 when it comes to AVF maturation. MMPs are a family of multi-domain endopeptidases that regulate physiological and pathological vascular remodeling under conditions such as atherosclerosis, arterial aneurysm, graft, and wound healing. The major function of MMPs is degrading extracellular matrix components thereby allowing circulating cells and smooth muscle cells to migrate into the vessel wall that undergoes outward remodeling. MMPs also target non-matrix substrates such as cytokines (TNFa, IL-1) and growth factors (VEGF, TGFb, bFGF).16 Both vascular cells (endothelium) and inflammatory cells (monocytes) can produce MMPs. In many cases, MMPs are secreted as pro-enzymes (latent) and then processed to active forms. Enzymatic activation requires removal of a pro-domain secondary to conformational changes that reveal the catalytic site, and hence the basis for detection of both latent and activated MMPs by zymography. In terms of activity, the best recognized regulators are cell-associated membrane-type MMPs (MT-MMPs) and tissue inhibitor of metalloproteinases (TIMPs). Using MMP-2 as an example, activation occurs via formation of a tertiary complex containing MT1-MMP (activator), TIMP-2 (inhibitor), and MMP-2 on endothelial cell surface. Equilibrium is maintained by balanced activities of these enzymes. Thus, the ratio of MMP/TIMP is often used as an indicator of MMP activity: a higher ratio means higher activity.13, 17
The endothelium is an important source of MMPs in the vascular wall.18 Endothelial injury can cause impaired MMP production and activity required for expansive remodeling.19 In particular, MMP-2 and MMP-9 activity has been established as a mechanistic step for the venous outflow segment of an arteriovenous fistula to mature in animal models.6-7, 15, 20 However, intimal hyperplasia has also been associated with increased MMP-221 and MMP-9 activity.15 The relative contribution of MMP activity to remodeling vs. intimal hyperplasia is not clearly known.4, 22 Given the critical role of expansive remodeling in preserving vessel lumen and patency in fistula maturation, the MMP effect is considered more beneficial than detrimental. Indeed, a recent study demonstrates that MMPs play a crucial role in the expansive remodeling of AVF in rats.7
This study is an attempt to evaluate biological serum markers to assess for the potential of AVF maturation in trying to predict initial AVF maturation. Biomarkers have demonstrated a great value in the diagnosis and treatment of many cardiovascular disease entities such as troponin in the diagnosis of acute myocardial infarction23 and C-reactive protein in predicting cardiac risk.24 More recently, circulating biomarkers of fibrinogen, D-dimer, and IL-6 have been suggested as areas of interest in detecting the presence and progression of abdominal aortic aneurysms25 and other types of vascular injury.26 Circulating biomarkers offer diagnostic or prognostic value by reflecting the disease state and predilection based on plasma measurements of molecules or proteins.24
The clinical impact of using preoperative markers resides with its use as a potential adjunct to other known clinical parameters that best maximize AVF maturation, such as a thorough physical examination, arterial inflow assessment as well as noninvasive vein mapping. The findings in this study, with a limited number of patients, point to the potential use of known enzymes and its inhibitors (MMP-2 and TIMP-2) that are necessary in vascular remodeling. The ELISA technique is readily available in many basic laboratories and the potential application to clinical practice is possible.
However, the data presented in this study demonstrates relative value differences between patients who matured their fistulae versus those who did not. Patient sample size was small and no significant differences were found in traditional clinical parameters in predicting success. A follow-up study designed to determine predictability is in progress so that in a prospective manner, an ELISA can be performed an estimate of successful maturation can be made.
Since the Fistula First Initiative, AVF prevalence has increased from 24% to 57.5% in the United States from 2004 to December, 2010.27 However, central vein catheter use for implementation of dialysis has risen 1.5 to 3.0 fold from 1996-2007 in the international community when evaluating trends of rising AVF prevalence and decreasing AV graft use.28 Others dispute the application of these numbers to the United States. Spergel states that despite the rise in AVF prevalence rate over the past several years since the Fistula First Initiative in 2004, catheter use has remained flat at 27-28%.29 This has left many difficulties in clinical care, such as the quandary of an AV graft versus an AVF and catheter in the face of marginal or unusuable veins.1 The use of MMPs in modulating practice patterns in deciding which treatment option is best is potentially promising and worth further research effort.
A severe limitation of this study has been the wide variation in the definition of AVF maturation.4 Previous surgical studies have defined adequate maturation as deemed by the vascular surgeon and nephrologist on the basis of thrill characteristics and AVF diameter.30 Other studies have defined a mature AVF as multiple successful cannulation attempts for dialysis. Nephrologists have defined maturation in other ways. Lok and associates define AVF maturation as the continuous use of the AVF for 1 month within 6 months of AVF creation, regardless of secondary interventions required to mature the AVF.31 However, the Dialysis Access Consortium had the strictest definition of AVF maturation where an AVF needs to have an 8 of 12 successful dialysis sessions during a 30 day “suitability” period within the first 150 days of AVF creation.3 The DOQI access group plans to publish guidelines for AVF maturation in 2011.
Serum MMP levels are markers of inflammation and are known to be increased in renal failure. Patients with renal failure, but not yet on dialysis have the highest levels of serum MMP-2. When patients are placed on dialysis, the MMP levels decrease, but do not fall to control levels.17 The data presented in this study utilized patients in various stages of kidney failure and were either pre-dialysis or on hemodialysis. Clearly, the data are confounded by patients in various stages of renal insufficiency to those patients on hemodialysis. Once a patient is on hemodialysis, the MMP levels have been shown to decrease, but not quite to levels seen in control patients without any renal insufficiency. Ironically, MMP levels could be serially tested to identify a peak increase to when the timing of initial AVF should be constructed. Further data should be collected and standardized in patients who have not yet begun dialysis in assessing the likelihood for AVF maturation.
Conclusions
MMP-2 and TIMP-2 levels were different among patients who mature their fistulae versus those who did not. Further follow-up studies to determine the predictability of AVF maturation using relative patient serum levels of MMP-2 and TIMP-2 are ongoing.
Acknowledgments
Supported in part by the Peripheral Vascular Surgery Society Academic Award and the Society for Vascular Surgery Seed Award.
Footnotes
Presented at the 25th Annual Meeting Western Vascular Society Sunriver, OR September 25-28, 2010
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James MT, Manns BJ, Hemmelgarn BR, Ravani P. What's next after fistula first: is an arteriovenous graft or central venous catheter preferable when an arteriovenous fistula is not possible? Semin Dial. 2009;22(5):539–44. doi: 10.1111/j.1525-139X.2009.00633.x. [DOI] [PubMed] [Google Scholar]
- 2.Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001;37(1 Suppl 2):S137–181. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 3.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299(18):2164–71. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon BS. Why don't fistulas mature? Kidney Int. 2006;70(8):1413–22. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli M. Randomized trials in hemodialysis patients: time to step up to the plate. JAMA. 2008;299(18):2205–7. doi: 10.1001/jama.299.18.2205. [DOI] [PubMed] [Google Scholar]
- 6.Berceli SA, Jiang Z, Klingman NV, Schultz GS, Ozaki CK. Early differential MMP-2 and -9 dynamics during flow-induced arterial and vein graft adaptations. J Surg Res. 2006;134(2):327–34. doi: 10.1016/j.jss.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Chan CY, Chen YS, Ma MC, Chen CF. Remodeling of experimental arteriovenous fistula with increased matrix metalloproteinase expression in rats. J Vasc Surg. 2007;45(4):804–11. doi: 10.1016/j.jvs.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 8.Chase AJ, Newby AC. Regulation of matrix metalloproteinase (matrixin) genes in blood vessels: a multi-step recruitment model for pathological remodelling. J Vasc Res. 2003;40(4):329–43. doi: 10.1159/000072697. [DOI] [PubMed] [Google Scholar]
- 9.Kargozaran H, Yuan SY, Breslin JW, Watson KD, Gaudreault N, Breen A, et al. A role for endothelial-derived matrix metalloproteinase-2 in breast cancer cell transmigration across the endothelial-basement membrane barrier. Clin Exp Metastasis. 2007;24(7):495–502. doi: 10.1007/s10585-007-9086-6. [DOI] [PubMed] [Google Scholar]
- 10.Goodall S, Crowther M, Hemingway DM, Bell PR, Thompson MM. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation. 2001;104(3):304–9. doi: 10.1161/01.cir.104.3.304. [DOI] [PubMed] [Google Scholar]
- 11.Lee ES, Shen Q, Pitts RL, Guo M, Wu MH, Yuan S. Vein Tissue Expression of Matrix Metalloproteinase as Biomarker for Hemodialysis Arteriovenous Fistula Maturation. Vasc Endovascular Surg. 2010 doi: 10.1177/1538574410377021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vascular Access 2006. Am J Kid Dis. 2006;48(Supp1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg. 2008;47(2):415–21. doi: 10.1016/j.jvs.2007.10.041. discussion 420-1. [DOI] [PubMed] [Google Scholar]
- 15.Misra S, Fu AA, Anderson JL, Sethi S, Glockner JF, McKusick MA, et al. The rat femoral arteriovenous fistula model: increased expression of matrix metalloproteinase-2 and -9 at the venous stenosis. J Vasc Interv Radiol. 2008;19(4):587–94. doi: 10.1016/j.jvir.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26(7):1503–9. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 17.Preston GA, Barrett CV, Alcorta DA, Hogan SL, Dinwiddie L, Jennette JC, et al. Serum matrix metalloproteinases MMP-2 and MMP-3 levels in dialysis patients vary independently of CRP and IL-6 levels. Nephron. 2002;92(4):817–23. doi: 10.1159/000065464. [DOI] [PubMed] [Google Scholar]
- 18.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48(3):504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20(12):E120–6. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- 20.Karwowski JK, Markezich A, Whitson J, Abbruzzese TA, Zarins CK, Dalman RL. Dose-dependent limitation of arterial enlargement by the matrix metalloproteinase inhibitor RS-113,456. J Surg Res. 1999;87(1):122–9. doi: 10.1006/jsre.1999.5707. [DOI] [PubMed] [Google Scholar]
- 21.Tummers AM, Mountain DJ, Mix JW, Kirkpatrick SS, Cassada DC, Stevens SL, et al. Serum levels of matrix metalloproteinase-2 as a marker of intimal hyperplasia. J Surg Res. 2010;160(1):9–13. doi: 10.1016/j.jss.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–62. [PubMed] [Google Scholar]
- 23.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 24.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47(8 Suppl):C19–31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 25.Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008;118(23):2382–92. doi: 10.1161/CIRCULATIONAHA.108.802074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo M, Daines D, Tang J, Shen Q, Perrin RM, Takada Y, et al. Fibrinogen-gamma C-terminal fragments induce endothelial barrier dysfunction and microvascular leak via integrin-mediated and RhoA-dependent mechanism. Arterioscler Thromb Vasc Biol. 2009;29(3):394–400. doi: 10.1161/ATVBAHA.108.180950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [Accessed February 21, 2011]; http://www.fistulafirst.org.
- 28.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23(10):3219–26. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spergel LM. Has the Fistula First Breakthrough Initiative caused an increase in catheter prevalence? Semin Dial. 2008;21(6):550–2. doi: 10.1111/j.1525-139X.2008.00501.x. [DOI] [PubMed] [Google Scholar]
- 30.Patel ST, Hughes J, Mills JL., Sr Failure of arteriovenous fistula maturation: an unintended consequence of exceeding dialysis outcome quality Initiative guidelines for hemodialysis access. J Vasc Surg. 2003;38(3):439–45. doi: 10.1016/s0741-5214(03)00732-8. discussion 445. [DOI] [PubMed] [Google Scholar]
- 31.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I) J Am Soc Nephrol. 2006;17(11):3204–12. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]


