Abstract
Background
Stress fractures commonly affect military recruits during basic training. Several lines of evidence suggest genetic factors are involved in stress fracture predisposition. As gender steroid hormone levels and activity have been implicated in affecting bone strength, one of the candidate genes likely to be involved is the androgen receptor gene.
Questions/purposes
We assessed the possible involvement of the androgen receptor gene in stress fracture predisposition in Israeli soldiers.
Patients and Methods
Between January 2007 and December 2009, we collected clinical and imaging data from 454 Israeli soldiers referred for bone scans with clinical symptoms compatible with stress fractures: 171 soldiers (154 men, 17 women) (patients) with bone scan-proven stress fractures and 283 soldiers (242 men, 41 women) with normal bone scans (control subjects). All participants were genotyped for the length of the CAG (cytosine-adenine-guanine) repeat in exon 1 of the androgen receptor gene using PCR and subsequent fragment analysis on sequence analyzer.
Results
The androgen receptor gene CAG repeat was ranged between six and 31 (mean ± SD, 20.6 ± 4.3) among patients and between 11 and 32 (mean ± SD, 20.0 ± 3.8) among control subjects. Smaller-sized (< 16) androgen receptor CAG repeats were more prevalent among control subjects (23%) than among patients (13%); the risk for having SFs was almost halved if the size of the repeat was shorter than 16 repeats.
Conclusions
The androgen receptor gene CAG repeat has a different allele distribution among Israeli soldiers with stress fractures than in control subjects. While our finding must be validated, it could be used for screening individuals at risk for stress fractures.
Level of Evidence
Level II, prognostic study. See the Guidelines for Authors complete description of levels of evidence.
Introduction
Stress fractures (SFs) are commonly encountered among young army recruits who undergo strenuous exercise. The underlying cause(s) of SFs are unknown, although various authors have suggested mechanical, environmental, behavioral, or genetic factors predispose individuals to subsequent SFs [8, 9, 28, 42].
Multiple lines of indirect evidence point to genetic factors contributing to SF pathogenesis, specifically, reports of multiple SFs affecting monozygotic twins [29], multiple lower limb SFs in single individuals [19, 23, 26], high recurrence rate (approximately 10% in a year) of SFs in different anatomic sites in Israel Defense Force (IDF) recruits [9], occurrence of SFs in the pediatric age group [2, 17, 20, 22, 25, 32, 36], and the interindividual variation in SF incidence given the similar training load among soldiers in the same unit [10, 21, 24]. In some studies relating to predisposing factors of SFs, little or no mention is given to family history of bone diseases [16, 24, 27]. However, in one study, known family history of osteoporosis was associated with an increased risk of stress fractures in females [7]. In approximately 8% to 13% of IDF soldiers with SFs, a family history of SFs or bone disease is elicited [13]. Taken together, these data support the notion of genetic factors involved in SF pathogenesis.
Although in all likelihood genetic susceptibility to SFs involves multiple genes, the exact identity of these genes remains elusive. Obvious candidate genes are those involved in bone formation, bone remodeling, bone structure, and bone-associated disorders, in humans and in animal models. One of these candidate genes is the androgen receptor (AR) gene. The AR gene (OMIM 313700) localizes to Xq11-q12 and encodes for the nuclear receptor whose ligand is dihydrotestosterone. A CAG (cytosine-adenine-guanine) triplet repeat sequence is present in exon 1 of the AR gene, which encodes a polyglutamine (CAG)n tract in the N-terminal transactivation domain of the protein. The number of CAG repeats is highly polymorphic, ranging from six to 39 repeats, with an average between 20 and 22 [6, 11]. Variations of the CAG repeat size have been linked to endocrine abnormalities. Reduced CAG sizes, shown in vitro to be associated with enhanced transactivation function of the AR [1, 3, 15], have been associated with clinical features similar to those observed with increased androgenic function, such as prostate cancer [12]. Conversely, long CAG sizes that are within the normal range (approximately 35–37 repeats) reportedly have been linked to clinical disorders characterized by reduced androgenic function: male infertility, hypogonadism, gynecomastia, and cryptorchidism [18, 27]. Some studies have investigated the association between the CAG repeat size and bone density, but the results were inconclusive [30, 31]. However, the AR may be considered a plausible candidate gene in SF predisposition.
We therefore assessed the AR gene CAG repeat length in soldiers in the IDF as an indirect measure of the putative contribution of the AR gene to SF pathogenesis.
Patients and Methods
Study participants were recruited from among soldiers referred to the Central Orthopaedic Clinic of the IDF with clinical symptoms compatible with SF from January 2007 to December 2009. All were active-duty IDF soldiers. All soldiers who were referred to the clinic with symptoms compatible with SF were eligible and were offered participation in our study. Overall, there were 454 participants in the study: 396 men and 58 women. The recruitment rate was approximately 25%, and there were no differences between participants and nonparticipants in terms of SF diagnosis rates, ethnicity, height, weight, or state at military training (data not shown). The primary reason for nonparticipation (68% of nonparticipants) was lack of will to undergo blood withdrawal requiring another venous puncture. Thirty-nine percent of participating men were in the basic training phase of their military service, ranging from 2 to 8 months, and all men were within the first 18 months initiation of their service. The demographic and relevant clinical features (height, weight, ethnic origin) of male (Table 1) and female (Table 2) patients and control subjects were recorded. Among male soldiers with SFs, 61 of 154 (40%) had severe SFs (Grade 3 or higher), 86 (56%) had moderate SFs (Grade 2), and for seven, the severity was unrecorded. The anatomic location of the SFs in male study participants were either at the femur (n = 43) tibia (n = 93), or the fibula (n = 11), and unrecorded for seven. For female soldiers with SFs, eight of 17 (47%) had severe SF and nine of 17 (53%) had moderate SF. The majority of female soldiers with SF (n = 13) had SFs concurrently diagnosed in the tibia and the femur whereas two had bilateral tibial fractures (12%) and two (12%) had bilateral femoral SFs.
Table 1.
Characteristics of the male participants by patient-control subject status assignment
| Group | Origin | Age (years)† | Height (cm)† | Weight (kg)† | Body mass index (kg/m2)† |
|---|---|---|---|---|---|
| Patients | AJ (n = 84) |
20.0 ± 1.5 (18–27) p = 0.004 |
176.8 ± 6.3 (160–190) p = 0.983 |
71.0 ± 8.7 (47–94) p = 0.711 |
22.7 ± 0.2 (18.4–26.0) p < 0.0001 |
| NAJ (n = 64) |
20.1 ± 1.7 (18–25) p = 0.007 |
175.6 ± 6.4 (160–192) p = 0.004 |
71.3 ± 10.9 (54–105) p = 0.291 |
23.1 ± 0.2 (21.1–28.5) p < 0.0001 |
|
| Unknown (n = 6) |
19.5 ± 1.9 (18–22) p = 0.120 |
183.5 ± 7.8 (178–189) p = 0.280 |
75.3 ± 10.0 (68–90) p = 0.246 |
22.2 ± 0.2 (21.5–25.2) p = 0.0001 |
|
| Total (n = 154) |
20.0 ± 1.6 (18–27) p = 0.0001 |
176.3 ± 6.4 (160–192) p = 0.052 |
71.3 ± 9.7 (47–105) p = 0.349 |
23.0 ± 0.2 (18.4–28.5) p < 0.0001 |
|
| Control subjects | AJ (n = 112) |
19.3 ± 2.0 (18–30) |
176.7 ± 6.5 (163–193) |
71.5 ± 11.7 (49–125) |
22.8 ± 0.2 (18.4–33.6) |
| NAJ (n = 87) |
19.4 ± 1.5 (18–25) |
178.7 ± 6.6 (163–197) |
73.2 ± 10.8 (50–110) |
22.9 ± 0.2 (18.8–28.3) |
|
| Unknown (n = 43) |
18.1 ± 0.2 (18–19) |
179.5 ± 7.8 (174–185) |
69.7 ± 10.8 (50–97) |
21.6 ± 0.2 (16.5–28.3) |
|
| Total (n = 242) |
19.3 ± 1.8 (18–30) |
177.6 ± 6.6 (163–197) |
72.3 ± 11.3 (49–125) |
22.9 ± 0.2 (16.5–33.6) |
|
| Total | n = 396 | 19.5 ± 1.7 (18–30) |
177.0 ± 6.5 (160–197) |
71.6 ± 10.6 (47–125) |
22.9 ± 0.2 (16.5–33.6) |
AJ = Ashkenazi Jews; NAJ = nonAshkenazi Jews; †values expressed as mean ± SD, with range in parentheses; p values reflect differences in parameter between patients and control subjects of the same ethnic origin or overall
Table 2.
Characteristics of the female participants by patient-control subject status assignment
| Group | Origin | Age (years)† | Height (cm)† | Weight (kg)† | Body mass index (kg/m2)† |
|---|---|---|---|---|---|
| Patients | AJ (n = 6) |
19.0 ± 0.8 (18–20) p = 0.409 |
166.8 ± 3.0 (163–170) p = 0.216 |
62.3 ± 6.3 (55–70) p = 0.370 |
22.4 ± 0.7 (20.7–24.2) p = 0.082 |
| NAJ (n = 11) |
19.7 ± 1.4 (18–23) p = 0.846 |
166.3 ± 5.5 (158–175) p = 0.586 |
60.3 ± 9.4 (50–84) p = 0.660 |
21.8 ± 0.3 (20.0–27.4) p = 0.022 |
|
| Total (n = 17) |
19.5 ± 1.3 (18–23) p = 1.0 |
166.4 ± 4.8 (158–175) p = 0.238 |
60.8 ± 8.5 (50–84) p = 0.452 |
22.0 ± 0.5 (20.0–27.4) p = 0.029 |
|
| Control subjects | AJ (n = 15) |
19.3 ± 0.7 (18–21) |
163.7 ± 7.8 (153–180) |
58.3 ± 13.1 (43–94) |
21.8 ± 0.2 (18.4–29.0) |
| NAJ (n = 25) |
19.6 ± 1.4 (18–25) |
165.1 ± 6.6 (148–180) |
58.8 ± 8.2 (47–79) |
21.6 ± 0.2 (21.5–24.4) |
|
| Unknown (n = 1) |
19.0 | 162.0 | 55.0 | 21.0 | |
| Total (n = 41) |
19.5 ± 1.3 (18–25) |
164.4 ± 7.7 (148–180) |
58.6 ± 10.6 (43–94) |
21.7 ± 0.2 (18.4–29.0) |
|
| Total | n = 58 | 19.5 ± 1.3 (18–25) |
165.1 ± 6.9 (148–180) |
60.1 ± 9.8 (43–94) |
22.0 ± 0.2 (18.4–19.0) |
AJ = Ashkenazi Jews; NAJ = nonAshkenazi Jews; †values expressed as mean ± SD, with range in parentheses; p values reflect differences in parameter between patients and control subjects of same ethnic origin or overall
Among women, there was a difference (p = 0.05) between patients and control subjects for body mass index (BMI) (mean ± SD, 22.0 ± 0.5 for patients versus 21.7 ± 0.2 for control subjects). Among men, there were differences (p = 0.05) between patients and control subjects in age (20.0 ± 1.6 years for patients versus 19.3 ± 1.8 years for control subjects) and BMI (23.0 ± 0.2 for patients versus 22.9 ± 0.2 for control subjects). There were no other differences between the patients and control subjects. Specifically, there were no differences in the ethnic makeup of the participants who revealed their ethnicity: 84 of 148 patients (57%) and 112 of 199 control subjects (56%) were Ashkenazim.
All participants were evaluated by various orthopaedic surgeons who obtained the history and performed a physical examination focusing on the lower limbs. Imaging included TC99 bone scanning as routinely practiced [14] in the Central Orthopaedic Clinic for IDF soldiers. The bone scans were interpreted by one physician (LT), and based on the results, the soldiers were classified as either having no evidence of SF or having Grade 1 to Grade 4 SF, according to practiced criteria and protocol [44]. Individuals with Grades 1 and single Grade 2 SFs and individuals with metatarsal SFs were excluded from the study. Participants classified as having no evidence of SF were considered control subjects, and individuals with Grades 3 and 4 SFs or multiple Grade 2 SFs were the patients. The study was approved by the IDF IRB and the Ministry of Health’s IRB for genetic studies, and each participant signed a written informed consent. After signing the informed consent, each participant completed a questionnaire detailing demographic data, personal and family history of SFs and bone diseases, engagement in sports, and smoking, among other parameters.
DNA was extracted from peripheral blood leukocytes using the PureGen® kit by Gentra Inc (Minneapolis, MN, USA), following the manufacturer’s recommended protocol. The primers sequences for the genotyping were retrieved from the Genome DataBase online database [www.gdb.org]. Genomic DNA from each subject was amplified by PCR. The forward primer was labeled with FAM™ for the analysis of the amplicons. Two microliters of each PCR product were mixed with 0.5 μL TAMRA™ 500 internal size standard (Applied Biosystems Inc, Foster City, CA, USA) and 12 μL formamide. Samples were read on an ABI Prism® 3100 Sequencer using GeneScan® software (Applied Biosystems). The GeneScan® raw data were analyzed using the Genotyper® software to obtain the number of CAG repeats in base pairs. The CAG repeat number ranged between six and 32 repeats for all study participants.
The statistical analysis included comparison of distributions of various parameters of interest between the patients and the control subjects. Student’s t test was used for continuous variables such as height and weight. Discrete variables such as origin and genotype were compared using Pearson chi square and Fisher exact tests. All calculations were performed using STATA® SE 10 software (StataCorp LP, College Station, TX, USA).
Results
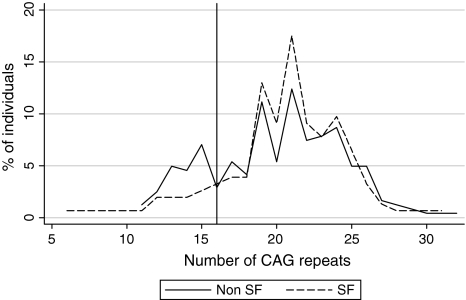
Fewer (p = 0.013; odds ratio, 0.49, 95% confidence interval, 0.27–0.89) male patients had a repeat CAG repeat size less than 16 compared with the control subjects: 20 of 154 (13%) versus 56 of 242 (23%) control subjects (Fig. 1). Among men, we observed no differences (p = 0.16) in the mean allele size between patients (20.6 ± 4.3) and control subjects (20.0 ± 3.76). In both groups, the most common alleles were repeats of 19 and 21 (11% and 12% in control subjects and 13% and 18% in patients, respectively; p = 0.204). Among women, there also were no differences (p = 0.12) in both mean alleles sizes (allele 1 and allele 2) between patients (20.2 ± 3.9 and 22.1 ± 4.3) and control subjects (18.7 ± 31.3 and 22.1 ± 3.2). The most common alleles were repeats 21, 22/24 (18%/24% in patients), and 19 (22% in control subjects).
Fig. 1.
A graph shows the association between AR CAG repeats and SF status in men. The vertical solid line = 16 CAG repeats. We found a difference in the rate of patients with CAG repeats ≤ 16 compared with control subjects, supporting the notion of an association (not causation) between SF and AR CAG repeats
Discussion
SFs are commonly encountered bone disorders affecting, in particular, military recruits during basic training. Several lines of evidence suggest genetic factors are involved in SF predisposition. One of the candidate genes likely to be involved is the AR gene. We therefore assessed the possible involvement of the AR gene in SF predisposition in Israeli soldiers.
The limitations of our study should be borne in mind. First, this is a study focusing on Israeli soldiers, in a narrow age range, with a very specific type of training. Thus these results may not apply to ethnically diverse populations or to other groups whose mechanisms of sustaining stress fractures are different (eg, elite athletes). Second, only one polymorphism was analyzed for a group of patients and control subjects. Although this is the largest study reporting genetic analysis in SFs, the study size is too limited for firm conclusions to be drawn. There is little doubt the genetic component in SFs would not be a simple Mendelian one and multiple genes converge to result in SFs in a genetically susceptible individual, given the appropriate environmental context, ie, military training. Despite this limitation, the fact that the CAG repeat size is related to androgen action which in turn may affect bone strength, the association reported herein remains intriguing and needs further confirmation. Finally, the reported association between CAG repeat size and SF risk is merely an association and does not imply a mechanism or even causation.
We found soldiers who were diagnosed with high-grade or multiple SFs had a nominally different CAG repeat in the first exon of the AR gene compared with soldiers in the IDF with no SFs. The risk for having SFs was almost halved if the size of the repeat was shorter than 16 repeats. This result is derived from an exploratory analysis and needs confirmation in additional studies. We are aware of only two published reports on genetic analysis of SFs. Välimäki et al. [37] genotyped a limited number of Finnish soldiers with SFs (n = 15) and limited their analysis to two polymorphisms in two genes (AR and estrogen receptor). Chatzipapas et al. [4] also reported the results of genetic analysis of 32 patients with SFs and genotyping was limited to three polymorphisms in the vitamin D receptor (VDR). Our current data add to this limited dataset and support the notion of genetic factors being operative in SF pathogenesis.
The possible mechanisms underlying the putative association between bone density and AR CAG are unknown. Furthermore, the role that the AR CAG repeat plays in determining bone density is controversial. Sowers et al. [33] reported, for premenopausal and perimenopausal women, there was an overrepresentation of women with the lowest bone mineral density (BMD) in women with AR CAG repeats ranging from 12 to 15 compared with women with higher BMD. Chen et al. [5] reported an association between AR CAG repeat size and osteoporosis in postmenopausal women, whereby an allele size longer than 20 CAG repeats was associated with an increased risk for osteoporosis and osteoporotic BMD at the femoral neck but not at the lumbar spine. Their conclusion was based purely on observational data, similar to our study, with no confirmation by biologic-experimental data. Moreover, the wide confidence interval (1.0-17.2) in the Chen et al. study may indirectly indicate that these results should be interpreted with caution.
Few studies have evaluated possible associations of CAG repeats in AR and BMD in men. Langdahl et al. [23] analyzed the association of the CAG repeat size to osteoporosis in Danish men aged 28 to 83 years and concluded there was a tendency among individuals with osteoporosis to have longer AR CAG repeats than control subjects. However, raw data show, of only men with osteoporosis, there was a CAG repeat size of 17 repeats or shorter. Zitzmann et al. [43] reported a similar relationship between BMD and CAG repeat size in men, whereby men with longer CAG repeat sizes (range, 22–31) had lower BMD and age-dependent bone loss than men with repeat sizes in the 14 to 21 range. Their interpretation of this observation is that an increased-size CAG attenuates the effect of testosterone on bone remodeling and turnover. In that study only four individuals (of the total 110) had CAG repeats of up to and including 16 repeats. Stiger et al. [34] reported an inverse association between BMD in healthy men (n = 229; age, 40–76 years), whereby men with longer AR repeats (> 24) had lower BMD at all sites compared with men with shorter repeats (< 21). Of the other studies focusing primarily on older men, most reported no association between AR CAG repeat length and BMD [16, 28, 33, 35]. Two studies focusing on younger individuals also did not show any consistent association between BMD and CAG repeat, although the numbers were limited [34, 39]. Recently Guadalupe-Grau et al. [14] analyzed the association between the AR CAG repeats and bone density in 282 healthy Spanish men (mean age, 28.6 ± 7.6 years) and reported that no associations between CAG repeat length and regional bone mineral content or BMD were observed after adjusting for age. However, femoral neck BMD was 4.8% greater in individuals with CAG length shorter than 20 repeats (who also have short GGN repeats in the same gene) compared with men with longer CAG (and CGN) repeats. Several mechanisms have been proposed to account for this association between BMD and CAG repeat size [27, 31, 35, 38], focusing on the feedback mechanisms and testosterone conversion to estrogen and altered aromatase activity. However, these reports remain speculative and inconsistent.
Moreover, even the effect of AR CAG repeat size on serum testosterone levels and their age-related decline are an unsettled issue. Krithivas et al. [21] reported individuals (men) with a shorter CAG repeat have lower total free and albumin-bound testosterone and faster rates of age-related testosterone decline compared with individuals with longer repeats. In contrast, Westberg et al. [41] reported, for premenopausal women, shorter CAG repeat size was associated with increased androgen levels, with higher levels for women with repeat sizes lower than 17. Goutou et al. [15] observed no association between AR CAG repeats and testosterone levels or other gonadal steroid levels in healthy men. Walsh et al. [40] reported men with longer CAG repeats (≥ 22) had lower fat-free mass and higher testosterone levels compared with individuals with shorter repeats. Using a cutoff of 17 CAG repeats, Ding et al. [6] showed the transactivation activity of the AR is 40% more efficacious compared with a CAG repeat of 21 in prostate cancer cell lines.
Our data support the notion that genetic factors are involved in SF pathogenesis, that one of the molecular pathways involved in SF pathogenesis involves an altered activity of the AR signaling cascade affecting bone structure, and that the CAG repeat in the AR gene may be used as a marker for such susceptibility, if the data are validated in subsequent studies.
Acknowledgments
This study was performed in part as a prerequisite for the graduate degree (PhD) for Ran Yanovich at The Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel. We thank Professor Laurence Freedman and Dr Illya Novikov from the Biostatistics Unit, The Gertner Institute of Epidemiology and Health Policy Research, Chaim Sheba Medical Center, for statistical advice, and the orthopaedic surgeons and staff at the Central Orthopaedic Clinic of the IDF for facilitating this study. In particular, we thank Dr. Leonardo Trejo, a Nuclear Medicine specialist, and Israel Cohen, the bone scan technician.
Footnotes
One or more of the authors (EF, DSM) have received funding from TATRC (Telemedicine and Advanced Technology Research Center, Fredrick MD) (Grant W81XWH-09-2-0054). Each of the other authors certifies that he or she has no commercial associations (eg, consultations, stock ownership, equity, interest, patent licensing, etc) that might pose a conflict of interest with the submitted manuscript.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Chaim Sheba Medical Center.
References
- 1.Beilin J, Ball EM, Favaloro JM, Zajac JD. Effect of the androgen receptor CAG repeat polymorphism on transcriptional activity: specificity in prostate and non-prostate cell lines. J Mol Endocrinol. 2000;25:85–96. doi: 10.1677/jme.0.0250085. [DOI] [PubMed] [Google Scholar]
- 2.Buckley SL, Robertson WW, Jr, Shalaby-Rana E. Stress fractures of the femoral diaphysis in young children: a report of 2 cases. Clin Orthop Relat Res. 1995;310:165–169. [PubMed] [Google Scholar]
- 3.Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzipapas C, Boikos S, Drosos GI, Kazakos K, Tripsianis G, Serbis A, Stergiopoulos S, Tilkeridis C, Verettas DA, Stratakis CA. Polymorphisms of the vitamin D receptor gene and stress fractures. Horm Metab Res. 2009;41:635–640. doi: 10.1055/s-0029-1216375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HY, Chen WC, Wu MC, Tsai FJ, Tsai CH. Androgen receptor (AR) gene microsatellite polymorphism in postmenopausal women: correlation to bone mineral density and susceptibility to osteoporosis. Eur J Obstet Gynecol Reprod Biol. 2003;107:52–56. doi: 10.1016/S0301-2115(02)00315-9. [DOI] [PubMed] [Google Scholar]
- 6.Ding D, Xu L, Menon M, Reddy GP, Barrack ER. Effect of a short CAG (glutamine) repeat on human androgen receptor function. Prostate. 2004;58:23–32. doi: 10.1002/pros.10316. [DOI] [PubMed] [Google Scholar]
- 7.Edwards A, Hammond HA, Lin J, Caskey CT, Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992;12:241–253. doi: 10.1016/0888-7543(92)90371-X. [DOI] [PubMed] [Google Scholar]
- 8.Friedl KE, Nuovo JA, Patience TH, Dettori JR. Factors associated with stress fracture in young army women: indications for further research. Mil Med. 1992;157:334–338. [PubMed] [Google Scholar]
- 9.Gehrmann RM, Renard RL. Current concepts review: stress fractures of the foot. Foot Ankle Int. 2006;27:750–757. doi: 10.1177/107110070602700919. [DOI] [PubMed] [Google Scholar]
- 10.Giladi M, Milgrom C, Kashtan H, Stein M, Chisin R, Dizian R. Recurrent stress fractures in military recruits: one-year follow-up of 66 recruits. J Bone Joint Surg Br. 1986;68:439–441. doi: 10.1302/0301-620X.68B3.3733811. [DOI] [PubMed] [Google Scholar]
- 11.Gill RM, Hopkins GO. Stress fractures in parachute regiment recruits. J R Army Med Corps. 1988;134:91–93. doi: 10.1136/jramc-134-02-06. [DOI] [PubMed] [Google Scholar]
- 12.Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Dahl D, Brufsky A, Talcott J, Hennekens CH, Kantoff PW. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci USA. 1997;94:3320–3323. doi: 10.1073/pnas.94.7.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Givon U, Friedman E, Reiner A, Vered I, Finestone A, Shemer J. Stress fractures in the Israeli Defense Forces in 1995 to 1996. Clin Orthop Relat Res. 2000;373:227–232. doi: 10.1097/00003086-200004000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Guadalupe-Grau A, Rodríguez-González FG, Ponce-González JG, Dorado C, Olmedillas H, Fuentes T, Pérez-Gómez J, Sanchís-Moysi J, Díaz-Chico BN, Calbet JA. Bone mass and the CAG and GGN androgen receptor polymorphisms in young men. PLoS One. 2010;5:e11529. doi: 10.1371/journal.pone.0011529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goutou M, Sakka C, Stakias N, Stefanidis I, Koukoulis GN. AR CAG repeat length is not associated with serum gonadal steroids and lipid levels in healthy men. Int J Androl. 2008;32:616–622. doi: 10.1111/j.1365-2605.2008.00908.x. [DOI] [PubMed] [Google Scholar]
- 16.Hod N, Ashkenazi I, Levi Y, Fire G, Drori M, Cohen I, Bernstine H, Horne T. Characteristics of skeletal stress fractures in female military recruits of the Israel defense forces on bone scintigraphy. Clin Nucl Med. 2006;31:742–749. doi: 10.1097/01.rlu.0000246632.11440.70. [DOI] [PubMed] [Google Scholar]
- 17.Jiang M, Boonen S, Borghs H, Vanderschueren D, Adams JE, Ward KA, Bartfai G, Casanueva F, Finn JD, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Silman AJ, Wu FC, European Male Ageing Study Group Increased estrogen rather than decreased androgen action is associated with longer androgen receptor CAG repeats. J Clin Endocrinol Metab. 2009;94:277–284. doi: 10.1210/jc.2008-0848. [DOI] [PubMed] [Google Scholar]
- 18.Kazemi-Esfarjani P, Trifiro MA, Pinsky L. Evidence for a repressive function of the long polyglutamine tract in the human androgen receptor: possible pathogenetic relevance for the (CAG)n-expanded neuronopathies. Hum Mol Genet. 1995;4:523–527. doi: 10.1093/hmg/4.4.523. [DOI] [PubMed] [Google Scholar]
- 19.Kenny AM, McGee D, Joseph C, Covault J, Abreu C, Raisz LG. Lack of association between androgen receptor polymorphisms and bone mineral density or physical function in older men. Endocr Res. 2005;31:285–293. doi: 10.1080/07435800500406221. [DOI] [PubMed] [Google Scholar]
- 20.Kozlowski K, Urbonaviciene A. Stress fractures of the fibula in the first few years of life (report of six cases) Australas Radiol. 1996;40:261–263. doi: 10.1111/j.1440-1673.1996.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 21.Krithivas K, Yurgalevitch SM, Mohr BA, Wilcox CJ, Batter SJ, Brown M, Longcope C, McKinlay JB, Kantoff PW. Evidence that the CAG repeat in the androgen receptor gene is associated with the age-related decline in serum androgen levels in men. J Endocrinol. 1999;162:137–142. doi: 10.1677/joe.0.1620137. [DOI] [PubMed] [Google Scholar]
- 22.Lambros G, Alder D. Multiple stress fractures of the tibia in a healthy adult. Am J Orthop (Belle Mead NJ) 1997;26:687–688. [PubMed] [Google Scholar]
- 23.Langdahl BL, Stenkjaer L, Carstens M, Tofteng CL, Eriksen EF. A CAG repeat polymorphism in the androgen receptor gene is associated with reduced bone mass and increased risk of osteoporotic fractures. Calcif Tissue Int. 2003;73:237–243. doi: 10.1007/s00223-002-0019-8. [DOI] [PubMed] [Google Scholar]
- 24.Linenger JM, Shwayhat AF. Epidemiology of podiatric injuries in US marine recruits undergoing basic training. J Am Pediatr Med Assoc. 1992;82:269–271. doi: 10.7547/87507315-82-5-269. [DOI] [PubMed] [Google Scholar]
- 25.Meany JE, Carty H. Femoral stress fractures in children. Skeletal Radiol. 1992;21:173–176. doi: 10.1007/BF00242131. [DOI] [PubMed] [Google Scholar]
- 26.Milgrom C, Chisin R, Giladi M, Stein M, Kashtan H, Margulies J, Atlan H. Multiple stress fractures: a longitudinal study of a soldier with 13 lesions. Clin Orthop Relat Res. 1985;192:174–179. [PubMed] [Google Scholar]
- 27.Milgrom C, Giladi M, Stein H, Kashtan H, Margulies J, Chisin R, Steinberg R, Aharonson Z. Stress fractures in military recruits: a prospective study showing an unusually high incidence. J Bone Joint Surg Br. 1985;67:732–735. doi: 10.1302/0301-620X.67B5.4055871. [DOI] [PubMed] [Google Scholar]
- 28.Murray SR, Reeder MT, Udermann BE, Pettitt RW. High-risk stress fractures: pathogenesis, evaluation, and treatment. Compr Ther. 2006;32:20–25. doi: 10.1385/COMP:32:1:20. [DOI] [PubMed] [Google Scholar]
- 29.Nielens H, Devogelaer JP, Malghem J. Occurrence of a painful stress fracture of the femoral neck simultaneously with six other asymptomatic localizations in a runner. J Sports Med Phys Fitness. 1994;34:79–82. [PubMed] [Google Scholar]
- 30.Palazzolo I, Gliozzi A, Rusmini P, Sau D, Crippa V, Simonini F, Onesto E, Bolzoni E, Poletti A. The role of the polyglutamine tract in androgen receptor. J Steroid Biochem Mol Biol. 2008;108:245–253. doi: 10.1016/j.jsbmb.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Remes T, Vaisanen SB, Mahonen A, Huuskonen J, Kroger H, Jurvelin JS, Penttila IM, Rauramaa R. Aerobic exercise and bone mineral density in middle-aged Finnish men: a controlled randomized trial with reference to androgen receptor, aromatase, and estrogen receptor alpha gene polymorphisms. Bone. 2003;32:412–420. doi: 10.1016/S8756-3282(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 32.Singer A, Ben-Yehuda O, Ben-Ezra Z, Zaltzman S. Multiple identical stress fractures in monozygotic twins: case report. J Bone Joint Surg Am. 1990;72:444–445. [PubMed] [Google Scholar]
- 33.Sowers M, Willing M, Burns T, Deschenes S, Hollis B, Crutchfield M, Jannausch M. Genetic markers, bone mineral density, and serum osteocalcin levels. J Bone Miner Res. 1999;14:1411–1419. doi: 10.1359/jbmr.1999.14.8.1411. [DOI] [PubMed] [Google Scholar]
- 34.Stiger F, Brändström H, Gillberg P, Melhus H, Wolk A, Michaelsson K, Kindmark A. Association between repeat length of exon 1 CAG microsatellite in the androgen receptor and bone density in men is modulated by sex hormone levels. Calcif Tissue Int. 2008;82:427–435. doi: 10.1007/s00223-008-9128-3. [DOI] [PubMed] [Google Scholar]
- 35.St Pierre P, Staheli LT, Smith JB, Green NE. Femoral neck stress fractures in children and adolescents. J Pediatr Orthop. 1995;15:470–473. doi: 10.1097/01241398-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Valero C, Zarrabeitia MT, Hernandez JL, Zarrabeitia A, Gonzalez-Macias J, Riancho JA. Bone mass in young adults: relationship with gender, weight and genetic factors. J Intern Med. 2005;258:554–562. doi: 10.1111/j.1365-2796.2005.01568.x. [DOI] [PubMed] [Google Scholar]
- 37.Välimäki VV, Alfthan H, Lehmuskallio E, Löyttyniemi E, Sahi T, Suominen H, Välimäki MJ. Risk factors for clinical stress fractures in male military recruits: a prospective cohort study. Bone. 2005;37:267–273. doi: 10.1016/j.bone.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Pottelbergh I, Lumbroso S, Goemaere S, Sultan C, Kaufman JM. Lack of influence of the androgen receptor gene CAG-repeat polymorphism on sex steroid status and bone metabolism in elderly men. Clin Endocrinol (Oxf) 2001;55:659–666. doi: 10.1046/j.1365-2265.2001.01403.x. [DOI] [PubMed] [Google Scholar]
- 39.Walker RN, Green NE, Spindler KP. Stress fractures in skeletally immature patients. J Pediatr Orthop. 1996;16:578–584. doi: 10.1097/01241398-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Walsh S, Zmuda JM, Cauley JA, Shea PR, Metter EJ, Hurley BF, Ferrell RE, Roth SM. Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J Appl Physiol. 2005;98:132–137. doi: 10.1152/japplphysiol.00537.2004. [DOI] [PubMed] [Google Scholar]
- 41.Westberg L, Baghaei F, Rosmond R, Hellstrand M, Landén M, Jansson M, Holm G, Björntorp P, Eriksson E. Polymorphisms of the androgen receptor gene and the estrogen receptor beta gene are associated with androgen levels in women. J Clin Endocrinol Metab. 2001;86:2562–2568. doi: 10.1210/jc.86.6.2562. [DOI] [PubMed] [Google Scholar]
- 42.Willing MC, Torner JC, Burns TL, Janz KF, Marshall T, Gilmore J, Deschenes SP, Warren JJ, Levy SM. Gene polymorphisms, bone mineral density and bone mineral content in young children: the Iowa Bone Development Study. Osteoporos Int. 2003;14:650–658. doi: 10.1007/s00198-003-1416-1. [DOI] [PubMed] [Google Scholar]
- 43.Zitzmann M, Brune M, Kornmann B, Gromoll J, Junker R, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene affects bone density and bone metabolism in healthy males. Clin Endocrinol (Oxf) 2001;55:649–657. doi: 10.1046/j.1365-2265.2001.01391.x. [DOI] [PubMed] [Google Scholar]
- 44.Zwas ST, Elkanovitch R, Frank G. Interpretation and classification of bone scintigraphic findings in stress fractures. J Nucl Med. 1987;28:452–457. [PubMed] [Google Scholar]