Abstract
Background
Because the injured joint has an actively inflammatory environment, the survival and repair potential of cartilage grafts may be influenced by inflammatory processes. Understanding the interactions of those processes with the graft may lead to concepts for pharmacologic or surgical solutions allowing improved cartilage repair.
Questions/purposes
We asked whether the maturation level of cartilaginous tissues generated in vitro by expanded human articular chondrocytes (HACs) modulate (1) the spontaneous production of cytokines and (2) the response to interleukin (IL)-1β.
Methods
Twelve pellets/donor prepared with monolayer-expanded HACs (n = 6 donors) were evaluated at six different culture times for mRNA expression (n = 72) and spontaneous baseline release of monocyte chemoattractant protein (MCP)-1, IL-8, and transforming growth factor (TGF)-β1 (n = 72). We cultured 24 pellets/donor from each of four donors for 1 or 14 days (defined as immature and mature, respectively) and exposed the pellets to IL-1β for 3 days. MCP-1, IL-8, TGF-β1, and metalloprotease (MMP)-1 and MMP-13 were quantified in pellets and culture supernatants.
Results
By increasing culture time, the spontaneous release of IL-8 and MCP-1 decreased (12.0- and 5.5-fold, respectively), whereas that of TGF-β1 increased (5.4-fold). As compared with immature pellets, mature pellets responded to IL-1β by releasing lower amounts of MMP-1 (2.9-fold) and MMP-13 (1.7-fold) and increased levels of IL-8, MCP-1, and TGF-β1 (1.5-, 5.0-, and 7.5-fold, respectively). IL-8 and MCP-1 promptly returned to baseline on withdrawal of IL-1β.
Conclusions
Our observations suggest more mature cartilaginous tissues are more resistant to IL-1β exposure and can activate chemokines required to initiate tissue repair processes.
Clinical Relevance
The implantation of more mature cartilaginous tissues might provide superior graft survival and improve/accelerate cartilage repair.
Introduction
Autologous chondrocytes are commonly used as therapeutic agents for the repair of articular cartilage defects [4, 10]. As compared with the transplantation of cell suspension, the grafting of chondrocyte-loaded three-dimensional biodegradable scaffolds immediately after loading (a technique named matrix-mediated autologous chondrocyte implantation) [5] or after a certain in vitro maturation time [30, 35] would hypothetically allow improved handling of the cell preparation and could offer the possibility of earlier postoperative loading. However, it is uncertain whether and to what extent engineered cartilage should be structurally and functionally developed before implantation.
Most cell-based cartilage repair techniques require monolayer expansion of chondrocytes, which is associated with cell dedifferentiation [1]. It is well established that dedifferentiated chondrocytes share some phenotypic and genotypic traits with chondrocytes derived from patients with osteoarthritis (OA) [2, 39, 49], including expression of typically fibroblastic markers (eg, versican, Type I collagen, and cathepsin B) [6, 20, 32, 45]. Moreover, similar to OA chondrocytes, ex vivo-cultured chondrocytes express a variety of proinflammatory chemokines/chemokine receptors and cartilage degenerative enzymes [8, 9, 13, 15, 36, 48], whose production is enhanced by increasing cell passaging and by stimulation with interleukin (IL)-1β and tumor necrosis factor-α [17, 26].
IL-1 isoforms have harmful effects on chondrocytes. They (1) reduce the synthesis of the major physiologic inhibitors of prodegradative enzymes [31]; (2) stimulate the production of prostaglandins, free radicals, and nitric oxide [23]; (3) inhibit the synthesis of matrix components such as Type II collagen and proteoglycans [19, 31, 42, 46]; (4) induce a dedifferentiated chondrocyte phenotype by suppressing the expression of Sox-9 [19, 28]; and (5) inhibit chondrocytes proliferation and induce cell death [28].
These IL-1-mediated effects have particular relevance in the context of cell-based cartilage repair, considering therapeutic cell preparations (single-cell suspension, cell-seeded matrices, or cartilaginous tissues) once grafted in the joint defect will become exposed to a biochemical environment that likely contains catabolic mediators derived from the diseased joint or from the surgical intervention itself, including IL-1β [12, 27, 28]. The presence of abundant extracellular matrix surrounding the chondrocytes in an engineered cartilage graft in principle may protect the cells from the IL-1 insult. Using native bovine chondrocytes, Lima et al. [27] recently showed mature engineered cartilage constructs (having a native level of glycosaminoglycan [GAG] content and Young’s modulus) were capable of counteracting IL-1α-mediated catabolic effects. However, this concept should be investigated using clinically relevant cells (ie, adult human chondrocytes after monolayer expansion), which have reduced chondrogenic capacity as compared with freshly harvested bovine chondrocytes and typically produce tissues of biochemical and biomechanical properties inferior to those of native cartilage.
We asked whether the extent of maturation of human-based cartilaginous tissues modulates the (1) spontaneous production of cytokines and (2) the response to IL-1β in terms of extracellular matrix loss, cytokine, and catabolic enzyme production.
Materials and Methods
We isolated human articular chondrocytes (HACs) from cartilage biopsies of six donors and expanded the cells in monolayer. HACs from all the donors were cultured in pellets without IL-1β for 3, 6, 9, 15, 21, and 27 days and analyzed for spontaneous release of IL-8, monocyte chemoattractant protein (MCP)-1, and transforming growth factor (TGF)-β1. HACs from four of the six donors were cultured in pellets for 1 or 14 days (defined as immature and mature, respectively) and then exposed to IL-1β and assessed for cartilage matrix loss and production of IL-8, MCP-1, TGF-β1, metalloprotease (MMP)-1, and MMP-13. Cytokine production by immature pellets from one of six donors was also assessed 3 or 7 days after IL-1β withdrawal (recovery culture) (Fig. 1). IL-8 and MCP-1 are the most commonly used monocyte and neutrophil chemoattractants, whereas TGF-β1 is one of the main factors stimulating cartilage matrix production. The selection of these cytokines and the degradative enzymes investigated was also based on partially published data [41] that these were among the most regulated by the process of in vitro maturation.
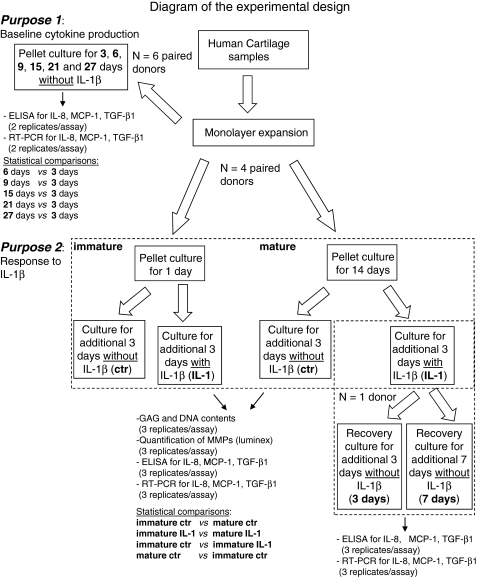
Fig. 1.
Diagram of the experimental design. HACs harvested from six donors were expanded in monolayer and then cultured in pellets. For Purpose 1, six donor primaries were used. The total number of pellets (per donor) was 24, four for each culture time: two were analyzed by real-time RT-PCR (total number of replicates: 12) and the others by histology/immunohistochemistry (total number of replicates: 12, not included in this scheme). ELISA quantification of MCP-1, TGF-β1, and IL-8 was instead performed in the same supernatants harvested from two pellets (total number of replicates: 12). For Purpose 2, four donor primaries were used. The total number of pellets generated per donor was 48, nine for each group: three were analyzed by RT-PCR, (total number of replicates: 12) three by biochemical test (GAGs, DNA, total number of replicates: 12), and three by histology/immunohistochemistry (not included in this scheme, total number of replicates: 12). ELISA and Luminex analyses were performed in the same supernatants harvested from three pellets (total number of replicates: 12). Differences in the cytokine expression and release, MMP release, GAG and DNA contents between the indicated groups (in bold) were assessed using the nonparametric two-tailed Wilcoxon test. Pellets generated from one donor were also exposed to 3 or 7 days of recovery culture after IL-1 removal. ctr = control (without IL-1β).
Macroscopically normal human articular cartilage samples (Mankin score 2 to 3) were obtained postmortem (within 24 hours after death) from the knees of four male and two female donors (mean age, 44 years; range, 32–65 years) with no clinical history of joint disorders after informed consent by relatives and in accordance with the local ethics committee (University Hospital Basel, Basel, Switzerland).
Cartilage tissues were minced in small pieces and digested with 22 hours’ incubation at 37°C in 0.15% Type II collagenase (10 mL solution/g tissue, 300 U/mg; Worthington Biochemical Corp, Lakewood, NJ). The isolated HACs were plated in tissue culture flasks at 104 cells/cm2 in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 4.5 mg/mL D-glucose, 0.1 mmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, 100 mmol/L HEPES buffer, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.29 mg/mL glutamine (complete medium) supplemented with 1 ng/mL TGF-β1 and 5 ng/mL fibroblast growth factor-2 (expansion medium) in a humidified 37°C/5% CO2 incubator. The growth factor combination was selected based on the previously reported ability to increase human chondrocyte proliferation and capacity to redifferentiate [3, 24].
After approximately 10 days, when cells were approximately 80% confluent, first-passage cells were rinsed with phosphate-buffered saline (PBS), detached using 0.05% trypsin/0.53 mmol/L EDTA, and replated at 5 × 103 cells/cm2. After an additional week, when cells were again approximately 80% confluent, second-passage cells (corresponding to a total of 8.1 ± 1.0 doublings) were detached and induced to redifferentiate in pellet cultures as described subsequently.
The chondrogenic capacity of expanded chondrocytes was investigated in pellet cultures using a defined serum-free medium, as previously described [28]. Cells were suspended in DMEM supplemented with ITS + 1 (10 μg/mL insulin, 5.5 mg/mL transferrin, 5 ng/mL selenium, 0.5 mg/mL bovine serum albumin, 4.7 mg/mL linoleic acid; Sigma-Aldrich Corp, St Louis, MO), 0.1 mmol/L ascorbic acid 2-phosphate, 1.25 mg/mL human serum albumin, 10−7 mol/L dexamethasone, and 10 ng/mL TGF-β1 (chondrogenic medium). Aliquots of 5 × 105 cells/0.5 mL were centrifuged at 250 × g for 5 minutes in 1.5-mL polypropylene conical tubes (Sarstedt, Nümbrecht, Germany) to form spherical pellets, which were placed onto a three-dimensional orbital shaker (Bioblock Scientific, Frenkendorf, Switzerland) at 30 rpm. Pellets were evaluated for spontaneous release and mRNA expression of cytokines at different experimental times: 3, 6, 9, 15, 21, and 27 days; we used six pellets for each time period.
In another experimental set, pellets were cultured for 1 or 14 days (nine pellets for each time period) and then exposed to 1 ng/mL IL-1β for 3 days [11]. Control pellets were cultured for 4 or 17 days (nine pellets for each time period) without IL-1β. Pellets generated with HACs from one donor were cultured for an additional 3 and 7 days (six pellets for each time period) in chondrogenic medium after exposure to IL-1β (recovery culture).
The IL-1β concentration was selected based on a preliminary study [12]. Pellets were processed for histologic, immunohistochemical, biochemical, and gene expression analyses, whereas supernatants were collected and evaluated at different time points for cytokines and MMP release.
Pellets were rinsed with PBS, fixed in 4% formalin for 24 hours, embedded in paraffin, and cross-sectioned (5 μm thick). Sections were stained with safranin O for sulfated GAGs [3]. The immunohistochemical analyses for IL-8, MCP-1, TGF-β1, and Type II collagen were performed using the following primary antibodies: mouse monoclonal antihuman IL-8 and TGF-β1 (R&D Systems, Minneapolis, MN), mouse monoclonal antihuman MCP-1 (PeproTech Inc, Rocky Hill, NC), and mouse monoclonal antihuman Type II collagen (II-II6B3; Hybridoma Bank, University of Iowa, Iowa City, IA). Immunostaining for Type II collagen was performed as previously described [3, 21]. Tissue sections for MCP-1 and TGF-β1 were treated with 0.1% hyaluronidase (Sigma) in PBS at 37°C for 10 minutes for epitope unmasking. After rinsing, slides were incubated for 1 hour at room temperature with primary antibodies diluted 1:10 for both MCP-1 and TGF-β1 in PBS with 1% bovine serum albumin (BSA). The slides for IL-8 after deparaffination and rehydration were incubated for 1 hour at room temperature with the primary antibodies diluted 1:10 in PBS with 1% BSA and then with biotinylated immunoglobulins against various animal species (Biogenex, San Ramon, CA) for 20 minutes. Samples were then incubated with a phosphatase-labeled streptavidin kit (Biogenex) for 20 minutes at room temperature and rinsed. The reactions were developed using fast red substrate (Biogenex). Negative controls were performed by omitting the primary antibody. Slides were counterstained with hematoxylin and mounted in glycerol gel. Two-three histologic sections for each condition were visualized with a Nikon Eclipse 90i microscope equipped with NIS (Nikon Imaging Software) elements (Nikon Instruments Europe BV, Amstelveen, The Netherlands).
Pellets were digested in 0.5 mL proteinase K (1 mg/mL proteinase K in 50 mmol/L Tris with 1 mmol/L EDTA, 1 mmol/L iodoacetamide, and 10 μg/mL pepstatin A) for 15 hours at 56°C. GAG amounts were measured spectrophotometrically after reaction with dimethylmethylene blue [16] with chondroitin-4-sulfate from bovine trachea (Sigma-Aldrich Chemie, Buchs San Gallen, Switzerland) as a standard. The amount of DNA was measured spectrofluorometrically using the CyQuant® Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR) with calf thymus DNA as a standard [22].
IL-8 and MCP-1 chemokines were determined by the use of specific immunoassays standardized in the laboratory of Istituto Ortopedico Rizzoli Bologna. Briefly, for each cytokine’s determination, two monoclonal antibodies of different epitope specificity were used to prepare the sandwich ELISA (IL-8: capture antibody: mouse antihuman IL-8 [Pharmingen, San Diego, CA]; detection antibody: biotinylated mouse antihuman IL-8 [Pharmingen]; MCP-1: capture antibody: mouse antihuman MCP-1 [Pharmingen]; detection antibody: biotinylated IgG fraction of rabbit antihuman MCP-1 [Pharmingen]). Ninety-six-well, polystyrene plates (EIA microplate; ICN, Costa Mesa, CA) were coated with 50 μL purified mouse antihuman IL-8 or MCP-1 adjusted at a concentration of 1 and 4 μg/mL, respectively, in sodium carbonate buffer pH 9.5 and incubated overnight at 4°C. After washing the plates, serial dilutions of recombinant human IL-8 or MCP-1 and appropriate diluted samples were added to the wells (100 μL/well) and incubated for 2 hours at room temperature followed by a 1-hour incubation with 100 μL biotinylated mouse antihuman IL-8 or rabbit antihuman MCP-1 per well. After a further 30 minutes’ incubation at room temperature with 100 μL streptavidin-horseradish peroxidase conjugate per well, the bound antibodies were detected by adding 1,2-o-phenylenediamine and H2O2 as substrate, running the reaction for 10 minutes before terminating it with 2 mol/L H2SO4, and the absorbance measured at 492 nm.
TGF-β1 concentrations in supernatants were evaluated by commercial ELISA kits following the manufacturer’s instructions (R&D Systems). Values of TGF-β1 measured in the chondrogenic medium were subtracted from those measured in the supernatants.
The amount of each released cytokine was normalized to the DNA content of the tissue and expressed as picogram per nanogram.
MMPs were quantified in media collected from cultured pellets by using the MultiAnalyte Profiling MMP Base Kit (Fluorokine MAP: LMP000) complemented with the specific MMPs (MMP-1: LMP901; MMP-13: LMP511; R&D Systems). The assay was performed on a Luminex 100TM Analyzer (Luminex Corp, Austin, TX) following the manufacturer’s instructions. The amount of released MMPs was normalized to the DNA content of the tissue.
RNA was extracted from expanded HACs and from pellets using 250 μL Trizol® (Life Technologies, Basel, Switzerland) according to the manufacturer’s protocol. RNA was treated with DNAse I using the DNA-free™ Kit (Ambion, Austin, TX) and quantified spectrofluorometrically. cDNA was generated from total RNA by using 500 μg/mL random hexamers (Promega AG, Zürich, Switzerland) and 1 μL 50 U/mL StrataScript™ reverse transcriptase (Stratagene, Amsterdam, The Netherlands) in the presence of dNTPs.
TGF-β1, IL-8, and MCP-1 gene expression was analyzed by real-time reverse-transcription (RT)-PCR (Table 1) using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene [7]. Real-time PCR was run in a LightCycler® Instrument (Roche Molecular Biochemicals, Mannheim, Germany) using the QuantiTect™ SYBR® Green PCR Kit (Qiagen, GmbH, Hilden, Germany) with the following protocol: initial activation of HotStarTaq™ DNA polymerase at 94°C for 15 minutes, 45 cycles of 94°C for 15 seconds, 56 to 60°C for 20 seconds, and 72°C for 10 seconds. The increase in PCR products was monitored for each amplification cycle by measuring the increase in fluorescence caused by the binding of SYBR® Green I dye to dsDNA. For each cDNA sample, the threshold cycle (Ct) value of GAPDH was subtracted from the Ct value of the target gene to derive ΔCt. The levels of expression of the target genes were calculated as 2ΔCt.
Table 1.
Real-time reverse-transcription PCR primer description
| RNA template | Primer sequences | Annealing temperature | Reference |
|---|---|---|---|
| GAPDH | 5′-TGG TAT CGT GGA AGG ACT CAT GAC 3′-ATG CCA GTG AGC TTC CCG TTC AGC |
60°C | Blanco et al. [7] |
| TGF-β1 | 5′-CTGAGGTATCGCCAGG 3′-CGCGTGCTAATGGTGGA |
58°C | PRIMER 3 [38] |
| IL-8 | 5′-ACTTCTCCACAACCT 3′-CCAAACCTTTCCACCC |
56°C | PRIMER 3 [38] |
| MCP-1 | 5’-AGCCACCTTCATTCC 3’-GCTTCTTTTGGGACACTTGCT |
56°C | PRIMER 3 [38] |
GAPDH = glyceraldehyde-3-phosphate dehydrogenase; TGF-β1 = transforming growth factor-β1; IL-8 = interleukin-8; MCP-1 = monocyte chemoattractant protein-1.
Two-three replicate samples were assessed for each donor and experimental group. Sample sizes in all groups compared were equal (Fig. 1). Values are presented as mean ± SD. Because the assumptions of a parametric test were not met for data collected for either purpose, we used a nonparametric two-tailed Wilcoxon test to compare two matched groups at a given time. For Purpose 1, we determined differences in the cytokine protein and mRNA expression levels between Days 6, 9, 15, 21, or 27 and Day 3. For Purpose 2, we determined differences in GAG, DNA, MMP amounts, and cytokine expression levels between the following groups: immature without IL-1β versus mature without IL-1β, immature with IL-1 versus mature with IL-1, immature without IL-1β versus immature with IL-1, mature without IL-1β versus mature with IL-1 (Fig. 1). Statistical evaluation was performed using SPSS® Version 7.5 software (SPSS Inc, Chicago, IL).
Results
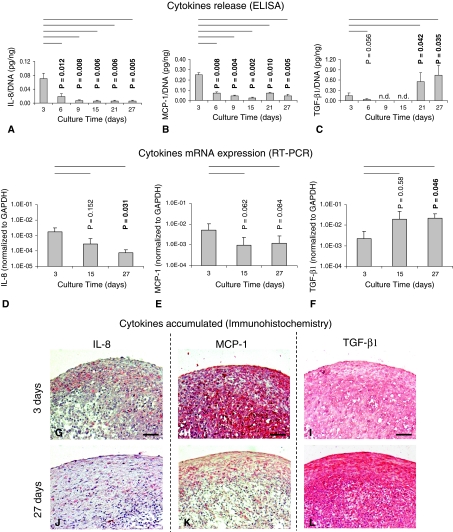
IL-8 and MCP-1 showed similar secretion kinetics during pellet culture. The amounts of these cytokines were highest at Day 3. They decreased (by 3.7- and 3.4-fold, respectively) after an additional 3 days’ culture and remained close to the limit of detection after additional culture time. In contrast, TGF-β1 amounts in the culture medium remained very low or close to the limit of detection up to 15 days’ culture, increased at 21 days, and remained at similar levels until Day 27 (Fig. 2A–C). RT-PCR analyses were in agreement with the ELISA results: IL-8 and MCP-1 mRNA levels decreased from 3 to 27 days of culture, respectively, by 22.9- and 5.1-fold, whereas TGF-β1 mRNA increased by 9.6-fold (Fig. 2D–F). Immunohistochemical analysis confirmed, as compared with pellets cultured for 3 days, those cultured for 27 days accumulated lower amounts of IL-8 and MCP-1 and higher amounts of TGF-β1 (Fig. 2G–L).
Fig. 2A–L.
Images show the quantification of IL-8, MCP-1, and TGF-β1 produced/accumulated by HACs at different time of pellet culture. Amounts of (A) IL-8, (B) MCP-1, and (C) TGF-β1 proteins are shown, normalized to the DNA contents of the corresponding pellets, released in the supernatants (six donors, 12 specimens). Real-time RT-PCR analysis of the expressions of (D) IL-8, (E) MCP-1, and (F) TGF-β1 mRNA by chondrocytes cultured in pellets at the different times (four donors, 12 specimens) is shown. Values are expressed as mean ± SD of measurements. Probability values (p) versus the 3-day groups are reported on the top of each bar. n.d. = not detectable. Immunohistologic assessment of representative pellets cultured for (G–I) 3 days and (J–L) 27 days is shown (staining with antibodies against [G, J] IL-8, [H, K] MCP-1, and [I, L] TGF-β1; original magnification, ×10). Scale bars = 100 μm. Results indicate a decrease of IL-8 and MCP-1 and an increase of TGF-β1 spontaneous production with increasing maturation extent.
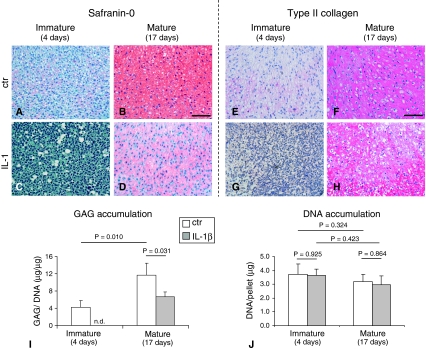
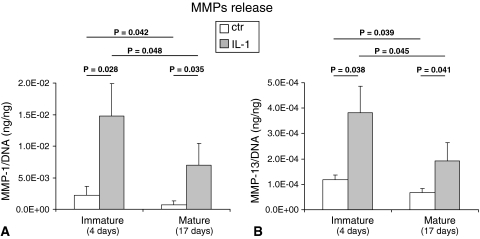
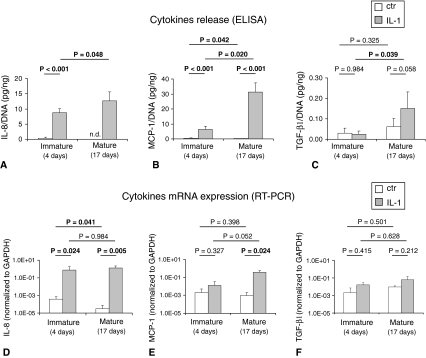
IL-1β exposure to immature pellets caused an extensive loss of cartilaginous matrix as evidenced (immuno)histologically by absence of GAG and Type II collagen staining (Fig. 3A–H) and biochemically by a reduction of GAG to undetectable levels (Fig. 3I). In mature pellets, IL-1β exposure also caused a loss of cartilaginous components (GAG content reduced by 1.7-fold). DNA contents were, instead, unaffected by IL-1β exposure (Fig. 3 J). All the cell primaries tested in this study (independently from donors’ age) similarly responded to IL-1β. In both immature and mature pellets, the exposure to IL-1β increased the release of MMP-1 (6.7- and 3.2-fold, respectively) and MMP-13 (9.3- and 2.8-fold, respectively). However, the amounts of both degradative enzymes after IL-1β exposure were higher in the media from immature versus mature pellets (2.1- and 2.0-fold, respectively, for MMP-1 and MMP-13) (Fig. 4). Stimulation with IL-1β drastically enhanced the release of IL-8 and MCP-1. Mature tissues responded to IL-1β by releasing higher amounts of both chemokines so that the final amounts of IL-8 and MCP-1 in the supernatants of such pellets were 1.5- and 5.0-fold higher than those of immature pellets. TGF-β1 release was also enhanced to a higher extent by mature pellets in response to IL-1β; the amount of this cytokine was 6.2-fold higher in the supernatants of mature versus immature pellets (Fig. 5A–C). RT-PCR results were in general agreement with ELISA results. Stimulation with IL-1β caused an upregulation of IL-8 and MCP-1 mRNA expression that was more pronounced in mature tissues. Consequently, IL-8 and MCP-1 mRNA expression were, respectively, 1.8- and 28.0-fold higher expressed in mature versus immature tissues. TGF-β1 mRNA expression was only slightly increased after IL-1β stimulation (Fig. 5D–F) in both mature and immature tissues. The intensity of staining for IL-8 and MCP-1 increased in both mature and immature tissues after IL-1β exposure (Fig. 6). The amounts of IL-8 and MCP-1 released by mature pellets strongly decreased (by 2.3- and 1.8-fold, respectively) after 3 days of recovery culture and reached levels measured before IL-1β exposure after 7 days of recovery culture. TGF-β1, instead, further increased by 5.8-fold after 3 days of recovery culture and then remained at similar levels at 7 days of recovery culture (Table 2).
Fig. 3A–J.
Images illustrate (A–D) histologic and (E–H) immunohistologic assessment of representative pellets maintained in culture for a total of (A, C, E, G) 4 days (immature) or (B, D, F, H) 17 days (mature) in which the last 3 days were (A, B, E, F) without IL-1β (ctr) or (C, D, G, H) with IL-1β ([A–D] safranin O and [E–H] Type II collagen staining; original magnification, ×2). Scale bars = 200 μm. (I) Sulfated GAG content normalized to the amount of DNA and (J) DNA content of mature and immature pellets are shown. Values are expressed as mean ± SD of measurements (four donors, 12 specimens). n.d. = not detectable. Probability values (p) (mature versus mature [same culture condition] and ctr versus IL-1 [same culture time]) are reported on the top of the bars. Results indicate a superior IL-1β-mediated cartilage loss by immature pellets.
Fig. 4A–B.
Graphs show the quantification of released MMPs by HACs. Amounts of (A) MMP-1 and (B) MMP-13 proteins are shown, normalized to the DNA contents of the corresponding pellets, released in the supernatants of pellets maintained in culture for a total of 4 days (immature) or 17 days (mature) in which the last 3 days with IL-1β or without IL-1β (ctr). Values are expressed as mean ± SD of measurements (four donors, 12 specimens). Probability values (p) (mature versus mature [same culture condition] and ctr versus IL-1β [same culture time]) are reported on the top of the bars. Results indicate a superior IL-1β-mediated MMPs production by immature pellets.
Fig. 5A–F.
Graphs show the quantification of IL-8, MCP-1, and TGF-β1 produced by HACs. Amounts of (A) IL-8, (B) MCP-1, and (C) TGF-β1 are shown, normalized to the DNA contents of the corresponding pellets, released in the supernatants of tissues maintained in culture for a total of 4 days (immature) or 17 days (mature) in which the last 3 days were with IL-1β or without IL-1β (ctr). Real-time RT-PCR analysis of the expression of (D) IL-8, (E) MCP-1, and (F) TGF-β1 mRNA by chondrocytes cultured in pellets at the different times is shown. Values are expressed as mean ± SD of measurements (four donors, 12 specimens). n.d. = not detectable. Probability values (p) (mature versus mature [same culture condition] and ctr versus IL-1β [same culture time]) are reported on the top of the bars. Results indicate a superior IL-1β-mediated production of IL-8, MCP-1, and TGF-β1 by immature pellets.
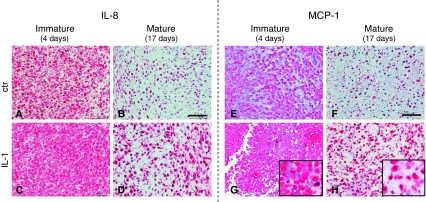
Fig. 6A–H.
Images illustrate the accumulation of (A–D) IL-8 and (E–H) MCP-1 in the tissues. Immunohistologic assessment of representative pellets maintained in culture for a total of (A, C, E, G) 4 days (immature) or (B, D, F, H) 17 days (mature) in which the last 3 days were (A, B, E, F) without IL-1β (ctr) or (C, D, G, H) with IL-1β is shown (staining with antibodies against [A–D] IL-8 and [E–H] TGF-β1; original magnification, ×20 for pictures and ×40 for inserts). Scale bars = 50 μm.
Table 2.
Amounts of IL-8, MCP-1, and TGF-β1 released during recovery culture of mature pellets
| Conditions | Amount released (pg/ng)* | ||
|---|---|---|---|
| IL-8 | MCP-1 | TGF-β1 | |
| IL-1β | 11.31 ± 0.92 | 20.84 ± 1.89 | 0.01 ± 0.00 |
| 3 days | 4.93 ± 0.41 | 1.23 ± 0.10 | 0.36 ± 0.02 |
| 7 days | 0.04 ± 0.01 | 0.32 ± 0.02 | 0.33 ± 0.03 |
* The amounts of cytokines are normalized to the DNA contents of corresponding pellets and presented as delta between baseline (control) values; values are expressed as mean ± SD; IL-8 = interleukin-8; MCP-1 = monocyte chemoattractant protein-1; TGF-β1 = transforming growth factor-β1; IL-1β = interleukin-1β.
Discussion
Chondrocyte interactions with inflammatory processes have been reported in native cartilage and freshly isolated chondrocytes of animal origin but not during chondrogenesis of expanded HACs. We therefore investigated whether and how the extent of maturation of cartilaginous tissues generated in vitro by monolayer-expanded HACs modulates the profile of chemokine production and the inflammatory/catabolic response to IL-1β.
Some limitations of this study must be considered. First, we investigated only a limited number of cytokines, and anabolic factors could show a different expression pattern. However, we selected what are considered to be the most relevant factors involved in cartilage anabolic and inflammatory processes. Second, we quantified only the amounts of MMP-1 and MMP-13 and thus could not exclude the involvement of other relevant proteases such as aggrecanase (ADAMTS-4, 5) in the observed IL-1β-mediated matrix reduction. Third, IL-1β was supplemented in cells cultured with medium containing dexamethasone and TGF-β1, necessary to maintain the chondrocytic phenotype [18] in pellet culture, and it is thus possible some of the observed effects can be partially mediated by these factors [29]. Fourth, the production of the investigated chemokines by monolayer-expanded HACs might differ using a model system different from the one implemented in this study (ie, high-density culture in the presence of TGF-β) and in an in vivo situation. In the absence of an alternative orthotopic model in which to test the performance of human chondrocytes for cartilage repair, further studies would be necessary to investigate the HAC performance under conditions better resembling the injured joint (eg, more physiologic oxygen tension, exposure to loading, coculture with mesenchymal progenitor cells).
Several studies suggest unstimulated cultured chondrocytes produce various chemokines involved in the recruitment of inflammatory cells [13, 33, 48]. However, it is unclear whether HACs at different stages of in vitro redifferentiation express differing levels of chemokines. Our finding that IL-8 and MCP-1 mRNA expression and protein release by HACs decreased whereas TGF-β1 mRNA and protein increased with increasing culture time indicates, under “unstressed” conditions (ie, absence of IL-1β), the more mature engineered grafts have the propensity to reduce inflammatory signals and to boost anabolic processes.
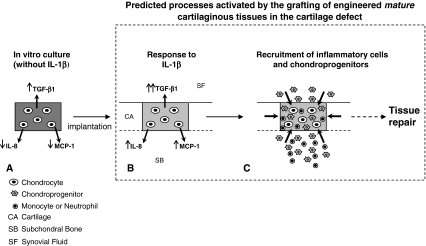
The responses of HACs to such proinflammatory cytokines have been investigated by several scientists [26, 40] to mimic processes occurring in degenerative cartilage diseases. It appears such molecules enhance the expression of a large number of mediators contributing to cartilage degradation such as MMPs [17, 33] and inflammatory cytokines [29, 44] and reduce the synthesis of key extracellular matrix proteins such as Type II collagen and proteoglycans [19, 31, 42, 46, 47]. For such studies, however, primary undifferentiated chondrocytes or cartilage explants derived from patients with OA have so far been used. Because the responses of chondrocytes to IL-1 isoforms vary from normal versus osteoarthritic chondrocytes and from differentiated versus passaged chondrocytes [26], the results reported in the aforementioned studies cannot be directly translated to a cell-based clinical scenario. In our study, treatment for 3 days with IL-1β mimics the duration of the inflammation caused by the surgical intervention and could thus be relevant to predict some traits of the response of chondrocyte-based grafts to the inflamed cartilage defects. We observed IL-1β treatment caused a reduction in the amount of cartilaginous matrix, which was more pronounced in the immature tissues compared with the mature ones. These results might be the consequence of an enhanced tissue degradation mediated by MMPs: our data show IL-1β treatment increased production of MMP-1 and MMP-13 that was more pronounced in immature pellets. Although the functional activity of such MMPs in our study was not assessed, previous studies reported it may be enhanced by IL-1β [17, 25, 43]. Mature pellets (versus immature pellets) responded to IL-1β by expressing higher amounts of IL-8 and MCP-1 mRNA and releasing a larger amount of these two chemokines. Importantly, the production of IL-8 and MCP-1 was not chronic but strongly reduced a few days after IL-1β withdrawal. The transient release of these chemokines would support recruitment to the grafted area not only of monocytes and neutrophils but also of chondroprogenitor cells [14, 34, 37], possibly resulting in a controlled activation of inflammatory-mediated repair processes (Fig. 7).
Fig. 7A–C.
Schematic view of the hypothesis and results is shown. (A) During the in vitro maturation (in the absence of IL-1β), chondrocyte-based constructs accumulate a large amount of cartilaginous matrix (dark gray) and express/release lower amounts of IL-8 and MCP-1 and higher amounts of TGF-β1. (B) When implanted in the cartilage defect, mature grafts respond to IL-1β by losing some of the accumulated cartilage matrix (light gray) and enhancing the expression/release of IL-8 and MCP-1 in a transient manner and of TGF-β1. (C) IL-8 and MCP-1 would attract neutrophils, monocytes, and chondroprogenitors from the surrounding tissues. Although the former cells would induce tissue remodeling, the chondroprogenitors, especially in the presence of elevated concentration of TGF-β1, would contribute to the deposition of extracellular matrix. Taken together, the contribution of the different recruited cell types would improve/accelerate cartilage repair processes (see dashed arrow), and the transient nature of the enhanced release of IL-8 and MCP-1 would not induce chronic inflammation processes.
In conclusion, the cytokine expression profile and response to IL-1β by HACs are modulated by their differentiation stage. Engineered tissues with higher extent of maturation after IL-1β treatment (1) better preserve the deposited cartilaginous matrix, (2) release lower amounts of MMPs, and (3) transiently release higher amounts of proinflammatory and chondrogenic chemokines. Our findings suggest more mature cartilaginous tissues might be more resistant to IL-1β exposure and more efficiently recruit/commit cells typically involved in tissue repair processes (Fig. 7).
Acknowledgments
We thank Professor Marcel Jakob for the procurement of cartilage samples. We are grateful to Mrs F. Wolf and Jennifer Frueh for their assistance with Luminex assay and to Dr. Sylvie Miot for her precious help in the culture of chondrocytes and in the biochemical analyses.
Footnotes
One or more of the authors (IM) received funding from the Swiss National Science Foundation (Grant 3200B0-110054).
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and informed consent for participation in the study was obtained.
References
- 1.Aulthouse AL, Beck M, Griffey E, Sanford J, Arden K, Machado MA, Horton WA. Expression of the human chondrocyte phenotype in vitro. In Vitro Cell Dev Biol. 1989;25:659–668. doi: 10.1007/BF02623638. [DOI] [PubMed] [Google Scholar]
- 2.Baici A, Lang A, Horler D, Knopfel M. Cathepsin B as a marker of the dedifferentiated chondrocyte phenotype. Ann Rheum Dis. 1988;47:684–691. doi: 10.1136/ard.47.8.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbero A, Grogan SP, Schaefer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 5.Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006;13:194–202. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 7.Blanco FJ, Geng Y, Lotz M. Differentiation-dependent effects of IL-1 and TGF-beta on human articular chondrocyte proliferation are related to inducible nitric oxide synthase expression. J Immunol. 1995;154:4018–4026. [PubMed] [Google Scholar]
- 8.Borzi RM, Mazzetti I, Cattini L, Uguccioni M, Maggiolini M, Facchini A. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 2000;43:1734–1741. doi: 10.1002/1529-0131(200008)43:8<1734::AID-ANR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 9.Borzi RM, Mazzetti I, Macor S, Silvestri T, Bassi A, Cattini L, Facchini A. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999;455:238–242. doi: 10.1016/S0014-5793(99)00886-8. [DOI] [PubMed] [Google Scholar]
- 10.Brittberg M. Autologous chondrocyte implantation—technique and long-term follow-up. Injury. 2008;39(Suppl 1):S40–S49. doi: 10.1016/j.injury.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Candrian C, Bonacina E, Frueh JA, VonWil D, Dickinson S, Wird D, Heberer M, Jakob M, Martin I, Barbero A. Intra-individual comparison of human ankle and knee chondrocytes in vitro: relevance for talar cartilage repair. Osteoarthritis Cartilage. 2009;17:489–496. doi: 10.1016/j.joca.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Darabos N, Hundric-Haspl Z, Haspl M, Markotic A, Darabos A, Moser C. Correlation between synovial fluid and serum IL-1beta levels after ACL surgery—preliminary report. Int Orthop. 2009;33:413–418. doi: 10.1007/s00264-008-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceuninck F, Dassencourt L, Anract P. The inflammatory side of human chondrocytes unveiled by antibody microarrays. Biochem Biophys Res Commun. 2004;323:960–969. doi: 10.1016/j.bbrc.2004.08.184. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O’Brien T, Kerin M. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 15.Fan Z, Bau B, Yang H, Soeder S, Aigner T. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1beta. Arthritis Rheum. 2005;52:136–143. doi: 10.1002/art.20725. [DOI] [PubMed] [Google Scholar]
- 16.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 17.Flannery CR, Little CB, Caterson B, Hughes CE. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 1999;18:225–237. doi: 10.1016/S0945-053X(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg AJ, Lee DA, Bader DL, Bentley G. Autologous chondrocyte implantation: culture in a TGF-beta-containing medium enhances the re-expression of a chondrocytic phenotype in passaged human chondrocytes in pellet culture. J Bone Joint Surg Br. 2005;87:128–134. doi: 10.2106/JBJS.D.01966. [DOI] [PubMed] [Google Scholar]
- 19.Goldring MB, Fukuo K, Birkhead JR, Dudek E, Sandell LJ. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem. 1994;54:85–99. doi: 10.1002/jcb.240540110. [DOI] [PubMed] [Google Scholar]
- 20.Grigolo B, Franceschi L, Roseti L, Cattini L, Facchini A. Down regulation of degenerative cartilage molecules in chondrocytes grown on a hyaluronan-based scaffold. Biomaterials. 2005;26:5668–5676. doi: 10.1016/j.biomaterials.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Grogan SP, Rieser F, Winkelmann V, Berardi S, Mainil-Varlet P. A static, closed and scaffold-free bioreactor system that permits chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11:403–411. doi: 10.1016/S1063-4584(03)00053-0. [DOI] [PubMed] [Google Scholar]
- 22.Handley CJ, Buttle DJ. Assay of proteoglycan degradation. Methods Enzymol. 1995;248:47–58. doi: 10.1016/0076-6879(95)48006-4. [DOI] [PubMed] [Google Scholar]
- 23.Hedbom E, Häuselmann HJ. Molecular aspects of pathogenesis in osteoarthritis: the role of inflammation. Cell Mol Life Sci. 2002;59:45–53. doi: 10.1007/s00018-002-8404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakob M, Demarteau O, Schaefer D, Hintermann B, Dick W, Heberer M, Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2<368::AID-JCB1051>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.Kozaci LD, Buttle DJ, Hollander AP. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum. 1997;40:164–174. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre V, Peeters-Joris C, Vaes G. Modulation by interleukin 1 and tumor necrosis factor alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim Biophys Acta. 1990;1052:366–378. doi: 10.1016/0167-4889(90)90145-4. [DOI] [PubMed] [Google Scholar]
- 27.Lima EG, Tan AR, Tai T, Bian L, Stoker AM, Ateshiam GA, Cook JL, Hung CT. Differences in interleukin-1 response between engineered and native cartilage. Tissue Eng Part A. 2008;14:1721–1730. doi: 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- 28.Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;391(Suppl):S108–S115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 29.Lotz M, Terkeltaub R, Villiger PM. Cartilage and joint inflammation: regulation of IL-8 expression by human articular chondrocytes. J Immunol. 1992;148:466–473. [PubMed] [Google Scholar]
- 30.Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, Kon E, Pederzini L, Rosa D, Sacchetti GL, Zanasi S. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;435:96–105. doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 31.Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22:351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Mayne R, Vail MS, Mayne PM, Miller EJ. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci USA. 1976;73:1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzetti I, Magagnoli G, Paoletti S, Uguccioni M, Olivotto E, Vitellozzi R, Cattini L, Facchini A, Borzi RM. A role for chemokines in the induction of chondrocyte phenotype modulation. Arthritis Rheum. 2004;50:112–122. doi: 10.1002/art.11474. [DOI] [PubMed] [Google Scholar]
- 34.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26:1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 35.Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57:3–8. doi: 10.1016/j.ejrad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Pulsatelli L, Dolzani P, Piacentini A, Silvestri T, Ruggeri R, Gualtieri G, Meliconi R, Facchini A. Chemokine production by human chondrocytes. J Rheumatol. 1999;26:1992–2001. [PubMed] [Google Scholar]
- 37.Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C, Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 38.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 39.Stokes DG, Liu G, Coimbra I, Piera-Valazquez S, Crowl RM, Jimenez SA. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis Rheum. 2002;46:404–419. doi: 10.1002/art.10106. [DOI] [PubMed] [Google Scholar]
- 40.Stove J, Huch K, Gunther KP, Scharf HP. Interleukin-1beta induces different gene expression of stromelysin, aggrecan and tumor-necrosis-factor-stimulated gene 6 in human osteoarthritic chondrocytes in vitro. Pathobiology. 2000;68:144–149. doi: 10.1159/000055915. [DOI] [PubMed] [Google Scholar]
- 41.Stroebel S, Loparic M, Wendt D, Schenk AD, Candrian C, Linberg RL, Moldovan F, Barbero A, Martin I. Anabolic and catabolic response of human articular chondrocytes to varying oxygen percentages. Arthritis Res Ther. 2010;12:R34. doi: 10.1186/ar2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem Biophys Res Commun. 1994;200:142–148. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 43.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 44.Villiger PM, Terkeltaub R, Lotz M. Monocyte chemoattractant protein-1 (MCP-1) expression in human articular cartilage: induction by peptide regulatory factors and differential effects of dexamethasone and retinoic acid. J Clin Invest. 1992;90:488–496. doi: 10.1172/JCI115885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watt FM. Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. J Cell Sci. 1988;89:373–378. doi: 10.1242/jcs.89.3.373. [DOI] [PubMed] [Google Scholar]
- 46.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Muller PE, Evans CH, Porter EM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60:801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu C, Oyajobi BO, Frazer A, Kozaci LD, Russel RG, Hollander AP. Effects of growth factors and interleukin-1 alpha on proteoglycan and type II collagen turnover in bovine nasal and articular chondrocyte pellet cultures. Endocrinology. 1996;137:3557–3565. doi: 10.1210/en.137.8.3557. [DOI] [PubMed] [Google Scholar]
- 48.Yuan GH, Masuko-Hongo K, Sakata M, Tsuruha J, Onuma H, Nakamura H, Aoki H, Kato T, Nishioka K. The role of C-C chemokines and their receptors in osteoarthritis. Arthritis Rheum. 2001;44:1056–1070. doi: 10.1002/1529-0131(200105)44:5<1056::AID-ANR186>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 49.Zwicky R, Baici A. Cytoskeletal architecture and cathepsin B trafficking in human articular chondrocytes. Histochem Cell Biol. 2000;114:363–372. doi: 10.1007/s004180000199. [DOI] [PubMed] [Google Scholar]