Abstract
The results of the current analyses present preliminary evidence of an association between putatively functional variation in the prodynorphin (PDYN) gene and a dimensional measure of disinhibited behavior. A 68 bp sequence in the core promoter region of the PDYN gene was genotyped in a community sample of 1021 adults aged 30–54. Participants were interviewed for lifetime history of DSM-IV alcohol dependence and completed two self-report measures of sensation seeking and impulsiveness. Fifteen percent (n=151) of the sample met DSM-IV criteria for alcohol dependence and while results did not support an association between the PDYN polymorphism and the diagnosis of alcohol dependence, we did observe an association between the “low” expressing L allele of the PDYN gene and a preference for engaging in disinhibited behavior. Additionally, people who had both a history of alcohol dependence and higher scores on this Disinhibited Behavior scale were most likely to carry an L allele. These results indicate that variation in the PDYN gene is associated with a dimensional trait or intermediate phenotype that reflects a preference for heavy drinking and engaging in related risky behaviors (e.g., drug use, sexual activity).
Introduction
Candidate gene studies have identified a number of genetic variants associated with alcohol dependence, including polymorphisms in genes that code for alcohol metabolism (e.g., alcohol dehydrogenase and aldehyde dehydrogenase) and the alpha 2 GABAA receptor (Gelernter & Kranzler, 2009; Mayfield et al., 2008). Polymorphic variation in dopamine-regulating genes is also frequently examined in association with alcohol dependence because of the involvement of dopamine in brain reward circuitry and substance use disorders (Ducci & Goldman, 2008; Gelernter & Kranzler, 2009). Another gene that has been linked more recently to alcohol use disorders is the PDYN gene located on chromosome 20. For example, Xuei et al (2006) reported that a haplotype block of 6 single nucleotide polymorphisms (SNPs) in the PDYN gene was associated with alcohol dependence in a sample of 1860 Caucasians from 219 families. Additionally, Yuferov et al (2008) reported that a PDYN haplotype associated with comorbid cocaine and alcohol dependence was related to lower mRNA expression of the PDYN gene in the human dorsal and ventral striatum.
Prodynorphin is the precursor protein to several opioid receptor ligands, such as dynorphin A, dynorphin B, and α/β-neo-endorphin (Horikawa et al., 1983). Dynorphins function in blocking dopamine release by binding to the k-opioid receptors (KORs) on presynaptic dopaminergic nerve terminals (Krebs et al., 1994). Infusion of dynorphin A into the caudate putamen results in diminished basal extracellular dopamine as well as diminished dopamine release following cocaine administration. This effect can be blocked by pre-infusion with the selective KOR antagonist nor-BNI, implicating KORs in mediating the effect of dynorphin on dopamine release (Zhang et al., 2004). Dynorphin thus appears to serve a modulatory or protective role in decreasing extracellular dopamine after drug-induced surges in synaptic dopamine (Shippenberg et al., 1996; Svensson & Hurd, 1998) and provides a mechanism for the phenomenon of drug addiction (Bruijnzeel, 2009; Wee & Koob, 2010) and tolerance (Yuferov et al., 2008). Importantly, high dynorphin levels function in negative reinforcement and produce the dysphoria and anhedonia associated with drug withdrawal (Bruijnzeel, 2009).
Numerous mutations have been identified in the human PDYN gene, including a commonly studied repeat polymorphism in the core promoter region. This polymorphism consists of 1–4 repeats of a 68 bp sequence, each containing one binding site for the transcription factor activator protein (AP)-1. In vitro, induction of reporter genes bound to the human PDYN promoter containing 3 or 4 copies of the repeat resulted in nearly 1.5 times greater levels of gene-expression activity, and thus presumably protein product, compared with constructs containing 1 or 2 copies (Zimprich et al., 2000; Babbitt et al., 2010). Direct studies of the human brain revealed that individuals with 1 or 2 copies have low PDYN expression (L) whereas individuals with 3 or 4 copies have high PDYN expression (H) in the striatum (Nikoshkov et al, 2008) and the frontal cortex and cerebellum in females (Babbitt et al., 2010).
There have been several reports that have examined the relationship between this tandem repeat element polymorphism and substance use disorders (SUDs) but results are mixed, with some studies showing that high PDYN expressing alleles are more common among people with SUDs (Nomura et al., 2006; Dahl et al, 2005; Wei et al., 2011, Williams et al, 2007), others showing low expression alleles in association with dependence (Chen et al 2002) and still others reporting no association (Ray et al., 2005; Nikoshkov et al, 2008; Zimprich, 2000). The inconsistent results across studies can be attributed to a variety of factors, including small sample sizes, variation in ethnic background of the samples and the examination of different SUDs (e.g., heroin, cocaine, and methamphetamine).
Because the dependence phenotype is a complex disorder, it is likely that multiple genes influence a range of quantitative or intermediate traits that are associated with dependence. Such traits may confer risk for substance use problems or, alternatively, may be modified by heavy or chronic use (Kreek et al., 2005; Schuckit, 2009). Prospective investigations have shown that higher levels of heritable aspects of disinhibited behavior, including self-reported traits of sensation seeking and impulsiveness, are associated with the development of alcohol use disorders (reviewed in Sher et al 2005). Notably, Kirisci et al (2005) reported that the risk for development of a substance use disorder, including alcohol abuse and dependence, was particularly high for men who had a family history of substance use problems and who reported high levels of a latent factor comprised of behavioral and self-reported aspects of behavioral undercontrol. Young et al (2000) reported that self-reported novelty seeking and substance experimentation shared common genetic variance in a twin sample of adolescents, providing further justification for the consideration of this trait as an intermediate phenotype associated with alcohol dependence (Schuckit, 2009). Accordingly, the aim of the current analyses was to evaluate the relationship between variation in the functional tandem repeat polymorphism in the promoter region of the PDYN gene and alcohol dependence and self-reported sensation seeking and impulsiveness in a community sample. We also evaluated sex differences in these associations following a recent report showing a sex-dependent association between opioid dependence and SNPs in the PDYN gene (Clarke et al., 2009).
Method
Participants
Participants were adults aged 30 and 54 who were enrolled in the Adult Health and Behavior (AHAB) project, a registry of behavioral and biological phenotypes among community volunteers. Recruitment and initial screening were conducted by the Recruitment Office in the Department of Epidemiology in the School of Public Health at the University of Pittsburgh by mass-mail solicitation from Western Pennsylvania (principally Allegheny County). Data were collected between 2001 and 2005. To be eligible, participants had to report being in good general health and were excluded on the basis of significant cardiovascular disease (e.g., myocardial infarction, coronary revascularization, angina), chronic renal or liver disease, neurological or autoimmune disorder (e.g., epilepsy, multiple sclerosis), treatment for cancer in the past year, psychosis, or bipolar (Type I) affective disorder. People reporting use of the following medications were not eligible for participation: cardiovascular (except antihypertensives and lipid lowering medications), psychotropic, glucocorticoid, diabetes, or prescription weight-loss drugs. Women who were pregnant were also ineligible. Participants were recruited without regard to race, sex or ethnicity, but only data from Caucasians are reported here. Additionally, AHAB participants were excluded from the current analyses if they had an IQ less than 80 on the two scale subtest of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Note that one additional subject was genotyped for the PDYN genotype but did not complete the self-report scales and 29 individuals could not be genotyped for the PDYN polymorphism. Participants were classified according to whether or not they had a lifetime DSM-IV diagnosis of alcohol dependence (see details below). Thirty individuals who were negative for an alcohol dependence diagnosis were excluded from the current analyses because they met lifetime diagnosis of substance dependence to another substance (e.g., marijuana, stimulants, etc). These exclusions resulted in 1021 participants who provided written informed consent following a protocol that was approved by the University of Pittsburgh Institutional Review Board.
Measures
Participants were interviewed for lifetime history of DSM-IV Axis I disorders, including substance use disorders using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First, Spitzer, Gibbon, & Williams, 2002) by masters or doctoral level clinicians and consensus diagnoses were determined by a licensed clinical psychologist. For data analyses, participants were classified as alcohol dependent if they had ever met criteria for the diagnosis.
On a separate occasion, all participants completed two self-report measures to assess sensation seeking and impulsivity with the Zuckerman Sensation Seeking Scale (ZSS – V) (Zuckerman, 1979) and the Barratt Impulsiveness Scale (BIS 11-A) (Barratt, 1985; 1994; Stanford, Mathias, Dougherty, Lake, Anderson, & Patton, 2009). The ZSS-V is a 40 item, true/false scale that measures an individual’s preference to seek novel and complex experiences. High scorers on this scale reliably report a preference for engaging in risky behaviors, including the use of alcohol and other drugs to get high and other thrill seeking behaviors. The scale has four subscales, with 10 questions per subscale and named Thrill and Adventure Seeking (e.g., I often wish I could be a mountain climber), Experience Seeking (e.g., I like to explore a strange city or section of town by myself, even if it means getting lost), Boredom Susceptibility (e.g., The worst social sin is to be a bore) and Disinhibited Behavior (e.g., I like “wild” uninhibited parties). Subscale total scores (i.e., sums) can range from 0 to 10. The BIS-11A contains 30 questions regarding individuals’ control of thoughts and behaviors and is comprised of three subscales including 12 nonplanning items (e.g., I spend or charge more than I earn), 10 motor impulsiveness items (e.g., I do things without thinking) and 8 cognitive impulsiveness items (e.g., I am a steady thinker). Items are rated on a four-point scale, ranging from “rarely/never” to “almost always/always” and were summed for analyses.
Genotyping
Genomic DNA was isolated from peripheral white blood cells using the PureGene kit (Gentra Systems, Minneapolis, MN). The PDYN 68 bp tandem repeat element polymorphism was typed by polymerase chain reaction (PCR) amplification of genomic DNA using the primers and conditions as described by Zimprich et al. (2000). Amplimers were separated on 2.5 % agarose, and visualized by UV-transillumination of ethidium bromide stained gels. Alleles were assigned by comparison to sequenced verified controls of known genotype, run in the same gel. Genotypes were read in duplicate by independent observers. Alleles were designated as 1, 2, 3 or 4 based on the number of repeat elements that were identified. The four alleles were categorized into two groups: L (low), which included alleles 1 and 2 and H (high), including alleles 3 and 4. The repeat element contains a transcription factor binding site that is associated with transcriptional efficiency of the human PDYN gene and higher gene expression is associated with a 3 or 4 repeat allele (Zimprich et al 2000).
Statistical analyses
Sex differences in age, years of education, income and marital status and the frequency of lifetime diagnoses of DSM-IV substance dependence were evaluated using t-test or X2 analyses as appropriate. The association of the PDYN genotype to frequency of lifetime alcohol dependence diagnosis was evaluated using X2 analyses. A series of 2 (genotype) X 2 (gender) X 2 (Alcohol dependence diagnosis) analyses of variance was conducted to compare groups on the total scores of the two self report scales with the aim of examining the association of the PDYN genotype to impulsiveness and sensation seeking. Following the observation of a significant association (p < .05) for the total score, analyses were conducted on the subscales to evaluate whether specific subscales could account for any association between genotype and the total score. Because the frequency of the LL genotype was relatively low, particularly when considered separately by gender, the LL and LH genotype groups were combined. The decision to combine such groups is supported by results from a human postmortem study (Nikoshkov et al; 2008) showing that the H/H genotype is associated with higher PDYN mRNA expression in the striatum (nucleus accumbens and caudate nucleus), relative to HL and LL genotypes. Finally, the relationship of alcohol dependence diagnosis and gender to impulsivity and sensation seeking was presented for descriptive purposes.
Results
Subject characteristics
As shown in Table 1, the sample included 1021 men and women who averaged 45 years of age. Men and women differed by years of education and the likelihood of having an alcohol use or other substance use disorder. Fifteen percent of the sample (n=151) had a lifetime history of alcohol dependence, a rate that is comparable to US prevalence rates of lifetime DSM-IV alcohol dependence (e.g., 12.5%; Hasin, Stinson, Ogburn, & Grant, 2007). Likewise, although the total number of individuals with alcohol dependence is relatively small, the sample size is in accord with previously published research with this polymorphism.
Table 1.
Demographic characteristics and DSM-IV Substance Dependence diagnoses by gender
| Men (n=496) |
Women (n=525) |
t or χ2 | p | ||
|---|---|---|---|---|---|
| Age | 44.52 (6.92) | 44.88 (6.72) | −0.85 | 0.40 | |
| Marital status (% married/partne red) | 47.3 | 52.70 | 1.47 | 0.23 | |
| Education (years) | 16.26 (2.60) | 15.82 (2.92) | 2.51 | 0.01 | |
| Alcohol Dependence (DSM-IV) | |||||
| Current # (%) | 35 (7.1) | 5 (1.0) | 25.25 | <.0001 | |
| Past # (%) | 82 (16.5) | 29 (5.5) | 31.90 | <.0001 | |
| Other Substance Depend ence | |||||
| Current # (%) | 10 (2.0) | 0 (0) | 10.69 | 0.001 | |
| Past # (%) | 39 (7.9) | 7 (1.3) | 25.27 | <.0001 | |
Hardy-Weinberg Equilibrium
Genotypic frequencies (LL, LH, HH) of the repeat polymorphism in the PDYN gene promoter region did not differ significantly from that predicted by Hardy Weinberg Equilibrium, X2 (1) = 0.34, p > 0.10, indicating independent assortment of alleles. Allelic frequencies in the sample were 31% for the L allele and 69% for the H allele.
PDYN genotype and alcohol dependence
Chi-squared analyses indicated that the PDYN genotype was not associated with a diagnosis of alcohol dependence in this sample, X2(1)=.63, p=.43. Sixteen percent of people with a LL or LH genotype had a lifetime diagnosis of alcohol dependence versus 14% of people who were homozygous for the H allele. Chi-squared analyses conducted separately for men and women did not show significant association within gender groups (p’s > .39).
PDYN genotype, self-reported impulsiveness, sensation seeking and alcohol dependence
The total scores on the two self-report scales were modestly correlated, r1019= .26, p.<.0001 and similarly associated in men, r494= .27, p.<.0001, and women, r523= .25, p.<.0001. Genotype was not related to total scores on the ZSS or BIS scales, but an interaction between sex and genotype was observed for the total score on the Sensation Seeking Scale (F1,1013 =4.49, p.=.03). Pairwise comparisons showed that this interaction reflected the fact that women with the HH genotype reported lower sensation seeking compared to LL + LH women (M’s = 13.21 and 14.36, respectively), but genotype was not associated with sensation seeking among men (M’s = 18.31 and 17.74, respectively).
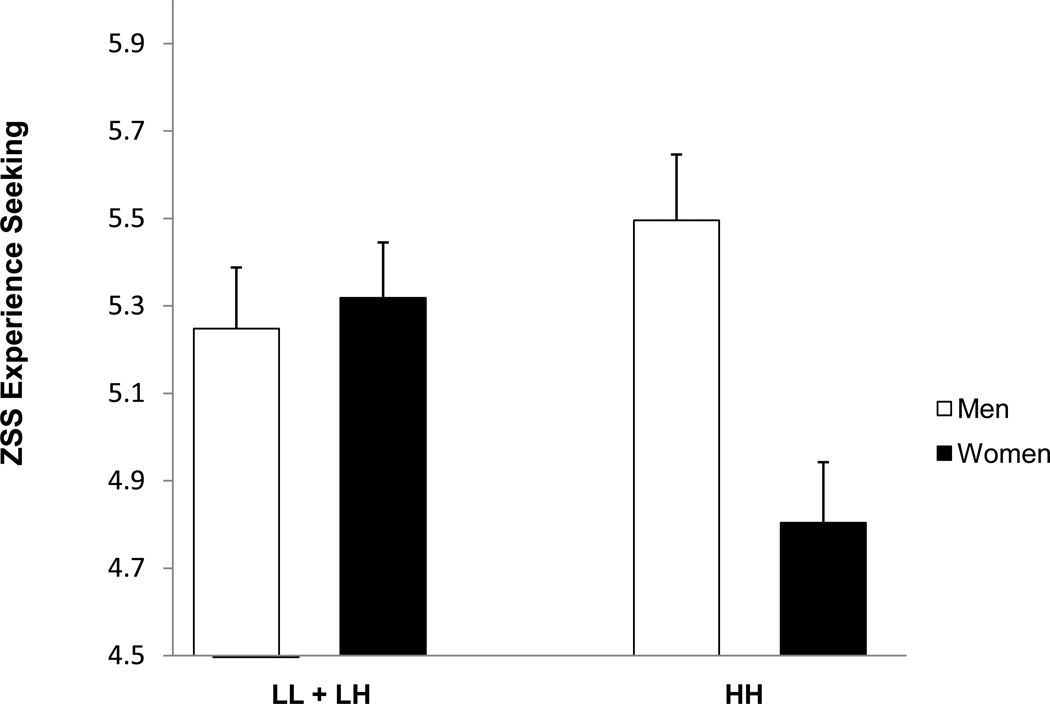
Follow up analyses conducted on the subscales of the Sensation Seeking Scale revealed a main effect of genotype group for the Disinhibited Behavior subscale (F1,1013 =4.51, p.=.03), indicating that people with the LL or LH genotype reported a preference for engaging in disinhibited behavior (See Table 2). Because this subscale includes items reflecting a preference for alcohol use in addition to engaging in other “disinhibited” behaviors, such as sexual activity, partying and getting high, the subscale was rescored by removing the 3 items that refer explicitly to alcohol use. Analyses were repeated using the rescored scale and revealed that the relationship was, in fact, strengthened when the scale did not include these items (F1,1013 =6.47, p.=.01). It was interesting to note, moreover, that the genotype group difference for Disinhibited Behavior was qualified by a genotype by alcohol dependence history interaction (F1,1013 =3.89, p.=.05), such that people who had the L allele and had a history of alcohol dependence reported the highest level of Disinhibited Behavior. This relationship is depicted in Figure 1. Analyses using the rescored scale also showed a significant interaction, (F1,1013 =5.34, p.=.02). Finally, an interaction between sex and genotype was also observed for the experience seeking subscale (F1,1013 =5.38, p.=.02) and follow up analyses indicated that the PDYN genotype was associated with experience seeking in women, but not men. Women with an L allele reported higher experience seeking than women with homozygous HH genotype.
Table 2.
Mean Scores (± standard error of the mean) for Zuckerman sensation seeking subscale scores as a function of genotype, gender and lifetime prevalence of DSM-IV alcohol dependence
| Thrill & Adventure Seeking |
Excitement Seeking |
Boredom Susceptibility |
Disinhibited Behavior |
|||
|---|---|---|---|---|---|---|
| n | ||||||
| Genotype | LL + LH | 524 | 5.96 (.21) | 5.70 (.16) | 2.13 (.12) | 4.07 (.17) |
| HH | 497 | 5.65 (.22) | 5.46 (.17) | 2.26 (.13) | 3.55 (.18)* | |
| Gender | Male | 496 | 6.31(.16) | 5.66 (.12) | 2.39 (.09) | 4.59 (.13) |
| Female | 525 | 5.30 (.26)** | 5.51 (.19) | 2.00 (.15)* | 3.03 (.21)*** | |
| Alcohol Dependence | AD− | 870 | 5.20 (.10) | 5.06 (.08) | 1.92 (.06) | 3.16 (.08) |
| AD+ | 151 | 6.41 (.29)*** | 6.11 (.21)*** | 2.47 (.17)*** | 4.45 (.23)*** |
p < .05
p ≤.01
p < .001
Figure 1.
Zuckerman Sensation Seeking subscale Disinhibited Behavior varies by DSM-IV Alcohol Dependence diagnosis and PDYN genotype.
Impulsiveness and Sensation Seeking: Gender and alcohol dependence
Men reported higher sensation seeking than women (F1,1013 =22.05, p.<.0001) and analyses on the sensation seeking subscales showed that men endorsed more thrill and adventure seeking (F1,1013 =11.23, p.=.01), boredom susceptibility (F1,1013 =5.00, p.=.03) and disinhibited behavior (F1,1013 =40.23, p.<.0001). Men and women did not differ in their reports of experience seeking (p.=52). As expected, lifetime alcohol dependence was associated with higher sensation seeking, including all subscales (F’s > 9.63, p’s ≤.002). Table 2 presents descriptive information about the relationship of gender and alcohol dependence diagnosis to Zuckerman Sensation Seeking scale subscale scores.
Discussion
Results showed that while there was no relationship between a polymorphic marker in the PDYN gene and categorical diagnoses of alcohol dependence, we observed an association between the LL or LH PDYN genotype and a preference for engaging in disinhibited behavior. Moreover, people who had one or two copies of the L allele had both a history of alcohol dependence and reported the highest level of Disinhibited Behavior. The findings reported here suggest that variation in the PDYN gene is associated with a dimensional trait or intermediate phenotype that reflects a preference for disinhibited behavior, and that this association is moderated by a lifetime history of alcohol dependence. It is notable that items on this scale reflect a preference for having “new and exciting” experiences with respect to alcohol use, but also extends to other drug use and sexual activity, suggesting a phenotype that underlies other types of risky behaviors. Notably, when the items reflecting alcohol use were removed from the subscale score, the results were unchanged, or even, strengthened.
Previous research has been mixed with respect to whether “low” or “high” expressing alleles are associated with dependence disorders. Indeed, it is possible to make contradictory predictions regarding the relationship between PDYN levels and dependence. On the one hand, low levels of dopamine release (H PDYN) may lead to compensatory behaviors (i.e., alcohol use), which have the result of increasing dopamine activity (Bruijnzeel, 2009). Alternatively, high dopamine release (L PDYN) associated with a strong reward may predictably increase the occurrence or likelihood of behaviors resulting in reward (i.e., alcohol use). Our results, showing an association between the “low” expressing allele and disinhibited behavior support the latter model and are consistent with work by Yuferov and colleagues (2008) who observed lower PDYN expression in the brains of people who had been addicted to cocaine and/or alcohol.
Like other drugs of abuse, ethanol increases the activity of dopaminergic neurons in the mesolimbic system (e.g. Trigo et al., 2010). As noted above, dynorphin is thought to diminish the increases in dopamine that follow alcohol intake, and does so by binding to KORs. Likewise, the KOR agonist U50,488H blocked the rewarding effect of ethanol in mice in place conditioning tasks (Logrip et al., 2009), and significantly reduced ethanol drinking in rats under a two-bottle choice paradigm (Lindholm et al., 2001). These findings support a role for the KOR ligand dynorphins in diminishing the rewarding effects of alcohol via interference with dopaminergic pathways of reinforcement. Consistent with this idea, pharmacologically blocking KORs increases ethanol intake (Mitchell et al., 2005). Thus, people with L activity alleles would be expected to have diminished dynorphin and less of an inability to attenuate surges in dopamine following alcohol use and rewarding behaviors. People with the L allele may be thus prone to develop dependence or otherwise seek dopamine releasing stimuli. Here we show that this allele is associated with a preference for disinhibited behavior across a range of behaviors (e.g., drug use, alcohol use, partying, and sexual behavior).
The results reported here are based on cross sectional data collected in a sample of middle aged adults. Thus, we are not able to make a causal interpretation of the results, for example, concluding that disinhibited behavior in association with the L allele confers risk for developing alcohol dependence. An alternative explanation is that the combination of alcohol dependence and the “risky” allele leads to increases in disinhibited behavior. In support of the latter view, recent research suggests that sensation seeking is not a stable trait among all people, increasing in association with increases in substance use behaviors among a subgroup of adolescents (Lynne-Landsman et al. 2011) or even possibly “normalizing” among long term abstinent alcoholics (Fein, Sclafani & Finn, 2010). Both directional explanations are plausible and can only be tested in opposition to each other in longitudinally collected data with people who have not yet developed an alcohol use disorder. This is also the first report of an association between variation in the PDYN gene and a dimensional phenotype reflecting a personality trait and thus, requires replication in independent samples. Finally, an additional limitation of the analyses reported here is that number of people with alcohol dependence in the sample was relatively small, particularly when considered by gender, which reduced the statistical power to examine whether the L allele exerts a dominant rather than additive effect. We note, however, the number of people in the sample with alcohol dependence is in accord with previously published research with the PDYN gene.
Sex differences in association with the PDYN genotype were also observed for the total sensation seeking score and the experience seeking subscale without respect to alcohol dependence diagnosis, suggesting that the PDYN genotype might be important for some sensation seeking phenotypes in women, but not men. Clarke et al (2009) reported that three SNPs in the PDYN gene were associated with opioid dependence in females only, but the functionality of these genetic variants is unknown making it difficult to compare the results to the current findings. The interaction effect involving gender that was observed was unexpected and there is no clear explanation for the effect. For women, the results paralleled the findings with the Disinhibited Behavior scale, but men with HH genotype did not differ from men with an L allele on experience seeking. One potential explanation for the lack of effect in men stems from the fact that the alcohol dependent men in our sample had a high rate of comorbid drug use disorders and it is possible that such comorbid drug use alters the rewarding effects of experience seeking. Regardless of gender differences in prior substance use in our sample, however, sex differences in the KORs are well documented with regard to noiciception and analgesia (e.g., Bodnar & Kest, 2009) and have been implicated in sex differences in prodynorphin mRNA in rats exposed to cocaine (Torres-Reveron, Hurd, & Dow-Edwards, 2007) and NMDA-mediated dopamine release in mice, together with sex differences in the ability of a kappa-opioid agonist to inhibit this release (Sershen et al., 1998). Sex differences in the prevalence of alcohol use disorders and sensation seeking (Cross, Copping, & Campbell, 2011) are reliably observed in the population. However, given that the current sample included only a small number of women who carried an alcohol dependence diagnosis, firm conclusions regarding the interpretation of the results reported here cannot be drawn and further examination of gender differences in association with variation in the PDYN gene is warranted.
In sum, we reported a relationship between a genetic variant in the PDYN gene and a preference for engaging in disinhibited behavior in association with alcohol dependence. Identifying such interactions within the complex architecture underlying vulnerability to alcohol dependence advances our understanding of predispositions to addiction, which may in turn contribute to personalizing prevention and treatment strategies for addictive disorders.
Highlights.
Prior research shows inconsistent relationships between variants in the prodynorphin (PDYN) gene and substance dependence disorders.
A 68 bp sequence in the core promoter region of the PDYN gene was genotyped in a community sample of 1021 adults aged 30–45. Fifteen percent of the sample was alcohol dependent.
The results of the current analyses present preliminary evidence of an association between putatively functional variation in the prodynorphin (PDYN) gene and alcohol dependence but only in association with a dimensional phenotype.
No association between the PDYN polymorphism and alcohol dependence, but there was an association between the “low” (L) expressing allele of the PDYN gene and a preference for engaging in disinhibited behavior.
Additionally, people who had both a history of alcohol dependence and higher scores on this scale were most likely to carry an L allele.
These results indicate that variation in the PDYN gene is associated with a dimensional trait or intermediate phenotype that reflects a preference for heavy drinking and engaging in related risky behaviors (e.g., drug use, sexual activity).
Figure 2.
Zuckerman Sensation Seeking subscale Experience Seeking varies by gender and PDYN genotype.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babbitt CC, Silverman JS, Haygood R, Reininga JM, Rockman MV, Wray GA. Multiple functional variants in cis modulate PDYN expression. Mol. Biol. Evol. 2010;27:465–479. doi: 10.1093/molbev/msp276. [DOI] [PubMed] [Google Scholar]
- Barratt E. Impulsiveness subtraits: Arousal and information processing. In: Spence JT, Izard CE, editors. Motivation, Emotion and Personality. Amsterdam: Elsevier Science Publishers; 1985. pp. 137–146. [Google Scholar]
- Bodnar RJ, Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: Central mechanisms of action and roles of gonadal hormones. Horm Behav. 2009 Sep 26; doi: 10.1016/j.yhbeh.2009.09.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Harris RA. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006;40:73–86. doi: 10.1016/j.alcohol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain Res Rev. 2009;62:127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, LaForge KS, Ho A, McHugh PF, Kellogg S, Bell K, Schluger RP, Leal SM, Kreek MJ. Potentially functional polymorphism in the promoter region of prodynorphin gene may be associated with protection against cocaine dependence or abuse. Am J Med Genet. 2002;114(4):429–435. doi: 10.1002/ajmg.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Krause K, Li T, Schumann G. An association of prodynorphin polymorphisms and opioid dependence in females in a Chinese population. Addict Biol. 2009;14(3):366–370. doi: 10.1111/j.1369-1600.2009.00151.x. [DOI] [PubMed] [Google Scholar]
- Claye LH, Unterwald EM, Ho A, Kreek MJ. Both dynorphin A(1–17) and [Des-Tyr1]dynorphin A(2–17) inhibit adenylyl cyclase activity in rat caudate putamen. J Pharmacol Exp Ther. 1996;277(1):359–365. [PubMed] [Google Scholar]
- Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137(1):97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- Dahl JP, Weller AE, Kampman KM, Oslin DW, Lohoff FW, Ferraro TN, O'Brien CP, Berrettini WH. Confirmation of the association between a polymorphism in the promoter region of the prodynorphin gene and cocaine dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;139B(1):106–108. doi: 10.1002/ajmg.b.30238. [DOI] [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103(9):1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Finn P. Sensation seeking in long-term abstinent alcoholics, treatment-naïve active alcoholics, and nonalcoholic controls. Alcohol Clin Exp Res. 2010 Jun;34(6):1045–1051. doi: 10.1111/j.1530-0277.2010.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of alcohol dependence. Hum Genet. 2009;126(1):91–99. doi: 10.1007/s00439-009-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Horikawa S, Takai T, Toyosato M, Takahashi H, Noda M, Kakidani H, Kubo T, Hirose T, Inayama S, Hayashida H, et al. Isolation and structural organization of the human preproenkephalin B gene. Nature. 1983;306(5943):611–614. doi: 10.1038/306611a0. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Vanyukov M, Tarter R. Detection of youth at high risk for substance use disorders: a longitudinal study. Psychol Addict Behav. 2005;19(3):243–252. doi: 10.1037/0893-164X.19.3.243. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Gauchy C, Desban M, Glowinski J, Kemel ML. Role of dynorphin and GABA in the inhibitory regulation of NMDA-induced dopamine release in striosome-and matrix-enriched areas of the rat striatum. J Neurosci. 1994;14(4):2435–2443. doi: 10.1523/JNEUROSCI.14-04-02435.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8(11):1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brené S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120(2):137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Blockade of ethanol reward by the kappa opioid receptor agonist U50,488H. Alcohol. 2009;43(5):359–365. doi: 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynne-Landsman SD, Graber JA, Nichols TR, Botvin GJ. Is sensation seeking a stable trait or does it change over time? J Youth Adolesc. 2011;40(1):48–58. doi: 10.1007/s10964-010-9529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology. 2005;182(3):384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Nikoshkov A, Drakenberg K, Wang X, Horvath MC, Keller E, Hurd YL. Opioid neuropeptide genotypes in relation to heroin abuse: dopamine tone contributes to reversed mesolimbic proenkephalin expression. Proc Natl Acad Sci U S A. 2008;105(2):786–791. doi: 10.1073/pnas.0710902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Ujike H, Tanaka Y, Otani K, Morita Y, Kishimoto M, Morio A, Harano M, Inada T, Yamada M, Komiyama T, Sekine Y, Iwata N, Sora I, Iyo M, Ozaki N, Kuroda S. Genetic variant of prodynorphin gene is risk factor for methamphetamine dependence. Neurosci Lett. 2006;400(1–2):158–162. doi: 10.1016/j.neulet.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Ray R, Doyle GA, Crowley JJ, Buono RJ, Oslin DW, Patkar AA, Mannelli P, DeMaria PA, Jr, O'Brien CP, Berrettini WH. A functional prodynorphin promoter polymorphism and opioid dependence. Psychiatr Genet. 2005;15(4):295–298. doi: 10.1097/00041444-200512000-00013. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Heidbreder C. Kappa-opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36(1):S5–S14. [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Rev Clin Psychol. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt impulsiveness scale: An update and review. Pers Indiv Diff. 2009;47:385–395. [Google Scholar]
- Svensson P, Hurd YL. Specific reductions of striatal prodynorphin and D1 dopamine receptor messenger RNAs during cocaine abstinence. Brain Res Mol Brain Res. 1998 May;56(1–2):162–168. doi: 10.1016/s0169-328x(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Hurd YL, Dow-Edwards DL. Gender differences in prodynorphin but not proenkephalin mRNA expression in the striatum of adolescent rats exposed to prenatal cocaine. Neurosci Lett. 2007;421(3):213–217. doi: 10.1016/j.neulet.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Marin-Garcia E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108:183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-K opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Zhu YS, Lai JH, Xue HX, Chai ZQ, Li SB. Association between heroin dependence and prodynorphin gene polymorphisms. Brain Res Bulletin. 2011 doi: 10.1016/j.brainresbull.2011.02.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Williams TJ, LaForge KS, Gordon D, Bart G, Kellogg S, Ott J, Kreek MJ. Prodynorphin gene promoter repeat associated with cocaine/alcohol codependence. Addict Biol. 2007;12(3–4):496–502. doi: 10.1111/j.1369-1600.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- Xuei X, Flury-Wetherill L, Bierut L, Dick D, Nurnberger J, Jr, Foroud T, Edenberg HJ. The opioid system in alcohol and drug dependence: family-based association study. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(7):877–884. doi: 10.1002/ajmg.b.30531. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96:684–695. [PubMed] [Google Scholar]
- Yuferov V, Ji F, Nielsen DA, Levran O, Ho A, Morgello S, Shi R, Ott J, Kreek M. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology. 2009;34(5):1185–1197. doi: 10.1038/npp.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1–17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psycholpharmacology (Berl) 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Kraus J, Wöltje M, Mayer P, Rauch E, Höllt V. An allelic variation in the human prodynorphin gene promoter alters stimulus-induced expression. J Neurochem. 2000;74(2):472–477. doi: 10.1046/j.1471-4159.2000.740472.x. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimal level of arousal. Hillsdale, NJ: Lawrence Erlbaum Associates; 1979. [Google Scholar]