Abstract
Background
Soluble factors released from chondrocytes can both enhance and induce chondrocyte-like behavior in cocultured dedifferentiated cells. The ability to similarly prime and modulate biosynthetic activity of differentiated cells encapsulated in a three-dimensional environment is unknown.
Questions/purposes
To understand the effect of coculture on engineered cartilage, we posed three hypotheses: (1) coculturing with a monolayer of chondrocytes (“chondrocyte feeder layer”) expedites and increases engineered tissue growth; (2) expedited growth arises from paracrine effects; and (3) these effects are dependent on the specific morphology and expression of the two-dimensional feeder cells.
Methods
In three separate studies, chondrocyte-laden hydrogels were cocultured with chondrocyte feeder layers. Mechanical properties and biochemical content were quantified to evaluate tissue properties. Histology and immunohistochemistry stains were observed to visualize each constituent’s distribution and organization.
Results
Coculture with a chondrocyte feeder layer led to stiffer tissue constructs (Young’s modulus and dynamic modulus) with greater amounts of glycosaminoglycan and collagen. This was dependent on paracrine signaling between the two populations of cells and was directly modulated by the rounded morphology and expression of the feeder cell monolayer.
Conclusions
These findings suggest a potential need to prime and modulate tissues before implantation and present novel strategies for enhancing medium formulations using soluble factors released by feeder cells.
Clinical Relevance
Determining the soluble factors present in the coculture system can enhance a chondrogenic medium formulation for improved growth of cartilage substitutes. The feeder layer strategy described here may also be used to prime donor cartilage allografts before implantation to increase their success in vivo.
Introduction
Tissue-engineered articular cartilage substitutes with functional properties of native cartilage tissue are needed to survive the harsh joint-loading environment after implantation into damaged articular cartilage. To produce functional tissues, our laboratory has tested both the addition of chemical cues such as growth factors (transforming growth factor [TGF]-β3, TGF-β1, insulin-like growth factor, fibroblast growth factor [11, 28, 29, 41], corticosteroids [4, 9], and interleukins [5, 25, 33]) and mechanical stimuli including dynamic loading, hydrostatic pressure, and osmotic loading to encourage tissue growth and development [24, 27]. For example, sequential application of physiologic deformational loading after culturing with TGF-β3 yielded chondrocyte-seeded agarose constructs [34] with both stiffness and glycosaminoglycan (GAG) content comparable to native tissue after 42 days in culture [24].
Alternatively, endogenous chemical factors can be introduced to the culture system through the addition of a second population of cells. Underlying cell monolayers, or feeder layers, introduce paracrine signaling between populations of cells and cell-cell interactions that can modulate cellular activity [14, 20, 35, 37, 43]. In particular, primary chondrocytes induce chondrocyte differentiation of embryonic stem cells and cause redifferentiation of cocultured passaged chondrocytes by releasing soluble signaling factors. When cocultured, these highly plastic stem cells adopt a more rounded phenotype and show increased production of aggrecan and Type II collagen, characteristic of native chondrocytes [10, 17, 43]. Similarly, coculture systems including primary chondrocytes with passaged chondrocytes can also induce stable redifferentiation [1, 13, 40]. Reversion in phenotype of the passaged cells toward articular chondrocytes was confirmed by increased expression of Type II collagen and cartilage oligomeric matrix protein (COMP) genes and decreased expression of the Type I collagen gene. In further support of these findings, other studies suggest articular chondrocytes expanded in monolayer cultures display characteristics similar to those of mesenchymal stem cells [6, 39]. This may explain the ease in which passaged cells are able to reacquire their former phenotype. Taken together, these studies suggest chondrocyte feeder layers have the potential to enhance and induce chondrocyte-like behavior in populations of dedifferentiated cells (passaged cells or stem cells). It is unclear, however, what effects coculture has on already differentiated cells, such as primary chondrocytes, when they are encapsulated in a hydrogel matrix.
To investigate the use of a coculture system on engineered cartilage, we tested three hypotheses: (1) coculturing of engineered cartilage constructs in the presence of a monolayer of primary chondrocytes (“chondrocyte feeder layer”) expedites and increases development of their material and biochemical properties; (2) the effects of coculture with a chondrocyte feeder layer arise from paracrine effects (soluble factors) rather than direct cell-cell contact with the feeder cells; and (3) the feeder layer effect depends on the specific morphology and expression of the two-dimensional (2D) cell monolayer.
Materials and Methods
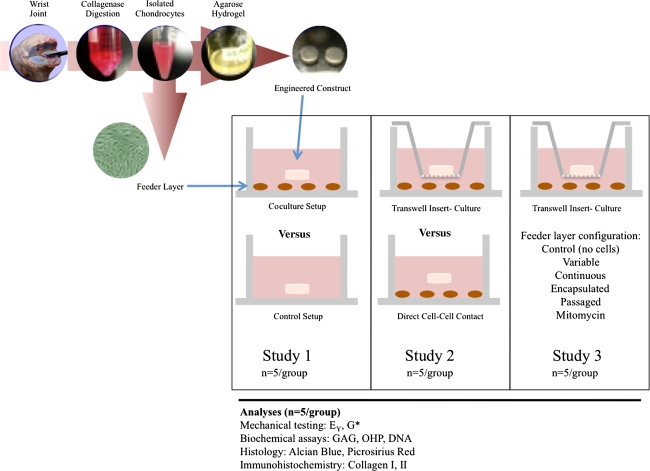
To investigate the effect of introducing a chondrocyte feeder layer to engineered cartilage constructs, we developed a coculture model system (Fig. 1). Briefly, to test Hypothesis 1, the effect of culturing engineered cartilage in the presence of subconfluent (low-density) primary chondrocyte monolayers was investigated (Study 1). To test Hypothesis 2, the dependence of this effect on cell-cell contact was investigated by eliminating physical contact as a means of interaction using tissue culture Transwell® inserts (Study 2). To test Hypothesis 3, the role of the monolayer’s phenotypic expression in influencing tissue development in cocultured constructs was studied (Study 3). We performed each study independently, using cell populations isolated and pooled from different animals.
Fig. 1.
A schematic shows the experimental design of the three studies described.
We isolated tissue from bovine carpometacarpal joints of freshly slaughtered 2- to 4-week-old calves. Six to eight joints were used per study and cells were pooled from all joints for each study described herein. Cartilage was digested in high-glucose Dulbecco’s modified Eagle’s medium (hgDMEM) with collagenase Type V (Sigma-Aldrich Corp, St Louis, MO) for 11 hours at 37°C with shaking. Cell suspensions were filtered through a 70-μm porous mesh and sedimented in a bench-top centrifuge for 15 minutes at 1500 g. Viable cells were counted with a hemocytometer and viability was assessed by trypan blue exclusion. Cell suspensions (60 × 106 cells/mL) were mixed in equal parts with 4% low-gelling agarose (Type VII; Sigma) to yield a final cell concentration of 30 × 106 cells/mL in 2% wt/vol agarose. The chondrocyte/agarose mixture was cast into slabs and later cored using a sterile disposable biopsy punch (Miltex Inc, York, PA) to final dimensions of 4-mm diameter and 2.34-mm thickness.
Cells from the same harvest and final suspension were plated into tissue-culture-treated 24-well plates at a density of 0.1 × 106 cells/well (5.6 × 104 cells/cm2) in the presence of hgDMEM supplemented with 5% fetal bovine serum to facilitate cell attachment. We exposed developing engineered cartilage constructs to different monolayer formulations in each of the three studies.
In Study 1, one construct was placed above the monolayer in each well and allowed to grow in culture for the duration of the study (Fig. 2A). In Study 2, to investigate the dependence on cell-cell contact, we physically separated chondrocyte monolayers from the three-dimensional (3D) engineered tissue by a Transwell® insert (Corning, Acton, MA) (Fig. 2B). Tissue-culture-treated membranes with a 3.0-μm pore size, which is orders of magnitude larger than the pore size of native cartilage [30] or 2% Type VII agarose [31], were used to permit free transport of diffusible signals but not direct cell-cell contact between the chondrocytes in the engineered constructs and feeder layer. In Study 3, we established five subgroups to examine the dependence on cell manipulation and presentation as described below. Primary chondrocyte monolayers were replated each week (“variable”) to contrast with monolayers plated only once at the start of the study (“continuous”) to assess the effect of the presentation of the feeder cells when introduced to the engineered construct. For the feeder layers in the variable group, fresh harvests and tissue digestions were performed each week to yield a new cell suspension for plating of primary cells at the same initial seeding density. Alternatively, to understand whether this effect was dependent on soluble factors released only transiently immediately after a fresh harvest, after each harvest, and tissue digestion, we plated cell suspensions in a high-density monolayer (0.4 × 106 cells/cm2) to preserve the chondrocyte phenotype [45]. This high-density monolayer was cultured for a week and then trypsinized and replated at a density of 0.1 × 106 cells/well (5.6 × 104 cells/cm2) the following week and used at Passage 1 (P1, “passaged”). Then, to assess the dependence of this effect on feeder cell presentation resulting from changes in morphology, we embedded cells (“encapsulated”) in low-gelling agarose at a final density of 0.5 × 106 cells/mL in 1% wt/vol agarose. An aliquot of the cell-agarose solution was then transferred to each well such that the same number of cells (0.1 × 106 cells) was entrapped in the hydrogel feeder layer to preserve cell morphology and replaced weekly to match the variable time course. Finally, to understand whether the effect was dependent on active processes of cellular division and proliferation in the feeder layer, monolayers were first treated with mitomycin C (10 μg/mL), as it inhibits cell proliferation and division, before use in the coculture setup (“mitomycin”).
Fig. 2A–B.
A diagram illustrates the experimental coculture setup for (A) Study 1 (direct contact between the construct and the feeder layer) and (B) Studies 2 and 3 (separation between the construct and the feeder layer by a Transwell® insert).
Two days after cell plating, one construct was transferred to each well for all the groups and cultured in 2 mL hgDMEM supplemented with 1× penicillin-streptomycin, 0.1 μmol/L dexamethasone, 50 μg/mL ascorbate 2-phosphate, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, and 1× ITS + premix (insulin, human transferrin, and selenous acid; Becton Dickinson, Franklin Lakes, NJ) for the remainder of the culture period. Medium was further supplemented with 10 ng/mL TGFβ-3 (Invitrogen, Carlsbad, CA) for the first 14 days of construct culture. Culture medium was changed every other day. A minimum ratio of 1 mL medium per 1 million cells was maintained throughout the duration of the study to encourage and promote tissue growth. As the total number of cells in each well has been previously estimated to range from 1 to 2 million cells, 2 mL medium was used per well.
We tested five whole samples from each group in unconfined compression to assess equilibrium (Young’s) modulus (EY) and dynamic modulus (G*) using a custom computer-controlled system [36]. An initial 0.02-N tare load was applied, followed by compression to 10% strain, at a strain rate of 0.05% s−1 to measure Young’s modulus after stress relaxation. Dynamic modulus was subsequently measured by superimposing a cyclical 2% peak-to-peak strain at 0.1 Hz.
After mechanical testing, we halved each sample, with one half dried and digested in proteinase K solution overnight at 56°C, as described previously [22], and the other half preserved for histology (see below). An aliquot was analyzed for GAG content via the 1,9-dimethylmethylene blue dye-binding assay [12]. A further aliquot was hydrolyzed in 12 N HCl at 110°C for 16 hours, dried, and resuspended in assay buffer [22]. Orthohydroxyproline (OHP) content was determined via a colorimetric assay in which chloramine T and dimethylaminobenzaldehyde reaction was quantified [38]. We calculated overall collagen content by assuming a 1:7.64 OHP-to-collagen mass ratio [18]. Double-stranded DNA content was also assessed by the PicoGreen® assay (Invitrogen) according to the manufacturer’s standard protocols. Each biochemical constituent was normalized to tissue wet weight (ww).
For histology, the other half of each sample was fixed in acid-formalin-ethanol [26], paraffin embedded, sectioned (8 μm thick), and stained for histology to assess both proteoglycan (alcian blue) and collagen (picrosirius red) distribution and organization. Immunohistochemistry was performed to confirm the development of collagen II in developing constructs. Briefly, tissue sections were digested in 5.0 mg/mL testicular hyaluronidase, swollen in 0.5 mol/L acetic acid, and blocked in 10% normal goat serum (NGS). We labeled the sections with 10% NGS containing primary antibody for Types I and II collagen (Abcam, Cambridge, MA). Alexa Fluor® 488-conjugated goat anti-rabbit secondary antibody labeling (Molecular Probes, Eugene, OR) and diamidino-2-phenylindole nuclear counterstaining (Molecular Probes) were performed to visualize the extracellular matrix and cells, respectively. Three independent observers (ART, EYD, JPA) examined tissue sections on an inverted confocal microscope (Leica Microsystems, Bannockburn, IL) to assess whether constructs exposed to the feeder layer exhibited more extensive biochemical content and distribution and to confirm the type of collagen produced.
All data are reported as the mean ± SD of four to five samples per time point and group. In Study 1, to assess differences in mechanical and material properties of constructs exposed to a feeder layer compared to control samples at each time point, a Student’s t test was performed. Similarly, in Study 2, differences at each time point between constructs cocultured in direct contact with the monolayer and constructs cocultured in the Transwell® setup were determined by a Student’s t test. In Study 3, to compare the properties of control constructs to those cultured in each experimental group, a one-way ANOVA was performed, and differences were confirmed with Tukey’s honestly significant difference post hoc test. Statistica® software (StatSoft, Inc, Tulsa, OK) was used to perform all statistical tests.
Results
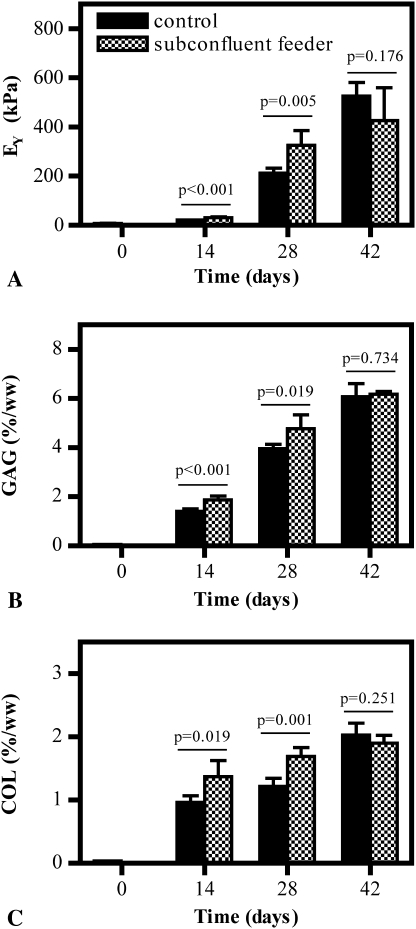
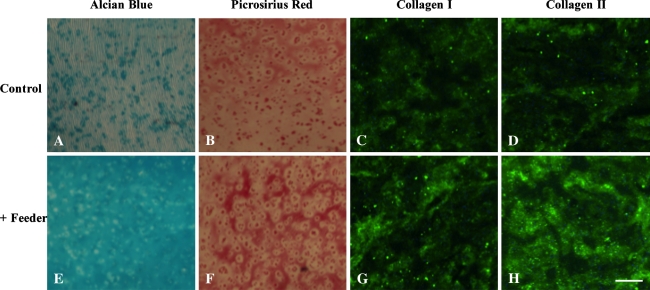
Study 1 showed the feeder layer stimulated growth of differentiated chondrocytes. For constructs cocultured with a subconfluent population of primary chondrocytes, a beneficial effect was noted on cells in the constructs: constructs exhibited higher (p = 0.005) Young’s modulus and greater (p = 0.019) GAG and (p = 0.001) collagen content than controls; however, this effect was only noted through Day 28 of culture (Fig. 3). By Day 42 in culture, Young’s modulus and GAG content were comparable (pEY = 0.176, pGAG = 0.734, pCollagen = 0.251) whether constructs were cocultured in the presence or absence of a cell monolayer (Fig. 3). Confirming these quantitative findings, compared to controls (Fig. 4A), histology sections of tissue from cocultured constructs (Fig. 4E) showed enhanced staining in alcian blue (for GAG) over time in culture, suggestive of advanced extracellular matrix development and deposition for sections exposed to a monolayer. Compared to controls (Fig. 4B), enhanced staining for picrosirius red (for nonspecific collagen) was also present for cocultured constructs (Fig. 4F). Similarly, when immunohistologic stains were analyzed, similar trends in Types I and II collagen distribution were observed in cocultured constructs (Fig. 4G–H) compared to controls (Fig. 4C–D), suggesting increased deposition of both types of collagen was present after coculture.
Fig. 3A–C.
Graphs show (A) Young’s modulus (EY), (B) GAG content, and (C) collagen (COL) content of constructs with time in culture in Study 1 (n = 5 per group). Increased mechanical properties and GAG content occurred up to Day 28 of culture.
Fig. 4A–H.
Representative constructs cocultured in the (A–D) absence and (E–H) presence of a feeder cell monolayer at Day 28 of culture stained with (A, E) alcian blue, (B, F) picrosirius red, (C, G) collagen I, and (D, H) collagen II are shown (scale bar = 200 μm). Cocultured constructs produced more extensive and organized tissue development, as assessed by staining intensity from histology and immunohistochemistry.
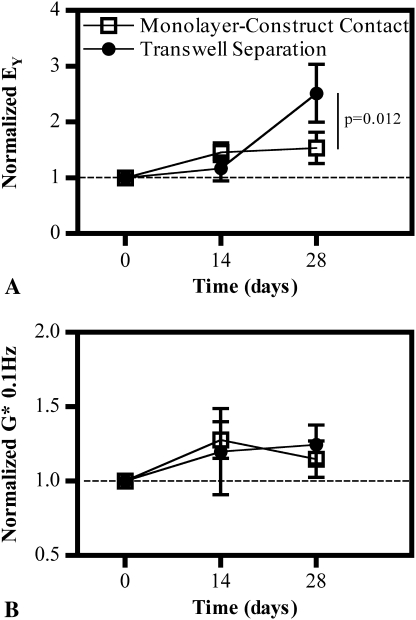
In Study 2, cell-cell contact was not necessary to produce the same beneficial effect of coculture as seen in Study 1, indicating the mechanism of intercellular signaling was primarily paracrine or soluble factors that can diffuse through the porous membrane. By the end of the culture period, constructs placed into Transwell® inserts and exposed to a subconfluent monolayer exhibited an increase (p = 0.012) in Young’s modulus compared to both control and contact conditions (Fig. 5A). This additional increase in stiffness was not paralleled in dynamic modulus; modest increases in dynamic modulus were seen compared to constructs cultured without a monolayer, but there were no differences (p = 0.286) between the constructs in contact with the monolayer and those separated with a Transwell® insert (Fig. 5B).
Fig. 5A–B.
Graphs show normalized (A) Young’s modulus (EY) and (B) dynamic modulus (G*) of constructs exposed to a feeder layer (in direct contact or with a Transwell® insert separation) compared to feeder-free controls in Study 2 (n = 5 per group). Increased mechanical properties at Day 28 are achieved when constructs are separated from the monolayer, confirming cell-cell contact is unnecessary.
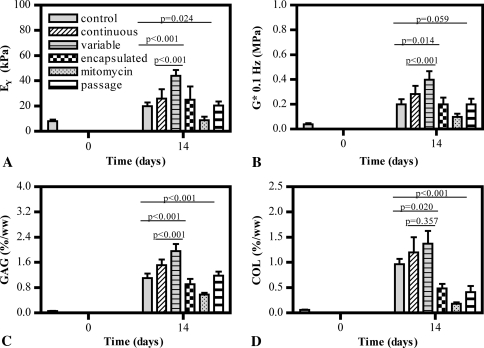
The specific presentation of the feeder cell monolayer was crucial in affecting the development of engineered cartilage tissue (Study 3). As early as 14 days in culture, cell monolayers replaced each week with a subconfluent population of cells freshly harvested from cartilage (“variable”) showed positive influences of coculture. These constructs developed superior mechanical properties and biochemical markers compared to control constructs cultured in the absence of any cells (pEY < 0.001, pG* < 0.001, pGAG < 0.001) or constructs cultured in the presence of a continuous monolayer (pEY < 0.001, pG* = 0.014, pGAG < 0.001) (Fig. 6). While collagen content for these constructs was increased over control (p = 0.020), it was comparable to levels seen in constructs exposed to the continuous monolayer (p = 0.357). The repeated replacement of the feeder layer with P1 cells did not have the same effect; constructs exposed to these monolayers developed tissue with properties comparable to control (pEY = 0.999, pG* = 1, pGAG = 0.945, pCollagen = 0.450) (Fig. 6). In further support of the finding that replacing the cells weekly was not the sole factor responsible for producing such an effect, cells encapsulated in 1% agarose and thus forced to adopt a rounded morphology due to the hydrogel also produced results comparable to control constructs (pEY = 0.668, pG* = 1, pGAG = 0.406, pCollagen = 0.543) (Fig. 6). Finally, when cell monolayers were mitotically inactivated by the application of mitomycin C, constructs exhibited delayed and stunted growth, yielding lower properties than controls (pEY = 0.024, pG* = 0.059, pGAG < 0.001, pCollagen < 0.001) (Fig. 6). DNA content in constructs from all groups at each time point was comparable (DNADay 14 ≈ 8 μg/construct; DNADay 28 ≈ 10 μg/construct, DNADay 42 ≈ 12 μg/construct), confirming the feeder layer did not adversely affect cellular viability.
Fig. 6A–D.
Graphs show (A) Young’s modulus (EY), (B) dynamic modulus (G*), (C) GAG content, and (D) collagen (COL) content of constructs with time in culture in Study 3 (n = 5 per group). When the cell monolayer is replaced weekly, increased mechanical properties and biochemical content are achieved by the cocultured tissue constructs.
Discussion
The principal focus of previous studies on the effects of coculture in cartilage tissue engineering has been on the effects of primary cells on dedifferentiated chondrocytes [1, 2, 13, 40] or various stem cells [17, 20, 43]. Cells that have undergone dedifferentiation are believed to be more plastic, similar to stem cells, allowing them to respond more efficiently to the chemical cues present during culture. Here, by coculturing engineered cartilage constructs with a chondrocyte feeder layer, we investigated whether differentiated chondrocytes already embedded in a 3D hydrogel scaffold exhibited a similar capacity to respond to these cues, and if so, if the mechanism of cellular signaling was dependent on direct cell-cell contact. Additionally, we looked at identifying specific plating conditions of the feeder layer, which may be necessary to elicit such a response.
Our studies are subject to some limitations. First, in our study, bovine chondrocytes from skeletally immature animals were utilized, as they are readily available, well characterized, and capable of robust tissue growth in culture (relative to adult cells). While their use facilitates comparison of current findings to our earlier work [19, 24] and to the extensive body of literature using bovine cartilage and chondrocytes for cartilage basic science and tissue engineering studies, it is unclear whether this technique would be applicable for an older population of cells whose release pattern of chemical factors may vary from that of younger, more robust cells. As such, further studies to identify an optimal cell source for the underlying feeder layer are warranted. Second, as the beneficial effects of a coculture system are heightened only when the feeder layer is replaced weekly with primary juvenile chondrocytes, the utility of this system in its current setup is limited due to a decreased availability of cells and may not be a clinically reasonable option. However, the data suggest soluble factors released by the underlying cell feeder layer when in a particular presentation can influence the biosynthetic activity of differentiated cells encapsulated in a 3D matrix. Our observations also suggest identifying one or more soluble mediators responsible for the beneficial feeder layer effects will be important to augmenting the coculture system and warrant future studies aimed at enhancing a chondrogenic medium formulation that could be used independent of a feeder layer.
Cell-cell communication has been identified as an important regulator in the behavior of cells and development of tissues [8, 13, 23, 44]. For the first time, we have identified the possibility for the biosynthetic activity of differentiated chondrocytes encapsulated in a 3D hydrogel matrix to be influenced by the presence of an underlying feeder chondrocyte monolayer, leading to enhanced tissue development and superior mechanical and material properties in the tissue construct (Study 1).
In Study 2, Transwell® inserts were used to provide physical separation between the developing engineered construct and the underlying cell monolayer, allowing the effects of direct cell-cell contact to be parsed out from paracrine signaling. While some studies have suggested direct cell-cell contact provides signaling factors that upregulate the biosynthetic activity of cells compared with the transfer of soluble factors between nonneighboring populations of cells [44], these experimental setups generally consist of cell populations plated together on a membrane or filter. In contrast, in our experimental setup, chondrocytes in the engineered cartilage tissue were singularly embedded within the agarose hydrogel and prohibited direct contact with one another and with the cells of the monolayer, even when directly placed upon it. Therefore, it is not surprising the effects seen to facilitate this increased tissue growth and development were present even when the construct was physically separated from the feeder layer and intercellular signaling relied on the diffusible passage of chemical cues. In support of our findings, many reports in the literature already utilize setups that physically separate the different populations of cells [21] or use conditioned media to provide chemical cues from one population of cells to another [37].
While our data indicate paracrine signaling is responsible for the expedited tissue growth in a 3D scaffold system, the type and degree of soluble factors released by the monolayer appear dependent on the specific presentation of the 2D feeder cell, which may be influenced by cellular morphology, harvest freshness (eg, primary versus passaged cells), and their phase within the cell division cycle (Study 3). Cell shape has been implicated in modulating the chondrocyte phenotype [7]; the feeder layers adopted in our study underwent an initial period of preconfluence where cell morphology was more stellate before reaching confluence whereupon chondrocytes assumed a more classical spherical morphology. Another factor known to influence the chondrocyte phenotype and therefore the release of particular soluble factors is passaging; with increased passage number, gene expression for collagen I increased while expression of collagen II, aggrecan, and cartilage oligomeric matrix protein (COMP), the major biomarkers present in cartilage, were downregulated [15]. It is feasible then downregulation of these genetic markers can adversely affect the soluble cues being emitted from the cell monolayer. Finally, mitomycin C, while used to halt the mitotic activity of cells, has been reported to induce premature aging of fibroblasts [32]. Aged cells are less biosynthetically active than juvenile cells and remain in the quiescent stage of the cell cycle rather than undergoing active division [42]. Taken together, these reports in the literature support our results and indicate the presentation of the 2D feeder layer is crucial to providing the correct chemical cues to the developing tissue construct.
Our observations suggest differentiated cells encapsulated in a 3D matrix have the potential to respond to soluble cues present in a coculture system and these chemical factors can prime and modulate the development of engineered cartilage constructs. Furthermore, while bovine cells were used in the engineered tissues and feeder layer of the current study, studies in the literature suggest the possibility of using cells from disparate species with beneficial effects as well [1]. In this manner, this work has the potential to be applied for donor cartilage allografts, which are currently in use clinically [3, 16], to increase their functional tissue properties to guarantee success in vivo after implantation. Our future work will also look to translate the current findings into other model systems of cartilage growth and injury repair to determine how universal and clinically useful they are.
Footnotes
One or more of the authors have received funding from the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the National Institutes of Health (AR46568, AR52871) (CTH); the National Institutes of Health (GAA, JCB); and the National Science Foundation Graduate Fellowship (ART).
Each author certifies that his or her institution waived approval for the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Columbia University.
References
- 1.Ahmed N, Gan L, Nagy A, Zheng J, Wang C, Kandel RA. Cartilage tissue formation using redifferentiated passaged chondrocytes in vitro. Tissue Eng Part A. 2009;15:665–673. doi: 10.1089/ten.tea.2008.0004. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed N, Taylor DW, Wunder J, Nagy A, Gross AE, Kandel RA. Passaged human chondrocytes accumulate extracellular matrix when induced by bovine chondrocytes. J Tissue Eng Regen Med. 2010;4:233–241. doi: 10.1002/term.235. [DOI] [PubMed] [Google Scholar]
- 3.Allen RT, Robertson CM, Pennock AT, Bugbee WD, Harwood FL, Wong VW, Chen AC, Sah RL, Amiel D. Analysis of stored osteochondral allografts at the time of surgical implantation. Am J Sports Med. 2005;33:1479–1484. doi: 10.1177/0363546505275010. [DOI] [PubMed] [Google Scholar]
- 4.Awad H, Halvorsen YD, Gimble JM, Guilak F. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003;9:1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 5.Aydelotte MB, Raiss RX, Caterson B, Kuettner K. Influence of interleukin-1 on the morphology and proteoglycan metabolism of cultured bovine articular chondrocytes. Connect Tissue Res. 1992;28:143–159. doi: 10.3109/03008209209014233. [DOI] [PubMed] [Google Scholar]
- 6.Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 7.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 9.Bian L, Stoker AM, Marberry KM, Ateshian GA, Cook JL, Hung CT. Effects of dexamethasone on the functional properties of cartilage explants during long-term culture. Am J Sports Med. 2010;38:78–85. doi: 10.1177/0363546509354197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bigdeli N, Karlsson C, Strehl R, Concaro S, Hyllner J, Lindahl A. Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem Cells. 2009;27:1812–1821. doi: 10.1002/stem.114. [DOI] [PubMed] [Google Scholar]
- 11.Byers BA, Mauck RL, Chiang I, Tuan RS. Temporal exposure of TGF-B3 under serum-free conditions enhances biomechanical and biochemical maturation of tissue-engineered cartilage. Trans Orthop Res Soc. 2006;31:43. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 13.Gan L, Kandel RA. In vitro cartilage tissue formation by coculture of primary and passaged chondrocytes. Tissue Eng. 2007;13:831–842. doi: 10.1089/ten.2006.0231. [DOI] [PubMed] [Google Scholar]
- 14.Guillotin B, Bourget C, Remy-Zolgadri M, Bareille R, Fernandez P, Conrad V, Amedee-Vilamitjana J. Human primary endothelial cells stimulate human osteoprogenitor cell differentiation. Cell Physiol Biochem. 2004;14:325–332. doi: 10.1159/000080342. [DOI] [PubMed] [Google Scholar]
- 15.Gunja NJ, Athanasiou KA. Passage and reversal effects on gene expression of bovine meniscal fibrochondrocytes. Arthritis Res Ther. 2007;9:R93. doi: 10.1186/ar2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennig A, Abate J. Osteochondral allografts in the treatment of articular cartilage injuries of the knee. Sports Med Athrosc. 2007;15:126–132. doi: 10.1097/JSA.0b013e31812e5373. [DOI] [PubMed] [Google Scholar]
- 17.Hoben GM, Willard VP, Athanasiou KA. Fibrochondrogenesis of hESCs: growth factor combinations and cocultures. Stem Cells Dev. 2009;18:283–292. doi: 10.1089/scd.2008.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, Poole AR. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/B:ABME.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 20.Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281–284. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- 21.Jikko A, Kato Y, Hiranuma H, Fuchihata H. Inhibition of chondrocyte terminal differentiation and matrix calcification by soluble factors released by articular chondrocytes. Calcif Tissue Int. 1999;65:276–279. doi: 10.1007/s002239900698. [DOI] [PubMed] [Google Scholar]
- 22.Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489–1497. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Kelly TA, Wang CC, Mauck RL, Ateshian GA, Hung CT. Role of cell-associated matrix in development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41:223–227. [PubMed] [Google Scholar]
- 24.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima EG, Tan AR, Tai T, Bian L, Stoker AM, Ateshian GA, Cook JL, Hung CT. Differences in interleukin-1 response between engineered and native cartilage. Tissue Eng Part A. 2008;14:1721–1730. doi: 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- 26.Lin W, Shuster S, Maibach HI, Stern R. Patterns of hyaluronan staining are modified by fixation techniques. J Histochem Cytochem. 1997;45:1157–1163. doi: 10.1177/002215549704500813. [DOI] [PubMed] [Google Scholar]
- 27.Mauck RL, Ho MM, Hung CT, Ateshian GA. Growth factor supplementation and dynamic hydrostatic pressurization for articular cartilage tissue engineering. Adv Bioeng. 2003:0283.pdf.
- 28.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 29.Mauck RL, Seyhan SL, Nicoll SB, Ateshian GA, Hung CT. Transforming growth factor B1 increases the mechanical properties and matrix development of chondrocyte-seeded agarose hydrogels. Adv Bioeng. 2001;50:691–692. [Google Scholar]
- 30.Mow VC, Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. Philadelphia, PA: Lippincott-Raven; 1997. pp. 113–177. [Google Scholar]
- 31.Ng KW, Wang CC, Mauck RL, Kelly TA, Chahine NO, Costa KD, Ateshian GA, Hung CT. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J Orthop Res. 2005;23:134–141. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Petri JB, Rott O, Wetzig T, Herrmann K, Haustein UF. The small proteoglycan fibromodulin is expressed in mitotic, but not in postmitotic fibroblasts. Mol Cell Biol Res Commun. 1999;1:59–65. doi: 10.1006/mcbr.1999.0113. [DOI] [PubMed] [Google Scholar]
- 33.Ratcliffe A, Tyler JA, Hardingham TE. Articular cartilage cultured with interleukin 1: increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem J. 1986;238:571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90:597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 35.Smola H, Thiekotter G, Fusenig NE. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol. 1993;122:417–429. doi: 10.1083/jcb.122.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/S0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 37.Solursh M, Meier S. A conditioned medium (CM) factor produced by chondrocytes that promotes their own differentiation. Dev Biol. 1973;30:279–289. doi: 10.1016/0012-1606(73)90089-4. [DOI] [PubMed] [Google Scholar]
- 38.Stegeman H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;19:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 39.Tallheden T, Dennis JE, Lennon DP, Sjogren-Jansson E, Caplan AI, Lindahl A. Phenotypic plasticity of human articular chondrocytes. J Bone Joint Surg Am. 2003;85(Suppl 2):93–100. doi: 10.2106/00004623-200300002-00012. [DOI] [PubMed] [Google Scholar]
- 40.Taylor DW, Ahmed N, Gan L, Gross AE, Kandel R. Proteoglycan and collagen accumulation by passaged chondrocytes can be enhanced through side by side culture with primary chondrocytes. Tissue Eng Part A. 2010;16:643–651. doi: 10.1089/ten.tea.2009.0236. [DOI] [PubMed] [Google Scholar]
- 41.Thorp BH, Anderson I, Jakowlew SB. Transforming growth factor-beta1, -beta2 and -beta3 in cartilage and bone cells during endochondral ossification in the chick. Development. 1992;114:907–911. doi: 10.1242/dev.114.4.907. [DOI] [PubMed] [Google Scholar]
- 42.Tran-Khanh N, Hoemann CD, McKee MD, Henderson JE, Buschmann MD. Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J Orthop Res. 2005;23:1354–1362. doi: 10.1016/j.orthres.2005.05.009.1100230617. [DOI] [PubMed] [Google Scholar]
- 43.Vats A, Bielby RC, Tolley N, Dickinson SC, Boccaccini AR, Hollander AP, Bishop AE, Polak JM. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 2006;12:1687–1697. doi: 10.1089/ten.2006.12.1687. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe T, Sakai D, Yamamoto Y, Iwashina T, Serigano K, Tamura F, Mochida J. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res. 2010;28:623–630. doi: 10.1002/jor.21036. [DOI] [PubMed] [Google Scholar]
- 45.Watt FM. Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. J Cell Sci. 1988;89(Pt 3):373–378. doi: 10.1242/jcs.89.3.373. [DOI] [PubMed] [Google Scholar]