Abstract
Background
Mechanical stimuli are of crucial importance for the development and maintenance of articular cartilage. For conditioning of cartilaginous tissues, various bioreactor systems have been developed that have mainly aimed to produce cartilaginous grafts for tissue engineering applications. Emphasis has been on in vitro preconditioning, whereas the same devices could be used to attempt to predict the response of the cells in vivo or as a prescreening method before animal studies. As a result of the complexity of the load and motion patterns within an articulating joint, no bioreactor can completely recreate the in vivo situation.
Questions/purposes
This article aims to classify the various loading bioreactors into logical categories, highlight the response of mesenchymal stem cells and chondrocytes to the various stimuli applied, and determine which data could be used within a clinical setting.
Methods
We performed a Medline search using specific search terms, then selectively reviewed relevant research relating to physical stimulation of chondrogenic cells in vitro, focusing on cellular responses to the specific load applied.
Results
There is much data pertaining to increases in chondrogenic gene expression as a result of controlled loading protocols. Uniaxial loading leads to selective upregulation of genes normally associated with a chondrogenic phenotype, whereas multiaxial loading results in a broader pattern of chondrogenic gene upregulation. The potential for the body to be used as an in vivo bioreactor is being increasingly explored.
Conclusions
Bioreactors are important tools for understanding the potential response of chondrogenic cells within the joint environment. However, to replicate the natural in vivo situation, more complex motion patterns are required to induce more physiological chondrogenic gene upregulation.
Introduction
Autologous chondrocyte transplantation procedures, which may or may not include supporting matrices, are increasingly being adopted for the repair of articular cartilage defects. Since the pioneering work of Brittberg et al., the technique has continuously been further developed [5, 6, 8]. Recent research has focused on tissue engineering (TE) solutions with the aim to generate in vitro functional constructs for implantation. One major drawback of all common TE approaches is the long and often complex in vitro preculture period before implantation of the graft. This is associated with immense requirements on the safety of such products and in turn very high costs. It is evident that both the manipulation steps and the culture time should be maintained at a minimum for eventual clinical application. In fact, one-step procedures in combination with appropriate rehabilitation programs have the greatest potential to be realized. The question remains how to test the performance of different strategies and optimize the procedure before initiating in vivo animal and clinical studies because a variety of parameters, including cell source, density, carrier, expansion, culture supplements, and postoperative physical and molecular therapy, are still a matter of debate.
A wide array of materials have been used in various in vivo [20, 81, 91, 94] and in vitro [2, 7, 12, 16, 35, 41, 48, 82] studies for chondrocytes and chondrogenesis of mesenchymal stem cells (MSCs). The most frequent synthetic foam structures used are poly(α-hydroxyacid)s, their copolymers, and poly(ester-urethane) [65, 81]. Hydrogels or highly hydrated polymeric scaffolds such as agarose [41, 48], alginate [7, 12, 16, 20, 61, 82], fibrin [54, 55], hyaluronan [2], and collagen [7, 91] have been extensively used together with hybrid systems incorporating both a hydrogel and a stiffer, macroporous synthetic foam [94]. We have found a combination of a biodegradable poly(ester-urethane) scaffold and fibrin hydrogel is suitable for chondrocyte culture [54] and chondrogenesis of human MSCs [55]. Clinically available scaffolds are almost exclusively natural polymers [34].
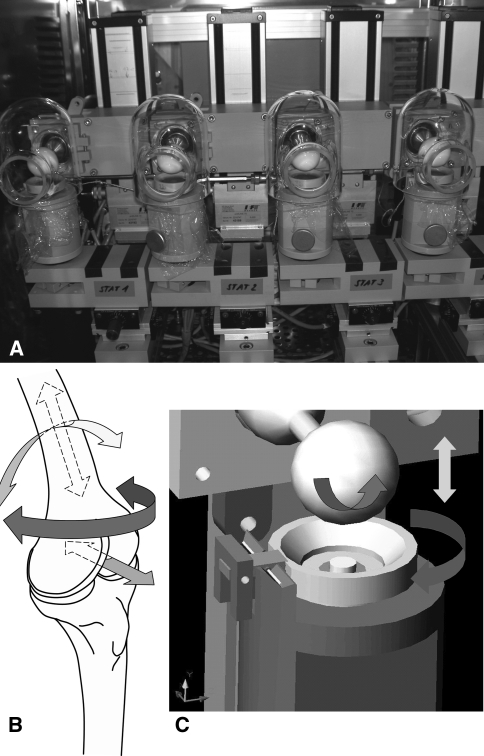
Bioreactor systems that closely reproduce the in vivo environment offer the possibility to evaluate novel therapeutic approaches. Bioreactors can provide the technical means to perform controlled studies taking into account specific biologic, biochemical, or biomechanical effects. Traditionally, bioreactors have been used with the idea of preconditioning implants to improve their quality before implantation. However, bioreactors also provide the opportunity to study the cellular response to mechanical stimulation under defined conditions. Many different groups have designed and built bioreactors that can apply compression [2, 12, 39, 69, 86] (for example see Fig. 1). Also, bioreactors have been developed that can apply shear, ranging from uncontrolled [84] to fully defined computer-controlled shear application, which allows for regulated frequency and amplitude [98] (Fig. 1). The use of these increasingly complex bioreactors can help to gain important insight into the role of biomechanics in chondrocyte phenotype and the regulation of stem cell fate, particularly of pluripotent adult MSCs [2, 39, 80].
Fig. 1A–C.
(A) A four-station bioreactor that is capable of applying multiaxial load. Each station can be individually programmed, and each sample can also be cultured in its own, individual medium. The bioreactor fits into a standard cell culture incubator and therefore also allows for the control of oxygen tension. The sample holders can be easily removed, allowing for multiple samples to be loaded. (B) Schematic showing the directions of movement found within a human knee and (C) the directions of movement that can be applied using a multiaxial bioreactor. In each case, 6 degree-of-freedom kinematics is possible. To simulate rotation around the femur, the sample holder can be rotated around its axis. To simulate knee flexion, the ceramic hip ball can be rotated. The ceramic hip ball can also be moved in the Z axis to apply compression of the sample. Each parameter can be modified for frequency and amplitude. This bioreactor was developed by Wimmer and is in use in Chicago, IL, and Davos, Switzerland. It has been used extensively both for chondrocytes, where it has been shown the requirement for shear to upregulate PRG4 expression, and with human mesenchymal stem cells, where it has demonstrated the role of transforming growth factor-β in mechanically induced chondrogenesis.
The purposes of our review are to: (1) highlight the response of MSCs and chondrocytes to the different specific stimuli applied; (2) detail conditions in which physical stimulation has been combined with other regulatory influences such as growth factors; and (3) determine whether the data could be used within a clinical setting.
Search Strategy and Criteria
We searched all published literature in the English language using MEDLINE. An original search of (Mesenchymal stem cells OR chondrocytes OR Mesenchymal stem cell OR chondrocyte or MSC) AND (chondrogenesis OR shear OR load OR compression OR hydrostatic) yielded 2698 references. Those not involving bioreactors were excluded (this search term was not used initially because a number of papers involving physical stimulation do not use this term). Those involving only explant culture, fibrocartilage, or meniscus were also excluded. Because the terminology used in studies involving physical stimulation can be quite varied, further references were obtained during the course of reading the initial references.
Regarding terminology used during the review, we have used the most commonly accepted terms when discussing the type of load applied. We understand that in some cases a more accurate term could be used, but we believe this would lead to confusion for many readers.
Hydrostatic Pressure
During loading of the joint, water from the synovial fluid is retained within the cartilage matrix by the presence of charged proteoglycans, resulting in increased hydrostatic pressure (HP). The collagen network functions to prevent swelling of the tissue. Experimental studies indicate intermittent application of HP in the physiological range of 7 to 10 MPa over longer time periods promotes matrix synthesis, whereas constant pressure seems unsuitable [22].
A number of bioreactors use HP as the means by which the cells are stimulated. However, because several parameters, including the magnitude, frequency, onset, and duration of application of HP, can be varied, a range of culture conditions have been used [22]. It has been demonstrated that 5 MPa load at 0.5 Hz inhibited GAG synthesis in the monolayer while stimulating GAG synthesis in explant culture [75]. Moreover, juvenile and adult chondrocytes cultured in polyglycolic acid meshes seem to react differently to intermittent pressurization [15]. Scaffold-free constructs were also exposed to cyclic HP with increases in their matrix accumulation compared with control constructs [38]. Interestingly, static HP in a physiological range of 3 to 10 MPa has in general produced stimulatory effects on chondrocytes in a three-dimensional environment. When cultured in collagen scaffolds, immature bovine chondrocytes increased their proteoglycan production on application of static HP [68]. Similar results were obtained with chondrocytes in agarose gel cultures and scaffold-free constructs [21, 87]. Because most studies on the effect of HP on TE cartilage have been performed with often immature bovine chondrocytes, the outcome should only be translated to human cells and tissue after careful evaluation.
In alginate beads, apoptosis in rabbit chondrocytes was induced by 10 MPa static pressure, whereas no apoptotic cells were observed at atmospheric pressure [71]. In a hyaluronan-based scaffold (Hyaff 11), a dynamic pressure of 5 MPa at 1 Hz for 4 hours leads also to an increase of apoptotic human chondrocytes [95]. Others have found that in Type I collagen gel, under 40 KPa at 0.0125 Hz, Type II collagen and aggrecan were upregulated at the gene expression level but not at the protein level for human chondrocytes cultured for 14 days [28]. Finally, the phenotypic and proteomic expression pattern of chondrocytes seeded in a polyurethane scaffold (Degrapol®, Abmedica, Italy) with HP of 10 MPa at 0.33 Hz 4 hours per day was similar to those from nonloaded cells, although the maintenance of cell viability was improved after 3 days [13].
HP has also been applied for MSC differentiation toward the chondrocytic phenotype [3, 67, 90]. From studies performed with human MSCs, it can be concluded that repetitive application of HP over several days is necessary to achieve a major effect. In addition, several studies suggest the peak effects of HP may be delayed until some weeks after termination of loading, which impedes comparison of loaded samples analyzed at different time points after stimulation [22, 60, 66].
Using human bone marrow-derived stem cells, Miyanishi et al. compared 0.1, 1, and 10 MPa using HP and demonstrated increased collagen II and aggrecan expression with 10 MPa leading to the greatest increase [66].
These data demonstrate that cells embedded within tissue-engineered constructs increase the expression and production of cartilage-specific matrix proteins in response to intermittent hydrostatic pressure applied at physiological loads.
Tension
Tensile loading is not generally regarded as physiologically relevant for articular cartilage and has therefore attracted little attention. The studies that have investigated dynamic tension on TE cartilage have mainly observed negligible or inhibitory effects. Compared with unloaded controls, 10% and 20% displacement inhibited proteoglycan synthesis in chondrocytes, whereas 5% displacement had no effect [18]. Cyclic tensile stretching of bovine chondrocytes inhibits protein kinase C activity [27]. On the other hand, it was suggested cyclic tension activates the hypertrophic pathway and increases the expression of markers typically associated with chondrocytes undergoing terminal differentiation to hypertrophic chondrocytes (eg, collagen X) [99]. Because there is evidence that articular cartilage in vivo is under a degree of static pretension, the effect of intermittent static biaxial tensile strains on cartilaginous constructs was studied [24]. Average magnitudes of 3.8% radial and 2.1% circumferential tensile strains for 30 minutes were identified as leading to the greatest increase in proteoglycan content.
Compression
By far the greatest number of studies involving mechanical load has been performed using bioreactors that apply uniaxial compression. Direct compression results from direct contact between joint surfaces and has been simulated in a diverse number of reactor systems. For articular cartilage of the major weightbearing joints in the hip and the knee, average loadings of approximately 0.5 to 7.7 MPa and average compression amplitudes of more than 13% have been measured during normal daily movements [1, 70, 89]. A wide range of loading frequencies (0.001–5 Hz) and amplitudes has been applied to TE cartilaginous constructs.
In one study comparing different loads and frequencies on native cartilage, increasing frequency produced an increased aggrecan synthesis in regions of high interstitial fluid flow [11]. Compression amplitudes [78] and shear [25] also play a role.
Many different biomaterial scaffolds have been used to study the effect of dynamic loading on chondrocytes and MSCs [32, 45, 56, 63, 74, 93, 100]. There is a strong dependency of the mechanical signal sensed by the cells on the material mechanical properties as shown by the wide range of compressive load applied. Tailored synthetic crosslinked poly(ethylene glycol) hydrogels have been prepared to study the interactions between chondrocytes and material in compressive static and dynamic culture systems [9, 72]. Over short culture periods, dynamic loading of 15% strain at 1 Hz did not affect considerably the chondrocyte extracellular matrix gene expression (Type I and II collagen and aggrecan) compared with the static mode when encapsulated in a nondegradable crosslinked pure poly(ethylene glycol) hydrogel [72]. At first glance, this seems to contradict others’ findings that demonstrate the importance of dynamic loading on chondrocyte gene expression and differentiation of MSCs encapsulated in a three-dimensional matrix. However, chondrocytes cultured in a similar poly(ethylene glycol) hydrogel decorated with RGD peptides, which can act as a binding site for chondrocytes, showed substantial gene expression upregulation under mechanical loading compared with static culture [88]. Even if the presence of RGD peptides is not beneficial to the conservation of chondrocyte gene expression, this demonstrates the importance of the interaction/binding between the encapsulating matrix (ie, poly[ethylene glycol]) and the chondrocytes for conveying mechanical signals [88]. The importance of scaffold binding sites or “bioactivity” to transmit mechanical signals to seeded chondrocytes has also been demonstrated for other matrices [4].
MSC survival and differentiation are markedly dependent on their ability to attach on the substrate they have been seeded on. Therefore, similarly as for chondrocytes, the ability of the cells to bind the material is critical to convey mechanical signals. This is one reason for the frequent use of fibrin gel, a favorable substrate for cell attachment with mechanical properties easily tuned by variation of concentration and gelling mechanism. The effect of the hydrogel stiffness on viability could be clearly demonstrated using fibrin gels of different concentrations [76].
A number of studies involving dynamic compression suggest a beneficial effect of load for chondrogenesis of MSCs, resulting in an increase in collagen II and aggrecan. Most of these studies used a frequency of 1 Hz and either 10% [39, 69, 86] or 15% [12, 40] compression. These are similar magnitudes to those that lead to the greatest increase in chondrogenic gene expression and GAG synthesis in chondrocytes [17, 63, 64].
Taken together these data suggest culture in a scaffold material that allows for cell attachment, combined with greater than 10% compression at a frequency of 1 Hz, may be a suitable starting point for the physical stimulation of both MSCs and chondrocytes.
Combined Compression and Shear
To use cartilage bioreactor systems as a supporting tool to simulate and predict in vivo processes, the applied load and motion pattern should attempt to mimic the in vivo conditions. In reality this is quite difficult to predict, because in vivo loads can vary greatly even within the same joint. During load, the lateral tibial plateau has a greater cartilage contact deformation, but lower cartilage contact area, than the medial compartment during the same loading cycle [37]. Both compartments demonstrated a cartilage contact deformation of between 10% and 15% and the bulk of this deformation was generated during the first 20 seconds of load [37]. The rotation of the femur with respect to the tibia varies during gait with twin peaks of external rotation being observed during early midstance and toe-off [50]. The same study demonstrated twin peak of valgus at similar points of the stance phase [50]. It is interesting to note this corresponds with the twin peaks of load measured in dogs during gait [29]. Taking these data into account it is clear the loads and pattern of motion in the tibiofemoral joint are complex during gait, and it is unlikely uniaxial loading devices can invoke the complete range of cellular responses. The data obtained under weightbearing gait are different from that under nonweightbearing flexion [50]. This, combined with the lack of clear data, makes it difficult to determine to what extent multiaxial load bioreactors mimic the in vivo environment. Shear stress is a potent modulator of the amount and type of extracellular matrix synthesized [30]. One group suggested the best way to envision the typical loading processes affecting articular cartilage is to recognize them as a rolling movement of direct compression in concert with a generation of shear and tensile forces and high hydrostatic pressure [36]. The complex kinematic environment to which cartilage is exposed has increasingly led to a more tribologic approach in the design of cartilage bioreactors. Tribology is defined as the science and technology of interacting surfaces in relative motion [19]. A number of groups have designed bioreactors that incorporate these features in their design [73, 84, 98] (Fig. 1).
Combinatory systems have been developed that enable the application of intermittent cyclic shearing forces and axial deformations. A biaxial tissue loading device capable of applying axial deformations and sinusoidal rotations or shear deformation was widely applied for stimulation of cartilage explants and engineered constructs [26, 42, 43, 47]. Dynamic combined compression-shear stimulation (5% compression and 5% shear strain amplitudes) increased both collagen and proteoglycan synthesis [92], and application of multiaxial loading over 4 weeks substantially improved matrix accumulation and mechanical properties of in vitro-formed tissues.
We have developed a bioreactor that mimics complex three-dimensional motion and presumably can more accurately mimic the in vivo situation [98]. This provides us with opportunities to compare various loading protocols, involving multidirectional load, in a controlled, defined system [33, 58, 98].
Mechanical stimuli play a crucial role for the development of both the articular surface and the underlying zones. For instance, the effect of sliding surface motion on proteoglycan 4 (PRG4, lubricin) expression was most pronounced in chondrocytes that originated from deep zones of the articular cartilage that did not physiologically express PRG4 [57]. Both PRG4 and cartilage oligomeric matrix protein gene expression are markedly enhanced by applying sliding motion to the surface of a three-dimensional scaffold, whereas the upregulation of collagen Type II and aggrecan was more associated with the application of compression [32, 33]. Further studies revealed the velocity magnitude is a critical determinant for the cellular responses to oscillating sliding motion [97].
Synergistic Effects of Compression and Shear With Other Factors
Combining the application of compression and shear with a microenvironment more similar to that found in the in vivo joint environment offers a potential in vitro model of the in vivo environment. In terms of stabilization of the chondrogenic phenotype, an additive effect of hypoxia, combined with compression and shear, has been observed [96]. Different approaches have been taken to engineer tissues that mimic the organization of natural articular cartilage [49]. Mechanical stimuli play a crucial role for the development of both the articular surface and the underlying zones. For instance, the effect of sliding surface motion on PRG4 expression was most pronounced in chondrocytes that originated from deep zones of the articular cartilage that did not physiologically express PRG4 [57]. This indicates a certain plasticity of differentiated chondrocytes and the potential to adapt to changing environmental conditions and mechanical requirements. In accordance with this observation, human nasal chondrocytes were responsive to physical forces recapitulating joint movement and upregulated molecules involved in joint lubrication that are normally not present in nasal cartilage [14].
One potential reason in vitro MSC chondrogenesis commonly leads to terminal hypertrophy is the fact that the developing tissue is not stimulated. Under these conditions, mathematical models developed for fracture repair predict bone will be formed [46, 52, 53, 77]. Addition of low amounts of tissue shear strain and fluid flow is predicted to result in cartilage. Indeed, the regulation of stem cell fate can be modulated by applying different loading regimes, supporting to an extent the predictions made by the model described. A comparison of loading protocols varying the frequency (0.1 Hz and 1 Hz) and dynamic compression amplitudes (5%, 10%, and 20%), in combination with shear, showed the chondrogenic response of human MSCs can be modulated by differing the loading patterns applied [58]. A similar relationship was found in human MSC pellets and hydrostatic pressure [66, 67], static-loaded mouse embryonic limb bud mesenchymal cells [85], and cyclic-loaded chick limb bud mesenchymal cells [23].
Various growth factors (ie, fibroblast growth factor-2, transforming growth factor-β, insulin-like growth factor-1, and osteogenic protein-1) have been used to modulate the chondrogenesis of MSCs, chondrocyte phenotype, proliferation, and biosynthesis rates; often additive or synergistic effects of mechanical and molecular stimuli have been observed [21, 31, 56, 62]. Chondrogenic medium containing dexamethasone and transforming growth factor-β1 has been developed to induce chondrogenic differentiation of chondroprogenitor cells [44]. However, when considering the natural in vivo repair environment, one must also consider what would be the natural source of these factors. Chondrogenesis can be induced in vitro in the absence of transforming growth factor-β when HP [67], compression [39], or compression and shear [56] are applied. An approach to remove the necessity of repeated dosing with growth factors is the use of gene therapy. Synergistic effects of gene therapy with bone morphogenetic protein-2 and joint-simulating mechanical loading were demonstrated, which may also have implications for in vivo growth factor treatment [79]. Compression and shear, in combination with adenoviral gene transfer of the coding sequence for the SOX9 transcription factor, also has the potential to increase the chondrogenic response in the complete absence of any further chondrogenic stimuli [51].
Discussion
There are large numbers of bioreactors that are increasingly being used to stimulate chondrogenic cells. The types of load, and loading patterns used, are varied. Within this review, we aimed to detail which types of loads are applied to chondrogenic cells in vitro and then determine how effective the load applied is in enhancing the chondrocytic phenotype. We hope to use this information to highlight the increasing complexity to mechanical stimulation devices. The purposes of our review are to: (1) highlight the response of MSCs and chondrocytes to the different specific stimuli applied; (2) detail conditions where physical stimulation has been combined with other regulatory influences such as growth factors; and (3) determine whether the data could be used within a clinical setting.
Our review is limited by a number of factors. First, the terminology used to describe the type of load applied in studies involving physical stimulation is vast. This could easily lead to papers not being discovered during the original search. Second, there is little consistency between loading parameters applied, which creates difficulties in comparing results. Third, groups wishing to embark on such studies often develop their own custom-built machines. This greatly limits the capacity to compare data and, unless each machine is carefully validated, the stated parameters applied might not be the actual parameters applied.
The cellular response to load does seem to be specific to the type of load applied. Also, cyclical load leads to a more favorable response than constant load. The application of shear is required for the expression of certain chondrogenic genes such as PRG4, demonstrating compression alone is not sufficient to upregulate all the required chondrogenic genes. Complex mechanical motion studies in bioreactors suggest it plays a major role in modifying the cell phenotype. Under these conditions, chondrogenic cells upregulate the synthesis of transforming growth factor-β, which is then responsible for the chondrogenic response [56]. Thus, in the natural in vivo environment, a suitable rehabilitation protocol could be used to increase the synthesis of transforming growth factor-β leading to an appropriate chondrogenic response.
This also suggests certain studies involving the application of load should be carried out in the absence of exogenous growth factors when trying to predict what effect would be achieved under in vivo conditions. Also, it may be possible to reduce the number of animal studies performed by testing various conditions in vitro in the presence of suitable mechanical stimuli. When the load should be applied is still open to discussion. Some studies have suggested a period of free swelling culture before the application of compression may be beneficial [10, 63]. This is potentially the result of the extent and maturity of the pericellular matrix.
More groups are becoming interested in cell-based strategies that are less cell culture-intensive, presuming cells can be implanted within a single surgical procedure and the body itself would be the bioreactor. This shift away from the classic style TE to the concept of an in vivo bioreactor is becoming more widespread, assuming the natural environment is the most suitable environment for the tissue development and as such cell culture and bioreactors may not be required. Already progress has been made within the bone field [83]. Currently, such studies would require animal models to determine the role of mechanics; however, this is complicated by the fact that it is difficult to ensure each animal receives the same stimulus. Some of the bioreactors now being used would provide an excellent screening method to determine some basic information, which can be used to extrapolate optimized rehabilitation protocols. An ideal scenario would provide the opportunity to implant cells within the scaffold material within one operation, thus removing the need for expensive cell culture. Because CaRes® already adopts an approach that does not include a monolayer proliferation step, it suggests sufficient cell numbers may already be available. Attempts to eliminate cell culture have been made using minced cartilage intraoperatively as a cell source, which led to enhanced repair [59]. Although this method used cartilage, and therefore chondrocytes, as a cell source, it is likely future single-surgery treatments will use stem cells that can be isolated during the operation and manipulated within the operating room before immediate implantation.
To conclude, complex motion that includes compression and shear should be used to provide a wide-ranging response that is more physiological. This would allow more physiologically relevant studies to be conducted in vitro, moving directly into patients to test the suitability of the rehabilitation protocol.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Afoke NY, Byers PD, Hutton WC. Contact pressures in the human hip joint. J Bone Joint Surg Br. 1987;69:536–541. doi: 10.1302/0301-620X.69B4.3611154. [DOI] [PubMed] [Google Scholar]
- 2.Angele P, Schumann D, Angele M, Kinner B, Englert C, Hente R, Fuchtmeier B, Nerlich M, Neumann C, Kujat R. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004;41:335–346. [PubMed] [Google Scholar]
- 3.Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21:451–457. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 4.Appelman TP, Mizrahi J, Elisseeff JH, Seliktar D. The differential effect of scaffold composition and architecture on chondrocyte response to mechanical stimulation. Biomaterials. 2009;30:518–525. doi: 10.1016/j.biomaterials.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett W, Gooding CR, Carrington RW, Skinner JA, Briggs TW, Bentley G. Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft. A preliminary report. J Bone Joint Surg Br. 2005;87:330–332. doi: 10.1302/0301-620X.87B3.15552. [DOI] [PubMed] [Google Scholar]
- 6.Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006;13:194–202. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 8.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 9.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004;32:407–417. doi: 10.1023/B:ABME.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 10.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108:1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 11.Buschmann MD, Kim YJ, Wong M, Frank E, Hunziker EB, Grodzinsky AJ. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366:1–7. doi: 10.1006/abbi.1999.1197. [DOI] [PubMed] [Google Scholar]
- 12.Campbell JJ, Lee DA, Bader DL. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology. 2006;43:455–470. [PubMed] [Google Scholar]
- 13.Candiani G, Raimondi MT, Aurora R, Lagana K, Dubini G. Chondrocyte response to high regimens of cyclic hydrostatic pressure in 3-dimensional engineered constructs. Int J Artif Organs. 2008;31:490–499. doi: 10.1177/039139880803100604. [DOI] [PubMed] [Google Scholar]
- 14.Candrian C, Vonwil D, Barbero A, Bonacina E, Miot S, Farhadi J, Wirz D, Dickinson S, Hollander A, Jakob M, Li Z, Alini M, Heberer M, Martin I. Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis Rheum. 2008;58:197–208. doi: 10.1002/art.23155. [DOI] [PubMed] [Google Scholar]
- 15.Carver SE, Heath CA. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol Bioeng. 1999;62:166–174. doi: 10.1002/(SICI)1097-0290(19990120)62:2<166::AID-BIT6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Caterson EJ, Nesti LJ, Li WJ, Danielson KG, Albert TJ, Vaccaro AR, Tuan RS. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57:394–403. doi: 10.1002/1097-4636(20011205)57:3<394::AID-JBM1182>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury TT, Bader DL, Shelton JC, Lee DA. Temporal regulation of chondrocyte metabolism in agarose constructs subjected to dynamic compression. Arch Biochem Biophys. 2003;417:105–111. doi: 10.1016/S0003-9861(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 18.Connelly JT, Vanderploeg EJ, Levenston ME. The influence of cyclic tension amplitude on chondrocyte matrix synthesis: experimental and finite element analyses. Biorheology. 2004;41:377–387. [PubMed] [Google Scholar]
- 19.Czichos H. Tribilogy: A Systems Approach to the Science and Technology of Friction, Lubrication, and Wear. Amsterdam: Elsevier; 1978. [Google Scholar]
- 20.Diduch DR, Jordan LC, Mierisch CM, Balian G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy. 2000;16:571–577. doi: 10.1053/jars.2000.4827. [DOI] [PubMed] [Google Scholar]
- 21.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev. 2009;15:43–53. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elder SH, Goldstein SA, Kimura JH, Soslowsky LJ, Spengler DM. Chondrocyte differentiation is modulated by frequency and duration of cyclic compressive loading. Ann Biomed Eng. 2001;29:476–482. doi: 10.1114/1.1376696. [DOI] [PubMed] [Google Scholar]
- 24.Fan JC, Waldman SD. The effect of intermittent static biaxial tensile strains on tissue engineered cartilage. Ann Biomed Eng. 2010;38:1672–1682. doi: 10.1007/s10439-010-9917-5. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald JB, Jin M, Grodzinsky AJ. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem. 2006;281:24095–24103. doi: 10.1074/jbc.M510858200. [DOI] [PubMed] [Google Scholar]
- 26.Frank EH, Jin M, Loening AM, Levenston ME, Grodzinsky AJ. A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J Biomech. 2000;33:1523–1527. doi: 10.1016/S0021-9290(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda K, Asada S, Kumano F, Saitoh M, Otani K, Tanaka S. Cyclic tensile stretch on bovine articular chondrocytes inhibits protein kinase C activity. J Lab Clin Med. 1997;130:209–215. doi: 10.1016/S0022-2143(97)90098-6. [DOI] [PubMed] [Google Scholar]
- 28.Gavenis K, Kremer A, Walter M, Hollander DA, Schneider U, Schmidt-Rohlfing B. Effects of cyclic hydrostatic pressure on the metabolism of human osteoarthritic chondrocytes cultivated in a collagen gel. Artif Organs. 2007;31:91–98. doi: 10.1111/j.1525-1594.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 29.Geffre CP, Bliss CL, Szivek JA, DeYoung DW, Ruth JT, Margolis DS. Sensate scaffolds coupled to telemetry can monitor in vivo loading from within a joint over extended periods of time. J Biomed Mater Res B Appl Biomater. 2008;84:263–270. doi: 10.1002/jbm.b.30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gemmiti CV, Guldberg RE. Shear stress magnitude and duration modulates matrix composition and tensile mechanical properties in engineered cartilaginous tissue. Biotechnol Bioeng. 2009;104:809–820. doi: 10.1002/bit.22440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Bursac PM, Vunjak-Novakovic G, Freed LE. IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem Biophys Res Commun. 2001;286:909–915. doi: 10.1006/bbrc.2001.5486. [DOI] [PubMed] [Google Scholar]
- 32.Grad S, Gogolewski S, Alini M, Wimmer MA. Effects of simple and complex motion patterns on gene expression of chondrocytes seeded in 3D scaffolds. Tissue Eng. 2006;12:3171–3179. doi: 10.1089/ten.2006.12.3171. [DOI] [PubMed] [Google Scholar]
- 33.Grad S, Lee CR, Wimmer MA, Alini M. Chondrocyte gene expression under applied surface motion. Biorheology. 2006;43:259–269. [PubMed] [Google Scholar]
- 34.Haasper C, Zeichen J, Meister R, Krettek C, Jagodzinski M. Tissue engineering of osteochondral constructs in vitro using bioreactors. Injury. 2008;39(Suppl 1):S66–S76. doi: 10.1016/j.injury.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 35.Hauselmann HJ, Aydelotte MB, Schumacher BL, Kuettner KE, Gitelis SH, Thonar EJ. Synthesis and turnover of proteoglycans by human and bovine adult articular chondrocytes cultured in alginate beads. Matrix. 1992;12:116–129. doi: 10.1016/s0934-8832(11)80053-3. [DOI] [PubMed] [Google Scholar]
- 36.Heath CA, Magari SR. Mini-review: mechanical factors affecting cartilage regeneration in vitro. Biotechnol Bioeng. 1996;50:430–437. doi: 10.1002/(SICI)1097-0290(19960520)50:4<430::AID-BIT10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 37.Hosseini A, Velde SK, Kozanek M, Gill TJ, Grodzinsky AJ, Rubash HE, Li G. In-vivo time-dependent articular cartilage contact behavior of the tibiofemoral joint. Osteoarthritis Cartilage. 2010;18:909–916. doi: 10.1016/j.joca.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12:1337–1344. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 39.Huang CY, Hagar KL, Frost LE, Sun Y, Cheung HS. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22:313–323. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- 40.Huang CY, Reuben PM, Cheung HS. Temporal expression patterns and corresponding protein inductions of early responsive genes in rabbit bone marrow-derived mesenchymal stem cells under cyclic compressive loading. Stem Cells. 2005;23:1113–1121. doi: 10.1634/stemcells.2004-0202. [DOI] [PubMed] [Google Scholar]
- 41.Huang CY, Reuben PM, D’Ippolito G, Schiller PC, Cheung HS. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec A Discov Mol Cell Evol Biol. 2004;278:428–436. doi: 10.1002/ar.a.20010. [DOI] [PubMed] [Google Scholar]
- 42.Jin M, Emkey GR, Siparsky P, Trippel SB, Grodzinsky AJ. Combined effects of dynamic tissue shear deformation and insulin-like growth factor I on chondrocyte biosynthesis in cartilage explants. Arch Biochem Biophys. 2003;414:223–231. doi: 10.1016/S0003-9861(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 43.Jin M, Frank EH, Quinn TM, Hunziker EB, Grodzinsky AJ. Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch Biochem Biophys. 2001;395:41–48. doi: 10.1006/abbi.2001.2543. [DOI] [PubMed] [Google Scholar]
- 44.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 45.Jung Y, Kim SH, Kim SH, Kim YH, Xie J, Matsuda T, Min BG. Cartilaginous tissue formation using a mechano-active scaffold and dynamic compressive stimulation. J Biomater Sci Polym Ed. 2008;19:61–74. doi: 10.1163/156856208783227712. [DOI] [PubMed] [Google Scholar]
- 46.Kelly DJ, Prendergast PJ. Mechano-regulation of stem cell differentiation and tissue regeneration in osteochondral defects. J Biomech. 2005;38:1413–1422. doi: 10.1016/j.jbiomech.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 47.Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595–604. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Kisiday JD, Kopesky PW, Evans CH, Grodzinsky AJ, McIlwraith CW, Frisbie DD. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008;26:322–331. doi: 10.1002/jor.20508. [DOI] [PubMed] [Google Scholar]
- 49.Klein TJ, Malda J, Sah RL, Hutmacher DW. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev. 2009;15:143–157. doi: 10.1089/ten.teb.2008.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kozanek M, Hosseini A, Liu F, Velde SK, Gill TJ, Rubash HE, Li G. Tibiofemoral kinematics and condylar motion during the stance phase of gait. J Biomech. 2009;42:1877–1884. doi: 10.1016/j.jbiomech.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kupcsik L, Stoddart MJ, Li Z, Benneker LM, Alini M. Improving chondrogenesis—potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng Part A. 2010;16:1845–1855. doi: 10.1089/ten.tea.2009.0531. [DOI] [PubMed] [Google Scholar]
- 52.Lacroix D, Prendergast PJ. A mechano-regulation model for tissue differentiation during fracture healing: analysis of gap size and loading. J Biomech. 2002;35:1163–1171. doi: 10.1016/S0021-9290(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 53.Lacroix D, Prendergast PJ, Li G, Marsh D. Biomechanical model to simulate tissue differentiation and bone regeneration: application to fracture healing. Med Biol Eng Comput. 2002;40:14–21. doi: 10.1007/BF02347690. [DOI] [PubMed] [Google Scholar]
- 54.Lee CR, Grad S, Gorna K, Gogolewski S, Goessl A, Alini M. Fibrin-polyurethane composites for articular cartilage tissue engineering: a preliminary analysis. Tissue Eng. 2005;11:1562–1573. doi: 10.1089/ten.2005.11.1562. [DOI] [PubMed] [Google Scholar]
- 55.Li Z, Kupcsik L, Yao S, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites. Tissue Eng Part A. 2009;15:1729–1737. doi: 10.1089/ten.tea.2008.0247. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Kupcsik L, Yao SJ, Alini M, Stoddart MJ. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-beta pathway. J Cell Mol Med. 2010;14:1338–1346. doi: 10.1111/j.1582-4934.2009.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z, Yao S, Alini M, Grad S. Different response of articular chondrocyte subpopulations to surface motion. Osteoarthritis Cartilage. 2007;15:1034–1041. doi: 10.1016/j.joca.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Li Z, Yao SJ, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A. 2010;16:575–584. doi: 10.1089/ten.tea.2009.0262. [DOI] [PubMed] [Google Scholar]
- 59.Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, Binette F. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261–1270. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- 60.Luo ZJ, Seedhom BB. Light and low-frequency pulsatile hydrostatic pressure enhances extracellular matrix formation by bone marrow mesenchymal cells: an in-vitro study with special reference to cartilage repair. Proc Inst Mech Eng H. 2007;221:499–507. doi: 10.1243/09544119JEIM199. [DOI] [PubMed] [Google Scholar]
- 61.Ma HL, Hung SC, Lin SY, Chen YL, Lo WH. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res A. 2003;64:273–281. doi: 10.1002/jbm.a.10370. [DOI] [PubMed] [Google Scholar]
- 62.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 63.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 64.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879–890. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Meinel L, Hofmann S, Karageorgiou V, Zichner L, Langer R, Kaplan D, Vunjak-Novakovic G. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnol Bioeng. 2004;88:379–391. doi: 10.1002/bit.20252. [DOI] [PubMed] [Google Scholar]
- 66.Miyanishi K, Trindade MC, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, Schurman DJ, Smith RL. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-beta3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 2006;12:2253–2262. doi: 10.1089/ten.2006.12.2253. [DOI] [PubMed] [Google Scholar]
- 67.Miyanishi K, Trindade MC, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, Schurman DJ, Smith RL. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 2006;12:1419–1428. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- 68.Mizuno S, Tateishi T, Ushida T, Glowacki J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. J Cell Physiol. 2002;193:319–327. doi: 10.1002/jcp.10180. [DOI] [PubMed] [Google Scholar]
- 69.Mouw JK, Connelly JT, Wilson CG, Michael KE, Levenston ME. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells. 2007;25:655–663. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
- 70.Mow VC, Wang CC. Some bioengineering considerations for tissue engineering of articular cartilage. Clin Orthop Relat Res. 1999;367(Suppl):S204–S223. doi: 10.1097/00003086-199910001-00021. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura S, Arai Y, Takahashi KA, Terauchi R, Ohashi S, Mazda O, Imanishi J, Inoue A, Tonomura H, Kubo T. Hydrostatic pressure induces apoptosis of chondrocytes cultured in alginate beads. J Orthop Res. 2006;24:733–739. doi: 10.1002/jor.20077. [DOI] [PubMed] [Google Scholar]
- 72.Nicodemus GD, Bryant SJ. The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation. J Biomech. 2008;41:1528–1536. doi: 10.1016/j.jbiomech.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nugent-Derfus GE, Takara T, O’Neill JK, Cahill SB, Gortz S, Pong T, Inoue H, Aneloski NM, Wang WW, Vega KI, Klein TJ, Hsieh-Bonassera ND, Bae WC, Burke JD, Bugbee WD, Sah RL. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthritis Cartilage. 2007;15:566–574. doi: 10.1016/j.joca.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paige KT, Vacanti CA. Engineering new tissue: formation of neo-cartilage. Tissue Engineering. 1995;1:97–107. doi: 10.1089/ten.1995.1.97. [DOI] [PubMed] [Google Scholar]
- 75.Parkkinen JJ, Ikonen J, Lammi MJ, Laakkonen J, Tammi M, Helminen HJ. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Arch Biochem Biophys. 1993;300:458–465. doi: 10.1006/abbi.1993.1062. [DOI] [PubMed] [Google Scholar]
- 76.Pelaez D, Huang CY, Cheung HS. Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev. 2009;18:93–102. doi: 10.1089/scd.2008.0030. [DOI] [PubMed] [Google Scholar]
- 77.Prendergast PJ, Huiskes R, Soballe K. ESB Research Award 1996. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech. 1997;30:539–548. doi: 10.1016/S0021-9290(96)00140-6. [DOI] [PubMed] [Google Scholar]
- 78.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 79.Salzmann GM, Nuernberger B, Schmitz P, Anton M, Stoddart MJ, Grad S, Milz S, Tischer T, Vogt S, Gansbacher B, Imhoff AB, Alini M. Physicobiochemical synergism through gene therapy and functional tissue engineering for in vitro chondrogenesis. Tissue Eng Part A. 2009;15:2513–2524. doi: 10.1089/ten.tea.2008.0479. [DOI] [PubMed] [Google Scholar]
- 80.Schumann D, Kujat R, Nerlich M, Angele P. Mechanobiological conditioning of stem cells for cartilage tissue engineering. Biomed Mater Eng. 2006;16:S37–S52. [PubMed] [Google Scholar]
- 81.Solchaga LA, Dennis JE, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res. 1999;17:205–213. doi: 10.1002/jor.1100170209. [DOI] [PubMed] [Google Scholar]
- 82.Steinert A, Weber M, Dimmler A, Julius C, Schutze N, Noth U, Cramer H, Eulert J, Zimmermann U, Hendrich C. Chondrogenic differentiation of mesenchymal progenitor cells encapsulated in ultrahigh-viscosity alginate. J Orthop Res. 2003;21:1090–1097. doi: 10.1016/S0736-0266(03)00100-1. [DOI] [PubMed] [Google Scholar]
- 83.Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. In vivo engineering of organs: the bone bioreactor. Proc Natl Acad Sci USA. 2005;102:11450–11455. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stoddart MJ, Ettinger L, Hauselmann HJ. Enhanced matrix synthesis in de novo, scaffold free cartilage-like tissue subjected to compression and shear. Biotechnol Bioeng. 2006;95:1043–1051. doi: 10.1002/bit.21052. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi I, Nuckolls GH, Takahashi K, Tanaka O, Semba I, Dashner R, Shum L, Slavkin HC. Compressive force promotes sox9, type II collagen and aggrecan and inhibits IL-1beta expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J Cell Sci. 1998;111:2067–2076. doi: 10.1242/jcs.111.14.2067. [DOI] [PubMed] [Google Scholar]
- 86.Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 87.Toyoda T, Seedhom BB, Yao JQ, Kirkham J, Brookes S, Bonass WA. Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheum. 2003;48:2865–2872. doi: 10.1002/art.11250. [DOI] [PubMed] [Google Scholar]
- 88.Villanueva I, Weigel CA, Bryant SJ. Cell-matrix interactions and dynamic mechanical loading influence chondrocyte gene expression and bioactivity in PEG-RGD hydrogels. Acta Biomater. 2009;5:2832–2846. doi: 10.1016/j.actbio.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eisenhart R, Adam C, Steinlechner M, Muller-Gerbl M, Eckstein F. Quantitative determination of joint incongruity and pressure distribution during simulated gait and cartilage thickness in the human hip joint. J Orthop Res. 1999;17:532–539. doi: 10.1002/jor.1100170411. [DOI] [PubMed] [Google Scholar]
- 90.Wagner DR, Lindsey DP, Li KW, Tummala P, Chandran SE, Smith RL, Longaker MT, Carter DR, Beaupre GS. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng. 2008;36:813–820. doi: 10.1007/s10439-008-9448-5. [DOI] [PubMed] [Google Scholar]
- 91.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 92.Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. Multi-axial mechanical stimulation of tissue engineered cartilage: review. Eur Cell Mater. 2007;13:66–73. doi: 10.22203/ecm.v013a07. [DOI] [PubMed] [Google Scholar]
- 93.Wang PY, Chow HH, Lai JY, Liu HL, Tsai WB. Dynamic compression modulates chondrocyte proliferation and matrix biosynthesis in chitosan/gelatin scaffolds. J Biomed Mater Res B Appl Biomater. 2009;91:143–152. doi: 10.1002/jbm.b.31384. [DOI] [PubMed] [Google Scholar]
- 94.Wayne JS, McDowell CL, Shields KJ, Tuan RS. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005;11:953–963. doi: 10.1089/ten.2005.11.953. [DOI] [PubMed] [Google Scholar]
- 95.Wenger R, Hans MG, Welter JF, Solchaga LA, Sheu YR, Malemud CJ. Hydrostatic pressure increases apoptosis in cartilage-constructs produced from human osteoarthritic chondrocytes. Front Biosci. 2006;11:1690–1695. doi: 10.2741/1914. [DOI] [PubMed] [Google Scholar]
- 96.Wernike E, Li Z, Alini M, Grad S. Effect of reduced oxygen tension and long-term mechanical stimulation on chondrocyte-polymer constructs. Cell Tissue Res. 2008;331:473–483. doi: 10.1007/s00441-007-0500-9. [DOI] [PubMed] [Google Scholar]
- 97.Wimmer MA, Alini M, Grad S. The effect of sliding velocity on chondrocytes activity in 3D scaffolds. J Biomech. 2009;42:424–429. doi: 10.1016/j.jbiomech.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Wimmer MA, Grad S, Kaup T, Hanni M, Schneider E, Gogolewski S, Alini M. Tribology approach to the engineering and study of articular cartilage. Tissue Eng. 2004;10:1436–1445. doi: 10.1089/ten.2004.10.1436. [DOI] [PubMed] [Google Scholar]
- 99.Wong M, Siegrist M, Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685–693. doi: 10.1016/S8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 100.Xie F, Weiss P, Chauvet O, Le Bideau J, Tassin JF. Kinetic studies of a composite carbon nanotube-hydrogel for tissue engineering by rheological methods. J Mater Sci Mater Med. 2010;21:1163–1168. doi: 10.1007/s10856-009-3984-x. [DOI] [PubMed] [Google Scholar]