Abstract
Silicon (Si) is the most abundant minerals in soil and exerts beneficial effects on plant growth by alleviating various stresses. The transport of Si from soil to the panicles is mediated by different transporters. Lsi1, belonging to a NIP group of the aquaporin family, is responsible for the uptake of Si from soil into the root cells in both dicots and monocots although its expression patterns and cellular localization differ with plant species. The subsequent transport of Si out of the root cells towards the stele is medicated by an active efflux transporter, Lsi2. Lsi1 and Lsi2 are polarly localized at the distal and proximal sides, respectively, of both exodermis and endodermis in rice root. Silicon in the xylem sap is presented in the form of monosilicic acid and is unloaded by Lsi6, a homolog of Lsi1 in rice. Lsi6 is also involved in the inter-vascular transfer of Si at the node, which is necessary for preferential Si distribution to the panicles.
Keywords: localization, distribution, uptake, silicon, transporter
Introduction
Silicon (Si) is the second most abundant element after oxygen in soil. Because of its strong affinity with oxygen, in nature Si always exists as silica or silicate, which are combined with various metals. Silicon dioxide comprises 50 to 70 percent of the soil mass. Most soils have Si in the solution between 100 to 500 µM in the form of monosilicic acid (H4SiO4).1) Therefore, all plants grown in soil will contain some Si in their tissues although the Si concentration in the shoots varies considerably among plant species, ranging from 0.1% to 10% Si in the dry weight.2–4)
Si has not been recognized as an essential element for plant growth. The major reason is that there is no evidence to show that Si is involved in the metabolism of plant, which is one of the three criteria required for essentiality established by Arnon and Stout.5) However, the beneficial effects of Si on the growth have been reported in a wide of plant species, which are characterized by protecting the plant from various biotic and abiotic stresses.
Si enhances resistance of plants to diseases caused by both fungi and bacteria in different plant species. For example, in rice, Si reduces the epidemics of both leaf and panicle blast at different growth stages.6) Si also decreases the incidence of powdery mildew in cucumber, barley and wheat; sheath blight in rice; ring spot in sugarcane; rust in cowpea; leaf spot in bermuda grass and gray leaf spot in St. Augustine grass and perennial ryegrass.7)
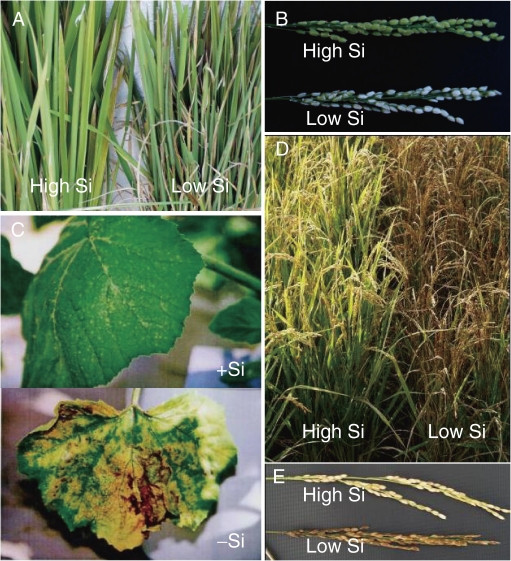
Silicon also suppresses insect pests such as stem borer, brown planthopper, rice green leafhopper, whitebacked planthopper and noninsect pests such as leaf spider and mites (Fig. 1A).3,8) Resistance to the damage by wild rabbit in wheat is also enhanced by an increased amount of Si in wheat.9)
Figure 1.
Examples showing beneficial effects of Si in plants. A, Effect of Si on pest damage. B, Protective role of Si in excess transpiration of panicles in rice. C, Alleviative effect of Si on Mn toxicity in pumpkin. The plants were exposed to high Mn in the presence (+Si) or absence (−Si) of Si. D–E, Decreased fertility due to low Si in rice. A–B, D–E, wild type rice (High Si) and a mutant defective in Si uptake (Low Si) were grown in the field. From Tamai and Ma (2008).15)
Si plays an important role in alleviating various abiotic stresses including physical stress and chemical stress.10) For example, Si can alleviate water stress, climatic stress such as typhoons, low temperature and insufficient sunshine during the summer season.2,3,11,12) Excess transpiration from the husk causes white head, resulting in low fertility in rice (Fig. 1B), however, Si is effective to protect the panicles from excess transpiration due to heavy deposition of Si on the husk. The beneficial effects of Si under chemical stresses including P-deficiency, P excess, Mn and salt toxicity have been observed in many plants.3) Mn toxicity in pumpkin is significantly reduced by Si application (Fig. 1C).13)
In addition to the role of Si in alleviating various stresses, Si improves light interception by keeping leaves erect, thereby stimulating canopy photosynthesis in rice.3) This is particularly important in dense plant stands and when nitrogen fertilizers are heavily applied, to minimize mutual shading.
Silicon is especially important for healthy growth and high production of rice,13–15) which is able to accumulate Si to over 10% of its dry weight in the shoots. When Si accumulation is not enough, the yield is greatly reduced mainly due to decreased fertility (Fig. 1D, E).15) For this reason, Si fertilizers are applied in paddy field in some countries including Japan.
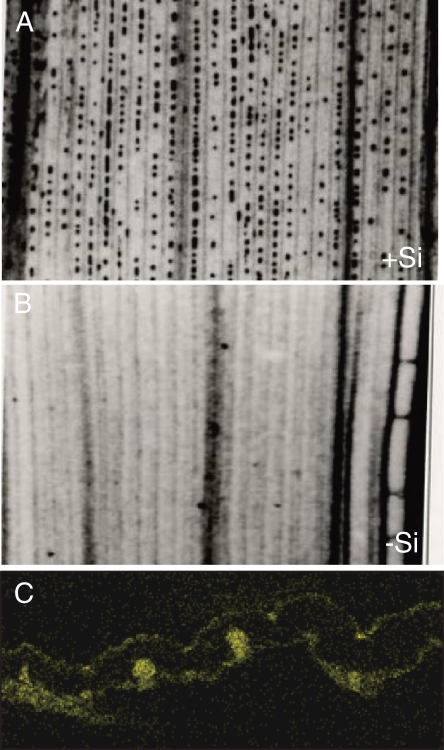
All beneficial effects of Si are mostly attributed to its deposition in different organs. For example, Si is deposited beneath the cuticle to form a cuticle-Si double layer in the leaf (Fig. 2).3,10) This layer can mechanically impede penetration by fungi and pest and thereby avoiding the infection process. Therefore, to benefit from Si, a high accumulation is required. In this review, we focus on Si transporters involved in the uptake, translocation and distribution, which are identified in our lab.
Figure 2.
Deposition of Si in rice leaf blade. A–B, soft X-ray image of rice leave with (A) or without (B) Si. Black dot shows silica bodies. C, SEM-EDX image of a cross section of rice leaf blade. Yellow color shows Si deposition under cuticle and bulliform motor cells known as plant opal.
Transporters involved in Si uptake
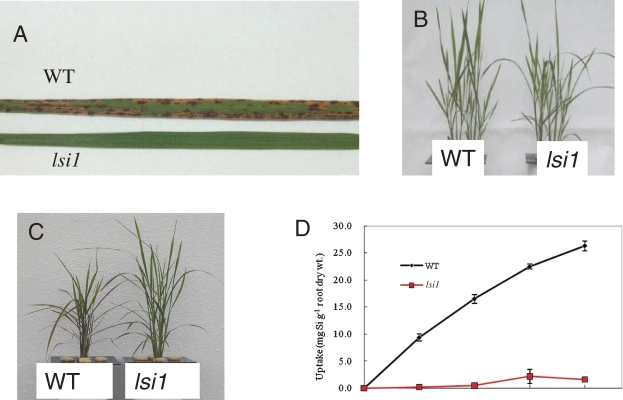
Plant roots take up Si in the form of silicic acid [Si(OH)4]. To identify genes that are responsible for Si uptake in rice, we isolated rice mutants with a low Si content. We used tolerance to germanium (Ge) as an indicator for screening rice mutants defective in Si uptake. Germanium is an analogue of Si and plant roots take up Si and Ge similarly. However, once it is taken up, Ge is toxic to plants, as shown by the characteristic brown spots on the leaf blades (Fig. 3A).16) By using this approach, we have isolated two independent mutants (designated lsi1 and lsi2 for low Si 1 and 2) showing tolerance to Ge from different mutagenized seeds.
Figure 3.
Phenotype of lsi1 mutant. A, Symptoms of Ge toxicity in the leaf of wild-type rice (WT) and mutant lsi1. B–C, Growth of WT and lsi1 in the absence (B) and presence (C) of Ge. D, Si uptake in the mutant and WT. From Ma et al. (2002).16)
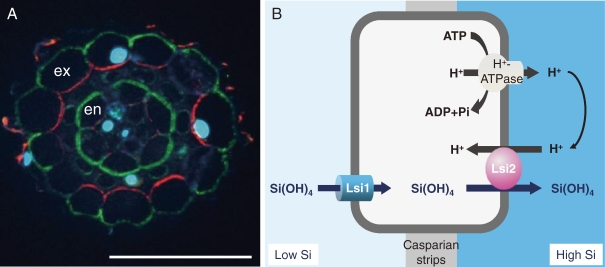
lsi1 mutant shows similar growth as wild-type rice in the absence of Ge (Fig. 3B).16) However, in the presence of Ge, the growth of lsi1 mutant is hardly inhibited, in contrast to the wild-type rice, whose growth is severely inhibited by Ge (Fig. 3C). The Si uptake of lsi1 is much lower than that of wild-type rice, indicating that this is a mutant defective in Si uptake. By using lsi1 mutant, the first Si transporter Lsi1 was identified by map-based cloning technique.17) Lsi1 belongs to a Nod26-like major intrinsic protein (NIP) subfamily of aquaporin-like proteins and shows influx activity for silicic acid in Xenopus oocyte. The predicted amino acid sequence has six transmembrane domains and two Asn-Pro-Ala (NPA) motifs, which is well conserved in typical aquaporins. Lsi1 is constitutively expressed in the roots, but its expression is decreased to one fourth by Si supply.17) Recently, it was found that Si accumulated in the shoots but not in the roots regulates the expression of Lsi1.18) Within a root, the expression of Lsi1 is much lower in the root tip region between 0–10 mm than in the basal regions of the root (>10 mm).19) Silicon uptake in the root tip region (0 to 10 mm) comprising both the apical meristem and the elongation zone is also much lower than that in the basal regions (>10 mm from the root tips). Therefore, the site of Si uptake is located in the mature regions of the roots rather than in the root tips. Lsi1 is localized in the main and lateral roots, but not in root hairs.17) In the roots including seminal, lateral and crown roots, the Lsi1 protein is localized to the plasma membrane of both exodermis and endodermis,17) where the Casparian strips prevents apoplastic transport into the root stele. Furthermore, Lsi1 shows polar localization at the distal side of both the exodermis and endodermis cells (Fig. 4A).
Figure 4.
Localization of Si transporters in rice. A, Polar localization of influx Si transporter Lsi1 (green) and efflux Si transporter Lsi2 (red) in lateral roots. Ex, exodermis; En, endodermis; nuclei stained by DAPI (cyan). From Yamaji and Ma (2011).18) B, Schematic presentation of Si uptake system in rice root exodermis/endodermis cells.
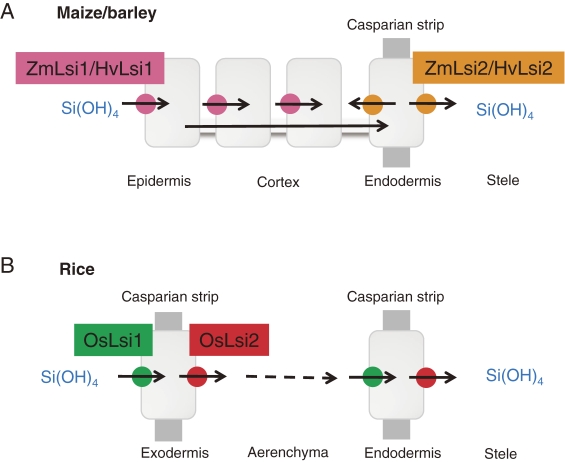
Homologs of rice Lsi1 (OsLsi1) have been identified in barley (HvLsi1), maize (ZmLsi1) and pumpkin (CmLsi1).20–22) However, different from OsLsi1, HvLsi1 from barley and ZmLsi1 from maize are localized at epidermal, hypodermal and cortical cells.20,21) CmLsi1 from the pumpkin is localized at all root cells.22) Interestingly, mutation at the position of 242 from proline to leucine in CmLsi1 resulted in significant decrease in Si uptake in some pumpkin cultivars due to failure to the localization at the plasma membrane.22) In contrast to OsLsi1, the expression levels of HvLsi1 and ZmLsi1 are unaffected by Si.20,21)
The second Si transporter Lsi2 in rice was also identified by mutant approach as Lsi1. However, different from Lsi1, Lsi2 is an efflux transporter of Si in rice.23) Lsi2 belongs to a putative anion transporter without any similarity with the silicon influx transporter Lsi1. The expression pattern and tissue- and cellular-localization of Lsi2 is the same as that of Lsi1,23) but in contrast to Lsi1, Lsi2 is localized at the proximal side of the exodermis and the endodermis cells (Fig. 4A). Transport of Si by Lsi2 is driven by the proton gradient (Fig. 4B).
Similar transporters of Lsi2 have also been identified in barley and maize.24) However, ZmLsi2 and HvLsi2 are localized only to the endodermis of roots in maize and barley without polarity. These differences result in different pathway of Si from external solution to the xylem between upland crops (barley and maize) and paddy crop (rice). In barley and maize, Si can be taken up from external solution (soil solution) by HvLsi1/ZmLsi1 at different cells including epidermal, hypodermal and cortical cells (Fig. 5A). In contrast, in rice, Si is only taken up at the exodermal cells by OsLsi1 (Fig. 5B). After being taken up into the root cells, Si is transported to the endodermis by symplastic pathway and then released to the stele by HvLsi2/ZmLsi2 in maize and barley (Fig. 5B). By contrast, in rice, Si taken up by OsLsi1 at the exodermal cells is released by OsLsi2 to the apoplast and then transported into the stele by both OsLsi1 and OsLsi2 again at the endodermal cells (Fig. 5B).
Figure 5.
Schematic presentation of different Si uptake system in barley/maize (A) and rice (B) roots. From Mitani et al. (2009).21)
The difference in the uptake system may be attributed to the root structures. In rice roots, there are two Casparian strips at the exodermis and endodermis, whereas one Casparian strip is usually present at the endodermis of maize and barley roots under non-stressed conditions. Moreover, mature roots in rice have a distinct structure, a highly developed aerenchyma, wherein almost all cortex cells between exodermis and endodermis are destructed. Therefore, Si transported into the exodermis cells by the influx transporter, OsLsi1, has to be released by the efflux transporter, OsLsi2, into the apoplast of a spoke-like structure across the aerenchyma. However, in maize and barley roots, there is no such structure or if any; it is developed poorly. These differences in the localization of transporters and polarity may be one of the reasons for the different Si uptake capacities among species.
Transporter involved in translocation of Si from roots to the shoots
Following uptake by the roots through Lsi1 and Lsi2, Si is translocated to the shoot by transpirational volume flow through the xylem. More than 90% of Si taken up by the roots is translocated to the shoots in rice.3) The Si concentration in the rice xylem sap could be as high as 20 mM. Chemically, silicic acid polymerizes into silica gel (SiO2·nH2O) when the concentration of silicic acid exceeds 2 mM. However, Si in the xylem sap is presented in the form of monosilicic acid.25,26) It seems that silicic acid in the xylem sap at high concentration is transiently present because it starts to polymerize in vitro.26) The transporter for xylem loading of Si has not been identified.
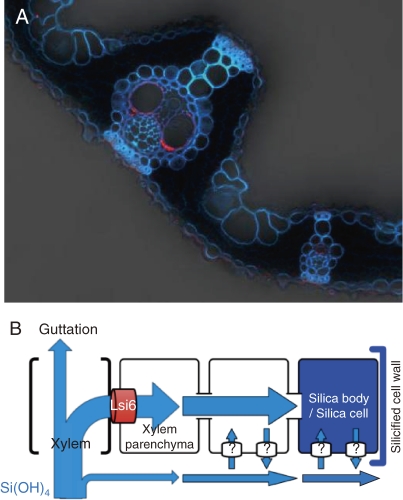
Silicic acid must be exported from the xylem to other leaf cells. Based on similarity with Lsi1, Lsi6 was isolated and found to be responsible for this process.27) Lsi6 also shows transport activity for silicic acid. However, different from Lsi1 and Lsi2, Lsi6 is also expressed in the leaf sheaths and leaf blades in addition to the root tips. Lsi6 is localized in the adaxial side of the xylem parenchyma cells in the leaf sheaths and leaf blades (Fig. 6A). Knockout of Lsi6 does not affect the uptake of Si by the roots, but affects the silica deposition pattern in the leaf blades and sheaths.27) There are two types of silicified cells in rice leaf blades; silica cells, and silica bodies or silicified motor cells (Fig. 2B).3,28) When lsi6 is knocked out, a part of abaxial epidermal cells was also observed to be silicified. Also in the leaf sheaths, the silicified epidermal cells were frequently observed in the knockout line, which are not present in the wild-type rice. These changes in the knockout lines are caused by altered pathway of Si due to loss of function of Lsi6 (Fig. 6B).
Figure 6.
Localization of Lsi6 in rice leaf blade. A, Immunostaining of Lsi6 (red) showing localization at xylem parenchyma cells. B, Pathway of Si from leaf xylem via Lsi6. From Yamaji et al. (2008).27)
Rice is known to produce guttation fluid during the night, a phenomenon of xylem sap exudation through hydathodes, which are located near terminal tracheids of the bundle ends around the margins of leaves due to positive xylem pressure.29) Knockout of lsi6 also causes increased excretion of Si in the guttation fluid in rice due to altered pathway (Fig. 6B).27)
All these observations indicate that Lsi6 is required for delivering Si to the specific cells. Therefore, cell-type specific silicification depends on the symplastic pathway of Si delivered by Lsi6 rather than apoplastic pathway.27)
Transporter for inter-vascular transfer of Si
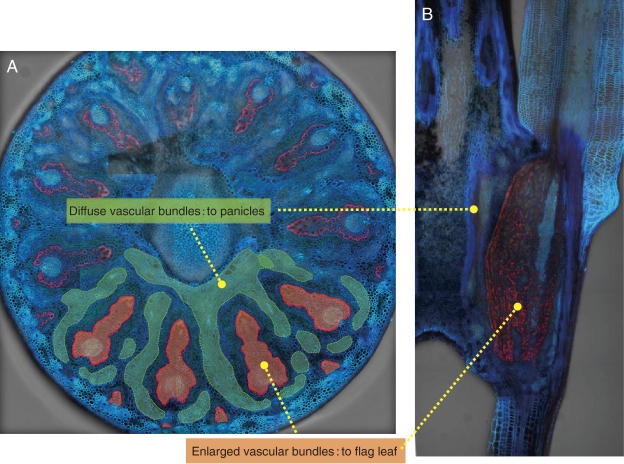
In gramineous plants, minerals taken up by the roots are not directly transported to the grains but are redirected at plant nodes.30–32) Therefore, the distribution of minerals at the nodes is considered to be a key step in the selective control of mineral accumulation in the panicles. At node I beneath the panicles of rice, there are enlarged large and small vascular bundles and diffuse vascular bundles (Fig. 7). Large and small vascular bundles come from the lower nodes and connect to the flag leaf and are markedly enlarged at the node. Diffuse vascular bundles are parallel to and surround the enlarged large vascular bundles. They also are assembled in the upper internode (peduncle) to form regular bundles, which connect toward the panicle tissues.30–32) Therefore, inter-vascular transfer of minerals from the enlarged vascular bundles to diffuse vascular bundles is required to deliver minerals taken up by the roots to developing seeds.
Figure 7.
Localization of Lsi6 at the node I in rice. A, Cross section showing localization of Lsi6 (red) at the transfer cells surrounding enlarged vascular bundles. Enlarged vascular bundles (orange) and diffuse vascular bundles (green) are indicated. B, Longitudinal section of rice node I. Modified from Yamaji and Ma (2009).33)
Recently, Lsi6 is found to be involved in the inter-vascular transfer of Si in rice.33) At the reproductive stage, Lsi6 is highly expressed at the node I below the panicles. Lsi6 is mainly localized at the xylem transfer cells with polarity facing toward the xylem vessel (Fig. 7). These cells are located at the outer boundary region of the enlarged vascular bundles and characterized by large surface area due to cell wall ingrowth. Knockout of lsi6 decreased Si accumulation in the panicles but increased Si accumulation in the flag leaf.33) These findings suggest that Lsi6 is required for transfer of Si from the large vascular bundles coming from the roots to the diffuse vascular bundles connected to the panicles.
Concluding remark
Three different transporters involved in uptake, xylem unloading and inter-vascular transfer of Si have been identified as described above. However, for transport of Si from soil to the panicles, other transporters are also required. For examples, transporters for xylem loading is necessary, but remain unknown. At the node, after unloading Si from enlarged vascular bundle by Lsi6, some transporters are required to re-load Si into the diffuse vascular. However, these transporters are unidentified.
Si taken up is finally deposited as silica in specific cells. For example, in rice leaves, silica is deposited in dumbbell-like vascular bundles cells, bulliform motor cells. In rice grain, silica is mostly deposited in the husk. Therefore, there must be other transporters for such cell-specific deposition of Si. There transporters remains to be identified in future.
Identification of more Si transporters from different plant species is also important. Si accumulation differs greatly with plant species. Comparison of the expression patterns and localization of Si transporters from different plant species may provide new insight into differential species-dependent Si accumulation.
Acknowledgements
Most work presented in this paper was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 22119002 to J.F.M.) and a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan, Genomics for Agricultural Innovation IPG-0006 (to J.F.M.). We thank Kozo Iwasaki for providing picture of pumpkin.
Profile
Jian Feng Ma was born in 1963. He earned his bachelor’s degree in Soil Science and Agrochemistry at Nanjing Agricultural University in 1984 and his master degree and Ph.D. in Agricultural Sciences from Kyoto University in 1998 and 1991. He started his research career with studies on the beneficial effects of silicon in rice and performed pioneering work on alleviative effects of silicon on rice growth and productivity under abiotic stress. He was employed as a postdoctoral fellow and researcher in Suntory Institute for Bioorganic Research during 1991–1995. He worked on iron acquisition in gramineous plants and demonstrated the biosynthetic pathway of various phytosiderophores in different plant species and a specific uptake system for phytosiderophore-ferric complex. In 1995, he was employed as an assistant professor in Research Institute for Bioresources of Okayama University and started to work on aluminum tolerance in plants. He found for the first time that high aluminum tolerance of buckwheat is achieved by both external and internal detoxification with oxalate. He moved to Faculty of Agriculture of Kagawa University as an associate professor in 1999 and expanded his work to cadmium uptake and accumulation in hyperaccumulating plants. In 2005, he moved to Research Institute for Bioresources of Okayama University as a full professor and leads a group of Plant Stress Physiology. His group has identified a number of important transporters involved in the uptake, distribution, and accumulation of different minerals including silicon, cadmium, aluminum, arsenic, iron, etc. He is currently a full professor and unit leader of Soil Stress Unit, Institute of Plant Science and Resources, Okayama University. He was awarded the JSPS Prize in 2006, Japan Academy Medal in 2006, and Awards of Japanese Society of Soil Science and Plant Nutrition in 2007.
References
- 1).Sommer M., Kaczorek D., Kuzyakov Y., Breuer J. (2006) Silicon pools and fluxes in soils and landscapes—a review. J. Plant Nutr. Soil Sci. 169, 310–329 [Google Scholar]
- 2).Epstein E. (1999) Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 641–664 [DOI] [PubMed] [Google Scholar]
- 3).Ma, J.F. and Takahashi, E. (2002) Soil, Fertilizer, and Plant Silicon Research in Japan. Elsevier, Amsterdam. [Google Scholar]
- 4).Hodson M.J., White P.J., Mead A., Broadley M.R. (2005) Phylogenetic variation in the silicon composition of plants. Ann. Bot. 96, 1027–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Arnon D.I., Stout P.R. (1939) The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physiol. 14, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Datnoff, L.E. and Rodrigues, F.A. (2005) The role of silicon in suppressing rice diseases. APSnet Features. http://www.apsnet.org/publications/apsnetfeatures/Pages/SiliconInRiceDiseases.aspx
- 7).Fauteux F., Remus-Borel W., Menzies J.G., Belanger R.R. (2005) Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 249, 1–6 [DOI] [PubMed] [Google Scholar]
- 8).Savant N.K., Snyder G.H., Datnoff L.E. (1997) Silicon management and sustainable rice production. Adv. Agron. 58, 151–199 [Google Scholar]
- 9).Cotterill J.V., Watkins R.W., Brennon C.B., Cowan D.P. (2007) Boosting silica levels in wheat leaves reduces grazing by rabbits. Pest Manag. Sci. 63, 247–253 [DOI] [PubMed] [Google Scholar]
- 10).Ma J.F., Yamaji N. (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11, 392–397 [DOI] [PubMed] [Google Scholar]
- 11).Ma J.F. (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 50, 11–18 [Google Scholar]
- 12).Richmond K.E., Sussman M. (2003) Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 6, 268–272 [DOI] [PubMed] [Google Scholar]
- 13).Iwasaki K., Matsumura A. (1999) Effect of silicon on alleviation of manganese toxicity in pumpkin (Cucurbita moschata Duch cv. Shintosa). Soil Sci. Plant Nutr. 45, 909–920 [Google Scholar]
- 14).Ma J.F., Nishimura K., Takahashi E. (1989) Effect of silicon on the growth of rice plant at different growth stages. Soil Sci. Plant Nutr. 35, 347–356 [Google Scholar]
- 15).Tamai K., Ma J.F. (2008) Reexamination of silicon effects on rice growth and production under field conditions using a low silicon mutant. Plant Soil 307, 21–27 [Google Scholar]
- 16).Ma J.F., Tamai K., Ichii M., Wu G.F. (2002) A rice mutant defective in Si uptake. Plant Physiol. 130, 2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Ma J.F., Tamai K., Yamaji N., Mitani N., Konishi S., Katsuhara M., Ishiguro M., Murata Y., Yano M. (2006) A silicon transporter in rice. Nature 440, 688–691 [DOI] [PubMed] [Google Scholar]
- 18).Yamaji N., Ma J.F. (2011) Further characterization of a rice Si efflux transporter, Lsi2. Soil Sci. Plant Nutr. 57, 259–264 [Google Scholar]
- 19).Yamaji N., Ma J.F. (2007) Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol. 143, 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Chiba Y., Mitani N., Yamaji N., Ma J.F. (2009) HvLsi1 is a silicon influx transporter in barley. Plant J. 57, 810–818 [DOI] [PubMed] [Google Scholar]
- 21).Mitani N., Yamaji N., Ma J.F. (2009) Identification of maize silicon influx transporters. Plant Cell Physiol. 50, 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Mitani N., Yamaji N., Ago Y., Iwasaki K., Ma J.F. (2011) Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant J. 66, 231–240 [DOI] [PubMed] [Google Scholar]
- 23).Ma J.F., Yamaji N., Mitani N., Tamai K., Konishi S., Fujiwara T., Katsuhara M., Yano M. (2007) An efflux transporter of silicon in rice. Nature 448, 209–212 [DOI] [PubMed] [Google Scholar]
- 24).Mitani N., Chiba Y., Yamaji N., Ma J.F. (2009) Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell 21, 2133–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Casey W.H., Kinrade S.D., Knight C.T.G., Rains D.W., Epstein E. (2003) Aqueous silicate complexes in wheat, Triticum aestivum L. Plant Cell Environ. 27, 51–54 [Google Scholar]
- 26).Mitani N., Ma J.F., Iwashita T. (2005) Identification of the silicon form in xylem sap of rice (Oryza sativa L.). Plant Cell Physiol. 46, 279–283 [DOI] [PubMed] [Google Scholar]
- 27).Yamaji N., Mitani N., Ma J.F. (2008) A transporter regulating silicon distribution in rice shoots. Plant Cell 20, 1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Prychid C.J., Rudall P.J., Gregory M. (2004) Systematics and biology of silica bodies in monocotyledons. Bot. Rev. 69, 377–440 [Google Scholar]
- 29).Taiz, L. and Zeiger, E. (2006) Plant Physiology. Fourth edition. Sinauer, Sunderland, MA. [Google Scholar]
- 30).Kawahara H., Chonan N., Matsuda T. (1974) Studies on morphogenesis in rice plants 7. The morphology of vascular bundles in the vegetative nodes of the culm. Jpn. J. Crop. Sci. 43, 389–401 [Google Scholar]
- 31).Chonan N., Kawahara H., Matsuda T. (1985) Ultrastructure of elliptical and diffuse bundles in the vegetative nodes of rice. Jpn. J. Crop. Sci. 54, 393–402 [Google Scholar]
- 32).Hoshikawa, K. (1989) The Growing Rice Plant. Nobunkyo, Tokyo. [Google Scholar]
- 33).Yamaji N., Ma J.F. (2009) Silicon transporter Lsi6 at the node is responsible for inter-vascular transfer of silicon in rice. Plant Cell 21, 2878–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]