Abstract
Background
The best strategy for delivering growth factors to cells for the purpose of cartilage tissue engineering remains an unmet challenge. Tethering biotinylated insulin-like growth factor-1 (bIGF-1) to the self-assembling peptide scaffold (RADA)4 effectively delivers bioactive bIGF-1 to cardiac tissue.
Questions/purposes
We therefore asked whether: (1) soluble bIGF-1 could stimulate proteoglycan production by chondrocytes; (2) bIGF-1 could be adsorbed or tethered to the self-assembling peptide scaffold (KLDL)3; (3) adsorbed or tethered bIGF-1 could stimulate proteoglycan production; and (4) transforming growth factor-β1 (TGF-β1) could be adsorbed or tethered and stimulate proteoglycan production by bone marrow stromal cells (BMSCs).
Methods
Chondrocytes or BMSCs were encapsulated in (KLDL)3. The growth factors were (1) delivered solubly in the medium; (2) adsorbed to (KLDL)3; or (3) tethered to (KLDL)3 through biotin-streptavidin bonds. Fluorescently tagged streptavidin was used to determine IGF-1 kinetics; sGAG and DNA content was measured.
Results
Soluble bIGF-1 stimulated comparable sGAG accumulation as soluble IGF-1. Tethering IGF-1 to (KLDL)3 increased retention of IGF-1 in (KLDL)3 compared with adsorption, but neither method increased sGAG or DNA accumulation above control. Adsorbing TGF-β1 increased proteoglycan accumulation above control, but tethering did not affect sGAG levels.
Conclusions
Although TGF-β1 can be effectively delivered by adsorption to (KLDL)3, IGF-1 cannot. Additionally, although tethering these factors provided long-term sequestration, tethering did not stimulate proteoglycan production.
Clinical Relevance
Tethering growth factors to (KLDL)3 results in long-term delivery, but tethering does not necessarily result in the same bioactivity as soluble delivery, indicating presentation of proteins is vital when considering a delivery strategy.
Introduction
Acute cartilage defects remain a challenge to repair. Currently, interventions such as microfracture or autologous chondrocyte implantation remain the standard of care [24], but these methods still result in mechanically inferior repair tissue [14]. Cartilage tissue engineering has emerged as a possible avenue for improving repair. As such, many combinations of scaffolds, cells, and growth factors have been proposed.
Growth factors including transforming growth factor-β1 (TGF-β1), insulin-like growth factor-1 (IGF-1), and members of the growth differentiation factor (GDF) and bone morphogenetic protein (BMP) families are important in inducing repair, attracting migration of repair cells, and stimulating chondrogenesis, proliferation, and production of matrix [4, 46, 47]. Because growth factors act on multiple tissues and can have detrimental side effects if applied systemically, local delivery is necessary. Although intra-articular injections present a simple approach for delivery to the joint, much higher than physiological concentrations must be used along with multiple injections to overcome the fact that growth factors have short half-lives and may be cleared rapidly from the synovial fluid. Therefore, delivery methods using scaffolds have been proposed to protect growth factors from degradation and to deliver them over longer periods of time [48]. Despite the advantages hydrogels offer as scaffolds, growth factors quickly diffuse out of them [28]. Therefore, most successful delivery strategies to date have incorporated growth factor-loaded microspheres within the hydrogel so release of the factors can be controlled by the degradation rate of the microspheres [46]. Although this has improved long-term delivery of factors, high loading concentrations of growth factors within these microspheres are still required, creating the possibility of high localized doses through bolus release in vivo. Therefore, a method for increasing retention of growth factors in hydrogels without the need for microspheres is of interest.
Hydrogel scaffolds made from the self-assembling peptide sequences (RADA)4 and (KLDL)3, which we refer to as RAD and KLD, respectively, support long-term maintenance of the chondrocyte phenotype [23, 33] and chondrogenesis of bone marrow stromal cells (BMSCs) [25], resulting in greater cell proliferation and sGAG production and less catabolic cleavage than agarose scaffolds [25]. These peptides assemble into hydrogels on contact with solutions of physiological pH and ionic strength [53], enabling them to be injected into tissues in vivo, where they encourage cell infiltration [7, 37]. KLD gels at a 1% concentration have a stiffness of approximately 120 Pa, whereas RAD gels at a 1% concentration have a stiffness of approximately 46 Pa [44]. Diffusion of proteins ranging in size from 14 to 150 kDa have diffusion constants ranging from 0.7 × 10−10 m2/s to 0.3 × 10−10 m2/s when premixed with 1% RAD gels [27]. Bioactive sequences have been appended to the RAD sequence without disrupting assembly [13, 16, 50]. RAD reportedly supports growth factor delivery: PDGF-BB and other proteins have been adsorbed to RAD [17, 27], SDF-1 was inserted directly onto the RAD peptide sequence [41], and IGF-1 was tethered to RAD through biotin-streptavidin-biotin bonds [6] successfully stimulating cardiomyocytes in vivo. Previous work established that TGF-β1 adsorbed to KLD stimulated chondrogenesis of BMSCs [26].
Based on the success of the use of biotinylated IGF-1 (bIGF-1) with RAD and cardiomyocytes, we therefore asked whether: (1) bIGF-1 could stimulate proteoglycan production by chondrocytes when delivered solubly; (2) bIGF-1 could be adsorbed or tethered to KLD; (3) bIGF-1 could stimulate proteoglycan production by chondrocytes when adsorbed or tethered to KLD; and (4) an alternative growth factor, TGF-β1, could be adsorbed or tethered to KLD and stimulate proteoglycan production by BMSCs.
Materials and Methods
To ensure bIGF-1 had the same biologic activity as unmodified IGF-1, chondrocytes encapsulated in KLD were stimulated with 100 ng/mL of soluble bIGF-1 or soluble IGF-1 or with soluble complexes of 100 ng/mL bIGF-1 premixed with an equimolar amount of streptavidin for 4 days and sGAG and DNA content was measured (n = 3–4). Next, bIGF-1 and streptavidin were either (1) premixed with the peptide solution before assembly to adsorb it to KLD; or (2) tethered to KLD through incorporation of biotinylated KLD (Fig. 1) [6]. Concentrations of bIGF-1 in the KLD hydrogel were varied for the adsorbed and tethered conditions (50, 300, and 1000 ng/mL) such that cells would be exposed to amounts known to stimulate proteoglycan synthesis [12]. We used fluorescently tagged streptavidin to track the streptavidin/bIGF-1 complex in KLD with encapsulated chondrocytes, and sGAG and DNA content were measured after 1, 2, 4, and 8 days in culture (n = 4). BMSCs were stimulated with soluble (10 ng/mL), adsorbed (100 ng/mL), or tethered (100 or 500 ng/mL) TGF-β1 for 7, 14, or 21 days and sGAG and DNA content were measured (n = 4–16).
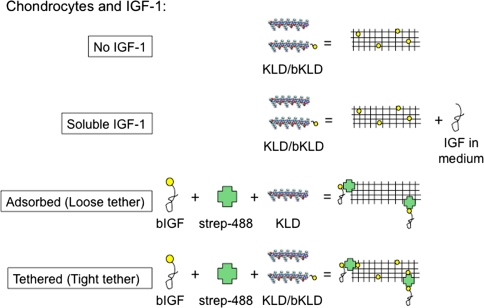
Fig. 1.
Experimental study design. Biotinylated insulin-like growth factor-1 (bIGF-1) was bound to a fluorescently labeled streptavidin molecule (strep-488) and adsorbed to the self-assembling peptide scaffold (KLDL)3 (eg, KLD) or tethered through incorporation of biotinylated-KLD (bKLD). In all cases, chondrocytes were encapsulated in the hydrogel.
KLD peptide with the sequence AcN-(KLDL)3-CNH2 and biotin-conjugated-KLD (bKLD) peptide with the sequence biotin-(aminocaproic acid)3-(KLDL)3 were synthesized by the MIT Biopolymers Laboratory (Cambridge, MA) using an ABI Model 433A peptide synthesizer with FMOC protection or received as a gift from 3DM (3DM, Inc, Cambridge, MA). In both cases, the purity of the peptide produced was confirmed by mass spectroscopy to be greater than 95%. Biotin-conjugated IGF-1 (bIGF-1) (Immunologic and Biochemical Test Systems GmbH, Reutlingen, Germany) and biotin-conjugated TGF-β1 (bTGF-β1) (R&D Systems, Minneapolis, MN) were purchased and used as described subsequently.
Chondrocytes were isolated from 1- to 2-week-old bovine calves (Research 87, Marlborough, MA) as described previously [40]. Chondrocytes were encapsulated in KLD using acellular agarose molds to initiate self-assembly as previously described [25], resulting in 6-mm diameter, 50-μL peptide gel disks. To verify bioactivity of bIGF-1, chondrocytes were encapsulated at 12 × 106 cells/mL in KLD peptide (0.35% w/v) alone and cultured in IGF-1-free basal medium supplemented with soluble IGF-1 (PeproTech Inc, Rocky Hill, NJ), soluble bIGF-1, or soluble bIGF-1/streptavidin at indicated concentrations (ng/mL) (n = 3–4). Basal medium consisted of serum-free high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 0.003% ITS + 1 (Sigma-Aldrich, St Louis, MO), 1 mM sodium pyruvate, 10 mM HEPES buffer, 0.1 mM nonessential amino acids, 0.4 mM proline, 20 μg/mL ascorbic acid, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B. This choice of concentration of ITS + 1 is equivalent to 5 nM insulin, termed mini-ITS [3, 30], and chosen to avoid crosstalk of insulin with the IGF-1 receptor.
Using a biotin-sandwich approach as previously described for a related peptide sequence, RAD [6], we tethered IGF-1 to KLD scaffolds by including 1:100 ratio by weight of bKLD:KLD and premixing streptavidin with bIGF-1. Multivalent streptavidin and bIGF-1 were mixed in equimolar amounts so the predominant complex formed would consist of a single biotinylated growth factor bound to a single streptavidin molecule, allowing streptavidin to bind bKLD with one of its three remaining binding sites. Excess bKLD (greater than 130:1 molar ratio bKLD:streptavidin) was used to ensure all of the delivered growth factor was tethered. For high-affinity tethering of IGF-1, control, soluble, and tethered gels were created by premixing KLD peptide (0.35% w/v) with 0.0035% w/v bKLD and encapsulating chondrocytes at 3 × 106 cells/mL (n = 4). For tethered gels, bIGF-1 and fluorescent streptavidin-AlexaFluor 488 (Invitrogen) were premixed at equimolar concentrations and added to the KLD/bKLD mixture before adding cells. For adsorbed gels, bIGF-1 and fluorescent-streptavidin were premixed as for the tethered gels, but no bKLD was used in this case. Chondrocyte gels were cultured in basal medium and supplemented with soluble IGF-1 for the soluble condition (soluble IGF-1 replenished at each medium change).
Bone marrow was harvested from 1- to 2-week-old bovine calves (Research 87) and stromal cells (BMSCs) were isolated as described previously [5, 25]. BMSCs were selected through differential adhesion and expanded two passages in low glucose-DMEM with 10% ES-FBS (Invitrogen), 10 mM HEPES, 100 U/mL penicillin G, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B, and 5 ng/mL FGF-2 (R&D Systems).
BMSCs were encapsulated in KLD mixtures at 3 × 106 cells/mL using acellular agarose molds to initiate self-assembly [25]. For no TGF (n = 8) and soluble TGF (n = 12) conditions, BMSCs were encapsulated in KLD peptide (0.35% w/v) alone. For adsorbed gels (n = 16), 0.35% KLD was premixed with 100 ng/mL TGF-β1 (R&D Systems) and 100 nM dexamethasone (Sigma-Aldrich) before adding cells. For tethered gels (n = 4), 0.35% w/v KLD was premixed with 0.0035% w/v bKLD, 2.1 μg/mL streptavidin (Pierce Biotechnology, Rockford, IL), and 100 or 500 ng/mL bTGF-β1 before adding cells. The resulting 6-mm diameter, 50-μL peptide gel disks were cultured in high glucose-DMEM (Invitrogen) supplemented with 1% ITS + 1 (Sigma-Aldrich), 1 mM sodium pyruvate, 37.5 μg/mL ascorbate-2-phosphate (Wako Chemicals, Richmond, VA), PSA (100 U/mL penicillin, 0.1 mg/mL streptomycin, 0.25 μg/mL amphotericin), 10 mM HEPES, 0.4 mM proline, and 0.1 mM nonessential amino acids. Soluble gels had additional supplementation of 10 ng/mL recombinant human TGF-β1 (R&D Systems) (replenished at each medium change). No TGF, soluble, or tethered gels had additional supplementation of 100 nM dexamethasone (replenished at each medium change).
Viability was determined by staining with FDA (live) and ethidium bromide (dead). There were no differences in viability among conditions for chondrocyte or BMSC experiments, and all experiments had greater than 75% viability. At each time point, gels were digested with 250 μg/mL proteinase-K (Roche Applied Science, Indianapolis, IN) overnight at 60ºC. sGAG in each hydrogel sample was assessed by DMMB dye binding [11]; DNA was quantified by Hoechst dye assay [22]. For chondrocyte gels, digests were analyzed for the presence of fluorescent streptavidin through fluorometer reading at 485/535 nm.
All data are presented as mean ± standard deviation of the mean. We determined differences in sGAG and DNA content among chondrocytes treated with no IGF-1, soluble IGF-1, and soluble bIGF-1 by one-way analysis of variance. We determined differences in sGAG and DNA content among chondrocytes treated with no IGF-1, soluble bIGF-1, and soluble bIGF-1/streptavidin complexes by one-way analysis of variance. At each time point, we determined differences in fluorescent counts between peptide gels loaded with 50, 300, or 100 ng/mL of adsorbed or tethered bIGF-1/fluorescent streptavidin complexes by one-way analysis of variance. At each time point, we determined differences in sGAG and DNA content among chondrocytes treated with no IGF-1, soluble, adsorbed, or tethered IGF-1 by one-way analysis of variance. At each time point, we determined differences in sGAG and DNA content among BMSCs treated with no TGF-β1, soluble, adsorbed, or tethered TGF-β1 by mixed model of variance with animal cell source as a random factor. For all these tests, residual plots were constructed for dependent variable data to test for normality and data were log-transformed if necessary to satisfy this assumption. Additionally, post hoc Tukey tests for significance of pairwise comparisons were performed with a threshold for significance of p < 0.05.
Results
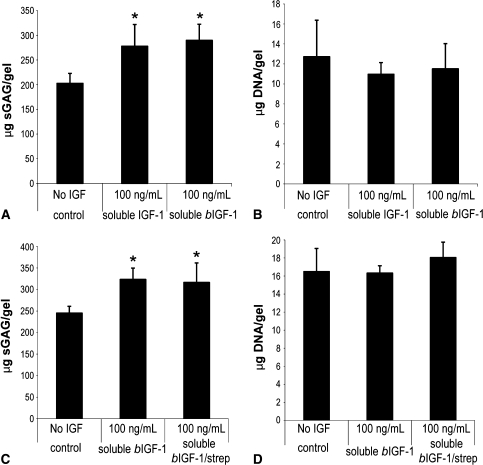
Chondrocytes encapsulated in KLD and treated with either soluble bIGF-1 or soluble IGF-1 for 4 days produced similar amounts of sGAG, and both conditions produced more sGAG than gels incubated without IGF-1 (Fig. 2A). DNA content was similar among the groups (Fig. 2B). Chondrocytes encapsulated in KLD and treated with soluble bIGF-1 with and without streptavidin produced similar amounts of sGAG, but more than gels incubated without IGF-1 (Fig. 2C). DNA was similar among the treatments (Fig. 2D).
Fig. 2A–D.
Biotinylated insulin-like growth factor-1 (bIGF-1) is bioactive. Chondrocytes were encapsulated in KLD at 12 × 106 cells/mL and cultured in medium with different soluble factors for 4 days. (A) sGAG and (B) DNA retained after culture in medium with soluble IGF-1 or soluble bIGF-1. (C) sGAG and (D) DNA retained after culture in medium with soluble bIGF-1 or soluble bIGF-1 + soluble streptavidin. Mean ± standard deviation. *Versus no IGF, p < 0.05.
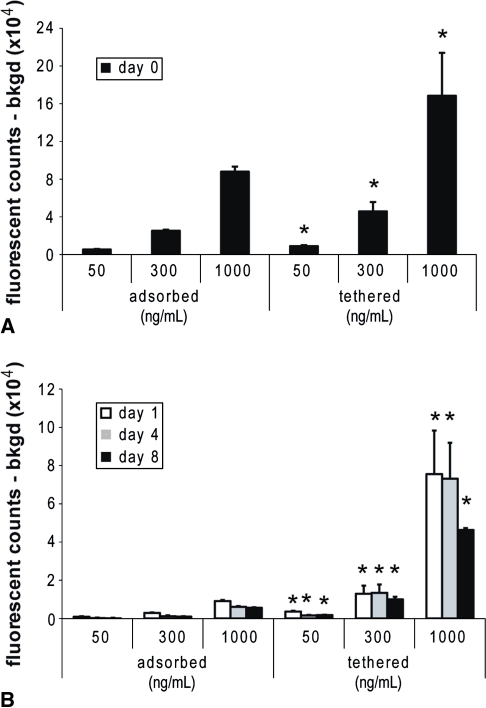
Because bIGF-1/streptavidin complexes were bioactive when delivered solubly, we progressed to adsorbing and tethering the complexes to KLD gels before encapsulation of chondrocytes. Although adsorbed and tethered gels were loaded with equivalent amounts of bIGF-1/streptavidin, tethered gels retained considerably more bIGF-1 immediately after encapsulation (Fig. 3A). After 1 day, adsorbed streptavidin/bIGF-1 had decreased to 10% to 17% of the Day 0 levels, and by Day 8, it was only 2% to 6% of Day 0 (Fig. 3B). Tethering allowed amounts to remain at 20% to 27% of the Day 0 levels even by Day 8 (Fig. 3B).
Fig. 3A–B.
High-affinity tethering prolongs retention of insulin-like growth factor-1 (IGF-1). Fluorescently labeled streptavidin and biotinylated-IGF-1 (bIGF-1) were either adsorbed or tethered to KLD before encapsulation of chondrocytes at 3 × 106 cells/mL. Background from the peptide was measured on gels without fluorescent streptavidin and subtracted from fluorescence counts. (A) Streptavidin retained immediately after gel assembly. (B) Streptavidin retained after 1, 4, or 8 days of culture. *Versus corresponding concentration adsorbed gel, p < 0.05. Mean ± standard deviation.
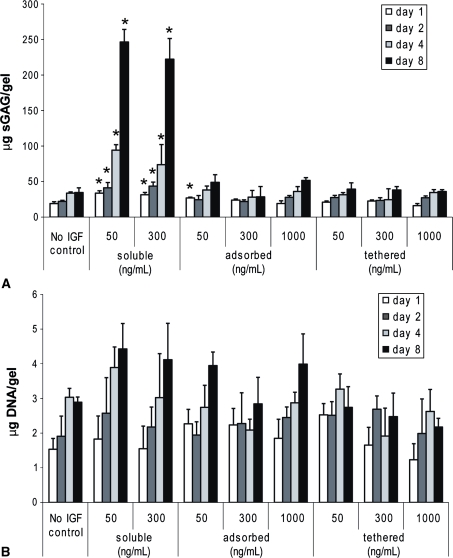
Although concentrations up to 1000 ng/mL were tested for adsorbed and tethered bIGF-1, neither method of delivering IGF-1 stimulated sGAG production compared to no IGF-1 levels (Fig. 4A). The lack of sGAG production resulting from adsorption of bIGF-1/streptavidin is consistent with the rapid diffusion out of KLD of adsorbed bIGF-1/streptavidin (Fig. 3). However, the lack of bioactivity for tethering was unexpected because sufficient levels were present throughout the culture period. Gels treated with 50 ng/mL and 300 ng/mL of soluble IGF-1 produced substantially greater amounts of sGAG than control IGF-1-free gels at all time points, as expected (Fig. 4A). DNA levels increased over time but were similar to those for control IGF-1-free gels for any treatment at any time point (Fig. 4B).
Fig. 4A–B.
Soluble insulin-like growth factor-1 (IGF-1), but not adsorbed or tethered IGF-1, stimulates sGAG production. Biotinylated IGF-1 and streptavidin were either adsorbed or tethered to KLD at the indicated concentrations before encapsulation of chondrocytes at 3 × 106 cells/mL. No IGF-1 control gels were cultured in IGF-1-free medium. (A) sGAG and (B) DNA retained in gel. Mean ± standard deviation. *Versus no IGF, p < 0.05.
In contrast to IGF-1, adsorbed TGF-β1 stimulated BMSC sGAG production higher than no TGF-β1 at Days 14 and 21. By Day 21, adsorbed TGF-β1 stimulated 31% as much sGAG as soluble TGF-β1 (Table 1). Tethering TGF-β1 at up to 500 ng/mL did not stimulate sGAG production by BMSCs, similar to that seen with chondrocytes exposed to tethered IGF-1. In addition, tethering at 500 ng/mL inhibited cell proliferation compared with TGF-β1-free gels at Days 14 and 21, seen as a lack of increase in DNA accumulation (Table 1). Soluble TGF-β1 stimulated an increase in DNA over TGF-β1-free gels by Day 21; adsorbed gels had similar DNA content to TGF-β1-free gels at all time points (Table 1).
Table 1.
Soluble and adsorbed transforming growth factor-β1 (TGF-β1), but not tethered, promotes sGAG production. sGAG and DNA content (µg/gel) of BMSCs encapsulated in KLD gels with or without TGF
| Time point | No TGF-β1 (n = 8) | Soluble TGF-β (n = 12) (10 ng/mL) | Adsorbed TGF-β1 (n = 16) (100 ng/mL) | Tethered TGF-β1 (n = 4) (100 ng/mL) | Tethered TGF-β1 (n = 4) (500 ng/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| sGAG | DNA | sGAG | DNA | sGAG | DNA | sGAG | DNA | sGAG | DNA | |
| Day 7 | 6.0 ± 2.2 | 1.8 ± 0.6 | 19.3 ± 10.9* | 2.3 ± 0.9 | 12.3 ± 9.5 | 1.6 ± 0.8 | 3.5 ± 0.7 | 1.2 ± 0.5 | 5.0 ± 0.6 | 1.4 ± 0.2 |
| Day 14 | 6.3 ± 1.9 | 2.0 ± 0.3 | 57.7 ± 33.5 | 3.2 ± 2.6 | 26.5 ± 17.9* | 2.2 ± 0.7 | 4.6 ± 0.7 | 1.9 ± 0.9 | 5.0 ± 1.5 | 1.0 ± 0.4* |
| Day 21 | 7.6 ± 2.2 | 2.3 ± 0.3 | 110.9 ± 49.6* | 4.8 ± 2.6* | 35.1 ± 21.3* | 2.3 ± 0.8 | 5.7 ± 0.5 | 3.1 ± 0.9 | 8.4 ± 1.2 | 1.7 ± 0.7* |
* Versus no TGF-β1, p < 0.05. Mean ± standard deviation.
Discussion
The optimal strategy for delivering growth factors to cells for the purposes of cartilage tissue engineering and cartilage repair remains an unmet challenge. Self-assembling peptides are a clinically relevant material that can be injected in vivo [6, 7] and assemble on contact with solutions of physiological pH and ionic strength [53]. Tethering bIGF-1 to the self-assembling peptide scaffold (RADA)4 effectively delivers bioactive bIGF-1 to cardiac tissue. We therefore asked whether: (1) bIGF-1 could stimulate proteoglycan production by chondrocytes when delivered solubly; (2) bIGF-1 could be adsorbed or tethered to KLD; (3) bIGF-1 could stimulate proteoglycan production by chondrocytes when adsorbed or tethered to KLD; and (4) an alternative growth factor, TGF-β1, could be adsorbed or tethered to KLD and stimulate proteoglycan production by BMSCs.
Readers should be aware of the limitations of the study. First, the growth factor of interest for the chondrocyte experiments was IGF-1, whereas that for the BMSC experiments was TGF-β1; therefore, the GAG and DNA contents from these two sets of experiments should not be compared directly, but instead interpreted to understand that the adsorbed versus tethered delivery methods may have different bioactivity outcomes depending on the particular growth factor used. The choice of growth factors was based on the widely accepted approach that TGF-β1 is essential to obtain BMSC chondrogenesis, whereas, by comparison, chondrocytes already possess the desired phenotype and IGF-1 is a widely accepted choice for matrix biosynthesis. Second, we studied a small number of constructs, although the variability of the assays was relatively small. Third, ours was an in vitro study without many of the variables that would occur in vivo. The findings would therefore require further confirmation.
As a result of the known biologic effects of biotin on cells [52] and the effects of modifying IGF-1 structure [1] as well as the possibility of steric effects resulting from streptavidin size relative to IGF-1, we first tested whether biotinylated IGF-1 and a complex of biotinylated IGF-1 with streptavidin would retain biologic activity measured by sGAG production. When delivered solubly in the medium, bIGF-1 induced similar amounts of sGAG as unmodified IGF-1, consistent with the observations of Davis et al. [6] measuring pAKT stimulation of cardiomyocytes. Similarly, supplementation of medium with the complex of bIGF-1 and streptavidin resulted in sGAG production comparable to soluble bIGF-1 alone, indicating that the molecules chosen did not hamper the absorption and tethering strategies.
Adsorption of bIGF-1 to KLD did not result in delivery of IGF-1 with the majority of the growth factor diffusing out of the gel within the first 24 hours. Nixon et al. showed an improvement in repair of an equine cartilage defect with delivery of IGF-1 premixed with a fibrin clot [39], although that study did not examine the release kinetics out of the gel, and so it is possible that an initial bolus release occurred that was sufficient in stimulating improved repair. In contrast, in our study tethering bIGF-1 to KLD through biotin-streptavidin bonds resulted in prolonged retention of IGF-1, although there was a loss of 70% to 80% of the growth factor by Day 8. As a result of the strength of the noncovalent biotin-streptavidin bond, KD = 4 × 10−14 M [15], it is unlikely the growth factors are released by this tether. Instead, the loss of bIGF-1 by Day 8 is probably the result of the degradation of the gel.
Other methods for slow release of IGF-1 and TGF-β1 have been developed [28, 48], but IGF-1 remains difficult to effectively deliver in vivo as a result of its small size and rapid diffusion out of tissue. A new fusion protein made by adding the heparin-binding domain of heparin-binding EGF to IGF-1 (ie, HB-IGF-1) is amenable to delivery in mature articular cartilage [36, 49]. A single dose of HB-IGF-1 resulted in sustained delivery and stimulation of proteoglycan synthesis for at least 8 days. The use of HB-IGF-1 with the KLD peptide system could be enabled by mixing in heparin, heparan sulfate, or the heparan sulfate proteoglycan, perlecan, with KLD [18, 19, 51]. Although adsorption of TGF-β1 to KLD effectively increased proteoglycan production in our study and a previous study [26], changing the degradation rates of KLD by changing the concentration or adding crosslinks may offer a way of improving delivery.
Although biotin-streptavidin tethering of IGF-1 reduces apoptosis of implanted cardiomyocytes [6], it did not deliver IGF-1 or TGF-β1 to stimulate proteoglycan production by chondrocytes or BMSCs, respectively, in the context of cartilage tissue engineering. A difference in peptide sequence, (RADA)4 versus (KLDL)3, or differences in growth factor-receptor binding in cardiomyocytes could partly explain the disparities between this study and that of Davis et al. [6]. Cartilage tissue also differs from myocardial tissue in that large amounts of extracellular matrix are produced as early as 1 day after encapsulation. This matrix synthesis and secretion may prevent the stimulation of chondrocytes and BMSCs by immobilized factors by sterically blocking receptor-ligand binding.
There has been a limited number of other attempts at delivering tethered growth factors for the purposes of cartilage tissue engineering [38]. Growth factors, including TGF-β1, have been covalently immobilized or tethered onto other polymer scaffolds or hydrogels with other cell types [2, 8, 20, 21, 29, 34], sometimes with altered signaling as a result [10, 35, 42]. The density of tethered growth factors can also affect bioactivity [45]; however, both IGF-1 and TGF-β receptors exist as functional complexes on cell surfaces, and binding of one ligand can initiate signaling [9, 43]. Consistent with this, increasing the amount of growth factors immobilized within the scaffold had no effect on bioactivity. Two possible solutions to increase bioactivity of our tethering may be to include matrix metalloproteinase-cleavable links in our peptide sequence to deliver growth factors on catabolic events [31, 32, 41] or to use tethers with a lower binding affinity.
Our study describes an initial evaluation of methods for delivering growth factors to cells within the self-assembling peptide hydrogel KLD for cartilage tissue engineering and showed that although TGF-β1 can be delivered by adsorption, IGF-1 cannot. Additionally, although tethering these factors provided long-term sequestration, tethered growth factors were not effective in stimulating proteoglycan production. Therefore, although peptide sequences are readily functionalized, the manner in which growth factors are delivered affects bioactivity and varies for specific growth factors and biologic systems of interest.
Acknowledgments
We thank Ms Emily Florine, Dr David Frisbie, Dr John Kisiday, Dr Bodo Kurz, Dr Richard Lee, and Dr Eric Vanderploeg for helpful discussions.
Footnotes
One or more authors (AJG) received funding from National Institutes of Health–NIBIB grant EB003805 and National Institutes of Health–NIAMS grant AR33236. One or more of the authors (AJG) is a former member of the 3DM Science Advisory Board. One or more of the authors (REM) received NSF and NDSEG graduate fellowships and a Siebel Foundation Scholar fellowship. One or more of the authors (PWK) received a National Institutes of Health MCTB training grant.
References
- 1.Ballard FJ, Wallace JC, Francis GL, Read LC, Tomas FM. Des(1–3)IGF-I: a truncated form of insulin-like growth factor-I. Int J Biochem Cell Biol. 1996;28:1085–1087. doi: 10.1016/1357-2725(96)00056-8. [DOI] [PubMed] [Google Scholar]
- 2.Bentz H, Schroeder JA, Estridge TD. Improved local delivery of TGF-beta2 by binding to injectable fibrillar collagen via difunctional polyethylene glycol. J Biomed Mater Res. 1998;39:539–548. doi: 10.1002/(SICI)1097-4636(19980315)39:4<539::AID-JBM6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Benya PD, Padilla SR. Dihydrocytochalasin B enhances transforming growth factor-[beta]-induced reexpression of the differentiated chondrocyte phenotype without stimulation of collagen synthesis. Exp Cell Res. 1993;204:268–277. doi: 10.1006/excr.1993.1033. [DOI] [PubMed] [Google Scholar]
- 4.Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243–262. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connelly JT, Garcia AJ, Levenston ME. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28:1071–1083. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Davis ME, Hsieh PCH, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis ME, Motion JPM, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 10.Fan VH, Au A, Tamama K, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 11.Farndale R, Sayers C, Barrett A. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 12.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–288. doi: 10.1302/0301-620X.84B2.11167. [DOI] [PubMed] [Google Scholar]
- 13.Genové E, Shen C, Zhang S, Semino CE. The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials. 2005;26:3341–3351. doi: 10.1016/j.biomaterials.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Getgood A, Brooks R, Fortier L, Rushton N. Articular cartilage tissue engineering: today’s research, tomorrow’s practice? J Bone Joint Surg Br. 2009;91:565–576. doi: 10.1302/0301-620X.91B5.21832. [DOI] [PubMed] [Google Scholar]
- 15.Holmberg A, Blomstergren A, Nord O, Lukacs M, Lundeberg J, Uhlen M. The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis. 2005;26:501–510. doi: 10.1002/elps.200410070. [DOI] [PubMed] [Google Scholar]
- 16.Horii A, Wang X, Gelain F, Zhang S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS One. 2007;2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh PCH, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005;105:249–259. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Jeon O, Song SJ, Kang SW, Putnam AJ, Kim BS. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28:2763–2771. doi: 10.1016/j.biomaterials.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Kapur TA, Shoichet MS. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J Biomed Mater Res A. 2004;68:235–243. doi: 10.1002/jbm.a.10168. [DOI] [PubMed] [Google Scholar]
- 21.Kim EJ, Kang IK, Jang MK, Park YB. Preparation of insulin-immobilized polyurethanes and their interaction with human fibroblasts. Biomaterials. 1998;19:239–249. doi: 10.1016/S0142-9612(98)00203-8. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y-J, Sah RLY, Doong J-YH, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 23.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci USA. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 25.Kopesky P, Vanderploeg E, Sandy J, Kurz B, Grodzinsky AJ. Self-assembling peptide hydrogels modulate in vitro chondrogenesis of bovine bone marrow stromal cells. Tissue Eng A. 2010;16:465–477. doi: 10.1089/ten.tea.2009.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopesky PW, Vanderploeg EJ, Kisiday JD, Frisbie DD, Sandy JD, Grodzinsky AJ. Controlled delivery of transforming growth factor beta1 by self-assembling peptide hydrogels induces chondrogenesis of bone marrow stromal cells and modulates Smad2/3 signaling. Tissue Eng Part A. 2011;17:83–92. doi: 10.1089/ten.tea.2010.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutsopoulos S, Unsworth LD, Nagai Y, Zhang S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc Natl Acad Sci USA. 2009;106:4623–4628. doi: 10.1073/pnas.0807506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S-H, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59:339–359. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Liu HW, Chen CH, Tsai CL, Lin IH, Hsiue GH. Heterobifunctional poly(ethylene glycol)-tethered bone morphogenetic protein-2-stimulated bone marrow mesenchymal stromal cell differentiation and osteogenesis. Tissue Eng. 2007;13:1113–1124. doi: 10.1089/ten.2006.0209. [DOI] [PubMed] [Google Scholar]
- 30.Loeser RF, Shanker G. Autocrine stimulation by insulin-like growth factor 1 and insulin-like growth factor 2 mediates chondrocyte survival in vitro. Arthritis Rheum. 2000;43:1552–1559. doi: 10.1002/1529-0131(200007)43:7<1552::AID-ANR20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 31.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 33.Maher SA, Mauck RL, Rackwitz L, Tuan RS. A nanofibrous cell-seeded hydrogel promotes integration in a cartilage gap model. J Tissue Eng Regen Med. 2010;4:25–29. doi: 10.1002/term.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann BK, Schmedlen RH, West JL. Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–444. doi: 10.1016/S0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 35.Marcantonio NA, Boehm CA, Rozic RJ, Au A, Wells A, Muschler GF, Griffith LG. The influence of tethered epidermal growth factor on connective tissue progenitor colony formation. Biomaterials. 2009;30:4629–4638. doi: 10.1016/j.biomaterials.2009.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller RE, Grodzinsky AJ, Cummings K, Plaas AH, Cole AA, Lee RT, Patwari P. Intra-articular injection of HB-IGF-1 sustains delivery of IGF-1 to cartilage through binding to chondroitin sulfate. Arthritis Rheum. 2010;62:3686–3694. doi: 10.1002/art.27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller RE, Grodzinsky AJ, Vanderploeg EJ, Lee C, Ferris DJ, Barrett MF, Kisiday JD, Frisbie DD. Effect of self-assembling peptide, chondrogenic factors, and bone marrow-derived stromal cells on osteochondral repair. Osteoarthritis Cartilage. 2010;18:1608–1619. doi: 10.1016/j.joca.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motoyama M, Deie M, Kanaya A, Nishimori M, Miyamoto A, Yanada S, Adachi N, Ochi M. In vitro cartilage formation using TGF-beta-immobilized magnetic beads and mesenchymal stem cell-magnetic bead complexes under magnetic field conditions. J Biomed Mater Res A. 2010;92:196–204. doi: 10.1002/jbm.a.32365. [DOI] [PubMed] [Google Scholar]
- 39.Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17:475–487. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- 40.Ragan PM, Chin VI, Hung HH, Masuda K, Thonar EJ, Arner EC, Grodzinsky AJ, Sandy JD. Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch Biochem Biophys. 2000;383:256–264. doi: 10.1006/abbi.2000.2060. [DOI] [PubMed] [Google Scholar]
- 41.Segers VFM, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local Delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 42.Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomaterialia. 2008;4:477–489. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Siddle K, Urso B, Niesler CA, Cope DL, Molina L, Surinya KH, Soos MA. Specificity in ligand binding and intracellular signalling by insulin and insulin-like growth factor receptors. Biochem Soc Trans. 2001;29:513–525. doi: 10.1042/BST0290513. [DOI] [PubMed] [Google Scholar]
- 44.Sieminski AL, Semino CE, Gong H, Kamm RD. Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. J Biomed Mater Res A. 2008;87:494–504. doi: 10.1002/jbm.a.31785. [DOI] [PubMed] [Google Scholar]
- 45.Sofia SJ, Kuhl PR, Griffith LG. Preparation and use of tethered ligands as biomaterials and tools for cell biology. In: Morgan JR, Yarmush ML, editors. Tissue Engineering Methods and Protocols. Totowa, NJ: Humana Press Inc; 1999. pp. 19–33. [DOI] [PubMed] [Google Scholar]
- 46.Sohier J, Moroni L, Blitterswijk C, Groot K, Bezemer JM. Critical factors in the design of growth factor releasing scaffolds for cartilage tissue engineering. Expert Opin Drug Deliv. 2008;5:543–566. doi: 10.1517/17425247.5.5.543. [DOI] [PubMed] [Google Scholar]
- 47.Steinert AF, Noth U, Tuan RS. Concepts in gene therapy for cartilage repair. Injury. 2008;39(Suppl 1):S97–113. doi: 10.1016/j.injury.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoop R. Smart biomaterials for tissue engineering of cartilage. Injury. 2008;39(Suppl 1):S77–87. doi: 10.1016/j.injury.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 49.Tokunou T, Miller R, Patwari P, Davis ME, Segers VF, Grodzinsky AJ, Lee RT. Engineering insulin-like growth factor-1 for local delivery. FASEB J. 2008;22:1886–1893. doi: 10.1096/fj.07-100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Horii A, Zhang S. Designer functionalized self-assembling peptide nanofiber scaffolds for growth, migration, and tubulogenesis of human umbilical vein endothelial cells. Soft Matter. 2008;4:2388–2395. doi: 10.1039/b807155a. [DOI] [Google Scholar]
- 51.Yang WD, Gomes RR, Alicknavitch M, Farach-Carson MC, Carson DD. Perlecan domain I promotes fibroblast growth factor 2 delivery in collagen I fibril scaffolds. Tissue Engineering. 2005;11:76–89. doi: 10.1089/ten.2005.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zempleni J, Helm RM, Mock DM. In vivo biotin supplementation at a pharmacologic dose decreases proliferation rates of human peripheral blood mononuclear cells and cytokine release. J Nutr. 2001;131:1479–1484. doi: 10.1093/jn/131.5.1479. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci USA. 1993;90:3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]