Abstract
The current status of screening for gastric cancer-risk (gastritis A, B, C, D) method using combined assay for serum anti-Helicobacter pylori (Hp) IgG antibody and serum pepsinogen (PG) levels, “ABC method”, was reviewed and the latest results of our ongoing trial are reported. It was performed using the following strategy: Subjects were classified into 1 of 4 risk groups based on the results of the two serologic tests, anti-Hp IgG antibody titers and the PG I and II levels: Group A [Hp(−)PG(−)], infection-free subjects; Group B [Hp(+)PG(−)], chronic atrophic gastritis (CAG) free or mild; Group C [Hp(+)PG(+)], CAG; Group D [Hp(−)PG(+)]), severe CAG with extensive intestinal metaplasia. Continuous endoscopic follow-up examinations are required to detect early stages of gastric cancer. Asymptomatic Group A, which accounts for 50–80% of all the subjects may be excluded from the secondary endoscopic examination, from the viewpoint of efficiency. Hp-infected subjects should be administered eradication treatment aimed at the prevention of gastric cancer.
Keywords: gastric cancer; cancer screening; pepsinogen (PG) I, II; anti-Helicobacter pylori (Hp) IgG antibody; serum pepsinogen test method (PG method); screening for gastric cancer-risk (gastritis A, B, C, D) method (ABC method)
Introduction
In 2010, gastric cancer remains one of the most important gastrointestinal cancers. It is the fourth most common cancer and second leading cause of cancer deaths (700,000 deaths annually) worldwide.1) In 2002, an estimated one million new cases of gastric cancer were diagnosed, with almost two-thirds occurring in developing countries. High-risk areas include East Asia (Japan, China), Eastern Europe and parts of Central and South America.2)
It is important to introduce an efficient and cost-effective practical mass screening method for early detection of gastric cancer. It may be possible to reconstitute the screening system of gastric cancer according to the risk level, so that unnecessary annual invasive screening examinations are avoided.
It is well established that gastric carcinogenesis is a continuous process starting from superficial gastritis to the development of glandular atrophy, metaplasia and dysplasia, and finally, adenocarcinoma.3) This process usually takes decades and seems to be initiated by infection with the gastric bacterium, Helicobacter pylori (Hp)4) in many, if not most cases. The long history of the disease process potentially provides opportunities for early detection of pre-cancerous lesions and consequent appropriate intervention. The application of strategies directed towards the elimination of risk factors is of paramount importance in the control of gastric cancer.5)
The high prevalence of intestinal metaplasia among Hp-infected patients suggests that the risk of development of gastric cancer will continue to remain high. Since gastric cancer is potentially curable if diagnosed early, it is insufficient to check for Hp antibody titers alone for the diagnosis of subjects with severe atrophic gastritis. The serum pepsinogen test method, or the “PG method”6–19) is also needed in Hp-positive subjects. Conversely, low-risk subjects who do not have atrophic gastritis or Hp infection can be screened by the combination of the PG method and Hp serology, or the screening for gastric cancer-risk (gastritis A, B, C, D) method or the “ABC method”.
Here, the author reviews the present status of gastric cancer screening using the ABC method, including the latest results of our ongoing trial.
Gastric cancer and Hp infection
The discovery of Hp in 1982 has not only changed the concept of upper gastrointestinal tract diseases, but also of clinical gastroenterological practice.4) Results of clinical and basic research accumulated over the last decade clearly demonstrate the existence of a close relationship between Hp infection and the risk of gastric cancer.20–34) Hp infection is now recognized as the main acquired factor involved in the pathogenesis of peptic ulcer disease and chronic gastritis, as also gastric cancer.20)
Gastric cancer almost never occurs in the absence of Hp infection. Observation of 1,526 individuals over a period of 10 years revealed that gastric cancer was found in 5% of all individuals infected with Hp and in none of the uninfected individuals.28)
Uemura N. et al.25) followed up patients who underwent endoscopic therapy for gastric cancer that resulted in complete cure, and compared the incidence rate of gastric cancer at another site in the stomach (metachronous gastric cancer) between patients who were and were not treated with an Hp eradication regimen. During the 54-month follow-up period, metachronous gastric cancer was not found in any of the patients administered Hp eradication treatment, but in 10% of those who did not receive the eradication therapy. Over longer periods, however, occurrence of gastric cancer was also detected among the patients who had received Hp eradication therapy, however, the incidence was clearly lower than that in the patients who had not received Hp eradication therapy. Other reports30) lend support to these findings.
Eradication of Hp decreases the severity of gastritis, producing significant changes in the serum PG levels; both serum PG I and PG II levels decrease, with elevation of the PG I to PG II ratio.35–38) Furuta T. et al.35) determined the optimal cutoff values for the percent change of the serum PG I/II ratio. The cutoff was tentatively set as +40%, +25%, and +10% when the serum PG I/II ratios before treatment were less than 3, equal to or greater than 3, but less than 5, and equal to or greater than 5, respectively. Since the method involving determination of the percent change of the serum pepsinogen levels has the advantage that no endoscopy is required, repeated examinations will be more acceptable to the patients. Thus, the serological method may be a useful non-invasive method for determining eradication of Hp.
Hp antibody titers39) vary greatly depending on the test kit used in Japan. Use of an Hp antibody test kit with Japanese strains without an indeterminate range is recommended.
The PG method
Serum pepsinogen (PG)40–42) is classified into two biochemically and immunologically distinct types, namely, PG I and PG II.43–47) PG I is produced by the chief and mucous neck cells in the fundic glands, while PG II is produced by these cells and also by the cells in the pyloric glands and Brunner’s glands. It is widely accepted that the serum PG levels reflect the functional and morphologic status of the stomach mucosa. As the fundic gland mucosal area reduces, the PG I levels gradually decrease, while the PG II levels remain fairly constant. As the result, a stepwise reduction of the PG I/II ratio is closely correlated with the progression from normal gastric mucosa to extensive atrophic gastritis.6–19)
Serum PG was used as a biomarker of the gastric mucosal status, including to detect atrophic changes and inflammation, before the discovery of Hp.
PG is a serum marker of atrophic gastritis, which is a precancerous change in the stomach, rather than being a tumor marker.13,14) The PG method6–19,48–74) allows the diagnosis of advanced atrophic gastritis, a high risk factor for gastric cancer, and can be applied to gastric cancer screening using the serum PG I level and PG I/II ratio as indices, based on the association between CAG and gastric cancer, and the correlation between the serum PG levels and the presence of CAG. Thus, for the detection of gastric cancer, patients at high risk for gastric cancer have been screened clinically by the PG method.11–15)
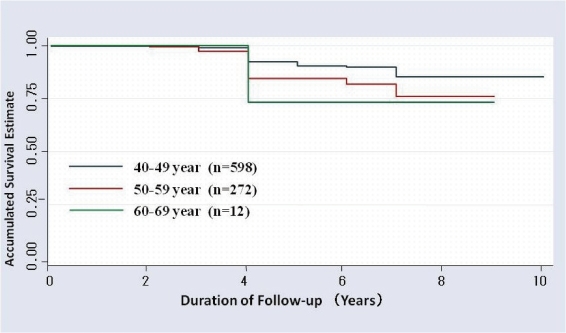
In 10-year follow-up studies, from 1992 to 2001, of 882 individuals from a Tokyo Teishin Hospital Health Care Center in Japan, where screening for gastric cancer by the PG method and endoscopy is practiced, Kaplan–Meier survival estimates stratified by age revealed no reduction of the cumulative survival estimate during 4 years in individuals in their 40’s, 50’s and 60’s63,64) (Fig. 1).
Figure 1.
Accumulated survival rate for each age-group by the PG method using Kaplan–Meier analysis at Tokyo Teishin Hospital Care Center in Tokyo for the ten-year period (1992–2001); n = 88263,64)
In cancer screening by the PG method in a municipality of Hiroshima prefecture in Japan, the number of reported deaths from gastric cancer was 41. In a case-control study based on the 41 deaths and 3 controls, the odds ratio (95% CI) for death within 3 years after screening by the PG method was 0.290 (0.0111–0.759), indicating the potential contribution of the PG method to a significant reduction of gastric cancer mortality within 3 years63) (Table 1).
Table 1.
Evidence of a decrease in gastric cancer mortality by the PG method at a municipality of Hiroshima Prefecture using Mantel–Hentzel estimate of the odds ratio63)
| Odds ratio | 95% of confidence intervals | Number of individuals | |
|---|---|---|---|
| Visit within 1 year | 0.238 | (0.061–0.929) | 41 |
| Visit within 2 years | 0.375 | (0.155–0.905) | 41 |
| Visit within 3 years | 0.290 | (0.111–0.759) | 36 |
| Visit within 4 years | 0.423 | (0.164–1.091) | 32 |
| Visit within 5 years | 0.440 | (0.171–1.135) | 31 |
The PG method identifies atrophic gastritis, a high-risk factor for gastric cancer, and therefore, may be called a mass screening test method for gastric-cancer-risk, rather than as one for gastric cancer itself.
It is of great significance to detect early gastric cancer by screening of high-risk patients detected using the PG method and following the patients up by endoscopy on a regular basis. As reported elsewhere,15) we have obtained good results of the mass screening system using the PG method alone.
However, a controversial and the most important weak point in the mass screening system by the PG method alone is the presence of the PG method-negative gastric cancer, especially, diffuse-type gastric cancer.
Although it is possible to detect such cancers by an endoscopic examination once in 5 years,15) patients will benefit from screening by the less invasive combination of the PG method and serum anti-Hp antibody assay, the “ABC method”,39,75,76) which can also detect PG method-negative gastric cancer.
We have confirmed that the ABC method is useful for the detection of both the intestinal and diffuse types of gastric cancer from the residual serum samples of 51 gastric cancer cases found among 43,438 subjects who had undergone screening as part of a health checkup at a certain workplace from the year 2000 to 2005;63) of the total, 7 cases were PG method-negative and Hp serology-positive. Thereafter, we decided to introduce the ABC method at this work place in Tokyo for the first time.
Stratification of the risk of gastric cancer by the ABC method39)
Results of much basic research suggest that Hp infection is closely associated with the development of gastric cancer.4,5) Domestic74,77–80) and foreign epidemiological studies also lend support to the notion that Hp infection is a risk factor for gastric cancer, except that the odds ratio varied from study to study. It has also become evident that atrophy of the gastric mucosa is a high-risk factor for the development of gastric cancer, and that the serum PG levels are correlate with atrophy of the gastric mucosa. The ABC method allows stratification of risk for the development of gastric cancer.24,26,29,34,57,72) Other reports69–71,81) from Japanese researchers also support these findings.
Cohort studies57) in 4,655 normal male individuals who could be followed up for at least 10 years show that gastric cancer developed only in individuals infected with Hp and did not develop in normal individuals testing negative for Hp. As chronic gastritis progressed, a gradual and significant increase in the incidence of gastric cancer and hazard ratio was noted (Table 2). The most advanced and severe cases of gastric atrophy judged by the pepsinogens assessment, when combined with a negative Hp serology, probably due to a Hp antibody spontaneous disappearance,54,57) was associated when an even greater progression to dysplasia and cancer.
Table 2.
Incidence of gastric cancer and hazard ratio associated with atrophic gastritis at a workplace in Wakayama Prefecture for the eight-year periods (1994–2002); n = 5,20939)
| Group* | A | B′ | B | C | C′ | D |
|---|---|---|---|---|---|---|
| Number of individuals | 966 | 501 | 2,327 | 1,329 | 53 | 33 |
| Person-year | 9,487 | 5,007 | 22,436 | 12,665.5 | 524.5 | 306 |
| Mean age (years) | 48.3 | 46.8 | 49.5 | 50.4 | 47.9 | 49.3 |
| Follow-up period (years) | 9.8 | 10.0 | 9.6 | 9.5 | 9.9 | 9.3 |
| Incidence of gastric cancer | 0 | 1 | 25 | 30 | 3 | 4 |
| Hazard ratio | 1 | 2.1 | 9.8 | 19.6 | 54.8 | 120.4 |
*Group: A: Hp(−)PG(−), B′: Hp(±)PG(−), B: Hp(+)PG(−), C: Hp(+)PG(+), C′: Hp(±)PG(+), D: Hp(−)PG(+).
Of 8,286 individuals of Matsue city in Japan who underwent endoscopic screening for gastric cancer during health checkup in which Hp antibodies were measured in addition to the PG method, 2,802 were classified as group A [Hp(−)PG(−)], 3,395 as group B [Hp(+)PG(−)], and 2,089 as group C [Hp(+)PG(+) and Hp(−)PG(+)].81) According to a follow-up over the subsequent 14 years, gastric cancer occurred in 46 individuals in Group C (1.87%) and 7 individuals in Group B (0.21%), while there were no cases from Group A. Therefore, we can discriminate between low-risk and high-risk groups for the development of gastric cancer using the ABC method.
The above results show that (1) the risk of gastric diseases is very low in individuals with a healthy gastric mucosa (Group A), (2) there is an elevated risk of peptic ulcer, etc., in Group B, (3) individuals in Group C are at a higher risk of developing diseases resulting from atrophy of the gastric mucosa, such as gastric cancer, gastric adenoma and hyperplastic polyps, and (4) individuals in Group D [Hp(−)PG(+)], with advanced atrophy, are at a higher risk of developing gastric cancer. The risk of gastric cancer is highest in Group D, followed by that in Groups C, B, and A, in descending order.34,57,63,64)
The ABC method allows stratification of the risk for the development of gastric cancer into four (A, B, C, and D) groups. The advantages of this examination are as follows: (1) Serum PG levels do not vary greatly within 10 years or so in more than 90% of adults, (2) Hp infection is originally acquired in childhood in most cases, (3) the antibody titer is relatively stable in people aged 40 years or older, and (4) this examination can be performed simultaneously with a regular health checkup.
If individuals are classified into a high-risk group or low-risk group through primary screening using the ABC method, it may be possible to reconstitute the screening system for gastric cancer according to the risk level of the patients, instead of implementing annual screening for all individuals.
Ongoing trial of the ABC method in Tokyo
Since 1991, we have been performing mass screening for gastric cancer risk.11,15) The mass screening system consists of primary screening of high-risk-employees for gastric cancer using serum samples and secondary examination by endoscopy among those presenting for a health-checkup.
We initiated the ABC method in 2007, in which people in group A are advised to have endoscopic examination every five years, those in group B every three years, those in group C every two years, and those in group D annually. During the three years from 2007 to 2009, we examined a total of 48,073 individuals after excluding those who met the exclusion criteria based on the information obtained from them by interview.76)
Serum samples collected at the time of the general health checkup were used to measure the serum PG I and II levels (LZ test, ‘Eiken’ Pepsinogen I and II: LA; latex agglutination method) and serum anti-Hp antibody (E-plate ‘Eiken’ H. pylori antibody: EIA; enzyme immunoassay method). Individuals with PG I levels of ≤70 µg/l and PG I/II ratio of ≤3 were classified as PG-positive, and those with a serum Hp antibody titer of ≥10 U/ml were classified as Hp-positive.
Based on the results of the above tests, the subjects were classified into the following four groups: group A [Hp(−)PG(−)], group B [Hp(+)PG(−)], group C [Hp(+)PG(+)], and group D [Hp(−)PG(+)], respectively. There were 35,177 individuals in group A, 7,883 individuals in group B, 4,489 individuals in group C, and 524 individuals in group D. Based on the time of the most recent endoscopic examination, 6,965 of all the individuals were advised to undergo endoscopic examination, and 3,921 (56%) actually underwent endoscopy76) (Table 3).
Table 3.
Subjects of “the ABC method” at a work place in Tokyo for three-year periods (2007–2009)76)
| Total number of 3 years | 2007 year | 2008 year | 2009 year | |
|---|---|---|---|---|
| Screened case | 48,073 (100%) | 15,043 (100%) | 16,080 (100%) | 16,950 (100%) |
| Group A* | 35,177 (73%) | 10,628 (71%) | 11,696 (73%) | 12,853 (76%) |
| B* | 7,883 (17%) | 2,911 (19%) | 2,681 (17%) | 2,291 (14%) |
| C* | 4,489 (9%) | 1,374 (9%) | 1,527 (9%) | 1,588 (9%) |
| D* | 524 (1%) | 130 (1%) | 176 (1%) | 218 (1%) |
| Requiring endoscopy | 6,965 (15%) | 3,346 (22%) | 2,154 (13%) | 1,465 (9%) |
| Underwent endoscopy | 3,921 (8%) | 1,627 (11%) | 1,535 (10%) | 759 (4%) |
*Group: A: Hp(−)PG(−), B: Hp(+)PG(−), C: Hp(+)PG(+), D: Hp(−)PG(+).
Group A is excluded from the secondary endoscopic examination from the view point of efficiency.
Of the 3,921 individuals, 23 were found to have gastric cancer (detection rate of gastric cancer: 0.05%, positive predictive value: 0.59%). Five of them were found to have advanced cancer, and it was the first time for all of them to have undergone screening for gastric cancer risk by the ABC method, for reasons such as mid-career hiring. The remaining 18 (78%) had early gastric cancer and 12 (52%) had intestinal-type gastric cancer. Endoscopic resection was performed in 12 of the patients (52%), and radical surgical resection was possible in the remaining 11 individuals, including those with advanced cancer. Histopathologically, 48% of the detected cancers were of the diffuse type, with the ratio of the diffuse type increasing gradually over the years (25% in 2007, 63% in 2008, and 100% in 2009).
From 2007 to 2009, individuals in group A (low-risk group), accounting for 73% of all the individuals, were excluded from the secondary endoscopic examination, to resolve the shortage of manpower, which resulted in successful examination of the risk of gastric cancer.
The percentages of people in group A in 2007, 2008, and 2009 were 71%, 73%, and 76%, and those in group B were 19%, 17% and 14%, respectively. Consequently, the annual rate of increase of group A subjects was estimated to be about 3% and the rate of decrease of group B was estimated to be about 3% per year. The number of individuals in group A will continue to increase at the rate of about 3% per year, which is expected to contribute to a further reduction in the number of individuals who are advised to undergo mass screening examination for gastric cancer in the future.76)
It remains to be seen whether the ABC method,39,75,76,82) which was introduced in 2007 and has been studied for only 3 years, is effective. Because 70% of the patients with gastric cancer have been found in group B, for which endoscopic examination would have been performed only once in 5 years by the conventional PG method, the ABC method is likely to detect PG-negative gastric cancer at an early stage.
It is also suggested that the ABC method, which also allows detection of diffuse-type gastric cancers and successful endoscopic resection, can reliably detect gastric cancer earlier. However, as I will mention the weak points and points of caution of the ABC method later in the appendix of this manuscript, there are several problems for adopting the ABC method in clinical practice for primary gastric cancer screening, especially to exclude Hp-infected individuals from group A.
At present, it is still too early for us to draw any definitive conclusions, but the ABC method should be positioned as an effective method for stratifying gastric cancer risk suitable for the circumstances in Japan, where the number of people infected with Hp or testing positive for PG is decreasing. It is hoped that more experience will be accumulated at many institutions besides this clinic in Japan, as well as in other countries across the world.
Conclusion
Although there is still room for improvement of the ABC method, I think it is useful to select high-risk and low-risk populations for development of gastric cancer, and to detect not only the intestinal type, but also the diffuse type of gastric cancers in the early stage. I believe it can be a step towards reaching the ultimate goal of mass screening, that is, eradication of gastric cancer.
Abbreviations
- PG method
serum pepsinogen test method
- ABC method
screening for gastric cancer-risk (gastritis A, B, C, D) method.
Profile
Professor Kazumasa Miki was born in 1942. He graduated and obtained his PhD from the School of Medicine at the University of Tokyo, Japan. He performed pioneering work on the basic and clinical application of pepsinogen for long time, over 30 years. He became Assistant Professor in 1981, and subsequently Senior Lecturer and Associate Professor at the First Department of Internal Medicine, Faculty of Medicine, University of Tokyo. He is currently Emeritus Professor at the Division of Gastroenterology and Hepatology of the Department of Internal Medicine (Omori), Toho University, Tokyo, as well as Consultant of the Cancer Institute, Ariake Hospital, of the Japanese Foundation for Cancer Research. He had also been Chairman of the Research Committee of Studies of the Ministry of Health, Labor and Welfare in Japan from 1996 to 2006.
Miki has won awards which includes Asahi Cancer Award in 2005, Award of Princess Takamatsu Cancer Research Fund in 2008, and 2009 ACG Governors Awards for Excellence in Clinical Research, and holds the post of Councilor/Director in many gastroenterological societies in Japan. These include the Japan Gastroenterological Endoscopy Society (JGES) and the Japanese Society of Cancer Screening and Diagnosis. He also sits on the editorial board of Digestive Endoscopy and President of Japan Research Foundation of Prediction Diagnosis Therapy for Gastric Cancer (JRF PDT GC), and Japanese Representative Councilor of China and Korea as well as President of Kanto District of JGES.
Appendix
Technical recommendations for adopting the ABC method39)
-
1.
According to the ABC method, it is recommended that the risk for gastric cancer be stratified into four groups according to the anti-Hp IgG antibody titer before eradication of Hp and the serum PG levels, as follows: group A [Hp(−)PG(−)], group B [Hp(+)PG(−)], group C [Hp(+)PG(+)], and group D [Hp(−)PG(+)]. For the PG method, the cutoff points for identifying the risk of gastric cancer should be ≤70 µg/l for pepsinogen I and ≤3 for the PG I/II ratio, and inquiries about a history of Hp eradication, previous treatment of peptic ulcer (especially, treatment with PPIs), previous gastric resection, and impairment of renal function are essential.
-
2.
PG levels do not vary from one test kit to another, whereas Hp antibody titers vary greatly depending on the test kit used in Japan. Use of an Hp antibody test kit with Japanese strains without an indeterminate range is recommended.
-
3.
To exclude Hp-infected individuals from group A: (1) Measures for individuals who have received Hp eradication: What is most important is an inquiry about a history of Hp eradication. If serum PG levels change significantly or both serum PG I and PG II levels are low, it may be assumed that the person has received Hp eradication therapy. (2) Measures for individuals who are false-negative for Hp antibody: It is highly likely that elevated PG levels, especially a PG II level of ≥15 µg/l, reflect the presence of histological gastritis associated with Hp infection. In this case, the presence of Hp should be checked using other antibodies or test methods. (3) Measures for individuals in whom Hp infection resolved spontaneously: low PG levels, especially a PG I level of ≤35 µg/l and PG I/II ratio of 4.0 to 3.1, may indicate the possibility of previous infection with Hp or spontaneous resolution of Hp infection.
-
4.
The interval for screening by the ABC method need not be yearly, and an interval of 5 years or so is recommended. It is recommended that endoscopic examination be performed at least once every 3 years for group B, at least once every 2 years for group C, and annually for group D, and that group A be excluded from the examination.
References
- 1).Melton S.D., Genta M., Souza R.F. (2010) Biomarkers and molecular diagnosis of gastrointestinal and pancreatic neoplasms. Nat. Rev. Gastroenterol. Hepatol. 7, 620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Parkin D.M., Bray F., Ferlay J., Pisani P. (2005) Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 [DOI] [PubMed] [Google Scholar]
- 3).Correa P., Piazuelo M.B., Wilson T. (2010) Pathology of gastric intestinal metaplasia: Clinical implications. Am. J. Gastroenterol. 105, 493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Asaka M., Kato M., Takahashi S., Fukuda Y., Sugiyama T., Ota H., Uemura N., Murakami K., Satoh K., Sugano K. (2010) Guideline for the management of Helicobacter pylori in Japan: 2009 revised edition. J. Helicobacter 15, 1–20 [DOI] [PubMed] [Google Scholar]
- 5).Asaka M., Kato M., Graham D.Y. (2010) Strategy for eliminating gastric cancer in Japan. Helicobacter 15, 486–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Miki K., Ichinose M., Shimizu A., Huang S.C., Oka H., Furihata C., Matsushima T., Takahashi K. (1987) Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol. Jpn. 22, 133–141 [DOI] [PubMed] [Google Scholar]
- 7).Miki K., Ichinose M., Kawamura N., Matsushima M., Ahmad H.B., Kimura M., Sano J., Tashiro T., Kakei N., Oka H., Furihata C., Takahashi K. (1989) The significance of low serum pepsinogen levels to detect stomach cancer associated with extensive chronic gastritis in Japanese subjects. Jpn. J. Cancer Res. 80, 111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Miki K., Ichinose M., Ishikawa K.B., Yahagi N., Matsushima M., Kakei N., Tsukada S., Kido M., Ishihama S., Shimizu Y., Suzuki T., Kurokawa K. (1993) Clinical application of serum pepsinogen I and II levels for mass screening to detect gastric cancer. Jpn. J. Cancer Res. 84, 1086–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Miki, K., Ichinose, M., Kakei, N., Yahagi, N., Matsushima, M., Tsukada, S., Ishihama, S., Shimizu, Y., Suzuki, T., Kurokawa, K. and Takahashi, K. (1995) The clinical application of the serum pepsinogen I and II levels as mass screening method for gastric cancer. In Aspartic Proteinases: Structure, Function, Biology and Biomedical Implications (ed. Takahashi, K.). Plenum Press, New York, pp. 139–143. [Google Scholar]
- 10).Ichinose, M., Yahagi, N., Oka, M., Ikeda, H., Miki, K. and Omata, M. (2001) Screening for gastric cancer in Japan. In Cancer Screening. A practical guide for physicians (eds. Wu, G.Y. and Aziz, K.). Humana Press, Potowa, NJ, pp. 87–102. [Google Scholar]
- 11).Miki K., Morita M., Sasasjima M., Hoshina R., Kanda E., Urita Y. (2003) Usefulness of gastric cancer screening using the serum pepsinogen test method. Am. J. Gastroenterol. 98, 735–739 [DOI] [PubMed] [Google Scholar]
- 12).Dinis-Ribeiro M., Yamaki G., Miki K., Costa-Pereira A., Matsukawa M., Kurihara M. (2004) Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J. Med. Screen. 11, 141–147 [DOI] [PubMed] [Google Scholar]
- 13).Miki K. (2006) Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 9, 245–253 [DOI] [PubMed] [Google Scholar]
- 14).Miki K., Urita Y. (2007) Using serum pepsinogens wisely in a clinical practice. J. Dig. Dis. 8, 8–14 [DOI] [PubMed] [Google Scholar]
- 15).Miki K., Fujishiro M., Kodashima S., Yahagi N. (2009) Long term results of gastric cancer screening using the serum pepsinogen test method among an asymptomatic middle-aged Japanese population. Dig. Endosc. 21, 78–81 [DOI] [PubMed] [Google Scholar]
- 16).Nomura A.M.Y., Stemmermann G.N., Samloff I.M. (1980) Serum Pepsinogen I as a predictor of stomach cancer. Ann. Intern. Med. 93, 537–540 [DOI] [PubMed] [Google Scholar]
- 17).Fukao A., Hisamichi S., Ohsato N., Fujino N., Endo N., Iha M. (1993) Correlation between the prevalence of gastritis and gastric cancer in Japan. Cancer Causes Control 4, 17–20 [DOI] [PubMed] [Google Scholar]
- 18).Miki, K. (ed.) (1998) The Pepsinogen Method. Igakushoin, Tokyo (in Japanese). [Google Scholar]
- 19).Correa P. (2010) Serum pepsinogen in gastric cancer screening. Dig. Dis. Sci. 55, 2123–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Hatakeyama M. (2004) Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4, 688–694 [DOI] [PubMed] [Google Scholar]
- 21).Nomura A., Stemmermann G.N., Chyou P.H., Kato I., Perez P.G., Blaser M.J. (1991) Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 325, 1132–1136 [DOI] [PubMed] [Google Scholar]
- 22).Parsonnet J., Friedman G.D., Vandersteen D.P., Chang Y., Vogelman J.H., Orentreich N., Sibley R.K. (1991) Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325, 1127–1131 [DOI] [PubMed] [Google Scholar]
- 23).International Agency for Research on Cancer (1994) Schistosomiasis, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 61, 177–2407715070 [Google Scholar]
- 24).Fukuda H., Saito D., Hayashi S., Hisai H., Ono H., Yoshida S., Oguro Y., Noda T., Sato T., Katoh M., Terada M., Sugimura T. (1995) Helicobacter pylori infection, serum pepsinogen level and gastric cancer: a case-control study in Japan. Jpn. J. Cancer Res. 86, 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Uemura N., Mukai T., Okamoto S., Yamaguchi S., Mashiba H., Taniyama K., Sasaki N., Haruma K., Sumii K., Kajiyama G. (1997) Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol. Biomarkers Prev. 6, 639–642 [PubMed] [Google Scholar]
- 26).Kitahara F., Shimazaki R., Sato T., Kojima Y., Morozumi A., Fujino M.A. (1998) Severe atrophic gastritis with Helicobacter pylori infection and gastric cancer. Gastric Cancer 1, 118–124 [DOI] [PubMed] [Google Scholar]
- 27).Graham D.Y. (2000) Helicobacter pylori infection is the primary cause of gastric cancer. J. Gastroenterol. 35 (Suppl. 12), 90–97 [PubMed] [Google Scholar]
- 28).Uemura N., Okamoto S., Yamamoto S., Matsumura N., Yamaguchi S., Yamakido M., Taniyama K., Sasaki N., Schlemper R.J. (2001) Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345, 784–789 [DOI] [PubMed] [Google Scholar]
- 29).Watabe H., Mitsushima T., Yamaji Y., Okamoto M., Wada R., Kokubo T., Doi H., Yoshida H., Kawabe T., Omata M. (2005) Predicting the development of gastric cancer from combing Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut 54, 764–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Fukase K., Kato M., Kikuchi S., Inoue K., Uemura N., Okamoto S., Terao S., Amagai K., Hayashi S., Asaka M., for the Japan Gast Study Group (2008) Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-lavel, randomized controlled trial. Lancet 372, 392–397 [DOI] [PubMed] [Google Scholar]
- 31).Graham D.Y. (2009) Gastric cancer surveillance or prevention plus targeted surveillance. Jpn. J. Helicobacter Res. 10, 9–14 [PMC free article] [PubMed] [Google Scholar]
- 32).Ito M., Takata S., Tatsugami M., Wada Y., Imagawa S., Matsumoto Y., Takamura A., Kitamura S., Matsuo T., Tanaka S., Haruma K., Chayama K. (2009) Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J. Gastroenterol. 44, 365–371 [DOI] [PubMed] [Google Scholar]
- 33).Graham D.Y., Asaka M. (2010) Eradication of gastric cancer and more efficient gastric cancer surveillance in Japan: two peas in a pod. J. Gastroenterol. 45, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Mizuno S., Miki I., Ishida T., Yoshida M., Onoyama M., Azuma T., Habu Y., Inokuchi H., Ozasa K., Miki K., Watanabe Y. (2010) Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig. Dis. Sci. 55, 3132–3137 [DOI] [PubMed] [Google Scholar]
- 35).Furuta T., Kaneko E., Baba S., Arai H., Futami H. (1997) Percentage changes in serum pepsinogens are useful as indices of eradication of Helicobacter pylori. Am. J. Gastroenterol. 92, 84–87 [PubMed] [Google Scholar]
- 36).Al-Assi M.T., Miki K., Walsh J.H., Graham D.P., Asaka M., Graham D.Y. (1999) Noninvasive evaluation of Helicobacter pylori therapy: Pole of fasting or postprandial gastrin, Pepsinogen I, Pepsinogen II, or serum IgG antibodies. Am. J. Gastroenterol. 94, 2367–2372 [DOI] [PubMed] [Google Scholar]
- 37).Kawai T., Miki K., Ichinose M., Kenji Y., Miyazaki I., Kawakami K., Kataoka M., Yamagishi T., Sofuni A., Itoi T., Moriyasu F., Takagi Y., Aoki T., Matsubayashi J., Mukai K. (2007) Changes in evaluation of the pepsinogen test result following Helicobacter pylori eradication therapy in Japan. Inflammopharmacology 15, 31–35 [DOI] [PubMed] [Google Scholar]
- 38).Suganuma T., Yamamoto Y., Uragami N., Fujisaki J., Hoshino E., Takahashi H., Miki K. (2009) A significant increase of fasting serum pepsinogen I/II ratio is a reliable biomarker for the successful Helicobacter pylori eradication in patients without peptic ulcer or gastric cancer. Gut 58 (Suppl. II), A401 [Google Scholar]
- 39).JRF PDT GC (ed.) (2009) Manual for the Gastric Cancer-Risk Screening (The ABC Method). Nanzan-do, Tokyo (in Japanese). [Google Scholar]
- 40).Ichinose M., Miki K., Furihata C., Kageyama T., Niwa H., Oka H., Oda T., Matsushima T., Takahashi K. (1982) Radioimmunoassay of group II pepsinogen in human serum. Clin. Chim. Acta 122, 61–69 [DOI] [PubMed] [Google Scholar]
- 41).Ichinose M., Miki K., Furihata C., Kageyama T., Hayashi R., Niwa H., Oka H., Matsushima T., Takahashi K. (1982) Radioimmunoassay of serum group I and group II pepsinogens in normal controls and patients with various disorders. Clin. Chim. Acta 126, 183–191 [DOI] [PubMed] [Google Scholar]
- 42).Huang S.C., Miki K., Furihata C., Ichinose M., Shimizu A., Oka H. (1988) Enzyme-linked immunosorbent assays for serum pepsinogens I and II using monoclonal antibodies—with data on peptic ulcer and gastric cancer. Clin. Chim. Acta 175, 37–50 [DOI] [PubMed] [Google Scholar]
- 43).Samloff I.M. (1971) Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology 61, 185–188 [PubMed] [Google Scholar]
- 44).Samloff I.M., Liebman W.M. (1973) Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology 65, 36–42 [PubMed] [Google Scholar]
- 45).Miki K., Ichinose M., Furihata C., Niwa H., Oka H., Oda T., Matsushima T. (1982) Potential peptic activity of pepsinogen of human gastroduodenal mucosa determined by fluorescent microassay method using albumin. Clin. Chim. Acta 121, 337–344 [DOI] [PubMed] [Google Scholar]
- 46).Huang S.C., Miki K., Sano J., Ichinose M., Kawamura N., Oka H., Hirano K., Furihata C., Masugi Y., Takahashi K. (1988) Pepsinogen I and II in gastric cancer: An immunohistochemical study using monoclonal antibodies. Jpn. J. Cancer Res. (Gann) 79, 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Sano J., Miki K., Ichinose M., Kimura M., Kurokawa K., Aida T., Ishizaki M., Asano G., Masugi Y., Wong R.N.S., Takahashi K. (1989) In situ localization of pepsinogens I and II mRNA in human gastric mucosa. Acta Pathol. Jpn. 39, 765–771 [DOI] [PubMed] [Google Scholar]
- 48).Kikuchi S., Wada O., Miki K., Nakajima T., Nishi T., Kobayashi O., Inaba Y. (1994) Serum pepsinogen as a new marker for gastric carcinoma among young adults. Cancer 73, 2695–2702 [DOI] [PubMed] [Google Scholar]
- 49).Kato I., Miki K., Muñoz N., Vivas J.H., Lopez G., Peraza S., Carillo E., Castro D., Andrade O., Sanchez V., Cano E., Ramirez H., Muggli R., Benz M., Oliver W. (1995) Determinants of plasma pepsinogen levels in population at high risk for stomach cancer in Venezuela. Int. J. Cancer 62, 512–518 [DOI] [PubMed] [Google Scholar]
- 50).Watanabe Y., Kurata J.H., Mizuno S., Mukai M., Inokuchi H., Miki K., Ozawa K., Kawai K. (1997) Helicobacter pylori infection and gastric cancer. A nested case-control study in a rural area of Japan. Dig. Dis. Sci. 42, 1383–1387 [DOI] [PubMed] [Google Scholar]
- 51).Yoshihara M., Sumii K., Haruma K., Kiyohira K., Hattori N., Kitadai Y., Komoto K., Tanaka S., Kajiyama G. (1998) Correlation of ratio of serum pepsinogen I and II with prevalence of gastric cancer and adenoma in Japanese subjects. Am. J. Gastroenterol. 93, 1090–1096 [DOI] [PubMed] [Google Scholar]
- 52).Ozasa K., Kurata J.H., Higashi A., Hayashi K., Inokuchi H., Miki K., Tada M., Kawai K., Watanabe Y. (1999) Helicobacter pylori infection and atrophic gastritis. A nested case-control study in a rural town in Japan. Dig. Dis. Sci. 44, 253–256 [DOI] [PubMed] [Google Scholar]
- 53).Namekata T., Miki K., Kimmey M., Fritsche T., Hughes D., Moore D., Suzuki K. (2000) Chronic atrophic gastritis and Helicobacter pylori infection among Japanese Americans in Seattle. Am. J. Epidemiol. 151, 820–830 [DOI] [PubMed] [Google Scholar]
- 54).Plummer M., Vivas J., Fauchère J.L., Giudice G.D., Peña A.S., Ponzetto A., Lopez G., Miki K., Oliver W., Muñoz N. (2000) Helicobacter pylori and stomach cancer: a case-control study in Venezuela. Cancer Epidemiol. Biomarkers Prev. 9, 961–965 [PubMed] [Google Scholar]
- 55).Miki K. (2000) Clinico-pathological characteristics of “the pepsinogen method” -positive and negative gastric cancer. J. Gastroenterol. Cancer Screening 38, 292–293(in Japanese) [Google Scholar]
- 56).Miki, K. (2004) Annual report 2001–2003 of the Research Committee of Effect and Efficiency of Gastric Cancer Screening, supported by a Grant-in-Aid for the Research from the Ministry of Health, Labour and Welfare, Japan, Tokyo (in Japanese).
- 57).Ohata H., Kitauchi S., Yoshimura N., Mugitani K., Iwane M., Nakamura H., Yoshikawa A., Yanaoka K., Arii K., Tamai H., Shimizu Y., Takeshita T., Mohara O., Ichinose M. (2004) Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int. J. Cancer 109, 138–143 [DOI] [PubMed] [Google Scholar]
- 58).Urita Y., Hike K., Torii N., Kikuchi Y., Kanda E., Sasajima M., Miki K. (2004) Serum pepsinogen as a predictor of the topography of intestinal metaplasia in patients with atrophic gastritis. Dig. Dis. Sci. 49, 795–801 [DOI] [PubMed] [Google Scholar]
- 59).Nomura A.M.Y., Kolonel L.N., Miki K., Stemmermann G.N., Wilkens L.R., Goodman M.T., Perez-Perez G.I., Blaser M.J. (2005) Helicobacter pylori, pepsinogen and gastric adenocarcinoma in Hawaii. J. Infect. Dis. 191, 2075–2081 [DOI] [PubMed] [Google Scholar]
- 60).Ohata H., Oka M., Yanaoka K., Shimizu Y., Mukoubayashi C., Mugitani K., Iwane M., Nakamura H., Tamai H., Arii K., Nakata H., Yoshimura N., Takeshita T., Miki K., Mohara O., Ichinose M. (2005) Gastric cancer screening of a high-risk population in Japan using serum pepsinogen and barium digital radiography. Cancer Sci. 96, 713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Oishi Y., Kiyohara Y., Kubo M., Tanaka K., Tanizaki Y., Ninomiya T., Doi Y., Shikata K., Yonemoto K., Shirota T., Matsumoto T., Iida M. (2006) The serum pepsinogen test as a predictor of gastric cancer: The Hisayama study. Am. J. Epidemiol. 163, 629–637 [DOI] [PubMed] [Google Scholar]
- 62).Sasazuki S., Inoue M., Iwasaki M., Otani T., Yamamoto S., Ikeda S., Hanaoka T., Tsugane S., the Japan Public Health Center Study Group (2006) Effect of Helicobacter pylori infection combined with CagA and pepsinogen status on gastric cancer development among Japanese men and women: A nested case-control study. Cancer Epidemiol. Biomarkers Prev. 15, 1341–1347 [DOI] [PubMed] [Google Scholar]
- 63).Miki, K. (2007) Annual report 2004–2006 of the Research Committee of High Risk Strategy of Gastric Cancer Screening, supported by a Grant-in-Aid for the Research from the Ministry of Health, Labour and Welfare, Japan, Tokyo (in Japanese).
- 64).Miki K. (2007) Endoscopic stomach cancer screening based on the high risk strategy. Gastroenterol. Endosc. 49, 2451–2461(in Japanese with English abstract) [Google Scholar]
- 65).Yoshihara M., Hiyama T., Yoshida S., Ito M., Tanaka S., Watanabe Y., Haruma K. (2007) Reduction in gastric cancer mortality by screening based on serum pepsinogen concentration: A case-control study. Scand. J. Gastroenterol. 42, 760–764 [DOI] [PubMed] [Google Scholar]
- 66).Yanaoka K., Oka M., Yoshimura N., Mukoubayashi C., Enomoto S., Iguchi M., Magari H., Utsunomiya H., Tamai H., Arii K., Yamamichi N., Fujishiro M., Takeshita T., Mohara O., Ichinose M. (2008) Risk of gastric cancer in asymptomatic middle-aged Japanese subjects based on serum pepsinogen and Helicobacter pylori antibody levels. Int. J. Cancer 123, 917–926 [DOI] [PubMed] [Google Scholar]
- 67).Yanaoka K., Oka M., Mukoubayashi C., Yoshimura N., Enomoto S., Iguchi M., Magari H., Utsunomiya H., Tamai H., Arii K., Ohata H., Fujishiro M., Takeshita T., Mohara O., Ichinose M. (2008) Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol. Biomarkers Prev. 17, 838–845 [DOI] [PubMed] [Google Scholar]
- 68).Yanaoka K., Oka M., Ohata H., Yoshimura N., Deguchi H., Mukoubayashi C., Enomoto S., Inoue I., Iguchi M., Maekita T., Ueda K., Utsunomiya H., Tamai H., Fujishiro M., Iwane M., Takeshita T., Mohara O., Ichinose M. (2009) Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int. J. Cancer 125, 2697–2703 [DOI] [PubMed] [Google Scholar]
- 69).Inui Y. (2009) Gastric cancer screening, past and future aspect: Reformation of gastric cancer screening, challenge from Takasaki city medical association. Gastro-Health Now 3, 1–3(in Japanese) [Google Scholar]
- 70).Ito F. (2009) Let’s introduce gastric cancer-risk screening into population-based screening. Gastro-Health Now 8, 1–4(in Japanese) [Google Scholar]
- 71).Tanaka H., Uemura N. (2010) Usefulness of gastric cancer screening using combination assay of the serum pepsinogen (PG) method and serum anti-Helicobacter pylori (H. pylori) IgG antibody, and the eradication therapy for all H. pylori infected subjects in Adachi ward. Gastroenterol. Endosc. 52 (Suppl. 2), 2411.(in Japanese) [Google Scholar]
- 72).Inoue K., Fujisawa T., Haruma K. (2010) Assessment of degree of health of the stomach by concomitant measurement of serum pepsinogen and serum Helicobacter pylori antibodies. Int. J. Biol. Markers 25, 207–212 [PubMed] [Google Scholar]
- 73).Yanaoka K., Oka M., Yoshimura N., Deguchi H., Mukoubayashi C., Enomoto S., Maekita T., Inoue I., Ueda K., Utsunomiya H., Iguchi M., Tamai H., Fujisiro M., Nakamura Y., Tsukamoto T., Inada K., Takeshita T., Ichinose M. (2010) Preventive effects of etodolac, a selective cyclooxygenase-2 inhibitor, on cancer development in extensive metaplastic gastritis, a Helicobacter pylori-negative precancerous lesion. Int. J. Cancer 126, 1467–1473 [DOI] [PubMed] [Google Scholar]
- 74).Asaka M., Kimura T., Kudo M., Takeda H., Mitani S., Miyazaki T., Miki K., Graham D.Y. (1992) Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology 102, 760–766 [DOI] [PubMed] [Google Scholar]
- 75).Miki, K., Kodashima, S., Fujishiro, M. and Yahagi, N. (2009) A more efficient system for gastric cancer screening using the combination assay of serum anti-Helicobacter pylori IgG antibody and the serum pepsinogen levels. ACG 2009 Annual Scientific Meeting Symposia Sessions Abstract (2009 ACG Governors Award Recipient for Excellence in Clinical Research) p. 156. [Google Scholar]
- 76).Kodashima S., Miki K., Fujishiro M., Yahagi N., Koike K. (2010) Usefulness of a new gastric cancer screening program using the combination assay of serum anti-Helicobacter pylori IgG antibody and the serum pepsinogen levels. Am. J. Gastroenterol. 105, S38 [Google Scholar]
- 77).Asaka M., Kimura T., Kato M., Kudo M., Miki K., Ogoshi K., Kato T., Tatsuta M., David Y.G. (1994) Possible role of Helicobacter pylori infection in early gastric cancer development. Cancer 73, 2691–2694 [DOI] [PubMed] [Google Scholar]
- 78).Asaka M., Kato M., Kudo M., Katagiri M., Nishikawa K., Yoshida J., Takeda H., Miki K. (1995) Relationship between Helicobacter pylori infection, atrophic gastritis and gastric carcinoma in a Japanese population. Eur. J. Gastroenterol. Hepatol. 7 (Suppl. 1), S7–S10 [PubMed] [Google Scholar]
- 79).Asaka M., Kato M., Kudo M., Katagiri M., Nishikawa K., Koshiyama H., Takeda H., Yoshida J., Graham D.Y. (1996) Atrophic changes of gastric mucosa are caused by Helicobacter pylori infection rather than aging; studies in asymptomatic Japanese adults. Helicobacter 1, 52–56 [DOI] [PubMed] [Google Scholar]
- 80).Asaka M., Kudo M., Kato M., Sugiyama T., Takeda H. (1998) Review article: long-term Helicobacter pylori infection—from gastritis to gastric cancer. Aliment. Pharmacol. Ther. 12 (Suppl. 1), 9–15 [DOI] [PubMed] [Google Scholar]
- 81).Inoue K. (2010) We can tell the “degree of health” of the stomach (gastric cancer risk) by blood test. Gastro-Health Now 12, 1–3(in Japanese) [Google Scholar]
- 82).Miki K. (2010) Present status and future aspects of gastric cancer-risk gastritis screening (ABC screening). Endoscopic Forum for digestive disease 26, 1–4(in Japanese with English abstract) [Google Scholar]