Abstract
In the nematode Caenorhabditis elegans, the zinc finger transcriptional regulator TRA-1A directs XX somatic cells to adopt female fates. The membrane protein TRA-2A indirectly activates TRA-1A by binding and inhibiting a masculinizing protein, FEM-3. Here we report that a part of the intracellular domain of TRA-2A, distinct from the FEM-3 binding region, directly binds TRA-1A. Overproduction of this TRA-1A-binding region has tra-1-dependent feminizing activity in somatic tissues, indicating that the interaction enhances TRA-1A activity. Consistent with this hypothesis, we show that tra-2(mx) mutations, which weakly masculinize somatic tissues, disrupt the TRA-2/TRA-1A interaction. Paradoxically, tra-2(mx) mutations feminize the XX germ line, as do tra-1 mutations mapping to the TRA-2 binding domain. We propose that these mutations render tra-2 insensitive to a negative regulator in the XX germ line, and we speculate that this regulator targets the TRA-2/TRA-1 complex. The intracellular domain of TRA-2A is likely to be produced as a soluble protein in vivo through proteolytic cleavage of TRA-2A or through translation of an XX germ line-specific mRNA. We further show that tagged derivatives of the intracellular domain of TRA-2 localize to the nucleus, supporting the hypothesis that this domain is capable of modulating TRA-1A activity in a manner reminiscent of Notch and Su(H).
Keywords: Sex determination, signal transduction, development, receptor, Gli, two-hybrid
A two-fold difference in X chromosome dosage distinguishes males (XO) and hermaphrodites (XX) of the nematode Caenorhabditis elegans at the beginning of embryogenesis. As adults, individuals of the two sexes differ in size, anatomy, and behavior. About one-third of their somatic cells express sex-specific characteristics. How the X chromosome to autosome (X : A) ratio determines sex has been the subject of detailed genetic investigation (for review, see Meyer 1997). These studies have defined a regulatory pathway that transduces information about the X : A ratio to a gene known as tra-1.
In all somatic tissues, tra-1 activity is necessary and sufficient to promote female differentiation (Hodgkin 1987). Inhibition of tra-1 activity results in male development. The tra-1 gene encodes a zinc finger transcription factor, TRA-1A, that is related to Drosophila Ci and the Gli proteins of vertebrates (Zarkower and Hodgkin 1992). Few targets of TRA-1A have been identified, but in at least two cell types, TRA-1A represses genes that would otherwise cause adoption of male-specific fates. The survival of the HSN neurons, which are required for egg laying in hermaphrodites, depends on the repression of the egl-1 gene by TRA-1A (Conradt and Horvitz 1999). Similarly, vitellogenin synthesis by intestinal cells in the hermaphrodite requires that TRA-1A repress the mab-3 gene (Yi et al. 2000).
The role of tra-1 in the germ line is less well understood. Mutants lacking tra-1 activity, whether XX or XO, often exhibit limited spermatogenesis followed by oogenesis, suggesting that tra-1 is needed to sustain spermatogenesis (Schedl et al. 1989). On the other hand, strong gain-of-function alleles of tra-1 completely feminize the germ line, indicating that unregulated tra-1 activity can suppress spermatogenesis (Hodgkin 1980; 1987). Recent observations suggesting that TRA-1A may serve as both an activator and a repressor of the fog-3 gene, which is required for spermatogenesis, reinforce the idea that the function of tra-1 in the germ line is complex (Chen and Ellis 2000).
Negative regulation plays a prominent role in C. elegans sex determination (Fig. 1A). Thus, male development in XO animals requires the inhibition of tra-1 activity by three fem genes (Doniach and Hodgkin 1984; Kimble et al. 1984; Hodgkin 1986). In XX animals, the tra-2 and tra-3 genes indirectly activate tra-1 by inhibiting fem activity. Support for this model comes from the observation that mutational inactivation of any of the fem genes renders tra-2 and tra-3 dispensable for female development. For example, both fem-1 mutants and tra-2; fem-1 double mutants develop as true females as a result of unregulated tra-1 activity (Doniach and Hodgkin 1984).
Figure 1.
Model of somatic sex determination in C. elegans. (A) Genetic pathway regulating somatic sex determination. Barred lines indicate negative interactions, and arrows indicate positive interactions. The X/A ratio controls X chromosome dosage compensation and sex determination via xol-1 and the sdc genes. The regulatory pathway branches at the level of the sdc genes, and only the branch that controls somatic sex determination is shown. In XX animals, tra-2 negatively regulates the fem genes, allowing tra-1 to promote female development. In XO animals, elevated her-1 activity inhibits tra-2 and permits the fem genes to bring about male development by negatively regulating tra-1. (B) Molecular model of somatic sex determination. (Left) A high X/A ratio prevents HER-1 synthesis, allowing TRA-2A to inhibit the FEM proteins by binding FEM-3. TRA-1A is thus free direct female somatic development. (Right) A low X/A ratio results in the synthesis of HER-1, a small, secreted protein that inactivates the membrane protein TRA-2A, thereby releasing the FEM proteins from negative regulation. The FEM proteins then inhibit the activity of TRA-1A to cause male development. TRA-3 is shown as activating TRA-2A. See text for possible mechanism. Whether it is subject to sex-specific regulation is not known.
The major product of tra-2 is a large membrane protein known as TRA-2A (Fig. 1B) (Kuwabara et al. 1992). A direct interaction between the intracellular domain of TRA-2A and FEM-3 inhibits the masculinizing activity of the FEM proteins, and although the mechanism of inhibition remains to be determined, this interaction would seem sufficient to explain the feminizing role of TRA-2A (Mehra et al. 1999). In support of this idea, overproduction of the intracellular domain of TRA-2A as a soluble protein in the somatic tissues of XO animals is strongly feminizing (Kuwabara and Kimble 1995; Mehra et al. 1999).
A 1.8 kb mRNA specific to the hermaphrodite germ line can encode a second TRA-2 protein known as TRA-2B, which is equivalent to the intracellular domain of TRA-2A (Okkema and Kimble 1991; Kuwabara et al. 1998). Genetic evidence suggests that TRA-2B may limit the extent of spermatogenesis in the hermaphrodite. Because TRA-2B includes the entire FEM-3-binding domain of TRA-2A, it is reasonable to expect that TRA-2B might also act by binding to and inhibiting FEM-3.
Although the mRNA encoding TRA-2B is restricted to the hermaphrodite germ line, a similar soluble protein might be produced in other tissues by cleavage of TRA-2A. The tra-3 gene, which acts at the same level in the sex-determining pathway as tra-2, encodes an atypical calpain protease (Barnes and Hodgkin 1996). When the two proteins are expressed together in insect cells, TRA-3 can cleave TRA-2A to release a fragment that includes part of the intracellular domain of TRA-2A (Sokol and Kuwabara 2000). This observation led to a suggestion that tra-3 might fulfill its role in sex determination by producing a soluble, FEM-3-binding fragment of TRA-2A. In this paper, we will use TRA-2c to refer to the intracellular domain of TRA-2A, whether it originates as part of TRA-2A or as TRA-2B.
Interaction with FEM-3 is the only effector function of TRA-2 that has been defined to date. FEM-3 binding does not require the C-terminal 200 amino acids of TRA-2c, but genetic evidence suggests that this region is nevertheless important for TRA-2 activity. Doniach (1986) identified tra-2 alleles that cause germ line feminization, a phenotype associated with increased tra-2 activity. Some of these alleles, now known as tra-2(mx) alleles, are unusual in that although they feminize the XX germ line, they appear to reduce tra-2 activity in somatic tissues. All of the tra-2(mx) alleles carry missense mutations that alter the sequence of a 22-amino acid region near the C-terminal of TRA-2c, outside the FEM-3-binding domain (Kuwabara et al. 1998). It has been suggested that this “MX region” might represent a domain required for interaction with a negative regulator of tra-2 in the germ line.
Here we report that TRA-2 directly interacts with TRA-1A and that the MX region of TRA-2 is critical for the interaction. The somatic effects of tra-2(mx) mutations and the results of overexpression experiments lead us to suggest that TRA-2 may promote somatic female development not only by inhibiting FEM-3, but also by directly stimulating the activity of TRA-1A. Mutations that alter either TRA-2 or TRA-1A so as to disrupt their interaction feminize the germ line. This observation raises the conundrum of why an interaction that enhances the feminizing activity of both proteins in somatic tissues should appear to have a role in hermaphrodite spermatogenesis.
Results
In vitro interaction between TRA-1A and a C-terminal fragment of TRA-2
To investigate the mechanisms regulating TRA-1A, we tested for interactions between TRA-1A and other known sex-determining proteins. We made use of nematodes carrying a myc epitope-tagged tra-1 minigene under the control of a heat-shock promoter (Fig. 2). On heat shock, these transgenic animals expressed a Myc-tagged protein of about 125 kD, which corresponds to the predicted molecular weight of TRA-1A (Fig. 3A). In Far Western experiments, we tested whether an in vitro translated, 35S-TRA-2c probe (Fig. 2) could specifically bind to MycTRA-1A in lysates of heat-shocked worms. As shown in Figure 3A, the TRA-2c probe specifically labelled a heat shock-induced band that comigrated with MycTRA-1A.
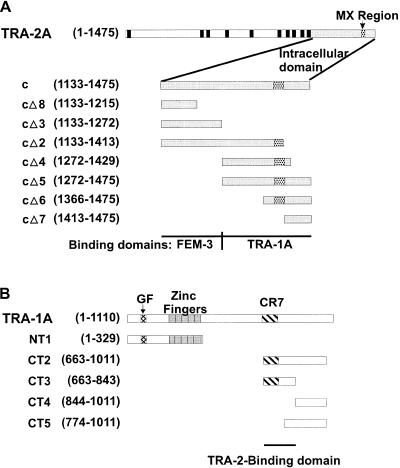
Figure 2.
Diagram of TRA-2A, TRA-1A, and the protein fragments used in this study. Numbers indicate the amino acids at the termini of each fragment. Tags (GFP, Myc, or HA, as described in Materials and Methods) were located at the N terminus of each protein. (A) TRA-2A and fragments. The predicted signal sequence and transmembrane domains of TRA-2A are indicated as black rectangles. The intracellular domain is shaded grey. Stippling indicates the MX region that is mutated in tra-2(mx) alleles. (B) TRA-1A and fragments. The zinc fingers of TRA-1A are indicated as grey boxes. The GF region, altered in gain-of-function alleles of tra-1, is indicated by cross-hatching. Hatching indicates a region of unknown function, referred to here as CR7, which is conserved in C. briggsae and C. elegans TRA-1A.
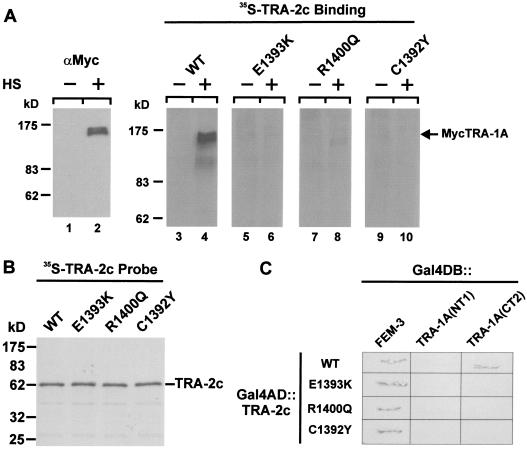
Figure 3.
Interaction between TRA-2c and TRA-1A. (A) TRA-2c binds to MycTRA-1A from nematode lysates in a Far Western assay. Lysates of worms carrying a HS-MycTRA-1A transgene were prepared before (−) or after (+) heat shock. They were analysed by SDS-PAGE, transferred to nitrocellulose, and probed either with the anti-Myc monoclonal antibody 9E10 (lanes 1,2) or with the indicated 35S-labelled fragments of TRA-2 (lanes 3–16). The positions of molecular weight markers are indicated. An asterisk indicates the position of MycTRA-1A. (B) Samples of the 35S-labelled TRA-2 probes used in A were analysed by SDS-PAGE and autoradiography to verify that they were of similar specific activity. (C,D) Yeast expressing the indicated protein fragments (see Fig. 2 for diagrams) as fusions with the Gal4 DNA-binding domain (Gal4 DB) or the Gal4 activation domain (Gal4 AD) were assayed on filters for β-galactosidase activity. TRA-2cΔ3 is the smallest fragment previously shown to interact with FEM-3.
To find out whether sequences required for binding to TRA-1A overlapped with the FEM-3-binding domain in TRA-2c (Mehra et al. 1999), we repeated the Far Western experiment using a series of labelled fragments of TRA-2c as probes (Figs. 2, 3B). Probes prepared from the FEM-3-binding domain of TRA-2c failed to interact with TRA-1A (probes cΔ2, cΔ3, Fig. 3A). In contrast, a C-terminal, 202 amino acid fragment of TRA-2, cΔ5, specifically detected MycTRA-1A. This fragment includes the 22-amino acid MX region (Fig. 2; see Introduction). A C-terminal probe lacking the MX region (cΔ7) did not detect MycTRA-1A, but neither did a C-terminally truncated probe that retained the MX region (cΔ4), suggesting that the MX region was required but not sufficient for the interaction. We infer that the intracellular domain of TRA-2 contains a C-terminal TRA-1A-binding domain that is distinct from the FEM-3-binding region.
A region near the C terminus of TRA-1A mediates TRA-2 binding in yeast
To confirm that TRA-2 and TRA-1A interact, and to locate a region in TRA-1A that mediates the interaction, we used the yeast two-hybrid system (Fields and Song 1989; Durfee et al. 1993). In the experiment shown in Figure 3C, we tested various Gal4 activation domain (Gal4 AD)::TRA-2 fusion proteins for the ability to interact with either of two different fragments of TRA-1A (see Fig. 2B for definition of fragments) fused to the Gal4 DNA-binding domain (Gal4 DB). None of the TRA-2 fusion proteins interacted with the N-terminal TRA-1A fragment NT1, but those including the C-terminal 202 residues of TRA-2 (c or cΔ5) interacted with a fusion protein containing a C-terminal fragment of TRA-1A, CT2. Controls showed that none of these fusion proteins activated reporter gene expression when expressed alone in yeast. A Gal4 DB::FEM-3 fusion protein interacted only with those TRA-2 fusion proteins that included the previously defined FEM-3-binding domain (c, cΔ2, cΔ3) (Mehra et al. 1999). The two-hybrid assay thus confirms the results of the Far Western assay in suggesting the existence of a C-terminal TRA-1A-binding domain within TRA-2. Moreover, our results indicate that the interaction depends on sequences within 450 residues of the C-terminal of TRA-1A.
To define more narrowly the TRA-2-binding region in TRA-1A, we repeated the experiment with smaller TRA-1::Gal4 DB fusion proteins (Fig. 3D). A 181 amino acid fragment of TRA-1A (CT3), extending from amino acid 663–843, was sufficient to interact with TRA-2c. No function had been assigned previously to this region of TRA-1A, but a notable feature of the sequence is a region of about 80 amino acids that exhibits significant sequence conservation with TRA-1 from Caenorhabditis briggsae (de Bono and Hodgkin 1996; CR7 in Fig. 2B).
tra-2(mx) point mutations disrupt the TRA-2/TRA-1A interaction
Because the TRA-1A-binding domain in TRA-2 included the 22 amino acid MX region, we tested whether the amino acid substitutions that result from mx mutations affect the TRA-2/TRA-1A interaction. We used three different MX variants of TRA-2c. The first corresponded to the product of the tra-2(e2021) allele and contained tyrosine in place of cysteine residue 1392. We refer to it by the amino acid substitution, C1392Y. We also tested the effects of the substitution E1393K, encoded by tra-2(e1939), and R1400Q, encoded by tra-2(e2019) (Kuwabara et al. 1998).
Each of the three MX substitutions severely reduced the TRA-1A-binding activity of 35S-TRA-2c in the Far Western assay. We were unable to detect specific MycTRA-1A binding by TRA-2 probes carrying either the C1392Y or the E1393K substitution, and the R1400Q variant exhibited only weak binding (Fig. 4A). Interestingly, of the five tra-2(mx) alleles characterized by Doniach (1986), those encoding the C1392Y and E1393K substitutions were the strongest with respect to their masculinizing effect on somatic development, whereas the allele encoding the R1400Q variant was the weakest.
Figure 4.
Effect of tra-2(mx) mutations on the TRA-2/TRA-1A interaction. (A) Far western analysis. Lysates were prepared from heat-shocked (+) or non-heat-shocked (−) nematodes carrying a HS-MycTRA-1A transgene, fractionated by SDS-PAGE, transferred to nitrocellulose, and probed either with monoclonal antibody 9E10 (lanes 1,2), with 35S-radiolabelled TRA-2c (lanes 3,4) or with 35S-TRA-2c carrying the indicated MX amino acid substitutions (lanes 5–10). After washing, bound antibody was detected by chemiluminescence, bound TRA-2c by autoradiography. (B) Samples of the 35S-TRA-2c probes were analysed by SDS-PAGE and autoradiography to verify that all were of similar specific activity. (C) tra-2(mx) mutations disrupt the TRA-2/TRA-1A interaction in the yeast two-hybridsystem. Yeast expressing the indicated protein fragments as fusion proteins with the Gal4 DNA-binding domain (Gal4 DB) or Gal4 activation domain (Gal4 AD) were assayed on filters for β-galactosidase activity. All three of the MX variants of TRA-2c that we tested retain the ability to interact with FEM-3, but none interacted with TRA-1A in this assay.
The mx mutations similarly disrupted the TRA-2/TRA-1A interaction in the yeast two-hybrid system (Fig. 4C). None of the three MX variants of a Gal4AD::TRA-2c fusion allowed reporter gene activation when coexpressed with a Gal4DB::TRA-1 fusion protein. All three mutant TRA-2c proteins, however, interacted with FEM-3 in this system, indicating that the MX mutations did not merely cause misfolding of TRA-2, but instead selectively interfered with the TRA-2/TRA-1A interaction.
Feminizing activity of the FEM-3 and TRA-1-binding domains of TRA-2
Having found TRA-2c to be capable of binding to TRA-1A, we wanted to establish whether this interaction contributed to the feminizing activity of TRA-2. To test the activity of the separate FEM-3 and TRA-1A binding domains in TRA-2, we produced a series of heat shock promoter-driven transgenes encoding GFP-Myc-tagged fragments of TRA-2. We subjected nematodes carrying these transgenes to periodic heat shock throughout development and scored feminization of sexually dimorphic tissues in adult XO animals. In these experiments, we used a host strain that produces 30% XO self-progeny (see Materials and Methods).
Overproduction of GFP-Myc-TRA-2c strongly feminized the somatic tissues of XO animals (Fig. 5A). Many of these animals were distinguishable from XX hermaphrodites only because their germ line remained male, presumably because of the poor expression of heat shock promoter transgenes in this tissue (Stringham et al. 1992). These results confirm that the feminizing activity of TRA-2A resides in its intracellular domain (Kuwabara and Kimble 1995; Mehra et al. 1999). Fragments of TRA-2c that bound FEM-3, but not TRA-1A, exhibited feminizing activity comparable to that of the complete TRA-2c (Fig. 5A, cΔ3, cΔ2). This observation supports our contention that TRA-2 promotes female development primarily by binding and inhibiting FEM-3 (Mehra et al. 1999).
Figure 5.
Feminizing activity of the FEM-3-binding and TRA-1A-binding domains of TRA-2c. (A) The indicated fragments of TRA-2c were expressed from the heat shock promoter throughout development in transgenic him-5 nematodes, and the adult animals were scored for sexual phenotype of the gonad, ventral hypodermis, and tail. Hermaphrodites homozygous for him-5(e1490) produce about 30% XO and 70% XX (somatically female) self-progeny. In those lines that produced morphologically normal males, we looked for yolk accumulation in the male pseudocoelom as an indicator of gut feminization. (B) Western blot verifying expression of TRA-2c fragments. Lysates were prepared from transgenic lines maintained continuously at 20° C (HS −) and from animals subjected to heat shock at 33° C followed by a 2 hr recovery period (HS +). The animals carried transgenes encoding GFP (lanes 1,2) or the indicated GFP-tagged fragments of TRA-2c. The lysates were analysed by SDS-PAGE and immunoblotting with anti-GFP antibody. The positions and relative molecular weights (in kD) of marker proteins are indicated to the left of the panel. (C) Western blot verifying that TRA-2cΔ5 was equally expressed in tra-1(+) (WT, lane 2) and tra-1(e1099) mutants (lane 3). Lysates were prepared and analysed as in B.
XO nematodes expressing the isolated TRA-1A binding domain (TRA-2cΔ5) from the heat shock promoter exhibited normal male tail and gonad morphology, but an accumulation of yolk droplets in their pseudocoelom indicated feminization of their gut cells (Fig. 5A). We confirmed that Myc-TRA-2cΔ5 induced the expression of a vitellogenin reporter gene (vit-2::GFP) (Yi and Zarkower 1999) in transgenic XO animals (see below; Fig. 6). Fragments of TRA-2 that did not bind to FEM-3 or TRA-1A in vitro (cΔ4, cΔ7) failed to feminize any tissue when overexpressed in XO animals (Fig. 5A), although they accumulated to levels comparable to cΔ5 (Fig. 5B). These overexpression experiments revealed that the feminizing activity of TRA-2 does not entirely reside in its ability to bind FEM-3 and support the hypothesis that the TRA-2-TRA-1A interaction is biologically significant.
Figure 6.
MX amino acid substitutions reduce the feminizing activity of TRA-2cΔ5. (A–H) Animals carrying a vit-2::gfp reporter transgene. Panels on the left are DIC images of the posterior body region, and those on the right show reporter expression. (A,B) XX hermaphrodite, expressing vit-2::gfp in the gut. (C,D) XO male does not express the reporter. (Autofluorescence of the gut is weakly visible in D.) (E,F) XO male, expressing MycTRA-2cΔ5 from the heat shock promoter. Note that although the tail morphology is that of a normal male (E), the gut expresses the vit-2::gfp reporter. (G,H) XO male expressing MycTRA-2cΔ5(E1393K) from the heat shock promoter. Overproduction of this MX mutant form of TRA-2cΔ5 seldom induced vit-2::gfp expression. (I) Frequency of gut feminization in XO animals by MycTRA-2cΔ5 and three MX mutant forms, as measured by vit-2::gfp expression and by the accumulation of yolk in the pseudocoelom. The E1393K and C1392Y mutants were much less effective at feminizing the gut than either the wild-type protein or the R1400Q mutant.
We verified that the feminizing activity of TRA-2cΔ5 depended on endogenous tra-1 activity by introducing the transgene into animals homozygous for the null allele tra-1(e1099) (Hodgkin 1987; Zarkower and Hodgkin 1992). No yolk accumulation was evident in these animals following repeated heat shock treatment (0/38 animals examined), although the TRA-2cΔ5 fusion protein accumulated to levels at least equivalent to those seen in wild-type animals (Fig. 5C). Therefore, the feminizing activity of TRA-2cΔ5 was dependent on tra-1 activity.
If TRA-2cΔ5 induced yolk synthesis as a result of its interaction with TRA-1A, then MX mutant forms of TRA-2cΔ5, which exhibit little or no TRA-1A-binding activity in vitro, should exhibit reduced feminizing activity. To test this prediction, we produced transgenic lines carrying both a vit-2::GFP reporter gene and a heat-shock-driven construct encoding either wild-type TRA-2cΔ5 or one of three MX variants. After heat shock induction of TRA-2, we assayed vitellogenin reporter expression and scored the accumulation of yolk droplets in the pseudocoelom. Whereas both wild-type TRA-2cΔ5 and the R1400Q mutant induced vit-2::GFP in the majority of transgenic XO animals, the other two MX mutant proteins, C1392Y and E1393K, were much less effective at inducing reporter expression, and neither caused visible accumulation of yolk (Fig. 6). We conclude that the gut-feminizing activity of TRA-2cΔ5 indeed depended on its ability to bind to TRA-1A. Yolk induction by TRA-2cΔ5(R1400Q) was consistent with our earlier observation that the R1400Q mutation affected TRA-1A binding less severely than either of the other MX mutations we tested.
Homozygous tra-2(e2021mx) XX mutants often have slightly masculinized tails, and in heterozygotes carrying e2021 in trans to the null allele e1095, the tail always shows some masculinization, often including male sensory structures such as rays and spicules (Doniach 1986). To find out if the isolated TRA-1A-binding domain might feminize the tail, we tested whether overproduction of TRA-2cΔ5 could rescue female somatic development in tra-2(e2021mx) or e2021mx/e1095 XX animals. Periodic expression of the wild type TRA-2cΔ5 from a heat-inducible transgene partially suppressed the development of male tail structures in mx/mx and mx/null animals (Table 1, data not shown). A comparable construct encoding TRA-2cΔ5 with the E1393K substitution did not have rescuing activity. These results support the conclusion that the isolated TRA-1A-binding domain of TRA-2c has feminizing activity in somatic tissues, and that tra-2(mx) mutations reduce or eliminate that activity.
Table 1.
Partial rescue of tra-2(mx)/tra-2(null)a somatic phenotype by HS-TRA-2cΔ5
| TRA-2AcΔ5 variantb
|
n
|
Percent Rolc with snub tail
|
Percent Rol with spicules
|
|---|---|---|---|
| noned | 51 | 100 | 63 |
| Wild type | 56 | 46 | 5 |
| E1393K | 58 | 100 | 74 |
The animals scored were of genotype tra-2(e2021mx)/tra2(e1095).
The indicated protein was expressed from the hsp-16.41 heat shock promoter. In each line, TRA-2cΔ5 carried an N-terminal GFP tag followed by a Myc tag.
The Rol phenotype marked animals carrying the transgene. See Materials and Methods.
Control nontransgenic animals were subjected to the same heat shock procedure as the transgenic animals. See Materials and Methods.
The intracellular domain of TRA-2 localizes to the nucleus
Our observations that TRA-2c directly interacts with TRA-1A led us to ask which subcellular compartment is the site of this interaction in vivo. As a transcription factor, TRA-1A must localize to nuclei in at least some cells in XX hermaphrodites. TRA-2c is expected to be soluble, but its subcellular localization is unknown. We made use of GFP-tagged derivatives of TRA-1A and TRA-2c to ask whether TRA-1A is restricted to the nucleus and to which compartment of the cell TRA-2c would localize.
Periodic expression of GFP-tagged TRA-1A from a heat shock inducible transgene partially rescued female somatic development in animals homozygous for the null allele, tra-1(e1099) (not shown). GFP-TRA-1A localized to nuclei in both XX and XO animals (Fig. 7A). Under the conditions we used (see Materials and Methods), the XO animals exhibited normal male morphology, indicating that the localization of TRA-1A to the nucleus is not sufficient to trigger female development. These observations suggest that TRA-1A constitutively localizes to nuclei, and we suggest that it is unlikely to interact with the intracellular domain of TRA-2A at the cell membrane.
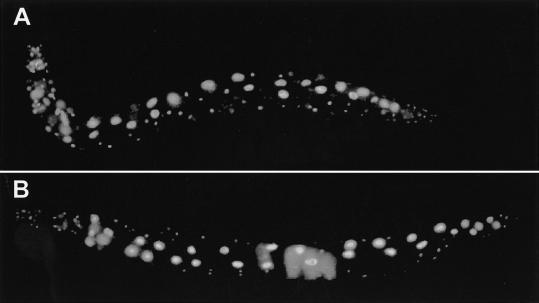
Figure 7.
Nuclear localisation of GFP-MycTRA-1A and GFP-TRA-2c. (A) Adult hermaphrodite expressing GFP-MycTRA-1A, showing predominantly nuclear fluorescence. (B) Adult hermaphrodite expressing GFP-TRA-2c, which also localized predominantly to nuclei.
The addition of a GFP tag to the N-terminal of TRA-2c did not interfere with its activity, because the tagged protein caused essentially complete somatic feminization of XO animals when overproduced from the heat shock promoter. Surprisingly, tagged TRA-2c localized to nuclei in all cells that expressed it in both XX and XO animals (Fig. 7B). TRA-2c localization did not depend on the presence of TRA-1A (not shown). We could not find an obvious nuclear localization signal (NLS) by inspecting the sequence of TRA-2c, but observations of various tagged fragments of TRA-2c suggested the presence of an NLS near the N terminus (data not shown). We conclude that TRA-2c would enter nuclei at least in somatic tissues, and that the interaction between TRA-2c and TRA-1A is likely to occur in the nucleus.
Several tra-1 alleles that cause germ line feminization have mutations affecting the TRA-2-binding domain
If loss of the TRA-2/TRA-1A interaction is responsible for the germ line feminization and mild somatic masculinization of tra-2(mx) mutants, then mutations in tra-1 that disrupt the interaction might cause similar phenotypes. Two kinds of tra-1 allele have been found to have feminizing effects. Alleles of the first type cause a gain of tra-1 function and feminize both germ line and somatic tissues (Hodgkin 1980; 1987). They alter the sequence of TRA-1A in a small, N-terminal region that is well outside the TRA-2-binding domain and is believed to be important for negative regulation of TRA-1A by the FEM proteins (de Bono et al. 1995). The second group includes mutations that have feminizing effects in an smg mutant background (Zarkower et al. 1994). The smg genes encode components of an RNA surveillance system that degrades aberrant transcripts (Pulak and Anderson 1993). In an otherwise wild-type background, most of these smg-sensitive tra-1 mutations cause weak somatic masculinization, suggestive of reduced function, or they produce no detectable phenotype. Zarkower et al. (1994) suggested that the mutations in smg-sensitive tra-1 alleles might disrupt a C-terminal negative regulatory domain in TRA-1A and also render tra-1 transcripts unstable in wild-type animals, so that the mutant products accumulate to levels sufficient to cause feminization only in an smg background.
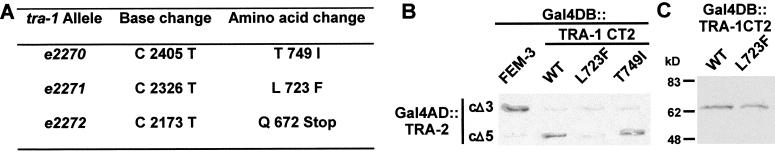
We sequenced the region encoding the TRA-2-binding domain in six smg-sensitive tra-1 alleles and found mutations in three (Fig. 8A). tra-1(e2272) carries a nonsense mutation that truncates TRA-1A at residue 672, deleting all but the first 10 residues of the minimal TRA-2-binding domain. Two other alleles, tra-1(e2270) and e2271, have missense mutations that cause nonconservative amino acid substitutions within the TRA-2 binding domain. The mutation in e2271 alters a residue within conserved region CR7 (Fig. 2B; see de Bono and Hodgkin 1996), whereas the residue affected by e2270 lies C-terminal to region CR7. Neither affected residue is itself conserved between C. elegans and C. briggsae.
Figure 8.
Effect of smg-sensitive tra-1 mutations on the TRA-2/TRA-1A interaction. (A) Three smg-sensitive alleles of tra-1 have mutations that affect the TRA-2c-binding domain. Nucleotide and amino acid numbering are from the sequence reported by Zarkower and Hodgkin (1992), GenBank accession no. M932256. We did not find mutations within the region encoding the TRA-2c-binding domain in the tra-1 alleles e2351, e2352, or e2368. (B) Yeast two-hybrid assay. Yeast coexpressing the indicated Gal4DB and Gal4AD fusion proteins were assayed for β-galactosidase activity. A TRA-1CT2 fusion protein carrying the L723F substitution fails to interact with TRA-2, whereas the T749I substitution does not affect the TRA-1/TRA-2 interaction in this assay. (C) Anti-HA Western blot verifying that the Gal4DB::TRA-1CT2(L723F) fusion protein is expressed in yeast at levels comparable to the corresponding wild-type fusion protein.
We used the yeast two-hybrid system to test the effects of the two missense mutations on the TRA-2c/TRA-1A interaction. A fragment of TRA-1A carrying the L723F amino acid substitution encoded by tra-1(e2271) failed to interact with TRA-2c (Fig. 8B). Western analysis confirmed that the mutant protein was stably expressed in yeast (Fig. 8C). We infer that the phenotypic defects in mutants homozygous for tra-1(e2271) or the nonsense mutation e2272 probably result from the failure of the mutant TRA-1A protein to bind TRA-2c. The tra-1(e2270) allele causes temperature-sensitive germ line feminization in a smg mutant background (Zarkower et al. 1994) consistent with the observation that its product retained the ability to bind TRA-2c in the two-hybrid assay (Fig. 8B).
Discussion
An unexpected protein-protein interaction between TRA-2 and TRA-1A
Our observations of a direct interaction between TRA-2c and TRA-1A were surprising for two reasons. First, genetic and molecular analyses of sex determination in C. elegans strongly support a model in which tra-2 indirectly activates tra-1 by inhibiting fem activity, thereby promoting female differentiation (Fig. 1; see Introduction). These results did not rule out the possibility of a direct interaction between tra-2 and tra-1, but neither did they suggest any requirement for such an interaction. Second, as TRA-2A is a membrane protein (Kuwabara et al. 1992; Sokol and Kuwabara 2000) and TRA-1A is a transcription factor (Conradt and Horvitz 1999; Chen and Ellis 2000; Yi et al. 2000), one might have expected differences in their subcellular localization to hinder their interaction.
Colocalization of TRA-2c with TRA-1A: interaction in the nucleus?
The finding that GFP-tagged derivatives of TRA-1A and TRA-2c localize to the nucleus suggests that the two proteins indeed have the opportunity to interact in vivo (Fig. 7). Although TRA-2c is only a fragment of the major tra-2 product, TRA-2A, we believe its localization to be biologically meaningful. First, GFP-TRA-2c is capable of completely feminizing the XO soma. Second, soluble TRA-2c protein may be produced in vivo by TRA-3-mediated cleavage of TRA-2A (Sokol and Kuwabara 2000) or by translation of a 1.8 kb mRNA specific to the hermaphrodite germ line (Kuwabara et al. 1998).
The generation of TRA-2c by cleavage of TRA-2A, its translocation to the nucleus, and its interaction with the transcription factor TRA-1A have precedents in the processing of the Notch receptor and the translocation of its intracellular domain to the nucleus, where it modulates the activity of the transcription factor Su(H) (Schroeter et al. 1998; Struhl and Adachi 1998). The proposed processing of TRA-2A seems mechanistically distinct from Notch processing, however, in that it is likely to involve a calpain protease (Barnes and Hodgkin 1996; Sokol and Kuwabara 2000) and is unlikely to be ligand-dependent, because the candidate ligand HER-1 (Hunter and Wood 1992; Perry et al. 1993) inhibits the activity of TRA-2A and indirectly, that of TRA-1A. Whether HER-1 might inhibit TRA-2A cleavage is not known, but inhibiting cleavage would not necessarily be sufficient to inhibit tra-2 activity. We would expect full-length TRA-2A to have FEM-3-binding activity similar to that of TRA-2c.
Evidence for the biological significance of the TRA-2/TRA-1A interaction
We have presented two types of evidence that the interaction between TRA-2c and TRA-1A is biologically significant. First, mutations that alter either protein so as to prevent its interaction with the other cause similar sex determination defects (Figs. 4, 8). The similarity in phenotypes caused by these mutations is most economically explained in terms of a shared defect in interaction between TRA-2c and TRA-1A.
The second kind of evidence for the significance of the interaction is that a fragment of TRA-2 that binds to TRA-1A but not to FEM-3 has tra-1-dependent somatic feminizing activity in overexpression assays, and that activity is abrogated by strong mx mutations (Figs. 5, 6). In otherwise wild type animals, feminization is limited to the gut, but the TRA-1A-binding domain of TRA-2c can feminize other tissues in tra-2(mx)/tra-2(null) animals, suggesting that its activity is weak but not intrinsically tissue-specific. Interestingly, the overproduction of TRA-3 has mild feminizing activity (Sokol and Kuwabara 2000) very similar to that of the TRA-1A-binding domain of TRA-2c, consistent with a role for TRA-3 in releasing TRA-2c to allow interaction with TRA-1A.
TRA-2 as a cofactor for TRA-1A in somatic tissues
If TRA-1A can promote female development in the absence of TRA-2 activity, what role does the interaction between the two proteins play in sex determination? We will address this question first for somatic tissues and then consider the germ line, as mutations that disrupt the interaction have different effects in germ line and soma.
Because tra-2(mx) mutations result in mild masculinization of the XX soma (Doniach 1986), we suggest that in somatic tissues, interaction with TRA-2c enhances the feminizing activity of TRA-1A. One simple model is that TRA-2c is a transcriptional coactivator or corepressor for TRA-1A (Fig. 9A). A second possibility is that by binding to TRA-1A, TRA-2c might partly counteract the inhibitory effect of the FEM proteins, perhaps by preventing a FEM-dependent modification of TRA-1A or by allowing TRA-1A to function despite such modification. Alternatively, or in addition, the binding of TRA-2 might stabilize TRA-1A, allowing it to promote female development more effectively.
Figure 9.
Model for the role of the TRA-2/TRA-1 interaction. (A) This model proposes that in somatic tissues, TRA-3 cleaves TRA-2A to release the intracellular domain, TRA-2c, as a soluble protein which then translocates to the nucleus and interacts with TRA-1A to stimulate its feminizing activity. TRA-2A also binds to FEM-3 as previously demonstrated to inhibit the masculinizing activity of the FEM proteins. (B) In the XX germ line, a 1.8 kb tra-2 transcript furnishes an additional source of TRA-2c. TRA-2c interacts with TRA-1A in the nucleus as above, but we hypothesize that a negative regulator in the XX germ line inhibits the feminizing activity of the complex to allow spermatogenesis.
We have shown that the isolated FEM-3-binding domain of TRA-2 has much stronger somatic feminizing activity than the isolated TRA-1A-binding domain. This observation strengthens our earlier conclusion that the primary role of TRA-2A is to bind and inhibit FEM-3 (Mehra et al. 1999). The TRA-2/TRA-1A interaction could be viewed, at least in somatic tissues, as a support mechanism that ensures that TRA-1A activity is adequate to prevent or overcome inhibition by residual FEM activity. Interestingly, tra-2 gain-of-function mutations that relieve translational repression of tra-2 (Goodwin et al. 1993) largely suppress the defects of tra-3 mutants (Doniach 1986). This observation has been interpreted as supporting a model in which TRA-3 inhibits a repressor of tra-2 translation (Goodwin et al. 1997), but it is also consistent with the idea that one role of TRA-3 is to generate TRA-2c, and that elevated levels of TRA-2A render TRA-2c and therefore TRA-3 dispensable.
Why do mutations that disrupt the TRA-2/TRA-1A interaction feminize the germ line?
In contrast to their weakly masculinized somatic phenotype, tra-2(mx) XX mutants exhibit germ line feminization, which implies that mx mutations render tra-2 less sensitive to negative regulation in the hermaphrodite germ line (Doniach 1986; Kuwabara et al. 1998). A similar proposal has been made to explain the germ line-feminizing effects of smg-sensitive tra-1 alleles (Zarkower et al. 1994). Why should disruption of the TRA-2c/TRA-1A interaction have opposite consequences for soma and germ line?
One possible answer is that the TRA-2/TRA-1A complex, which by itself is feminizing, is a target for a negative regulator of oogenesis in the XX germ line (Fig. 9B). Mutations that disrupt the complex could result in germ line feminization if the proposed negative regulator were less effective against the dissociated proteins than against the complex, because unregulated TRA-2 and TRA-1A would promote oogenesis. The Cip/Kip family of cyclin/Cdk inhibitors and the IκBα inhibitor of p65/p50 NFκB are examples of regulatory proteins that bind to their target protein complexes with greater affinity than to the subunits of those complexes (Russo et al. 1996; Huxford et al. 1998; Jacobs and Harrison 1998). Mutations that inactivated the proposed TRA-2c/TRA-1A regulator would also feminize the XX germ line, unless the regulator was also required for other processes.
Of those genes known for their roles in germ line sex determination in C. elegans, only fog-2 is specifically and exclusively required for hermaphrodite spermatogenesis, and its inactivation transforms XX animals into females (Schedl and Kimble 1988). Although the genetic properties of fog-2 are consistent with those expected for the negative regulator pictured in Figure 9B, molecular analysis suggests that FOG-2 interacts with the RNA-binding protein GLD-1 to regulate the translation of tra-2 mRNA (T. Schedl, pers. comm.), making a role for FOG-2 in regulating the TRA-2/TRA-1A complex seem unlikely.
Why does disruption of the interaction between TRA-2 and TRA-1A have more dramatic consequences for the germ line than for the soma of the C. elegans hermaphrodite? Sex determination in the germ line is more complex than in the soma, because the hermaphrodite germ line, which develops within a female soma, must transiently adopt a male fate and later switch to a female fate (for review, see Schedl 1997). It seems that the need to balance masculinizing and feminizing activities in the germ line makes it much more sensitive to perturbation than the soma. Mutations that interfere with translational regulation of either tra-2 or fem-3 (Ahringer et al. 1992; Goodwin et al. 1993; Zhang et al. 1997; Jan et al. 1999), for example, cause sexual transformation of the germ line but have little effect on somatic tissues (Doniach 1986; Barton et al. 1987), although the translational controls for both genes are active in both soma and germ line (Goodwin et al. 1997; Gallegos et al. 1998).
Conservation of the domains involved in the TRA-2c/TRA-1A interaction
It is noteworthy that the domains involved in the TRA-2/TRA-1A interaction include some of the more conserved regions in both proteins. The C-terminal half of TRA-1A is poorly conserved between C. elegans and C. briggsae, except for a region of about 80 residues, most of which lies within the TRA-2-binding domain (de Bono and Hodgkin 1996). In TRA-2, a region of about 100 residues that includes the MX region and lies within the TRA-1A-binding domain, is relatively well conserved between C. elegans, C. briggsae, and the male-female species Caenorhabditis remanei (Kuwabara 1996; Haag and Kimble 2000). Sequence conservation in the regions that mediate the TRA-2/TRA-1A interaction suggests that the interaction itself is a conserved feature of sex determination in Caenorhabditis. This seems surprising in view of its apparently secondary role in sex determination in C. elegans. In striking contrast, the FEM-3-binding region of TRA-2 shows no significant sequence conservation between C. elegans, C. briggsae, and C. remanei. Further study will be required to determine the contribution of the TRA-2/TRA-1A interaction to sex determination in other Caenorhabditis species and to understand why the domains that mediate this interaction appear to have been more tightly constrained during evolution than those involved in the TRA-2/FEM-3 interaction.
Materials and methods
C. elegans strains and culture methods
C. elegans was cultured and manipulated as described (Brenner 1974) on MYOB medium (Church et al. 1995) at 20° C unless otherwise noted. We used the standard wild-type strain of C. elegans var. Bristol, N2, and strains derived from N2 that carried the following mutant alleles (unless otherwise noted, described in Hodgkin 1997): LGII, tra-2(e1095), tra-2(e2021), LGIII, tra-1(e1099), eDp6, LGV, and him-5(e1490). Strain CB3300 has genotype tra-1(e1099); eDp6. Strain CB3779 is a male-female strain that is homozygous for tra-2(e2021mx) (Doniach 1986).
Plasmids
Plasmids were constructed using standard methods (Sambrook et al. 1989) and are available on request. DNA sequencing was carried out at the Centre for Applied Genomics at the Hospital for Sick Children, Toronto.
Plasmids for in vitro transcription/translation of fragments of TRA-2c were derived from pPK126 (Mehra et al. 1999). For yeast two-hybrid assays, TRA-2c fragments were expressed as Gal4 activation domain fusion proteins from plasmid AS#1191 or its derivatives (Mehra et al. 1999).
Plasmids derived from fragments of pPK126 and the C. elegans heat shock expression vector pPD49.83 (Mello and Fire 1995) directed the heat-inducible expression of fragments of TRA-2c in worms. TRA-2c fragments encoded by these plasmids carried an N-terminal Myc or GFP-Myc tag. The GFP coding sequence was from vector pPD95.02 (A. Fire), and the Myc tag was from T7plinkTag.
Plasmid pDZ48 contains a myc-tagged full-length tra-1 cDNA in pPD49.83. To produce AS#DL35, which encodes GFP-Myc-TRA-1A, the GFP coding sequence from plasmid pPD95.02 was ligated into pDZ48. Subcloning fragments of pDZ48 into the two-hybrid vector pAS1 (Durfee et al. 1993) produced plasmids directing the expression of fragments of TRA-1A as Gal4 DNA-binding domain fusion proteins.
Nematode transformation
DNA microinjection was carried out as described by Mello (1995). Unless otherwise noted, the injected DNA mixture contained 50 μg/ml each of pRF4 and the plasmid to be tested. Plasmid pRF4 carries rol-6(su1006dm), which confers a dominant Roller phenotype and serves as a transformation marker (Mello et al. 1991). The total DNA concentration in the injected mixture was kept at 100 μg/ml in all experiments by adding Bluescript DNA (Stratagene) where necessary. In most experiments, a him-5(e1490) strain was used as host. Hermaphrodites homozygous for him-5(e1490) produce about 30% XO animals among their self-progeny (Hodgkin et al. 1979). Extrachromosomally transformed lines were established from the F2 Roller progeny of injected animals. At least three independent lines were analyzed for each plasmid tested.
In tests of the ability of variants of TRA-2cΔ5 to induce vitellogenin reporter expression, the plasmids encoding wild-type and mutant MycTRA-2cΔ5 proteins were injected at a concentration of 25 μg/ml with the vit-2::GFP reporter plasmid, pCR2, (Yi and Zarkower 1999) at the same concentration, and pRF4 at 50 μg/ml.
Tests of the rescuing activity of HS-GFP-MycTRA-1A were carried out on transgenic lines derived from the strain CB3300 by injecting AS#DL35 (2 μg/ml) with pRF4 (50 μg/ml). The ability of TRA-2cΔ5 to rescue the somatic phenotype of tra-2(e2021mx) XX animals was tested by establishing transgenic lines in strain CB3779.
Heat shock experiments
In most experiments to test the feminizing activity of TRA-2c and its derivatives, adult transgenic hermaphrodites were transferred to seeded plates to let them lay eggs and were removed after 4–6 hr. After a further incubation of 1–3 hr, their progeny were heat-shocked at 33° C for 1 hr. The heat shock was repeated at intervals of 10–13 hr until the animals reached adulthood. Adult transgenic animals were scored for sexual phenotype using differential interference contrast microscopy. In experiments that tested for induction of vit-2::gfp, embryos were incubated for 18–20 hr before the first heat shock. In testing for rescue of tra-1(e1099) by HS-GFP-Myc-TRA-1A, we used daily 20 min heat shocks in a 33° C water bath to induce transgene expression.
To examine the subcellular localisation of GFP-MycTRA-1A and GFP-MycTRA-2c, we induced the expression of each protein from the relevant heat shock transgene with a 1–2 hr, 33° C heat shock followed by a 1–2 hr recovery period at 20° C.
Far Western experiments
One of two populations of transgenic animals carrying pDZ48 (HS-MycTRA-1A) was subjected to a 2 hr heat shock at 33° C and allowed to recover at 20° C for 2 hr, while the second was continuously kept at 20° C . The animals were rinsed off the plates in water, collected by centrifugation, and lysed by boiling in SDS sample buffer. Lysates were fractionated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and either silver-stained or transferred to nitrocellulose. TRA-2c binding was tested using the procedure of Guichet and coworkers (1997). Filters were blocked in AC buffer (10% glycerol, 100 mM NaCl, 20 mM Tris-HCl at pH 7.5, 0.5 mM EDTA, 0.1% Tween 20) containing 2% skim milk powder.
TRA-2c probes were synthesized by coupled in vitro transcription/translation (Promega TNT with T7 RNA polymerase) in the presence of [35S]-methionine. Reactions were diluted five-fold in AC buffer and passed over a Sephadex G-25 spun-column to remove unincorporated methionine. Ninety-five percent of the purified probe was diluted sixfold in AC buffer containing 2% skim milk powder, and the mixture was incubated with the blocked filters overnight at room temperature. Filters were washed three times with 20 ml AC buffer for 10 min each. After the final wash, the filters were allowed to dry and exposed to X-ray film (Kodak MR-1). The remaining 5% of the probe was mixed with SDS sample buffer, denatured by boiling, and analysed by SDS-PAGE. Gels were fixed, dried, and exposed to X-ray film.
Yeast two-hybrid assay
Yeast strain Y153 (Durfee et al. 1993) was cultured on standard complete and synthetic selective media (Sherman 1991). Transformations were performed as described by Schiestl and coworkers (1994). Standard methods were used to assay β-galactosidase activity (Ausubel et al. 1989).
Western analysis
To assay the expression of TRA-1 and TRA-2 fusion proteins in worms, we lysed samples by boiling in SDS sample buffer after a 2 hr heat shock at 33° C and a 2 hr recovery period at 20° C. Lysates were analysed by SDS-PAGE and transferred to nitrocellulose. TBST (25 mM Tris-HCl at pH 7.5, 150 mM NaCl, 0.2% Tween 20) with 5% skim milk powder was used to block filters and dilute antibodies. Filters were washed in TBST. Chicken anti-GFP (Chemicon) was detected with peroxidase-conjugated donkey anti-chicken Ig (Jackson Laboratories). Cell culture supernatant containing anti-Myc monoclonal 9E10 (Evan et al. 1985) was used at a 1 : 20 dilution and was detected with peroxidase-conjugated donkey anti-mouse IgG (Jackson Laboratories). Antibody binding was visualized using chemiluminescence (ECL, Amersham).
Acknowledgments
We thank Jonathan Hodgkin for sending nematode strains, Andy Fire for plasmids, John Copeland and Henry Krause for advice on Far Western blotting, Tim Schedl for communicating results before publication, and Brenda Andrews for comments on the manuscript. Some of the nematode strains used in this study were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). This research was supported by a grant from the Canadian Institutes of Health Research (CIHR) to A.M.S. Work in the laboratory of P.E.K is supported by the MRC, that in the laboratory of D.Z. by a grant from the NIH.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL andrew.spence@utoronto.ca; FAX (416) 978-6885.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.853700.
References
- Ahringer J, Rosenquist TA, Lawson DN, Kimble J. The Caenorhabditis elegans sex determining gene fem-3 is regulated post-transcriptionally. EMBO J. 1992;11:2303–2310. doi: 10.1002/j.1460-2075.1992.tb05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Wiley-Interscience; 1989. [Google Scholar]
- Barnes TM, Hodgkin J. The tra-3 sex determination gene of Caenorhabditis elegans encodes a member of the calpain regulatory protease family. EMBO J. 1996;15:4477–4484. [PMC free article] [PubMed] [Google Scholar]
- Barton MK, Schedl TB, Kimble J. Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics. 1987;115:107–119. doi: 10.1093/genetics/115.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Ellis RE. TRA-1A regulates transcription of fog-3, which controls germ cell fate in C. elegans. Development. 2000;127:3119–3129. doi: 10.1242/dev.127.14.3119. [DOI] [PubMed] [Google Scholar]
- Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 1995;121:2525–2535. doi: 10.1242/dev.121.8.2525. [DOI] [PubMed] [Google Scholar]
- Conradt B, Horvitz HR. The TRA-1A sex determination protein of C. elegans regulates sexually dimorphic cell deaths by repressing the egl-1 cell death activator gene. Cell. 1999;98:317–327. doi: 10.1016/s0092-8674(00)81961-3. [DOI] [PubMed] [Google Scholar]
- de Bono M, Hodgkin J. Evolution of sex determination in Caenorhabditis: Unusually high divergence of tra-1 and its functional consequences. Genetics. 1996;144:587–595. doi: 10.1093/genetics/144.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono M, Zarkower D, Hodgkin J. Dominant feminizing mutations implicate protein-protein interactions as the main mode of regulation of the nematode sex-determining gene tra-1. Genes & Dev. 1995;9:155–167. doi: 10.1101/gad.9.2.155. [DOI] [PubMed] [Google Scholar]
- Doniach T. Activity of the sex-determining gene tra-2 is modulated to allow spermatogenesis in the C. elegans hermaphrodite. Genetics. 1986;114:53–76. doi: 10.1093/genetics/114.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniach T, Hodgkin J. A sex-determining gene, fem-1, required for both male and hermaphrodite development in Caenorhabditis elegans. Dev Biol. 1984;106:223–235. doi: 10.1016/0012-1606(84)90077-0. [DOI] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen PL, Yeh SH, Yang YZ, Kilburn AE, Lee WH, Elledge SJ. The retinoblastoma protein associates with the protein phosphatase type-1 catalytic subunit. Genes & Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Gallegos M, Ahringer J, Crittenden S, Kimble J. Repression by the 3′ UTR of fem-3, a sex-determining gene, relies on a ubiquitous mog-dependent control in Caenorhabditis elegans. EMBO J. 1998;17:6337–6347. doi: 10.1093/emboj/17.21.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Goodwin EB, Okkema PG, Evans TC, Kimble J. Translational regulation of tra-2 by its 3′ untranslated region controls sexual identity in C. elegans. Cell. 1993;75:329–339. doi: 10.1016/0092-8674(93)80074-o. [DOI] [PubMed] [Google Scholar]
- Goodwin EB, Hofstra K, Hurney CA, Mango S, Kimble J. A genetic pathway for regulation of tra-2 translation. Development. 1997;124:749–758. doi: 10.1242/dev.124.3.749. [DOI] [PubMed] [Google Scholar]
- Guichet A, Copeland JWR, Erdelyi M, Hlousek D, Zavorszky P, Ho J, Brown S, Percival-Smith A, Krause HM, Ephrussi A. The nuclear receptor homologue Ftz-F1 and the homeodomain protein Ftz are mutually dependent cofactors. Nature. 1997;385:548–552. doi: 10.1038/385548a0. [DOI] [PubMed] [Google Scholar]
- Haag ES, Kimble J. Regulatory elements required for development of Caenorhabditis elegans hermaphrodites are conserved in the tra-2 homologue of C. remanei, a male/female sister species. Genetics. 2000;155:105–116. doi: 10.1093/genetics/155.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J. More sex-determination mutants of Caenorhabditis elegans. Genetics. 1980;96:649–664. doi: 10.1093/genetics/96.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Sex determination in the nematode C. elegans: Analysis of tra-3 suppressors and characterization of fem genes. Genetics. 1986;114:15–52. doi: 10.1093/genetics/114.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— A genetic analysis of the sex-determining gene, tra-1, in the nematode Caenorhabditis elegans. Genes & Dev. 1987;1:731–745. doi: 10.1101/gad.1.7.731. [DOI] [PubMed] [Google Scholar]
- ————— . Genetics. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 881–1047. [Google Scholar]
- Hodgkin J, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CP, Wood WB. Evidence from mosaic analysis of the masculinizing gene her-1 for cell interactions in C. elegans sex determination. Nature. 1992;355:551–555. doi: 10.1038/355551a0. [DOI] [PubMed] [Google Scholar]
- Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IκBα/NF-κB complex reveals mechanisms of NF-κB inactivation. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- Jacobs MD, Harrison SC. Structure of an IκBα/NF-κB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- Jan E, Motzny CK, Graves LE, Goodwin EB. The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J. 1999;18:258–269. doi: 10.1093/emboj/18.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Edgar L, Hirsh D. Specification of male development in Caenorhabditis elegans: The fem genes. Dev Biol. 1984;105:234–239. doi: 10.1016/0012-1606(84)90279-3. [DOI] [PubMed] [Google Scholar]
- Kuwabara PE. Interspecies comparison reveals evolution of control regions in the nematode sex-determining gene tra-2. Genetics. 1996;144:597–607. doi: 10.1093/genetics/144.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara PE, Kimble J. A predicted membrane protein, TRA-2A, directs hermaphrodite development in Caenorhabditis elegans. Development. 1995;121:2995–3004. doi: 10.1242/dev.121.9.2995. [DOI] [PubMed] [Google Scholar]
- Kuwabara PE, Okkema PG, Kimble J. tra-2 encodes a membrane protein and may mediate cell communication in the Caenorhabditis elegans sex determination pathway. Mol Biol Cell. 1992;3:461–473. doi: 10.1091/mbc.3.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Germ-line regulation of the Caenorhabditis elegans sex-determining gene tra-2. Dev Biol. 1998;204:251–262. doi: 10.1006/dbio.1998.9062. [DOI] [PubMed] [Google Scholar]
- Mehra A, Gaudet J, Heck L, Kuwabara PE, Spence AM. Negative regulation of male development in Caenorhabditis elegans by a protein-protein interaction between TRA-2A and FEM-3. Genes & Dev. 1999;13:1453–1463. doi: 10.1101/gad.13.11.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA Transformation. In: Epstein HF, Shakes DC, editors. Methods in Cell Biology: Caenorhabditis elegans: Modern Biological Analysis of an Organism. San Diego, CA: Academic Press; 1995. pp. 451–482. [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BJ. Sex determination and X chromosome dosage compensation. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 209–240. [PubMed] [Google Scholar]
- Okkema PG, Kimble J. Molecular analysis of tra-2, a sex determining gene in C. elegans. EMBO J. 1991;10:171–176. doi: 10.1002/j.1460-2075.1991.tb07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MD, Li WQ, Trent C, Robertson B, Fire A, Hageman JM, Wood WB. Molecular characterization of the her-1 gene suggests a direct role in cell signaling during Caenorhabditis elegans sex determination. Genes & Dev. 1993;7:216–228. doi: 10.1101/gad.7.2.216. [DOI] [PubMed] [Google Scholar]
- Pulak R, Anderson P. Messenger RNA surveillance by the Caenorhabditis elegans smg genes. Genes & Dev. 1993;7:1885–1897. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
- Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schedl T. Developmental genetics of the germ line. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 241–269. [PubMed] [Google Scholar]
- Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T, Graham PL, Barton MK, Kimble J. Analysis of the role of tra-1 in germline sex determination in the nematode Caenorhabditis elegans. Genetics. 1989;123:755–769. doi: 10.1093/genetics/123.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Meth Enzym. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sokol SB, Kuwabara PE. Proteolysis in Caenorhabditis elegans sex determination: Cleavage of TRA-2A by TRA-3. Genes & Dev. 2000;14:901–906. [PMC free article] [PubMed] [Google Scholar]
- Stringham EG, Dixon DK, Jones D, Candido EPM. Temporal and spatial expression patterns of the small heat shock (hsp-16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Nuclear access and action of Notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- Yi WS, Zarkower D. Similarity of DNA binding and transcriptional regulation by Caenorhabditis elegans MAB-3 and Drosophila melanogaster DSX suggests conservation of sex determining mechanisms. Development. 1999;126:873–881. doi: 10.1242/dev.126.5.873. [DOI] [PubMed] [Google Scholar]
- Yi W, Ross JM, Zarkower D. mab-3 is a direct tra-1 target gene regulating diverse aspects of C. elegans male sexual development and behavior. Development. 2000;127:4469–4480. doi: 10.1242/dev.127.20.4469. [DOI] [PubMed] [Google Scholar]
- Zarkower D, Hodgkin J. Molecular analysis of the C. elegans sex-determining gene tra-1: A gene encoding two zinc finger proteins. Cell. 1992;70:237–249. doi: 10.1016/0092-8674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Zarkower D, DeBono M, Aronoff R, Hodgkin J. Regulatory rearrangements and smg-sensitive alleles of the C. elegans sex-determining gene tra-1. Dev Genet. 1994;15:240–250. doi: 10.1002/dvg.1020150306. [DOI] [PubMed] [Google Scholar]
- Zhang BL, Gallegos M, Puoti A, Durkin E, Fields S, Kimble J, Wickens MP. A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature. 1997;390:477–484. doi: 10.1038/37297. [DOI] [PubMed] [Google Scholar]