Abstract
Background
Oral fluid, a promising alternative matrix for drug monitoring in clinical and forensic investigations, offers noninvasive sample collection under direct observation. Cannabinoid distribution into oral fluid is complex and incompletely characterized due to the lack of controlled drug administration studies.
Methods
To characterize cannabinoid disposition in oral fluid, we administered around-the-clock oral Δ9-tetrahydrocannabinol (THC) (Marinol®) doses to 10 participants with current daily cannabis use. We obtained oral fluid samples (n = 440) by use of Quantisal™ collection devices before, during, and after 37 20-mg THC doses over 9 days. Samples were extracted with multiple elution solvents from a single SPE column and analyzed by 2-dimensional GC-MS with electron-impact ionization for THC, 11-hydroxy-THC (11-OH-THC), cannabidiol, and cannabinol and negative chemical ionization for 11-nor-9-carboxy-THC (THCCOOH). Linear ranges were 0.5–50 μg/L, with the exception of cannabinol (1–50 μg/L) and THCCOOH (7.5–500 ng/L).
Results
THCCOOH was the most prevalent analyte in 432 samples (98.2%), with concentrations up to 1117.9 ng/L. In contrast, 11-OH-THC was not identified in any sample; cannabidiol and cannabinol were quantified in 3 and 8 samples, respectively, with maximum concentrations of 2.1 and 13 μg/L. THC was present in only 20.7% of samples, with highest concentrations near admission (median 4.2 μg/L, range 0.6–481.9) from previously self-administered smoked cannabis.
Conclusions
Measurement of THCCOOH in OF not only identifies cannabis exposure, but also minimizes the possibility of passive inhalation. THCCOOH may be a better analyte for detection of cannabis use.
Drug analysis using nontraditional matrices such as oral fluid (OF),5 sweat, and hair provides valuable information about an individual's drug exposure history. The demand for OF drug testing in cases of driving under the influence of drugs (DUID), the workplace, and drug treatment continues to increase (1). Samples are collected noninvasively, under direct observation, reducing adulteration and substitution without loss of privacy. Additionally, parent drug is frequently prominent in OF and may reflect recent drug use. OF is a greater analytical challenge than other matrices, because of limited sample volume, lower metabolite concentrations, and reduced salivation after drug consumption (1, 2).
Cannabis (marijuana), the most widely used illicit drug, is primarily smoked, although illicit oral administration and licit synthetic Δ9-tetrahydrocannabinol (THC) oral pharmacotherapy are common. THC, the primary psychoactive cannabis component, undergoes extensive phase 1 metabolism to produce the equipotent metabolite 11-hydroxy-THC (11-OH-THC), with further oxidation to the inactive metabolite 11-nor-9-carboxy-THC (THCCOOH) (3, 4). Phase 2 metabolism involves formation of glucuronide and sulfate conjugates that are eliminated in feces and urine (5).
Several pharmacokinetic studies describe cannabinoid OF distribution after smoked cannabis (6–8). THC contaminates the oral mucosa during cannabis smoking, yielding >1000 μg/L THC in OF for approximately 15–30 min (7, 8). Thereafter, OF concentrations correlate temporally with those in plasma, but because of high variability it is not possible to accurately predict concurrent plasma or blood concentrations (9). There are few data on the OF disposition of additional cannabinoids found in the cannabis plant, cannabidiol (CBD) and cannabinol (CBN), (10). Initially, it was thought that cannabinoids did not pass into OF from blood, as there was no measurable radio-activity in OF after intravenous administration of radiolabeled THC (11). In addition, no quantifiable 11-OH-THC or THCCOOH was found in OF after smoked THC, with a 0.5 μg/L limit of quantification (LOQ) (12, 13). However, detection limits greatly decreased with 2-dimensional (2D) GC-MS and tandem MS, making low OF THCCOOH concentration measurement possible. Two published studies reported ng/L THCCOOH OF concentrations after cannabis smoking (14, 15).
Cannabis also is abused by the oral route through THC ingestion in food, tea, and hemp oil (16–18). Synthetic THC (dronabinol; Marinol®) is approved by the US Food and Drug Administration for nausea and vomiting after cancer chemotherapy and for anorexia in AIDS patients (19). Oral ingestion generally produces lower and later peak blood concentrations and effects than smoked THC (20). Owing to first-pass hepatic metabolism and rapid tissue uptake, only 6%–20% of an orally administered THC dose reaches the systemic circulation (21–23). Plasma cannabinoid disposition after single and multiple oral THC doses was recently characterized (24–26).
To our knowledge, OF cannabinoid pharmacokinetics after controlled, multiple, high oral THC dosing has not been evaluated. We investigated THC and metabolite OF disposition after single and multiple around-the-clock oral synthetic THC administration to chronic cannabis smokers using a newly developed 2D GC-MS assay for THC, 11-OH-THC, CBD, CBN, and THCCOOH (27). We also determined cannabinoid detection windows after multiple THC doses.
Materials and Methods
Chemicals And Reagents
We purchased THC, 11-OH-THC, THCCOOH, CBD, and CBN for calibrators and quality control samples and corresponding internal standards (d3-THC, d3-11-OH-THC, d3-THCCOOH, d3-CBD) from Cerilliant; N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane from Thermo Fisher Scientific; trifluoroacetic anhydride (TFAA) and hexafluoroisopropanol (HFIP) from Campbell Science; and CEREX® Polycrom™ THC (3 cc/35 mg) extraction columns from SPEware. Quantisal™ OF collection devices and Quantisal transport buffer for diluting calibrators and quality control samples were obtained from Immunalysis.
Participants
Daily cannabis smokers resided on a secure clinical research facility, under continuous medical supervision, while participating in a protocol designed to investigate cannabis tolerance and withdrawal. The National Institute on Drug Abuse, University of Maryland, and Maryland Department of Health and Mental Hygiene Institutional Review Boards approved the study, and all participants provided written informed consent. Participants' inclusion criteria were age between 18 and 45 years, self-reported cannabis smoking ≥1 years, averaging daily cannabis use ≥3 months before study entry, and having a cannabinoid-positive urine sample on admission. Exclusion criteria were any clinically significant current medical or psychiatric disease, history of seizures or cannabis-related psychosis or other adverse effect, consuming more than 6 alcoholic drinks/day ≥4 times/week, or allergy to sesame oil. Participants were admitted the evening before the first oral THC dose and discharged 23 h after the final dose.
Oral Thc Dosing And Sample Collection
Over 8 days, 37 doses of 20 mg synthetic THC (dronabinol, Marinol®; Unimed Pharmaceuticals) were orally administered with increasing frequency (every 4–8 h around the clock) for total daily dosages of 40–120 mg/day. Dose frequency was increased rather than dose amount to minimize adverse events previously reported with 30-mg THC doses (28). This dosing regimen was designed to standardize cannabis tolerance in daily, heavy cannabis smokers.
We obtained 4 OF samples before the first THC dose: duplicates on admission and single samples 7.0 h before and 0.0 h after the first oral THC dose. THC dosing preceded collection when both were scheduled. The first dose (20 mg) was administered at 1500 on day 1 (16.9–19.3 h after admission), followed by 5 hourly samples to examine single-dose cannabinoid pharmacokinetics. During continuous dosing, we collected OF samples daily at about 1000, 2000, and 2200. We administered 5 daily doses on days 2–4 (total 100 mg) and 6 on days 5–7 (total 120 mg). After the final dose, collections occurred every 90–180 min for 23 h. Eight duplicate samples were simultaneously collected 2.2 h after the final daily evening dose. Duplicate OF samples were collected to evaluate reproducibility of the collection and analytical methods. Supplemental Table 1, which accompanies the online version of this article at http://www.clinchem.org/content/vol56/issue8, further details study design.
OF was collected by use of the Quantisal collection device, which has an absorptive cellulose pad on a polypropylene stem with a volume adequacy indicator. OF cannabinoid concentrations collected in this manner were highly reproducible, and high recoveries of THC and THCCOOH (81.3%–91.4%) were achieved (29). The pad was placed into the participant's mouth until 1.0 (0.1) mL [mean (SD)] was collected, then put into a plastic tube containing 3 mL buffer, yielding a 1:4 OF dilution. Pads were removed from stems and squeezed dry with a serum separator. OF was stored in Nunc cryotubes at −20 °C until analysis.
Of Analysis
We analyzed OF samples using a validated 2D GC-MS method for THC, 11-OH-THC, CBD, CBN, and THCCOOH, with separate injections on 2 analytical systems with different ionization techniques (27). LOQ and dynamic ranges for THC, 11-OH-THC, and CBD were 0.5–50 μg/L; quantification limits for CBN and THCCOOH were 1–50 μg/L and 7.5–500 ng/L, respectively. Samples exceeding the linear range were diluted with blank OF/Quantisal buffer mixture and reanalyzed. Intra- and interassay imprecision were <6.6%, and analytical recovery was within 13.8% of target.
Data Analysis
For statistical calculations, we used SPSS 14.0 for Windows. Calculation of the %THC decrease from admission to predosing baseline was determined as [(admission – baseline)/admission] × 100. We averaged duplicate samples before proceeding to descriptive statistical analysis. Median concentration reflects positive samples only (≥LOQ). Concentration ranges of positive samples are presented without averaging duplicate sample results. For comparative statistical analysis only, values of 0.5*LOQ for THC and THCCOOH, 0.25 μg/L and 3.75 ng/L, respectively, were included if concentrations were <LOQ. We examined intersubject variations for samples collected before the first oral dose, after the first and last dose, and on each of days 2–7 by use of nonparametric Friedman ANOVA (χ2) after verifying failure in normal distribution (Kolmogorov–Smirnov normality test) and homogeneity (Levene test) of variances. We tested differences between concentrations on the first and last day by nonparametric Wilcoxon test. We used a paired t-test for comparison between each day's results. For concentration differences in duplicate samples, we evaluated 2 sets of data (n = 80 each) by paired t-test analysis. A 2-tailed P < 0.05 defined significance for all comparisons.
Results
We collected 440 OF samples from 10 male participants over 9 days. Participants (range 18–32 years) self-reported daily cannabis smoking of 1–24 joints/day. All participants self-reported cannabis smoking in the 24 h before admission and had positive cannabinoid urine tests on admission (Table 1).
Table 1.
Demographic and self-reported drug use characteristics of 10 chronic cannabis smokers at the time of study qualification.
| Participant |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| Age, years | 20 | 32 | 26 | 18 | 23 | 21 | 25 | 27 | 25 | 22 |
| BMI, kg/m2 | 17.8 | 29.8 | 25.1 | 23.7 | 21.1 | 22.9 | 30.6 | 28.7 | 30.5 | 29.7 |
| Cannabis joints, n/day | 6 | 3 | 24 | 3 | 6 | 1 | 3 | 9 | 21 | 3 |
| Cannabis use in past 14 days, n days | 13 | 13 | 14 | 12 | 14 | 14 | 14 | 14 | 13 | 14 |
| Time since last cannabis use, days | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| Lifetime cannabis use, years | 4 | 18 | 13 | 4 | 10 | 4 | 11 | 16 | 13 | 10 |
| Age at first cannabis use, years | 16 | 14 | 13 | 14 | 13 | 17 | 14 | 11 | 12 | 12 |
| Alcohol occasions, n/week | 0.5 | 0.08 | 1 | 0.25 | 2 | 3 | 0.25 | 2 | 0.5 | 0.25 |
| Tobacco smoking occasions, n/week | 70 | 3 | 76 | 10.5 | 0 | 21 | 105 | 42 | 140 | 14 |
| Other drug use | NAa | NA | A | O | O | O,C,T,H | A,O,H, | A,O,C | A,O | NA |
| Other drug use frequency | NA | NA | Once past 3 months | Once past 3 months | 1.5 years ago | Each once in past 2 years | Each once in past 6 years | Each once in past 7 years | Once past 3 months | NA |
NA, none admitted; A, amphetamines; O, opiates; C, cocaine; T, tranquilizers; H, hallucinogens.
THCCOOH was the primary analyte in 432 samples (98.2%), with concentrations up to 1117.9 ng/L. THCCOOH concentrations gradually increased throughout the dosing period and remained unchanged 23 h after the final dose (see online Supplemental Fig. 1). In contrast, THC was present in only 20.7% of samples (n = 91), and concentrations generally decreased from admission (Table 2). 11-OH-THC was not identified in any sample at an LOQ of 0.5 μg/L, whereas CBD and CBN were present in 3 and 8 samples, with maximum concentrations of 2.1 and 13 μg/L, respectively.
Table 2.
THC and THCCOOH OF quantitative data from 44 OF samples per participant collected during around-the-clock controlled oral THC administration over 9 days.a
| THC |
THCCOOH |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive (≥LOQ) | Detection rate, % | Tmax' hb | Last (≥LOQ), μg/L | Time (≥LOQ), h | Time first negative, h | Positive (≥LOQ) | Detection rate, % | Tmax' h | Last (≥LOQ), ng/L | Time (aLOQ), h | Time first negative, h | |
| A | 12 | 27 | −7.0 | 0.9 | 17.0 | 29.0 | 44 | 100 | 161.0 | 197.3 | 185.0 | ND |
| B | 13 | 30 | −18.0 | 0.8 | 29.0 | 31.0 | 44 | 100 | 167.0 | 137.3 | 185.0 | −18 |
| C | 6 | 14 | 0.0 | 0.6 | 5.0 | −18.0 | 40 | 91 | 127.5 | 42.1 | 185.0 | ND |
| D | 4 | 9 | −18.0 | 0.5 | −7.0 | 1.0 | 44 | 100 | 164.0 | 137.3 | 185.0 | 149.5 |
| E | 5 | 11 | 17.0 | 8.0 | 17.0 | 0.0 | 43 | 98 | 161.0 | 88.8 | 185.0 | ND |
| F | 1 | 2 | −18.0 | 0.6 | −18.0 | −18.0 | 44 | 100 | 170.0 | 156.4 | 185.0 | ND |
| G | 10 | 23 | −18.0 | 0.9 | 17.0 | 2.0 | 44 | 100 | 162.5 | 167.3 | 185.0 | ND |
| H | 8 | 18 | −18.0 | 0.5 | 4.0 | 5.0 | 44 | 100 | 103.5 | 79.1 | 185.0 | ND |
| I | 20 | 45 | −18.0 | 0.7 | 149.5 | 43.0 | 44 | 100 | 103.5 | 965.9 | 185.0 | ND |
| J | 12 | 27 | −18.0 | 0.6 | 17.0 | 29.0 | 41 | 93 | 161.0 | 25.4 | 185.0 | −18 |
| Total or mean | 91c | 20.7 | −11.6 | 1.41 | 23.05 | 10.4 | 432c | 98.2 | 148.1 | 199.7 | 185.0 | 37.8 |
Detection rate, number of OF samples ≥LOQ divided by total number of samples tested; last (≥LOQ), concentration of last sample ≥LOQ; time (≥LOQ), time to last sample ≥LOQ; time first negative, time to first sample ≤LOQ;ND, none detected.
Time is relative to first oral THC dose.
Total number of ≥LOQ samples.
All 10 subjects were initially THC-positive at admission from previously self-administered smoked cannabis, with 2 subjects having only 1 of the duplicate samples above the LOQ (0.5 μg/L). THC was present in 35 of 40 predose samples, with a wide range of concentrations, from 0.5–481.9 μg/L (Table 3). There was a significant decrease in mean THC concentrations during the predose period (χ2 = 9.1, df = 2, P = 0.011). Mean THC concentrations decreased 75.1% (19.0%) in 9 participants during the first 20 h after admission, although in the subject C, THC concentrations increased 348%, from 0.7 μg/L on admission to 3.1 μg/L at the time of first dose.
Table 3.
Median concentrations of positive samples (and range including duplicate samples) of THC in OF predose, after the first dose (20 mg), during 36 doses, and after the last THC dose per subject (n = 10); CBD and CBN concentrations in OF from self-administered smoked cannabis.a
| THC, μg/L |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predose |
After first dose |
During 36 doses |
After last dose n = 8 | CBD, μg/L |
CBN, μg/L |
||||||
| Positive | n = 3b | Positive | n = 5 | Positive | n = 20 | Positive | n = 36 | Positive | n = 36 | ||
| A | 100 | 4.1 (2.6−7.6) | 100 | 1.5 (0.8−3.3) | 10 | 1.2 (0.9−1.8) | NDb | 0 | ND | 3 | 1.5 (1.4−1.7) |
| B | 100 | 19.3 (4.4−481.9) | 100 | 5.3 (4.4−6.2) | 15 | 2.0 (0.8−4.0) | ND | 3 | 1.8 (1.4−2.1) | 6 | 6.6 (1.3−13.0) |
| C | 100 | 1.4 (0.7−3.1) | 60 | 1.0 (0.6−3.0) | 0 | ND | ND | 0 | ND | 0 | ND |
| D | 100 | 0.5 (0.5−2.2) | 0 | ND | 0 | ND | ND | 0 | ND | 0 | ND |
| E | 67 | 2.2 (1.4−3.5) | 20 | 1.1 | 5 | 8.0 | ND | 0 | ND | 0 | ND |
| F | 33 | 0.6 | 0 | ND | 0 | ND | ND | 0 | ND | 0 | ND |
| G | 100 | 1.1 (0.6−5.8) | 80 | 1.0 (0.6−2.6) | 10 | 1.1 (0.9−1.3) | ND | 0 | ND | 0 | ND |
| H | 100 | 2.0 (0.7−3.1) | 80 | 0.6 (0.5−0.8) | 0 | ND | ND | 0 | ND | 0 | ND |
| I | 100 | 23.4 (8.4−55.8) | 100 | 4.3 (1.3−6.8) | 45 | 1.1 (0.6−4.5) | ND | 3 | 0.5 | 6 | 2.5 (1.7−3.6) |
| J | 100 | 4.3 (1.0−11.3) | 100 | 0.7 (0.6−0.9) | 10 | 1.1 (0.6−1.7) | ND | 0 | ND | 0 | ND |
| Mean | 90 | 2.4 (0.5−481.9) | 56 | 1.3 (0.5−6.8) | 9.5 | 1.1 (0.6−8.0) | ND | 0.6 | 1.2 (0.5−2.1) | 1.4 | 1.7 (1.3−13.0) |
Data are median (range) unless noted otherwise. Predose, data obtained before first 20-mg THC dose; after first dose, data obtained after first 20-mg THC dose over 5 h; during 36 doses, data obtained during 36 around-the-clock THC administrations; after last dose, data obtained after final THC dose (n = 37 doses) over 23 h to discharge; n, number of collections in each period.
ND, none detected.
Nine participants were positive for THCCOOH at admission. THCCOOH was measurable in 37 of 40 predose collections at an LOQ of 7.5 ng/L (Table 4), with concentrations up to 499.4 ng/L. Predose concentration changes for THCCOOH varied substantially, with a mean decrease of 37.7% (18.1%) in 4 participants, a mean increase of 44.6% (38.8%) in 3 participants, and no consistent pattern in 2 participants. Interestingly, in participant C, a continuous increase in THCCOOH concentrations from <LOQ at admission to 24.0 ng/L predose was observed. No significant differences were observed for mean THCCOOH concentrations during the predose period (χ2 = 1.8, df = 2, P = 0.408).
Table 4.
Median concentrations of positive samples (and range including duplicate samples) of THCCOOH in OF per subject (n = 10), predose, after first (20-mg) dose, during (100–120 mg/day), and after the final oral THC dose.a
| THCCOOH, ng/L |
|||||
|---|---|---|---|---|---|
| Predose (n = 3) | First dose (n = 5) | 3 days 100 mg/d (n = 9) | 3 days 120 mg/d (n = 9) | Last dose (n = 8) | |
| A | 24.7 (9.5–32.1) | 27.3 (22.3–52.0) | 53.1 (17.7–166.5) | 170 (100.4–397) | 147 (98.4–281.1) |
| B | 83.3 (59.6–143.3) | 60.8 (52.2–82.6) | 112.9 (47.4–148.4) | 161.8 (80.7–315.8) | 236 (69.8–358.7) |
| Cb | 17.0 (10.0–24.0) | 22.3 (14.0–47.6) | 50.5 (18.2–157.1) | 149.4 (50.3–333) | 61.7 (25.5–94.9) |
| D | 47.8 (44.1–52.4) | 44.3 (24.3–59.8) | 158.6 (59.8–185.4) | 221.9 (164–359.4) | 259.1 (137.3–364.5) |
| Ec | 14.3 (7.6–21.9) | 10.4 (7.5–18.7) | 21.2 (14.8–67.3) | 64.9 (16.8–108.6) | 62.9 (28.1–94.6) |
| F | 20.2 (11.8–27.1) | 24.8 (7.6–52.7) | 90.6 (33.2–167.7) | 140.3 (65.4–251.1) | 230.8 (150.7–371.7) |
| G | 14.3 (7.7–29.8) | 20.3 (10.2–52.1) | 46.8 (33.4–108.2) | 65.6 (35.3–159.7) | 145.3 (67.5–381) |
| H | 23.0 (14.2–28.1) | 32.3 (17.4–54.7) | 92.4 (46.2–154.4) | 80.9 (26.3–427.5) | 83.1 (73.7–107.4) |
| I | 211.3 (158.3–499.4) | 262.2 (129.9–557.1) | 475.4 (319.9–861.8) | 838 (397.9–1117.9) | 719.3 (441–1055.5) |
| Jd | 10.1 (7.9–30.0) | 8.7 (7.8–9.1) | 29.1 (11.8–63.6) | 23.8 (9.7–99.8) | 46.0 (25.4–102) |
| Mean | 24.0 (7.6–499.4) | 29.1 (7.5–557.1) | 81.7 (11.8–861.8) | 140.7 (9.7–1117.9) | 134.7 (25.4–1055.5) |
Data are median (range). Predose, data obtained before first 20-mg THC dose; first dose, data obtained after first 20-mg THC dose over 5 h; 3 days 100 mg/d, data obtained over 3 days (2–4) of 100 mg/day THC; 3 days 120 mg/d, data obtained over 3 days (5–7) of 120 mg/day THC; final dose, data obtained after final THC dose (n = 37 doses) over 23 h to discharge; n, number of collections in each period.
Subject C had 2 positive predose samples, 4 after the first dose, and 8 over the 3-day 100 mg/d THC.
Subject E had 8 positive samples over the 3-day 120 mg/day THC.
Subject J had 8 positive samples over the 3-day 100 mg/day THC.
CBD was present in only 2 subjects' OF. Three of 20 duplicate samples acquired immediately on admission were ≥LOQ for CBD (range 0.5–2.1 μg/L). Similarly, CBN was quantifiable in only 8 of 30 samples collected within 11 h of admission (range 1.3–13.0 μg/L). No other samples were positive for CBD or CBN during the remainder of the study (Table 3).
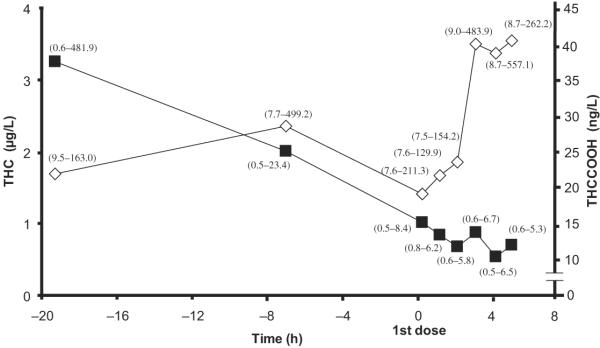
Samples collected at the time of the first 20-mg THC dose (1500 on the day after admission) had THC and THCCOOH concentrations of 2.7 (2.6) and 42.3 (61.8) ng/L, respectively. Thirty-two (64.0%) THC (range 0.5–6.7 μg/L) and 49 (98.0%) THCCOOH (range 7.5–557.1 ng/L) positive OF samples were obtained within the first 5 hourly collections after the first dose. Significant increases in mean THCCOOH concentrations were observed (χ2 = 9.8, df = 4, P = 0.045) over the 5 h after the first oral dose, although no changes in THC (χ2 = 6.6, df = 4, P = 0.157) concentrations were observed during this time period (Fig. 1). On days 2–7, THC was measurable in 15 of 240 (6.3%) samples from 6 participants (Table 4). Three participants' OF were continuously THC positive, and those of 2 others were occasionally positive until the beginning of day 2 (35 h since admission), with last positive THC concentrations ranging from 0.6 to 8.0 μg/L. One participant (subject I) produced consecutive THC positive samples for 49.0 h (3.9 μg/L) and was thereafter occasionally positive until day 7 (0.7 μg/L). The remaining 4 participants' OF samples were consistently THC negative after the first dosing day.
Fig. 1. Median (range) THC (∎) and THCCOOH (◇) OF concentrations collected on admission and after the first 20-mg THC dose.
The first point is the mean of the first duplicate collections. Time on x axis is related to the first dose administration.
THCCOOH was positive in 98.3% of samples throughout days 2–7, with concentrations of 9.7–1117.9 ng/L. Median Tmax for THCCOOH was 161 h (range 104–170 h) after the first dose. The dose increase from 100 to 120 mg/day on day 5 was associated with a significant increase in daily overall mean THCCOOH (t = −3.10, df = 29, P = 0.004) OF concentration, although there was no significant change (t = −1.03 to −0.59, df = 29, P = 0.309–0.934) on days with the same THC dose. Median OF THCCOOH concentration on day 4 (100 mg/day) was 102.8 ng/L (range 18.0–607.5), whereas on day 5 (120 mg/day) it was 133.0 ng/L (range 8.3–1087.7).
THCCOOH was present in all samples collected over the 23 h after the final THC dose (0930 on day 8) at 25.4–1055.5 ng/L (Table 4). The median THCCOOH concentration was 114.3 ng/L (range 25.5–922.5) at the time of final dose and 137.3 ng/L (range 25.4–965.9) 23 h later, with no significant change (χ2 = 4.9, df = 7, P > 0.05) over the interval. There was a significant increase in THCCOOH (Wilcoxon) concentrations between the first and last study days (Table 4).
In total, 80 OF duplicates were collected and analyzed for collection and analytical method reproducibility. If the quantitative result was less than the LOQ, a value of half the LOQ was substituted for paired t-test analysis. THC was measureable in only 29 of 160 samples. No significant differences (t = −0.9, df = 79, P = 0.331) were found in duplicate THC samples. THCCOOH was measureable in 157 of 160 samples, with no significant difference (t = −1.9, df = 79, P = 0.060) in duplicate THCCOOH concentrations.
Discussion
Cannabinoid OF quantification is an analytical challenge because of the wide range of concentrations encountered, from tens to hundreds of μg/L for THC (8, 10) to a few ng/L for THCCOOH (15, 30). Our fully validated method for quantifying THC, 11-OH-THC, THCCOOH, CBD, and CBN enabled us to capture these low cannabinoid concentrations (27).
This research was the first to characterize major cannabinoids and metabolites in OF after around-the-clock controlled oral THC administration. Measurable THC, CBD, CBN, and THCCOOH at admission reflected self-administered smoked cannabis, with variable concentrations due to different times since last administration (and possibly different doses). Participants' self-reported drug use history indicated that their last smoking episode occurred from just before admission up to 1 day earlier, explaining in part the wide range in initial concentrations. These predose concentrations were similar to those reported within 4 h after smoking a single cannabis cigarette, with THC concentrations from 3.6 to 5800 μg/L (7). Kauert et al. (8) reported mean peak OF THC concentrations of 1041 (652) μg/L 0.25 h after smoking, with a 50-fold decrease to 18 (12) μg/L over 6 h. Others observed OF THCCOOH concentrations from 2 to 352 ng/L in 109 randomly collected samples (30).
In the present study, CBD and CBN were detected in only 11 samples with the highest THC concentrations and were measurable on average for 11 h after admission, indicating recent cannabis smoking. Others found CBN only up to 4 h (range 0.9–4.1 μg/L) after a single smoking session in OF from habitual cannabis smokers (10). No measurable 11-OH-THC (LOQ 0.5 μg/L) was found in OF collected pre- and postdose during 8 days of oral THC administration in our study, despite high THCCOOH concentrations up to 1117.9 ng/L.
THC concentrations decreased variably during the study, possibly because of differences in frequency and amount of cannabis smoked. Subject F, who was negative for THC immediately after admission, self-reported smoking 1 joint/day for 1.5 years. Subject I, however, who self-reported smoking 21 joints/day for 10 years, was still positive for THC on day 7. Samples from subject C collected during the first dosing day were occasionally positive for THC (0.59–3.1 μg/L), possibly due to residual THC from self-reported heavy cannabis smoking (Table 1). In 6 study participants, THC concentrations continuously decreased, whereas in 4 others there were negative or only occasional THC-positive OF samples for 5 h after the first 20-mg THC dose. THC, a lipophilic substance, forms a depot in the oral mucosa during smoking, which serves as the main source of THC in OF (11). Tissue stores increase with frequency of use and amount of exposure, leading to prolonged THC excretion in chronic cannabis users (31). Some THC in OF also may be due to equilibration with unbound drug in blood (7).
In contrast to THC, THCCOOH was detected in almost all samples (98.2%), albeit at a much lower LOQ. LOQ differences were critical to the percentage of samples positive for these analytes; if THC had been quantified with an LOQ similar to THCCOOH (7.5 ng/L), a much longer detection window might have been achieved, but it is unknown if detection at this concentration could differentiate active cannabis smoking from passive inhalation.
At admission, relatively low THCCOOH concentrations were noted, with decreasing concentrations in 4 participants before the first THC dose. Four subjects displayed the opposite pattern, with THCCOOH increasing before dosing. This increase might indicate recent cannabis smoking before admission. Subject C, a heavy chronic user, self-reported smoking 24 joints/day for 8 years, with the most recent being 1 day before the study. Although below the LOQ (<7.5 ng/L) at admission, THCCOOH concentrations for subject C increased to 24 ng/L before the first oral THC dose. Despite chronic cannabis self-administration, his THCCOOH concentrations were low to moderate compared with those of other participants, possibly indicating fast metabolism and elimination patterns.
Mean THCCOOH concentrations increased significantly from the first to the last day of the study (Table 4). Mean THCCOOH concentrations varied during continuous oral THC dosing (days 2–7) but were not significantly different on days with the same total THC dose. In contrast, there was a statistically significant increase in mean THCCOOH concentration between days 4 and 5, when the total THC dose increased from 100 to 120 mg/day. This increase could be due to the increase in THC dose and/or accumulation of THCCOOH following around-the-clock THC dosing. Moore et al. (32) reported a similar pattern of increasing THCCOOH concentrations in OF after smoking of a single cannabis cigarette in a single participant. This THCCOOH concentration increase was observed in OF samples collected with the Quantisal device from the subject who self-reported smoking cannabis every other day for >20 years. Before, immediately after, and 48 h after a single cannabis cigarette, THCCOOH OF concentrations were 33, 46, and 77 ng/L, respectively.
Moore et al. (14) reported 2 THCCOOH maxima in samples collected for 8 h after a single smoked dose in only 1 participant, with a first maximum 45 min after smoking. Although a different route of drug administration was used in our study, 2 THCCOOH maxima were observed in only 2 of 10 participants after oral administration of the first 20-mg oral THC dose, with the first maximum occurring within 1 h. Moore et al. postulated that 2 maxima could be reflective of THCCOOH disposition from the blood, due to previously self-administered smoked cannabis. However, in our heavy, chronic cannabis smokers, 2 THCCOOH maxima were not noted in the majority of participants' OF. Further research is needed to determine if 2 THCCOOH OF maxima typically occur after smoked cannabis.
Simultaneously collected Quantisal OF samples produced equivalent qualitative results for THC and THCCOOH, satisfying the requirement for “split specimens,” as defined in the Department of Health and Human Services Substance Abuse and Mental Health Services Administration mandatory guidelines for federal workplace drug testing programs (33).
This study expands our knowledge of cannabinoid OF pharmacokinetics after cannabis smoking, single oral THC dosing, and around-the-clock multiple THC doses. We show that THC in OF appears to primarily reflect previously self-administered smoked cannabis rather than diffusion from the bloodstream after controlled oral administration. THCCOOH concentrations gradually increased with continuous oral THC doses and were detected in relatively high concentrations 23 h after the last dose. THCCOOH measurement not only identifies cannabis exposure, but also minimizes the possibility of passive inhalation of cannabis smoke and consequently may serve as a good biomarker for detection of cannabis use.
Acknowledgments
We acknowledge the National Institute on Drug Abuse, Intramural Research Program, the Behavioral Pharmacology Research Unit, and Maryland Psychiatric Research Center clinical research teams.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Research Funding: The research was funded by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), NIH, and NIDA Residential Research Support Services Contract HHSN271200599091 CADB Contract No. N01Da-5-9909, and Sanofi-Aventis, Inc. The research was approved by the Institutional Review Board, NIDA.
Footnotes
Nonstandard abbreviations: OF, oral fluid; DUID, driving under the influence of drugs; THC, Δ9-tetrahydrocannabinol; 11-OH-THC, 11-hydroxy-THC; THCCOOH, 11-nor-9-carboxy-THC; CBD, cannabidiol; CBN, cannabinol; LOQ, limit of quantification; 2D, 2-dimensional; BSTFA, N,O-bis(trimethylsilyl)trifluoroacetamide; TFAA, trifluoroacetic anhydride; HFIP, hexafluoroisopropanol.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors' Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: D.L. Kelly, Solvay, Bristol-Myers Squibb, and Janssen.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
References
- 1.Verstraete AG. Oral fluid testing for driving under the influence of drugs: history, recent progress and remaining challenges. Forensic Sci Int. 2005;150:143–50. doi: 10.1016/j.forsciint.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55:1910–31. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halldin MM, Widman M, Bahr CV, Lindgren JE, Martin BR. Identification of in vitro metabolites of delta-tetrahydrocannabinol formed by human livers. Drug Metab Dispos. 1982;10:297–301. [PubMed] [Google Scholar]
- 4.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, Δ9-tetrahydrocannabinol, cannabidiol and cannabinol. In: Pertwee RG, editor. Cannabinoids. vol. 168. Springer; New York: 2005. pp. 657–90. Handbook of experimental pharmacology. [DOI] [PubMed] [Google Scholar]
- 5.Harvey DJ, Paton WDM. Metabolism of the cannabinoids. Rev Biochem Toxicol. 1986;6:221–64. [Google Scholar]
- 6.Perez-Reyes M, Di Guiseppi S, Davis KH, Schindler VH, Cook CE. Comparison of effects of marijuana cigarettes of three different potencies. Clin Pharmacol Ther. 1982;31:617–24. doi: 10.1038/clpt.1982.86. [DOI] [PubMed] [Google Scholar]
- 7.Huestis MA, Cone EJ. Relationship of delta 9-tetrahydrocannabinol concentrations in oral fluid and plasma after controlled administration of smoked cannabis. J Anal Toxicol. 2004;28:394–9. doi: 10.1093/jat/28.6.394. [DOI] [PubMed] [Google Scholar]
- 8.Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. Pharmacokinetic properties of delta9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol. 2007;31:288–93. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- 9.Cone EJ, Huestis MA. Interpretation of oral fluid tests for drugs of abuse. Ann N Y Acad Sci. 2007;1098:51–103. doi: 10.1196/annals.1384.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore C, Rana S, Coulter C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J Chrom B-Anal Tech Biomed Life Sci. 2007;852:459–64. doi: 10.1016/j.jchromb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Reyes M. Marijuana smoking: factors that influence the bioavailability of tetrahydrocannabinol. In: Chiang CN, Hawks RL, editors. Research findings on smoking of abused substances. National Institute on Drug Abuse; Rockville (MD): 1990. pp. 42–62. NIDA research monograph nr 99. [PubMed] [Google Scholar]
- 12.Kintz P, Cirimele V, Ludes B. Detection of cannabis in oral fluid (saliva) and forehead wipes (sweat) from impaired drivers. J Anal Toxicol. 2000;24:557–61. doi: 10.1093/jat/24.7.557. [DOI] [PubMed] [Google Scholar]
- 13.Huestis MA, Cone EJ. Alternative testing matrices. In: Karch SB, editor. Drug abuse handbook. CRC Press; Boca Raton, FL: 1998. pp. 799–857. [Google Scholar]
- 14.Moore C, Coulter C, Rana S, Vincent M, Soares J. Analytical procedure for the determination of the marijuana metabolite 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid specimens. J Anal Toxicol. 2006;30:409–12. doi: 10.1093/jat/30.7.409. [DOI] [PubMed] [Google Scholar]
- 15.Day D, Kuntz DJ, Feldman M, Presley L. Detection of THCA in oral fluid by GC-MS-MS. J Anal Toxicol. 2006;30:645–50. doi: 10.1093/jat/30.9.645. [DOI] [PubMed] [Google Scholar]
- 16.Huestis MA, Elsohly M, Nebro W, Barnes A, Gustafson RA, Smith ML. Estimating time of last oral ingestion of cannabis from plasma THC and THCCOOH concentrations. Ther Drug Monit. 2006;28:540–4. doi: 10.1097/00007691-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Cone EJ, Johnson RE, Paul BD, Mell LD, Mitchell J. Marijuana-laced brownies: behavioral effects, physiologic effects, and urinalysis in humans following ingestion. J Anal Toxicol. 1988;12:169–75. doi: 10.1093/jat/12.4.169. [DOI] [PubMed] [Google Scholar]
- 18.Leson G, Pless P, Grotenhermen F, Kalant H, ElSohly MA. Evaluating the impact of hemp food consumption on workplace drug tests. J Anal Toxicol. 2001;25:691–8. doi: 10.1093/jat/25.8.691. [DOI] [PubMed] [Google Scholar]
- 19.Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25. doi: 10.1016/j.jep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Lemberger L, Weiss JL, Watanabe AM, Galanter LM, Wyatt RJ, Cardon PV. Delta-9-tetrahydrocannabinol temporal correlation of the psychologic effects and blood levels after various routes of administration. N Engl J Med. 1972;286:685–8. doi: 10.1056/NEJM197203302861303. [DOI] [PubMed] [Google Scholar]
- 21.Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352–63. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- 22.Sporkert F, Pragst F, Ploner CJ, Tschirch A, Stadelmann AM. Pharmacokinetic investigations and delta-9-tetrahydrocannabinol and its metabolites after single administration of 10 mg Marinol in attendance of a psychiatric study [Poster]. Annual meeting of The International Association of Forensic Toxicologists; Prague, Czech Republic. 2001 Aug 26–30.p. 62. [Google Scholar]
- 23.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–16. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- 24.Brenneisen R, Egli A, ElSohly MA, Henn V, Spiess Y. The effect of orally and rectally administered delta-9-tetrahydrocannabinol on spasticity: a pilot study with 2 patients. Int J Clin Pharmacol Ther. 1996;34:446–52. [PubMed] [Google Scholar]
- 25.Goodwin RS, Gustafson RA, Barnes A, Nebro W, Moolchan ET, Huestis MA. Delta(9)-tetrahydrocannabinol, 11-hydroxy-delta(9)-tetrahydrocannabinol and 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol in human plasma after controlled oral administration of cannabinoids. Ther Drug Monit. 2006;28:545–51. doi: 10.1097/00007691-200608000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Schwilke EW, Schwope DM, Karschner EL, Lowe RH, Darwin WD, Kelly DL, et al. Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin Chem. 2009;55:2180–9. doi: 10.1373/clinchem.2008.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chrom A. 2010;1217:1513–21. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999;141:385–94. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- 29.Quintela O, Crouch DJ, Andrenyak DM. Recovery of drugs of abuse from the Immunalysis Quantisal oral fluid collection device. J Anal Toxicol. 2006;30:614–6. doi: 10.1093/jat/30.8.614. [DOI] [PubMed] [Google Scholar]
- 30.Moore C, Ross W, Coulter C, Adams L, Rana S, Vincent M, et al. Detection of the marijuana metabolite 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid specimens and its contribution to positive results in screening assays. J Anal Toxicol. 2006;30:413–8. doi: 10.1093/jat/30.7.413. [DOI] [PubMed] [Google Scholar]
- 31.Johansson E, Halldin MM, Agurell S, Hollister LE, Gillespie HK. Terminal elimination plasma half-life of delta-1-tetrahydrocannabinol (delta-1-THC) in heavy users of marijuana. Eur J Clin Pharmacol. 1989;37:273–7. doi: 10.1007/BF00679783. [DOI] [PubMed] [Google Scholar]
- 32.Moore C, Rana S, Coulter C, Day D, Vincent M, Soares J. Detection of conjugated 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid. J Anal Toxicol. 2007;31:187–94. doi: 10.1093/jat/31.4.187. [DOI] [PubMed] [Google Scholar]
- 33.DHHS Mandatory guidelines for federal work-place drug testing. Fed Regist. 2008;73:71858–907. [Google Scholar]