Abstract
Background
Intraarticular injections of corticosteroids combined with local anesthetics are commonly used for management of chronic pain symptoms associated with degenerative joint diseases and after arthroscopic procedures. Several studies suggest chondrotoxicity of local anesthetics whereas others report chondroprotective and cytotoxic effects of corticosteroids on cartilage. Given the frequency of use of these agents, it is important to know whether they are in fact toxic.
Questions/purposes
We asked whether (1) bupivacaine and triamcinolone acetonide, alone and combined, were chondrotoxic to chondrocytes in culture; (2) buffering of the reagents diminished toxicity of the bupivacaine and triamcinolone; and (3) the presence of the superficial layer of articular cartilage protects against toxicity.
Materials and Methods
We obtained cartilage from three patients undergoing arthroplasty. To address triamcinolone acetonide, bupivacaine, and combinatorial toxicity to human chondrocytes, we set up monolayer chondrocyte cultures (n = 8 wells per condition). The question of buffering was addressed by performing the same assays as above, but the reagents were buffered. An MTT assay was used to assess chondrocyte survival in the monolayer. We harvested 21 articular plugs from each of three patients (total 63 plugs) and exposed them to the same reagents as above, including the buffered reagents. A Live/Dead assay was used to determine chondrocyte survival.
Results
Triamcinolone acetonide, bupivacaine, and their combination were toxic to human chondrocytes in the monolayer comparisons. The addition of buffering did not mitigate chondrocyte death. With the intact superficial layer in the plug group, bupivacaine was not toxic as compared with for the control group; all the other reagents (triamcinolone, combination bupivacaine/triamcinolone, buffered bupivacaine, buffered triamcinolone, and buffered combination) produced chondrotoxicity.
Conclusions
Triamcinolone induced chondrotoxicity in the articular plug and monolayer culture, whereas bupivacaine induced chondrotoxicity only in monolayer culture. The combined used of triamcinolone and bupivacaine did not show additive chondrocyte death in any arm. Buffering of bupivacaine increased its chondrotoxicity.
Clinical Relevance
Although not necessarily reflecting in vivo conditions, our data suggest physicians should be cognizant of the potential in vitro chondrotoxicity of bupivacaine and triamcinolone when contemplating intraarticular administration.
Introduction
Intraarticular injections of corticosteroids, combined with local anesthetics, are commonly used for treating pain in patients with degenerative joint diseases and postoperatively [28]. Clinical studies generally show corticosteroids help control short-term pain in patients with degenerative joints [3, 13, 21, 26]. Triamcinolone, a commonly used intraarticular corticosteroid [8, 42], is clinically nondeleterious [37]. Although some studies show slowing of mechanically and chemically induced degeneration on a gross level [33, 34, 43], others have found histologic evidence of chondrotoxicity from corticosteroids in experimental models [7, 19, 32, 39]. However, these studies did not evaluate buffering of the reagents to a physiologic pH and they were not performed on human cartilage. Also, there is heightened concern of potential chondrotoxicity of local anesthetics, with several case reports of patients having glenohumeral chondrolysis develop after placement of continuous intraarticular bupivacaine infusion [23, 25, 29, 35]. Additionally, multiple in vitro studies suggest a cytotoxic effect of bupivacaine on articular chondrocytes [1, 11, 12, 27, 36]. Some authors have speculated on the potential chondrotoxicity of the local anesthetics from their acidic pH effect alone [16, 22], although buffered local anesthetics have not been tested on articular cartilage directly. None of these prior studies examined the combined effects of local anesthetics and corticosteroids on human articular cartilage.
We asked whether (1) bupivacaine and triamcinolone acetonide, alone and combined, were chondrotoxic to chondrocytes in culture; (2) buffering of the reagents diminished toxicity of the bupivacaine and triamcinolone; and (3) the presence of the superficial layer of articular cartilage protects against toxicity.
Materials and Methods
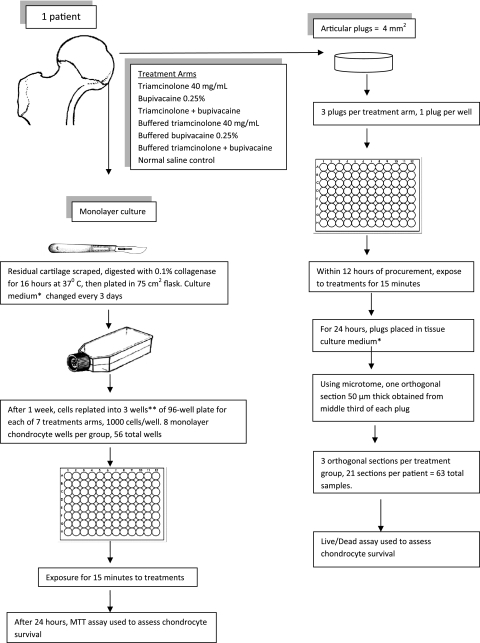
We collected human articular cartilage from the femora of four patients and the humeral head of one patient undergoing hemiarthroplasty for fractures. Two of the patient samples (one femoral head and humeral head) could not be processed owing to prolonged time from the operating room to the laboratory. We excluded patients with severe degenerative joint disease, history of intraarticular local anesthetic or steroid injections in the joint being replaced, or those who were on chronic systemic corticosteroid regimens. Institutional review board approval was obtained for harvesting of patient cartilage. The articular plug and monolayer chondrocyte assays were established from each patient (Fig. 1).
Fig. 1.
The flow diagram shows the methodology of setting up the articular plug and monolayer chondrocyte assay from the femoral articular cartilage of one patient. A total of three patients were used and the cartilage from each patient was kept separate. * Dulbecco’s Modified Eagle Medium/F12, 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% fungizone and were kept in an incubator at 37°C with 5% CO2; **except one patient who had enough cartilage for two wells per treatment arm.
To address triamcinolone acetonide toxicity to human chondrocytes, we set up monolayer chondrocyte cultures. We harvested the residual articular cartilage left behind on the femoral head after the articular plugs were removed. This remaining cartilage then was scraped off the femoral head using a scalpel and sharply minced into small pieces. Each patient’s cartilage was kept separate and was not mixed in creating the chondrocyte culture. We digested the minced cartilage in sterile 0.1% collagenase for 16 hours at 37°C. The chondrocytes initially were plated in a 75 cm3 flask in fresh tissue culture media (at a density of 104 cells/cm2) and maintained in an incubator at 37°C with 5% CO2. During this time the tissue culture medium (Dulbecco’s Modified Eagle Medium/F12, 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% fungizone) was changed every 3 days. Cells were allowed to digest for 1 week; one of the authors (LG) visually inspected the chondrocytes, using phase microscopy to verify that the chondrocytes in the monolayer cultures consisted of differentiated chondrocytes based on morphologic features of the cell. The method was based on that described by Piper and Kim [36]. The chondrocytes then were transferred to a 96-well plate 24 hours before experimental treatment with a cell density of 1000 per well and eight wells per condition. Each patient’s chondrocytes were replated in three wells, except for those of one patient who had only enough chondrocytes for two wells per treatment arm. We aspirated the culture medium, added 40 mg/mL triamcinolone acetonide (Kenalog®-40, Bristol-Meyers Squibb, Princeton, NJ, USA) in a 1:4 dilution to each well for 15 minutes, during which we incubated the samples in 5% CO2 at 37°C, aspirated the treatment solution, rinsed the cells with normal saline, and added fresh culture medium. Twenty-four hours after exposure, we used a colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay to assess chondrocyte viability.
To determine bupivacaine toxicity to human chondrocytes, we performed the same procedure as above, except that the treatment solution was bupivacaine 0.25% (Marcaine®, Hospira Inc, Lake Forest, IL, USA), which was used without additional dilution. To assess combinatorial toxicity, the same procedure was performed on the monolayer using a 1:4 triamcinolone to bupivacaine ratio in the combination arms (0.04 mL triamcinolone to 0.16 mL bupivacaine). The question of buffering was addressed by performing the same assays as above, but the reagents were buffered. This includes bupivacaine, triamcinolone, and combination bupivacaine/triamcinolone in 1:4 ratio, all buffered to the physiologic pH of synovial fluid at pH 7.4 [14], using a minute amount of 1 N sodium hydroxide.
As the monolayer is without the specific architecture as in the native joint, we also harvested articular plugs, which preserves the superficial layer. This examines the effect of the superficial layer. For this part of the experiment, we obtained 21 full thickness 4 mm2 articular cartilage plugs from each of the three patient’s femoral heads cartilage using skin punch biopsy devices, for a total of 63 articular cartilage plugs. We maintained the articular cartilage plugs in tissue culture media (Dulbecco’s Modified Eagle Medium/F12, 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% fungizone) and, within 12 hours of procurement, exposed it to the same experimental treatments (control, triamcinolone, bupivacaine, combination, and buffered) for 15 minutes. We rinsed the articular cartilage plugs with normal saline before transferring them to new tissue culture media. After 24 hours, we used a vibratory microtome (Vibratome, St Louis, MO, USA) to obtain one 50-μm thick slice from the middle third of each articular plug, orientated perpendicular to the articular surface. One of us (LG) quantified the viability of chondrocytes by staining with calcein-AM and ethidium homodimer-1 (EthD-1) in the live/dead viability/cytotoxicity kit (Molecular Probes, West Eugene, OR). Concentrations of dye were modified from the manufacturer’s protocol to optimize the sensitivity and specificity of the assay. An Axiocam digital camera captured fluorescence images (Carl Zeiss, Thornwood, NY, USA). The area for analysis was 1.0 mm deep from the articular surface and 2.0 mm wide. We performed semiautomated data collection and analysis using Adobe Photoshop (Adobe Systems Inc, San Jose, CA, USA) and a public domain Java image processing program (ImageJ; National Institutes of Health, Bethesda, MD, USA).
We performed ANOVA and post hoc pairwise comparison against the control to compare the chondrocyte viability for the means of the different treatment arms. Relative chondrocyte viability was defined as the mean luminescence of treated cells divided by the mean luminescence of the normal saline control cells. We used NCSS Statistical & Power Analysis (Software, Kaysville, UT, USA).
Results
Relative chondrocyte viability of the monolayer culture was measured after exposure to the control and treatment reagents (Table 1). Triamcinolone acetonide is toxic to human chondrocytes as seen in the monolayer comparison. Furthermore, bupivacaine also exhibited substantial toxicity. The combination thereof also appeared chondrotoxic. Additionally, buffering of the reagents did not mitigate chondrotoxic effects.
Table 1.
Monolayer
| Treatment arms | Relative viability | p Value |
|---|---|---|
| Normal saline control | 100.0 ± 9.8% | |
| Triamcinolone 40 mg/mL | 14.5 ± 1.8% | < 0.001 |
| Bupivacaine 0.25% | 71.8 ± 13.5% | < 0.001 |
| Triamcinolone + bupivacaine | 13.7 ± 1.0% | < 0.001 |
| Buffered triamcinolone 40 mg/mL | 14.0 ± 1.1% | < 0.001 |
| Buffered bupivacaine 0.25% | 22.2 ± 0.1% | < 0.001 |
| Buffered triamcinolone + bupivacaine | 13.3 ± 0.6% | < 0.001 |
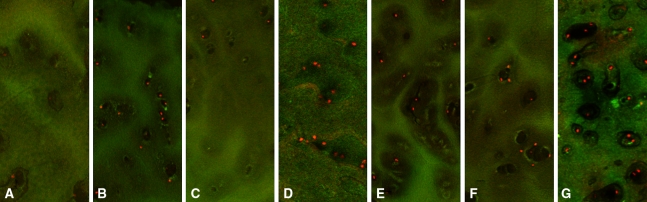
The intact superficial layer present in the articular plug assay was protective against the treatment reagents (Table 2). The bupivacaine arm of the articular plug group, in which the superficial layer was intact, did not show a difference in chondroctye death compared with the control group (Fig. 2); whereas in the monolayer culture, the bupivacaine arm showed significantly more chondrocyte death than the control arm. In addition, the magnitude of chondrocyte death was greater in all the monolayer arms as compared with their corresponding treatment arms in the articular plug group, again suggesting that the superficial layer may provide some protection.
Table 2.
Articular plugs
| Treatment arms | Relative viability | p Value |
|---|---|---|
| Normal saline control | 100.1 ± 3.7% | |
| Triamcinolone 40 mg/mL | 66.1 ± 16.3% | < 0.001 |
| Bupivacaine 0.25% | 101.0 ± 17.9% | 0.88 |
| Triamcinolone + bupivacaine | 54.9 ± 13.1% | < 0.001 |
| Buffered triamcinolone 40 mg/mL | 53.9 ± 19.4% | < 0.001 |
| Buffered bupivacaine 0.25% | 76.3 ± 21.8% | 0.003 |
| Buffered triamcinolone + bupivacaine | 54.3 ± 8.6% | < 0.001 |
Fig. 2A–G.
Articular plug fluorescence microscopy stained with Live/Dead assay is shown after 24 hours after 15-minute treatments with (A) normal saline, (B) triamcinolone 40 mg/mL, (C) bupivacaine 0.25%, (D) triamcinolone and bupivacaine, (E) buffered triamcinolone, (F) buffered bupivacaine, and (G) buffered combination.
Discussion
The widespread use of corticosteroids and local anesthetics for joint disease is one reason there has been concern regarding potential toxicity to articular cartilage. In vitro studies suggest chondrotoxicity of local anesthetics and corticosteroids in various animal models [11, 12, 22, 27, 32, 41]. However, combinational effects of corticosteroids and local anesthetics or the buffering of these agents have not been studied on human cartilage. We asked whether (1) bupivacaine and triamcinolone acetonide, alone and combined, were chondrotoxic to chondrocytes in culture; (2) buffering of the reagents diminished toxicity of the bupivacaine and triamcinolone; and (3) the presence of the superficial layer of articular cartilage protects against toxicity.
Limitations of our study include the following. First, our studies were performed in vitro and the observations cannot necessarily be applied directly in vivo; therefore they would need to be confirmed clinically. Second, as we used one concentration and exposure duration for triamcinolone and bupivacaine, our findings may be difficult to apply to in vivo conditions given the potential dilutional effects in the joint. Third, even though dose effects on cells may be different between the in vivo and in vitro settings, we attempted to use a clinically relevant dose [18, 37, 42] and a typical one to four, corticosteroid to anesthetic ratio; however, we recognize dosing variability exists among physicians [6, 40, 42]. Fourth, although using human articular cartilage introduces patient variability, we controlled for this by determining relative chondrocyte viability of each patient’s experimentally treated cartilage against their own normal, saline-treated cartilage. Further experimentation with different durations and longer end points may uncover other dose/time-dependent responses to triamcinolone, similar to those reported regarding the chondrotoxicity of local anesthetics, which are time- and dose-dependent [11, 27]. In a human and bovine chondrocyte alginate bead culture study, bupivacaine caused a time- and dose-dependent loss of chondrocyte viability [11]. In our investigation, chondrocyte viability decreased in the bupivacaine arm when compared with the control arm in the chondrocyte monolayer culture group, whereas there was essentially no difference in viability between the bupivacaine and control arms in the articular plug group. At first glance, the difference in chondrocyte viability in the articular cartilage plug and monolayer culture groups treated with unbuffered bupivacaine appears different than that reported in previous studies [30, 36]. However, the difference is likely attributable to the dose and length of exposure of the bupivacaine in our study. Although we initially chose higher concentrations and longer exposure times, meaningful comparison was not possible between groups owing to near-complete chondrocyte loss. Therefore, we chose exposure times of 15 minutes and 0.25% bupivacaine strength. Our study’s trends in the monolayer culture are similar to the 24-hour chondrocyte viability reported in a previous study using 0.25% bupivacaine exposure for 15 minutes [11].
Like other studies [7, 31], we found that triamcinolone exhibits chondrotoxicity. However, three systematic reviews suggest that, in knee osteoarthritis, corticosteroids, when compared with placebo, have short-term clinical relief lasting between 1 and 4 weeks [2, 3, 26]. In a randomized, double-blind, placebo controlled trial, repeated triamcinolone acetonide 40-mg knee injections every 3 months for 2 years had no deleterious radiographic changes, and patients trended toward greater symptom improvements at 1 year [37]. An in vivo canine osteoarthritis model found triamcinolone hexacetonide reduced osteophyte formation, histologic severity of the cartilage lesions, and amounts of metalloproteinase stromelysin [33]. However, other studies [31, 32] have shown cytotoxic effects of corticosteroids. In rabbit knees, repeated intraarticular hydrocortisone injections with different doses resulted in degradation of the cartilage in a dose-dependent fashion [32]. Single dosing of triamcinolone acetonide can cause substantial chondrocyte apoptosis in implanted human cartilage in severe, combined immune-deficient mice, and in a human chondrocyte culture [31]. In the current study where a single dose was used, our findings are consistent with those reported in these studies, showing that triamcinolone treatment caused substantial loss of chondrocyte viability. Although clinical studies in humans do not show deleterious effects of the corticosteroids radiographically or symptomatically, the histologic effects on the cartilage were not examined.
In the presence of corticosteroids, the addition of local anesthetics can affect chondrocyte viability. In a study evaluating equine articular cartilage exposed to known chondrotoxic agents, cartilage treated with triamcinolone acetonide showed higher chondrocyte viability when compared with the local anesthetic mepivacaine. In cartilage treated with triamcinolone acetonide and mepivacaine, chondrocyte viability was intermediate between the mepivacaine-only and triamcinolone acetonide-only groups. The investigators concluded that triamcinolone acetonide attenuated the chondrotoxicity of mepivacaine [5]. In contrast, a study using a bovine cartilage found that, although the methylprednisolone showed time- and dose-dependent chondrotoxic effects, the combination of lidocaine and methylprednisolone showed a synergistic chondrocidal effect; there was no single-treatment lidocaine group for direct comparison to methylprednisolone [41]. In our study on human cartilage, triamcinolone caused a substantial loss of chondrocyte viability in the articular plug and monolayer culture groups and was more chondrotoxic than bupivacaine. Addition of bupivacaine to triamcinolone did not synergize chondrocyte loss. The differences in our observations compared with those of the others may be multifactorial. Generalization regarding nonprimate cartilage used in the above studies of human cartilage may not be possible [20]. The cytoarchitecture of cartilage in rabbit medial femoral condyles, for example, has a much higher density of chondrocytes when compared with human cartilage [17]. Also, different corticosteroids and local anesthetics were used in experiments in the above-mentioned, making it to difficult to distinguish the individual effects of the drugs.
Buffering of bupivacaine to a pH of 7.4 resulted in more chondrocyte death in the monolayer culture and articular cartilage plug groups when compared with the unbuffered bupivacaine groups. Buffering of local anesthetics is a recommended way of reducing pain for intradermal injections [9, 10, 15, 24], although one study evaluating buffering of intraarticular prilocaine for pain control was inconclusive regarding its efficacy [38]. Others have considered whether the acidic pH of the solutions was partly responsible for its toxicity. Anesthetics containing epinephrine had lower pHs and consistently showed chondrotoxicity, although the increased cell death could be attributed to other factors, such as preservatives [16]. Another in vitro investigation found no difference in chondrocyte viability after exposure to saline solutions with pH of 5.0, 7.0, and 7.4 [27]. When buffering the bupivacaine solutions used in our study, these solutions became slightly cloudy, indicating the formation of a free base precipitate of bupivacaine [4]. The bupivacaine free base may be more chondrotoxic than the soluble form. From the toxicity we observed, alkalinization of intraarticular bupivacaine cannot be recommended.
The major difference between the chondrocyte environment in the articular plug group and monolayer cultures is that the chondrocytes in the plug are surrounded by a more similar cytoarchitecture as in a native joint and the superficial layer is intact. We observed no difference in chondrotoxicity between the control and bupivacaine arms in the articular plug group as was the case in the monolayer group. In addition, the magnitude of chondrocyte death between the corresponding treatment arms between the articular plug and monolayer groups compared with the respective control arms was less in the articular plug group. In a previous study that examined chondrocyte viability in bovine articular cartilage exposed to bupivacaine, an intact articular surface was associated with greater chondrocyte viability [11]. The authors suggested that this may be attributable to a “partial barrier” that the intact superficial layer of cartilage affords. Our observations are similar to those in that study.
Our observations show substantial chondrotoxicity of triamcinolone in articular cartilage plug and monolayer models. Viability decreased in the chondrocyte monolayer with exposure to bupivacaine, although the combined used of triamcinolone and bupivacaine did not increase chondrotoxicity. Buffering of bupivacaine cannot be recommended given the increased chondrotoxicity observed. As corticosteroids are commonly administered intraarticularly with local anesthetics, further studies are needed to evaluate the effects of these agents on cartilage.
Acknowledgments
We thank Drs. Thomas Donaldson, Alan Afsari, and Wesley Phipatanakul for providing tissue samples for this study.
Footnotes
One or more of the authors (HMS) received funding from grant of the Arthroscopy Association of North America (AANA). Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Anz A, Smith MJ, Stoker A, Linville C, Markway H, Branson K, Cook JL. The effect of bupivacaine and morphine in a coculture model of diarthrodial joints. Arthroscopy. 2009;25:225–231. doi: 10.1016/j.arthro.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869. doi: 10.1136/bmj.38039.573970.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;19:CD005328. doi: 10.1002/14651858.CD005328.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Bigeleisen PE, Wempe M. Identification of the precipitate in alkalinized solutions of mepivacaine and bupivacaine at 37 degrees C. J Clin Pharm Ther. 2001;26:171–173. doi: 10.1046/j.1365-2710.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- 5.Bolt DM, Ishihara A, Weisbrode SE, Bertone AL. Effects of triamcinolone acetonide, sodium hyaluronate, amikacin sulfate, and mepivacaine hydrochloride, alone and in combination, on morphology and matrix composition of lipopolysaccharide-challenged and unchallenged equine articular cartilage explants. Am J Vet Res. 2008;69:861–867. doi: 10.2460/ajvr.69.7.861. [DOI] [PubMed] [Google Scholar]
- 6.Cardone DA, Tallia AF. Diagnostic and therapeutic injection of the hip and knee. Am Fam Physician. 2003;67:2147–2152. [PubMed] [Google Scholar]
- 7.Céleste C, Ionescu M, Robin Poole A, Laverty S. Repeated intraarticular injections of triamcinolone acetonide alter cartilage matrix metabolism measured by biomarkers in synovial fluid. J Orthop Res. 2005;23:602–610. doi: 10.1016/j.orthres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Centeno LM, Moore ME. Preferred intraarticular corticosteroids and associated practice: a survey of members of the American College of Rheumatology. Arthritis Care Res. 1994;7:151–155. doi: 10.1002/art.1790070309. [DOI] [PubMed] [Google Scholar]
- 9.Cheney PR, Molzen G, Tandberg D. The effect of pH buffering on reducing the pain associated with subcutaneous infiltration of bupivicaine. Am J Emerg Med. 1991;9:147–148. doi: 10.1016/0735-6757(91)90177-L. [DOI] [PubMed] [Google Scholar]
- 10.Christoph RA, Buchanan L, Begalla K, Schwartz S. Pain reduction in local anesthetic administration through pH buffering. Ann Emerg Med. 1988;17:117–120. doi: 10.1016/S0196-0644(88)80293-2. [DOI] [PubMed] [Google Scholar]
- 11.Chu CR, Izzo NJ, Coyle CH, Papas NE, Logar A. The in vitro effects of bupivacaine on articular chondrocytes. J Bone Joint Surg Br. 2008;90:814–820. doi: 10.2106/JBJS.G.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu CR, Izzo NJ, Papas NE, Fu FH. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693–699. doi: 10.1016/j.arthro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Cole BJ, Schumacher HR., Jr Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13:37–46. doi: 10.5435/00124635-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Cummings NA, Nordby GL. Measurement of synovial fluid pH in normal and arthritic knees. Arthritis Rheum. 1966;9:47–56. doi: 10.1002/art.1780090106. [DOI] [PubMed] [Google Scholar]
- 15.Davies RJ. Buffering the pain of local anaesthetics: a systematic review. Emerg Med (Fremantle) 2003;15:81–88. doi: 10.1046/j.1442-2026.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- 16.Dragoo JL, Korotkova T, Kanwar R, Wood B. The effect of local anesthetics administered via pain pump on chondrocyte viability. Am J Sports Med. 2008;36:1484–1488. doi: 10.1177/0363546508318190. [DOI] [PubMed] [Google Scholar]
- 17.Eggli PS, Hunziker EB, Schenk RK. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat Rec. 1988;222:217–227. doi: 10.1002/ar.1092220302. [DOI] [PubMed] [Google Scholar]
- 18.Frías G, Caracuel MA, Escudero A, Rumbao J, Pérez-Gujo V, del Carmen Castro M, Font P, Gonzalez J, Collantes E. Assessment of the efficacy of joint lavage versus joint lavage plus corticoids in patients with osteoarthritis of the knee. Curr Med Res Opin. 2004;20:861–867. doi: 10.1185/030079904125003656. [DOI] [PubMed] [Google Scholar]
- 19.Fubini SL, Todhunter RJ, Burton Wurster N, Vernier-Singer M, Macleod JN. Corticosteroids alter the differentiated phenotype of articular chondrocytes. J Orthop Res. 2001;19:688–695. doi: 10.1016/S0736-0266(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 20.Gibson T, Burry HC, Poswillo D, Glass J. Effect of intra-articular corticosteroid injections on primate cartilage. Ann Rheum Dis. 1977;36:74–79. doi: 10.1136/ard.36.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godwin M, Dawes M. Intra-articular steroid injections for painful knees: systematic review with meta-analysis. Can Fam Physician. 2004;50:241–248. [PMC free article] [PubMed] [Google Scholar]
- 22.Gomoll AH, Kang RW, Williams JM, Bach BR, Cole BJ. Chondrolysis after continuous intra-articular bupivacaine infusion: an experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813–819. doi: 10.1016/j.arthro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Greis PE, Legrand A, Burks RT. Bilateral shoulder chondrolysis following arthroscopy: a report of two cases. J Bone Joint Surg Am. 2008;90:1338–1344. doi: 10.2106/JBJS.G.01004. [DOI] [PubMed] [Google Scholar]
- 24.Hanna MN, Elhassan A, Veloso PM, Lesley M, Lissauer J, Richman JM, Wu CL. Efficacy of bicarbonate in decreasing pain on intradermal injection of local anesthetics: a meta-analysis. Reg Anesth Pain Med. 2009;34:122–125. doi: 10.1097/AAP.0b013e31819a12a6. [DOI] [PubMed] [Google Scholar]
- 25.Hansen BP, Beck CL, Beck EP, Townsley RW. Postarthroscopic glenohumeral chondrolysis. Am J Sports Med. 2007;35:1628–1634. doi: 10.1177/0363546507304136. [DOI] [PubMed] [Google Scholar]
- 26.Hepper CT, Halvorson JJ, Duncan ST, Gregory AJ, Dunn WR, Spindler KP. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009;17:638–646. doi: 10.5435/00124635-200910000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621–1627. doi: 10.1177/0363546507304719. [DOI] [PubMed] [Google Scholar]
- 28.Koyonos L, Yanke AB, McNickle AG, Kirk SS, Kang RW, Lewis PB, Cole BJ. A randomized, prospective, double-blind study to investigate the effectiveness of adding DepoMedrol to a local anesthetic injection in postmeniscectomy patients with osteoarthritis of the knee. Am J Sports Med. 2009;37:1077–1082. doi: 10.1177/0363546508331204. [DOI] [PubMed] [Google Scholar]
- 29.Levy JC, Virani NA, Frankle MA, Cuff D, Pupello DR, Hamelin JA. Young patients with shoulder chondrolysis following arthroscopic shoulder surgery treated with total shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17:380–388. doi: 10.1016/j.jse.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Lo IK, Sciore P, Chung M, Liang S, Boorman RB, Thornton GM, Rattner JB, Muldrew K. Local anesthetics induce chondrocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25:707–715. doi: 10.1016/j.arthro.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Nakazawa F, Matsuno H, Yudoh K, Watanabe Y, Katayama R, Kimura T. Corticosteroid treatment induces chondrocyte apoptosis in an experimental arthritis model and in chondrocyte cultures. Clin Exp Rheumatol. 2002;20:773–781. [PubMed] [Google Scholar]
- 32.Papachristou G, Anagnostou S, Katsorhis T. The effect of intraarticular hydrocortisone injection on the articular cartilage of rabbits. Acta Orthop Scand Suppl. 1997;275:132–134. doi: 10.1080/17453674.1997.11744766. [DOI] [PubMed] [Google Scholar]
- 33.Pelletier JP, DiBattista JA, Raynauld JP, Wilhelm S, Martel-Pelletier J. The in vivo effects of intraarticular corticosteroid injections on cartilage lesions, stromelysin, interleukin-1, and oncogene protein synthesis in experimental osteoarthritis. Lab Invest. 1995;72:578–586. [PubMed] [Google Scholar]
- 34.Pelletier JP, Mineau F, Raynauld JP, Woessner JF, Jr, Gunja-Smith Z, Martel-Pelletier J. Intraarticular injections with methylprednisolone acetate reduce osteoarthritic lesions in parallel with chondrocyte stromelysin synthesis in experimental osteoarthritis. Arthritis Rheum. 1994;37:414–423. doi: 10.1002/art.1780370316. [DOI] [PubMed] [Google Scholar]
- 35.Petty DH, Jazrawi LM, Estrada LS, Andrews JR. Glenohumeral chondrolysis after shoulder arthroscopy: case reports and review of the literature. Am J Sports Med. 2004;32:509–515. doi: 10.1177/0363546503262176. [DOI] [PubMed] [Google Scholar]
- 36.Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986–991. doi: 10.2106/JBJS.G.01033. [DOI] [PubMed] [Google Scholar]
- 37.Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, Uthman I, Khy V, Tremblay JL, Bertrand C, Pelletier JP. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–377. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 38.Richmond CE. Alkalinization of local anaesthetic for intra-articular instillation during arthroscopy. Br J Anaesth. 1994;73:190–193. doi: 10.1093/bja/73.2.190. [DOI] [PubMed] [Google Scholar]
- 39.Robion FC, Doizé B, Bouré L, Marcoux M, Ionescu M, Reiner A, Poole AR, Laverty S. Use of synovial fluid markers of cartilage synthesis and turnover to study effects of repeated intra-articular administration of methylprednisolone acetate on articular cartilage in vivo. J Orthop Res. 2001;19:250–258. doi: 10.1016/S0736-0266(00)90008-1. [DOI] [PubMed] [Google Scholar]
- 40.Saunders S. Injection techniques: lower limb. In: Saunders S, editor. Injection Techniques in Orthopaedic and Sports Medicine. 2. Philadelphia, PA: WB Saunders; 2002. pp. 69–90. [Google Scholar]
- 41.Seshadri V, Coyle CH, Chu CR. Lidocaine potentiates the chondrotoxicity of methylprednisolone. Arthroscopy. 2009;25:337–347. doi: 10.1016/j.arthro.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skedros JG, Hunt KJ, Pitts TC. Variations in corticosteroid/anesthetic injections for painful shoulder conditions: comparisons among orthopaedic surgeons, rheumatologists, and physical medicine and primary-care physicians. BMC Musculoskelet Disord. 2007;8:63. doi: 10.1186/1471-2474-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams JM, Brandt KD. Triamcinolone hexacetonide protects against fibrillation and osteophyte formation following chemically induced articular cartilage damage. Arthritis Rheum. 1985;28:1267–1274. doi: 10.1002/art.1780281111. [DOI] [PubMed] [Google Scholar]