Abstract
Purpose
The cornea is sensitive to ultraviolet B (UV-B) radiation-induced oxidative stress and inflammation. Its clinical manifestations are photokeratitis and climatic droplet keratopathy. Urocanic acid (UCA) is a major endogenous UV-absorbing chromophore in the epidermis and it is also an efficacious immunosuppressant. We have previously shown that cis-UCA can suppress UV-B-induced interleukin-6 and −8 secretion and cytotoxicity in human corneal epithelium (HCE) cells. In the current study, we further wanted to investigate the effects of cis-UCA on UV-B-induced inflammatory and apoptotic responses in HCE-2 cells, focusing on the nuclear factor kappa B (NF-κB) and AP-1 (subunits c-Fos and c-Jun) signaling pathways.
Methods
After exposing HCE-2 cells to UV-B and cis-UCA, DNA binding of c-Fos, c-Jun and NF-κB was measured with ELISA. In addition, the endogenous levels of phosphorylated stress-activated protein kinase/c-Jun N-terminal kinase (phospho-SAPK/JNK) and phospho-c-Jun were determined. The proliferative capacity of HCE-2 cells was also quantified, and the cytotoxicity of the cis-UCA and UV-B treatments was monitored by measuring the release of lactate dehydrogenase enzyme in the culture medium.
Results
UV-B irradiation induced the binding of transcription factors c-Jun, c-Fos, and NF-κB to DNA. Cis-UCA inhibited the binding of c-Jun and c-Fos but not that of NF-κB. Moreover, UV-B increased the levels of phospho-c-Jun and phospho-JNK, and the expression of both was attenuated by cis-UCA. Cis-UCA also alleviated the UV-B-induced apoptosis and proliferative decline in human corneal cells.
Conclusions
The results from this study suggest that cis-UCA suppresses JNK signaling pathway, which provides potential for treating UV-B-induced inflammatory defects in human corneal cells.
Introduction
In addition to skin and its epithelial cells, keratinocytes, the eye and its corneal epithelial cells are constantly exposed to ultraviolet (UV) radiation. The acute clinical effect of UV radiation on the cornea is photokeratitis, also known as “snow blindness” or “welder’s flash.” It is a painful inflammatory damage of corneal epithelium caused by UV-B [1,2]. UV radiation accelerates the physiologic loss of surface cells [3,4]. Exfoliation takes place by two mechanisms; shedding where whole cells detach into the tear film and apoptosis in which cells disintegrate into the tear film [1]. Suprathreshold radiant exposure results in full-thickness loss of the stratified epithelium to the basement membrane and, consequently, exposed nerve fiber endings result in severe pain [1].
Climatic droplet keratopathy (CDK) is a degenerative condition characterized by the accumulation of translucent material in the superficial corneal stroma within the interpalpebral strip [5]. The corneal deposits are thought to be derived from plasma proteins which diffuse into cornea, and may become photochemically damaged by excessive exposure to UV [5]. Corneal deposits have been shown to contain various oxidative stress and inflammation–related agents [6-9].
The transcription factors activator protein-1 (AP-1) and nuclear factor-kappaB (NF-κB) are known to be induced by UV-B [10-12]. These two transcription factor families have been identified to be involved in the processes of cell proliferation, cell differentiation and cell survival as well as having important roles in tumorigenesis [12].
The transcription factor NF-κB comprises a family of proteins that are activated in response to inflammatory signals or cellular stress. In NF-κB-dependent gene expression analyses with human keratinocytes, tumor necrosis factor-alpha (TNF-α) and UV-B treatments resulted in the activation and inhibition of different genes, evidence of the stimuli and cell-type specific nature of NF-κB function [13]. NF-κB is activated by direct UV-B exposure and in different pathological conditions of the cornea [14]. During aging, the cellular capacity to respond to environmental stress via NF-κB-mediated signaling can be attenuated [15].
The heterodimeric AP-1 is a transcription factor that is composed of proteins belonging to several families, the Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra1, and Fra2) subfamilies being the major AP-1 proteins [16]. The AP-1 regulation has been shown to be affected by all forms of mitogen-activated protein kinase (MAPK) cascades, e.g., p38 and JNK (c-Jun N-terminal kinase) [16,17], which activate in response to cellular stress. Study results with human keratinocytes suggest that the activation of p38 MAPK is required for UV-B-induced AP-1 activation. A potential mechanism of UV-B-induced AP-1 activation through p38 is to enhance the binding of the AP-1 complex to its target DNA [18]. Besides p38 activation, a potential UV-B signaling cascade for AP-1 activation in human keratinocytes involves c-Fos gene expression [19,20]. The role of JNK in UV-induced apoptosis is still controversial, studies suggesting either an anti-apoptotic or a pro-apoptotic effect. The biphasic function of JNK can be dependent on cell type, type of stimuli, crosstalk with other signaling pathways, and the intensity and duration of activation [21-23].
UV-B has been shown to induce dose-dependent oxidative stress as well as MAP kinase activation, including JNK, in human corneal epithelium (HCE) cells [10]. In addtion, reactive oxygen species can induce phosphorylation of cell surface receptors, which results in the activation of the MAPK signaling pathway [24]. JNK phosphorylates c-Jun (Ser63/73 and Thr91/93) and potentiates the transcriptional capacity of c-Jun [25-28]. The JNK-initiated phosphorylation of c-Jun has been suggested to increase the half-life of c-Jun by protein stabilization, thus enabling potent and prolonged expression under stressful conditions such as UV irradiation [25,26,29-32]. However, this mechanism seems to depend on the cell type [32,33].
Urocanic acid (UCA) is the major UV-absorbing chromophore in the skin and it has been proposed to function as a regulator of UV-induced damage [34]. Cis-UCA, formed from trans-UCA upon UV-B exposure, has been implicated in the down-regulation of hypersensitivity reactions [35,36], in the actions of epidermal antigen-presenting cells [37,38], in the activation of neutrophils [39,40], and in the prolonged survival of organ transplants [41,42]. Moreover, cis-UCA neither photobinds to DNA [43,44] nor is able to enhance UV photocarcinogenesis [45], whereas it may suppress immunological recognition of tumor antigens in specific experimental conditions [46]. In our previous study, we have showed that cis-UCA suppresses UV-B-induced interleukin (IL)-6 and IL-8 secretion and cytotoxicity in human corneal and conjunctival cells in vitro [47]. However, the molecular targets of cis-UCA action remain to be resolved.
In this study we explored the hypothesis that UV-B radiation causes cell damage through an increase in transcription factor activity and that cis-UCA may protect the exposed corneal epithelial cells through alterations in this activity.
Methods
Cell culture
Human corneal epithelial (HCE-2) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). The cells were cultivated on 6-well cell culture plates (Cellstar®, Greiner Bio-One, Frickenhausen, Germany) in Keratinocyte-SFM medium (Gibco, Invitrogen, Paisley, UK) supplemented with 50 µg/ml bovine pituitary extract, 5 ng/ml human recombinant epidermal growth factor, 100 U/ml penicillin, 100 µg/ml streptomycin (all from Gibco), 5 µg/ml insulin (Sigma-Aldrich, St. Louis, MO), and 10% fetal bovine serum (HyClone, Logan, UT). Confluent cell cultures were treated with 100 µg/ml of cis-UCA (BioCis Pharma, Turku, Finland) when indicated in Results, and/or exposed to a UV-B irradiation dose of 153 mJ/cm2 (four TL 20W/12 tubes; Philips, Eindhoven, The Netherlands) at room temperature for 1 min using a source-to-target distance of 30 cm. Thereafter, the cell cultures were incubated in a humidified 10% CO2 incubator at 37 °C for 3, 6, or 24 h.
ELISA assays
For determining the DNA binding of transcription factors and for analyzing the activation of AP-1, cell lysates were prepared by scraping the cells into Buffer C (25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 20 mM Hepes in double-distilled water). To detect the binding of AP-1 and NF-κB to DNA, c-Fos and c-Jun TransAM™ kits (Active Motif, Rixensart, Belgium), and NF-κB p65 ELISA Kit (Enzo Life Sciences, Farmingdale, NY) were used. Phosphorylated c-Jun and phosphorylated stress-activated protein kinase/Jun-N-terminal kinase were measured using PathScan® Phospho-c-Jun (Ser63), and PathScan® Phospho-SAPK/JNK (Thr183/Tyr185) Sandwich ELISA Kits (Cell Signaling Technology, MA), respectively. All assays were performed according to the manufacturers’ protocols. The absorbance values were measured at 450 nm with a reference wavelength of 655 nm using a BIO-RAD Model 550 microplate reader (BIO-RAD, Hercules, CA).
Proliferation assay
For the proliferation test, 100,000 cells/ml were plated in 200 µl on 96-well flat-bottomed cell culture plates (Cellstar®, Greiner Bio-One). After 3 h of incubation in a humidified 10% CO2 incubator at 37 °C, cells in eight replicate wells were treated with cis-UCA and UV-B irradiation as described above. The cell cultures were incubated in a humidified 10% CO2 incubator at 37 °C for 24 or 48 h, and the proliferation of HCE-2 cells was quantified using the CyQUANT® Cell Proliferation Assay Kit (Invitrogen) according to the manufacturer’s instructions. After 2 min incubation at room temperature, fluorescence intensity of the samples was measured at the ex/em wavelength of 485/530 nm using VICTOR™ 1420 multilabel counter (PerkinElmer/Wallac, Turku, Finland).
Cytotoxicity assay
Cytotoxicity of the cis-UCA and UV-B treatments was monitored by measuring the amount of lactate dehydrogenase (LDH) enzyme in duplicate from the culture medium samples. Cyto-Tox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI) was used for detection according to the instructions of the manufacturer. Absorbance values after the colorimetric reaction were measured at the wavelength of 490 nm with a reference wavelength of 655 nm using a BIO-RAD Model 550 microplate reader (BIO-RAD).
Statistical analysis
Statistical differences between groups were assessed using the Kruskall-Wallis test, and post hoc comparisons were made using the Mann–Whitney U-test. P values below 0.05 were considered significant.
Results
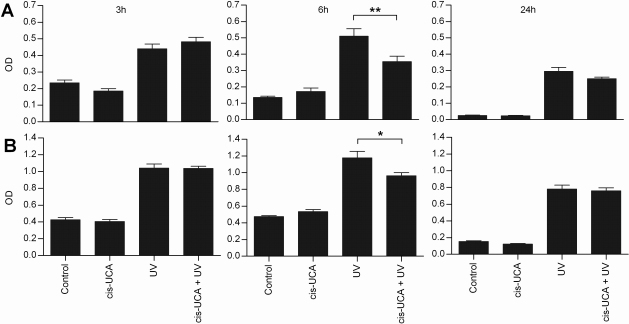
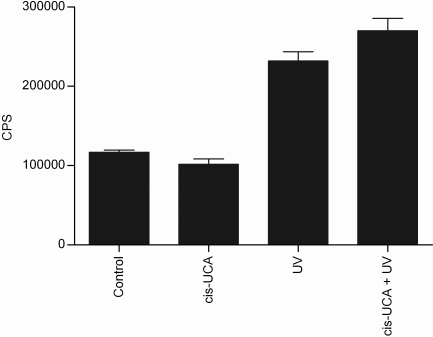
To examine the activity of central transcription factors following UV-B and cis-UCA treatments, the DNA binding of AP-1 and NF-κB were determined. The DNA binding of c-Fos and c-Jun subunits of the transcription factor AP-1 heterodimer increased following UV-B irradiation (Figure 1). After 6 h of incubation, cis-UCA significantly decreased the UV-B-induced binding of both subunits (Figure 1). The decrease was not yet observed at 3 h of incubation, and it was negligible after 24 h. UV-B irradiation also approximately doubled the binding activity of the p65 subunit of NF-κB when compared to non-irradiated control cells after 6 h of incubation. However, cis-UCA did not affect this activation at any of the 3, 6 or 24 h time points studied (Figure 2 and data not shown). No significant change in the activity of AP-1 or NF-κB was observed in non-irradiated cells treated with cis-UCA (Figure 1 and Figure 2).
Figure 1.
DNA binding of c-Fos and c-Jun subunits of the transcription factor AP-1 heterodimer. Binding of c-Fos (A) and c-Jun (B) to DNA. Results are presented as mean optical density (OD) ± SEM cis-UCA concentration was 100 µg/ml. Seven parallel samples were measured in control and cis-UCA, and nine parallel samples in UV and cis-UCA + UV treatments. *p<0.05; **p<0.01 (Mann–Whitney).
Figure 2.
Binding of NF-κB (p65) to DNA 6 h after stimulation. Results are presented as mean counts per second (CPS) ±SEM cis-UCA concentration was 100 µg/ml. Five parallel samples were measured in control and cis-UCA, and seven parallel samples in UV and cis-UCA + UV treatments.
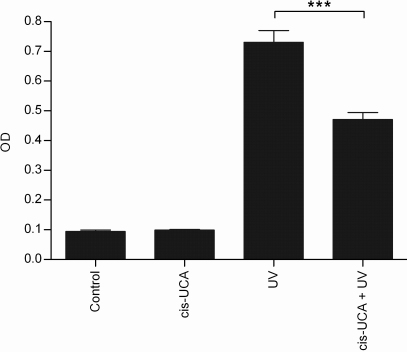
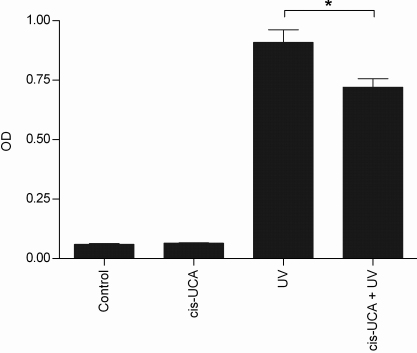
To verify our observation that cis-UCA inhibits the activity of AP-1 in UV-B-irradiated HCE-2 cells, we measured phosphorylated c-Jun from the cell extracts. As shown in Figure 3, cis-UCA significantly decreased the level of phospho-c-Jun in UV-B-irradiated HCE-2 cells after 6 h of incubation. Moreover, cis-UCA also significantly decreased the amount of phosphorylated JNK in those cells (Figure 4). In non-irradiated cells cis-UCA had no effect on the expression of these phosphoproteins.
Figure 3.
Phosphorylation of c-Jun (Ser63) 6 h after stimulation. Results are presented as mean optical density (OD) ±SEM cis-UCA concentration was 100 µg/ml. Seven parallel samples were measured in control and cis-UCA, and nine parallel samples in UV and cis-UCA + UV treatments. ***p<0.001 (Mann–Whitney).
Figure 4.
Phosphorylation of SAPK/JNK (Thr183/Tyr185) 6 h after stimulation. Results are presented as mean optical density (OD) ±SEM cis-UCA concentration was 100 µg/ml. Seven parallel samples were measured in control and cis-UCA, and nine parallel samples in UV and cis-UCA + UV treatments. *p<0.05 (Mann–Whitney).
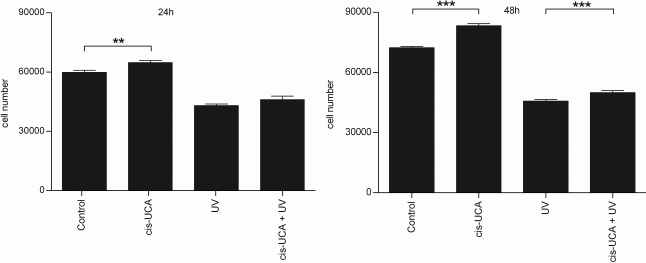
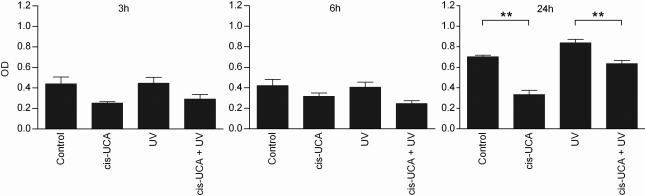
Since JNK signaling can result in either cellular proliferation or apoptosis [48], we examined the influence of cis-UCA on cell survival. As shown in Figure 5, cis-UCA significantly prevented the loss of viability of HCE-2 cells in normal cell culture conditions. Also after UV-B irradiation, cell survival was increased by cis-UCA after 24 h of incubation and reached statistical significance after 48 h (Figure 5). Concomitantly, cis-UCA decreased cell damage. The decreased release of LDH from cis-UCA-treated cells was observed at 3 and 6 h of incubation and was statistically significant after 24 h both in non-irradiated and in UV-B-exposed HCE-2 cells (Figure 6).
Figure 5.
Proliferation of HCE-2 cells. Results are presented as mean cell numbers ±SEM cis-UCA concentration was 100 µg/ml. Eight parallel samples were measured in all groups. **p<0.01; ***p<0.001 (Mann–Whitney).
Figure 6.
Release of lactate dehydrogenase (LDH). Results are presented as mean optical density (OD) ±SEM cis-UCA concentration was 100 µg/ml. Six parallel samples were measured in all groups. **p<0.01 (Mann–Whitney).
Discussion
We have previously shown that cis-UCA suppresses UV-B-induced cytokine expression and improves cell viability against UV-B irradiation in human ocular cells [47]. In the present study, we further investigated the mechanisms of these actions. As the cornea is frequently exposed to solar UV radiation, we wanted to elucidate the role of JNK in apoptotic regulation in HCE-2 cells. Our data demonstrates that cis-UCA inhibits the phosphorylation of c-Jun (Ser63) and JNK (Thr183/Tyr185) as well as the binding of c-Fos and c-Jun to DNA in response to UV-B stimulation. The findings that cis-UCA reduced the phosphorylation of both JNK and c-Jun, and had no effect on basal level of these phosphoproteins in non-irradiated cells, suggests that the molecular target of cis-UCA action is up-stream of JNK in UVB-stressed cells.
UV-induced generation of reactive oxygen species and subsequent TNF-α formation activates, besides JNK signaling, also the NF-κB pathway [49,50]. JNK activation by TNF- α activates pro-apoptotic effects; however, TNF-α-induced NF-κB activation prevents apoptosis through the suppression of the JNK pathway and the activation of antioxidant genes, such as manganese-superoxide dismutase (MnSOD) [49,50]. Interestingly, in epidermal cells, JNK activates cell proliferation, and the inhibition of JNK by NF-κB has a tumor-suppressing function [51].
Conversely, following UV stimulus, p65/RelA directly results in the expression of protein kinase C delta (PKCδ), which leads to activation of JNK [52]. In addition, after UV stimulation, MnSOD expression has been shown to be UV dose-dependent, exerting diminishing expression in high UV-B doses [10]. However, at the same time, UV-B exposure induces the NF-κB-related proinflammatory cytokines IL-6 and IL-8 in HCE-2 cells [47]. Our research shows that both JNK and NF-κB pathways are activated by UV-B. However, cis-UCA suppresses solely the JNK pathway, not NF-κB. In response to UV-B stress, HCE-2 cells showed decreased proliferation and increased LDH release, implying cell death, which could be alleviated by cis-UCA. Consistently with an earlier study with epidermal cells [51], the inhibition of JNK pathway seems to be a critical target in the regulation of apoptosis also in human corneal epithelial cells. While cis-UCA was present in the culture medium during UV-B irradiation of the cells, it was inferred from previous experience [47] that the cis-UCA concentration used in the current experiment does not appreciably block the transmission of UV-B photons.
Although acute and chronic damage and inflammation caused by UV radiation to the epithelial cells of the cornea are well known ophthalmologic diseases (e.g., photokeratitis and CDK), their precise mechanisms are still unclear. Current clinical therapy for ocular surface inflammation consists of anti-inflammatory agents that do not offer any protection against UV radiation-induced damage [53]. The present in vitro data show that cis-UCA regulates the JNK signaling pathway and it has at both anti-inflammatory and cytoprotective capacity against UV radiation on the corneal epithelial cells. Our results are supported by previous observations [54]. cis-UCA may be useful also in other inflammatory conditions of the cornea [55,56]. It would be worthwhile to examine cis-UCA effect on these diseases as well. Therefore, further in vivo studies are required.
Acknowledgments
This study was funded by the Academy of Finland (grant 133567), the EVO fund of the Kuopio University Hospital, the Finnish Cultural Foundation and its North Savo Fund, the Finnish Eye Foundation, the Finnish Funding Agency for Technology and Innovation and the Sakari and Päivikki Sohlberg Foundation. We thank technician Anne Kontkanen for technical assistance.
References
- 1.Cullen AP. Photokeratitis and other phototoxic effects on the cornea and conjunctiva. Int J Toxicol. 2002;21:455–64. doi: 10.1080/10915810290169882. [DOI] [PubMed] [Google Scholar]
- 2.Dolin PJ, Johnson GJ. Solar ultraviolet radiation and ocular disease: a review of the epidemiological and experimental evidence. Ophthalmic Epidemiol. 1994;1:155–64. doi: 10.3109/09286589409047224. [DOI] [PubMed] [Google Scholar]
- 3.Ren H, Wilson G. The effect of ultraviolet-B irradiation on the cell shedding rate of the corneal epithelium. Acta Ophthalmol (Copenh) 1994;72:447–52. doi: 10.1111/j.1755-3768.1994.tb02794.x. [DOI] [PubMed] [Google Scholar]
- 4.Kitaichi N, Shimizu T, Yoshida K, Honda A, Yoshihisa Y, Kase S, Ohgami K, Norisugi O, Makino T, Nishihira J, Yamagishi S, Ohno S. Macrophage migration inhibitory factor ameliorates UV-induced photokeratitis in mice. Exp Eye Res. 2008;86:929–35. doi: 10.1016/j.exer.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Gray RH, Johnson GJ, Freedman A. Climatic droplet keratopathy. Surv Ophthalmol. 1992;36:241–53. doi: 10.1016/0039-6257(92)90093-9. [DOI] [PubMed] [Google Scholar]
- 6.Menegay M, Lee D, Tabbara KF, Cafaro TA, Urrets-Zavalía JA, Serra HM, Bhattacharya SK. Proteomic analysis of climatic keratopathy droplets. Invest Ophthalmol Vis Sci. 2008;49:2829–37. doi: 10.1167/iovs.07-1438. [DOI] [PubMed] [Google Scholar]
- 7.Kaji Y, Nagai R, Amano S, Takazawa Y, Fukayama M, Oshika T. Advanced glycation end product deposits in climatic droplet keratopathy. Br J Ophthalmol. 2007;91:85–8. doi: 10.1136/bjo.2006.099812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaji Y, Oshika T, Takazawa Y, Fukayama M, Fujii N. Immunohistochemical localisation of D-beta-aspartic acid-containing proteins in climatic droplet keratopathy. Br J Ophthalmol. 2009;93:977–9. doi: 10.1136/bjo.2008.144212. [DOI] [PubMed] [Google Scholar]
- 9.Holopainen JM, Serra HM, Sánchez MC, Sorsa T, Zalentein WN, Barcelona PF, Moilanen JA, Tervahartiala T, Tervo TM, Cafaro TA, Virtanen I, Urrets-Zavalia EA, Bhattacharya SK, Urrets-Zavalia JA. Altered expression of matrix metalloproteinases and their tissue inhibitors as possible contributors to corneal droplet formation in climatic droplet keratopathy. Acta Ophthalmol. 2009 doi: 10.1111/j.1755-3768.2009.01764.x. [DOI] [PubMed] [Google Scholar]
- 10.Black AT, Gordon MK, Heck DE, Gallo MA, Laskin DL, Laskin JD. UVB light regulates expression of antioxidants and inflammatory mediators in human corneal epithelial cells. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L, Wang L, Shell B. UV-induced signaling pathways associated with corneal epithelial cell apoptosis. Invest Ophthalmol Vis Sci. 2003;44:5102–9. doi: 10.1167/iovs.03-0591. [DOI] [PubMed] [Google Scholar]
- 12.Cooper SJ, Bowden GT. Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr Cancer Drug Targets. 2007;7:325–34. doi: 10.2174/156800907780809714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis DA, Spandau DF. UVB activation of NF-kappaB in normal human keratinocytes occurs via a unique mechanism. Arch Dermatol Res. 2007;299:93–101. doi: 10.1007/s00403-006-0729-2. [DOI] [PubMed] [Google Scholar]
- 14.Alexander G, Carlsen H, Blomhoff R. Corneal NF-kappaB activity is necessary for the retention of transparency in the cornea of UV-B-exposed transgenic reporter mice. Exp Eye Res. 2006;82:700–9. doi: 10.1016/j.exer.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Helenius M, Makelainen L, Salminen A. Attenuation of NF-kappaB signaling response to UVB light during cellular senescence. Exp Cell Res. 1999;248:194–202. doi: 10.1006/excr.1999.4393. [DOI] [PubMed] [Google Scholar]
- 16.Shaulian E. AP-1–The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22:894–9. doi: 10.1016/j.cellsig.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Bowden GT. Role of p38 mitogen-activated protein kinases in ultraviolet-B irradiation-induced activator protein 1 activation in human keratinocytes. Mol Carcinog. 2000;28:196–202. doi: 10.1002/1098-2744(200008)28:4<196::aid-mc2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Borchers AH, Dong Z, Powell MB, Bowden GT. UVB irradiation-induced activator protein-1 activation correlates with increased c-fos gene expression in a human keratinocyte cell line. J Biol Chem. 1998;273:32176–81. doi: 10.1074/jbc.273.48.32176. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Bowden GT. Activation of p38 MAP kinase and ERK are required for ultraviolet-B induced c-fos gene expression in human keratinocytes. Oncogene. 1999;18:7469–76. doi: 10.1038/sj.onc.1203210. [DOI] [PubMed] [Google Scholar]
- 21.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–36. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 22.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–95. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 23.Wisdom R, Johnson RS, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999;18:188–97. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assefa Z, Garmyn M, Bouillon R, Merlevede W, Vandenheede JR, Agostinis P. Differential stimulation of ERK and JNK activities by ultraviolet B irradiation and epidermal growth factor in human keratinocytes. J Invest Dermatol. 1997;108:886–91. doi: 10.1111/1523-1747.ep12292595. [DOI] [PubMed] [Google Scholar]
- 25.Dérijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 26.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–48. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 27.Morton S, Davis RJ, McLaren A, Cohen P. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. EMBO J. 2003;22:3876–86. doi: 10.1093/emboj/cdg388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayakawa J, Depatie C, Ohmichi M, Mercola D. The activation of c-Jun NH2-terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2)-dependent enhanced DNA repair. J Biol Chem. 2003;278:20582–92. doi: 10.1074/jbc.M210992200. [DOI] [PubMed] [Google Scholar]
- 29.Musti AM, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–2. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs SY, Dolan L, Davis RJ, Ronai Z. Phosphorylation-dependent targeting of c-Jun ubiquitination by Jun N-kinase. Oncogene. 1996;13:1531–5. [PubMed] [Google Scholar]
- 31.Shaulian E, Schreiber M, Piu F, Beeche M, Wagner EF, Karin M. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell. 2000;103:897–907. doi: 10.1016/s0092-8674(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 32.Anzi S, Finkin S, Shaulian E. Transcriptional repression of c-Jun's E3 ubiquitin ligases contributes to c-Jun induction by UV. Cell Signal. 2008;20:862–71. doi: 10.1016/j.cellsig.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–5. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 34.Gibbs NK, Tye J, Norval M. Recent advances in urocanic acid photochemistry, photobiology and photoimmunology. Photochem Photobiol Sci. 2008;7:655–67. doi: 10.1039/b717398a. [DOI] [PubMed] [Google Scholar]
- 35.Prater MR, Blaylock BL, Holladay SD. Molecular mechanisms of cis-urocanic acid and permethrin-induced alterations in cutaneous immunity. Photodermatol Photoimmunol Photomed. 2003;19:287–94. doi: 10.1046/j.1600-0781.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 36.Lauerma AI, Aioi A, Maibach HI. Topical cis-urocanic acid suppresses both induction and elicitation of contact hypersensitivity in BALB/C mice. Acta Derm Venereol. 1995;75:272–5. doi: 10.2340/0001555575272275. [DOI] [PubMed] [Google Scholar]
- 37.Hart PH, Grimbaldeston MA, Finlay-Jones JJ. Mast cells in UV-B-induced immunosuppression. J Photochem Photobiol B. 2000;55:81–7. doi: 10.1016/s1011-1344(00)00032-4. [DOI] [PubMed] [Google Scholar]
- 38.el-Ghorr AA, Norval M. The effect of chronic treatment of mice with urocanic acid isomers. Photochem Photobiol. 1997;65:866–72. doi: 10.1111/j.1751-1097.1997.tb01936.x. [DOI] [PubMed] [Google Scholar]
- 39.Kivistö K, Punnonen K, Toppari J, Leino L. Urocanic acid suppresses the activation of human neutrophils in vitro. Inflammation. 1996;20:451–9. doi: 10.1007/BF01487038. [DOI] [PubMed] [Google Scholar]
- 40.Rinaldi M, Moroni P, Leino L, Laihia J, Paape MJ, Bannerman DD. Effect of cis-urocanic acid on bovine neutrophil generation of reactive oxygen species. J Dairy Sci. 2006;89:4188–201. doi: 10.3168/jds.S0022-0302(06)72464-X. [DOI] [PubMed] [Google Scholar]
- 41.Guymer RH, Mandel TE. Urocanic acid as an immunosuppressant in allotransplantation in mice. Transplantation. 1993;55:36–43. doi: 10.1097/00007890-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Filipec M, Letko E, Hasková Z, Jenícková D, Holler P, Jancárek A, Holán V. The effect of urocanic acid on graft rejection in an experimental model of orthotopic corneal transplantation in rabbits. Graefes Arch Clin Exp Ophthalmol. 1998;236:65–8. doi: 10.1007/s004170050044. [DOI] [PubMed] [Google Scholar]
- 43.Yarosh DB, Gettings SD, Alas LG, Kibitel JT, San RH, Wagner VO, 3rd, McEwen GN., Jr The biological interaction of cis- and trans-urocanic acid and DNA. Photodermatol Photoimmunol Photomed. 1992;9:121–6. [PubMed] [Google Scholar]
- 44.IJland SA, Noonan FP, Ceryak S, Steenvoorden DP, Bouscarel B, Hug D, Beijersbergen van Henegouwen GM, De Fabo EC. Urocanic acid does not photobind to DNA in mice irradiated with immunosuppressive doses of UVB. Photochem Photobiol. 1998;67:222–6. doi: 10.1562/0031-8655(1998)067<0222:uadnpt>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 45.Macve JC, Norval M. The effects of UV waveband and cis-urocanic acid on tumour outgrowth in mice. Photochem Photobiol Sci. 2002;1:1006–11. doi: 10.1039/b208247k. [DOI] [PubMed] [Google Scholar]
- 46.Beissert S, Rühlemann D, Mohammad T, Grabbe S, El-Ghorr A, Norval M, Morrison H, Granstein RD, Schwarz T. IL-12 prevents the inhibitory effects of cis-urocanic acid on tumor antigen presentation by Langerhans cells: implications for photocarcinogenesis. J Immunol. 2001;167:6232–8. doi: 10.4049/jimmunol.167.11.6232. [DOI] [PubMed] [Google Scholar]
- 47.Viiri J, Jauhonen HM, Kauppinen A, Ryhänen T, Paimela T, Hyttinen J, Sorri I, Laihia JK, Leino L, Kaarniranta K. Cis-urocanic acid suppresses UV-B-induced interleukin-6 and −8 secretion and cytotoxicity in human corneal and conjunctival epithelial cells in vitro. Mol Vis. 2009;15:1799–805. [PMC free article] [PubMed] [Google Scholar]
- 48.Pimienta G, Ficarro SB, Gutierrez GJ, Bhoumik A, Peters EC, Ronai Z, Pascual J. Autophosphorylation properties of inactive and active JNK2. Cell Cycle. 2007;6:1762–71. doi: 10.4161/cc.6.14.4434. [DOI] [PubMed] [Google Scholar]
- 49.Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006;13:730–7. doi: 10.1038/sj.cdd.4401830. [DOI] [PubMed] [Google Scholar]
- 50.Papa S, Bubici C, Zazzeroni F, Pham CG, Kuntzen C, Knabb JR, Dean K, Franzoso G. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–29. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- 51.Zhang JY, Tao S, Kimmel R, Khavari PA. CDK4 regulation by TNFR1 and JNK is required for NF-kappaB-mediated epidermal growth control. J Cell Biol. 2005;168:561–6. doi: 10.1083/jcb.200411060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Yang D, Minemoto Y, Leitges M, Rosner MR, Lin A. NF-kappaB is required for UV-induced JNK activation via induction of PKCdelta. Mol Cell. 2006;21:467–80. doi: 10.1016/j.molcel.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 53.Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137:337–42. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 54.Matsui M, Tashiro T. Protective effect of urocanic acid eye-drops to the ocular inflammation induced by the ultraviolet radiation. Nippon Ganka Gakkai Zasshi. 1967;71:571–6. [PubMed] [Google Scholar]
- 55.Cook EB. Tear cytokines in acute and chronic ocular allergic inflammation. Curr Opin Allergy Clin Immunol. 2004;4:441–5. doi: 10.1097/00130832-200410000-00018. [DOI] [PubMed] [Google Scholar]
- 56.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]