Abstract
Background
Rotator cuffs heal with an interposed layer of scar tissue that makes repairs prone to failure. Cell-based biologic therapies have the potential to augment this healing process. Scleraxis (Scx) is a transcription factor that is involved in tendon development during embryogenesis, and may help drive stem cells toward tenocyte differentiation in adults.
Questions/Hypothesis
(1) Overexpression of Scx with adenoviral-mediated gene transfer in stem cells will drive pluripotent stem cells toward tenoblastogenic lineages in vitro; (2) the application of these genetically modified cells will result in improved histologic and biomechanical healing of rotator cuff repairs.
Method of study
For the first hypothesis, we will determine whether stem cells derived from various sources can differentiate into tenocytes when genetically modified with Scx in vitro. We will assess morphologic features of cells with light microscopy, and gene expression analyses to confirm phenotypes consistent with tenocyte differentiation. For the second hypothesis, we will determine whether these genetically modified cells augment rotator cuff repairs in a rat model based on histology and biomechanical outcomes.
Significance
Development of this technology may substantially advance our ability to repair large to massive rotator cuff tears while limiting the rates of anatomic failure.
Questions/Hypothesis
(1) Overexpression of Scx in stem cells will drive pluripotent stem cells toward tenoblastogenic lineages in vitro and (2) application of these genetically modified cells will result in improved histologic and biomechanical healing of rotator cuff repairs in vivo.
Background
Rotator cuff repairs are one of the most commonly performed surgeries in the United States [1]. Despite its prevalence, several studies suggest that rotator cuff repairs fail to heal at an alarming rate [11, 13, 15, 18, 28]. Biologic therapies may be able to augment repairs and reduce anatomic failures [3, 9, 10, 16, 17, 27, 29]. Although bone marrow-derived mesenchymal stem cells (MSCs) reportedly improve tendon healing in a bone tunnel [7, 19, 22], we recently found they did not improve healing in a rotator cuff repair model [14]. In the complex biomechanical environment of the shoulder, the simple addition of MSCs appears to be insufficient to augment repairs. This led us to hypothesize a signal is required to increase their effectiveness.
Scx is a basic helix-loop-helix transcription factor that has been implicated in tendon development and regeneration [2, 23, 26]. Endogenous expression of Scx defines a zone between sclerotomal and myotomal cells in the developing somite, and persists throughout tendon formation in the mouse embryo [4, 5, 24, 26]. Scx deficiency results in severe tendon defects in mice [21]. Scx also is believed to direct tendinous attachments to bone through its effects on chondrogenic lineage formation [2, 4, 20]. The simultaneous role for Scx in tendon and cartilage development suggests it has the potential to improve regeneration of the rotator cuff insertion site consisting of the tendon and its cartilaginous transition zone into the bone [6, 8, 12, 17, 25].
The ultimate goal of our ongoing work is to develop a technique to direct the differentiation of stem cells into tenocytes with a specific emphasis on promoting regeneration of the tendon insertion into bone. We propose overexpression of Scx in stem cells will enable us to accomplish this goal. If established, this technology may improve our ability to repair massive rotator cuff tears.
Proposed Program
The proposed work consists of two experiments: The first experiment will be to induce tenoblastogenic differentiation of pluripotent stem cells by overexpression of Scx. Multiple human and mouse stem cell sources will be evaluated to determine the cell line with the most efficient tenoblastogenic differentiation potential. To induce differentiation, stem cells will be modified genetically with the mouse Scx gene in vitro. Tenocyte differentiation will be assessed with microscopic and gene expression analyses. The genes analyzed will include tendon markers (Col1A2, Col3A2, Scx, tenascin-c, cartilage oligomeric matrix protein [COMP], decorin, biglycan, and tenomodulin), cartilage markers (Col2A1, Col10A1, and Sox9), and bone markers (osteopontin, Runx2, and osteomodulin). This will be done with quantitative polymerase chain reaction (Q-PCR). These studies are important because they will determine the signals necessary to induce rotator cuff regeneration.
The second experiment will be to determine the role of Scx expression in rotator cuff repairs. We hypothesize that Scx-transduced stem cells will improve repairs when grown in three-dimensional depots in vitro and implanted in a rat rotator cuff repair model. Mouse Scx is homologous with rat Scx, and therefore we expect full metabolic activity in this model. Outcomes will include histologic and biomechanical evaluations. The Scx gene has been inserted into the adenoviral vector in frame with the FLAG tag. This FLAG tag will be used for immunostaining to prove Scx gene expression at the repair site. This is important because it will be the first step in developing a clinically useful therapy that can regenerate the rotator cuff after injury. The ability to perform the second experiment is not dependent on the results of the first, and can be performed concomitantly. The goal of the second experiment is to determine how these modified cells behave in vivo, whereas the goal of the first experiment is to determine how they behave in vitro. The in vivo environment may provide additional signals that allow differentiation of the applied cells, even if in vitro conditions show that differentiation is sparse. This information would be useful for future studies of the differentiation potential of MSCs into tenocytes.
The use of Scx to improve rotator cuff healing introduces a novel paradigm to the field of biologic augmentation. By recreating the molecular events that occur during the development of tendons during embryogenesis, we intend to drive the healing process toward regeneration and away from scar formation. As outlined, Scx is credited as the prime initiator and director of tendon development. Therefore, we believe it is the best factor to investigate in our ongoing studies.
Limitations
A potential pitfall in conducting the first experiment is that none of the lines of stem cells differentiate into tenocytes with Scx overexpression in vitro. In that case, we will use three strategies. The first will be to subject the cells to tensile loads using collagen gels and the FlexCell loading apparatus (FlexCell Inc, Hillsborough, NC, USA). The next will be to coculture the stem cells with mature tenocytes. And the third will be to add the tenogenetic growth factors BMP-12 and basic fibroblast growth factor (bFGF) to the culture medium. The potential pitfall with the second experiment is rejection of the stem cells once implanted. If this is a problem, we will use nude mice for the rest of the study. Also, dosage and timing of MSC application may affect the outcomes. If the null hypothesis is found for the second experiment, we will conduct a study using different dosages at different times. There are potential limitations with the clinical application of this technology. Overexpression of Scx could lead to cancer, or have other unforeseen affects on the body that are not anticipated at this time. Furthermore, it is possible that one factor will not be sufficient to improve the complex biologic nature of the healing process. Studies would need to be done on larger mammals before its use in humans can be justified.
Next Steps
For this technology to become a viable clinical tool, three things will need to be determined. The first is that we will need to develop a method to maintain tenocyte differentiation through several passages, as these cells have a propensity to dedifferentiate into fibroblasts. The next will be to develop a clinically relevant way to either harvest pluripotent stem cells from the patient, or to develop a way to massively expand other stem cell sources if it ultimately is determined that those cells have no antigenicity. This is challenging as these cells remain pluripotent only for a few passages, so the shelf-life needs to be determined and optimized. The final step will be to develop an adequate carrier for these cells so that they can be implanted at the repair site, preferably using arthroscopic techniques. This step will require the assistance of biomaterials engineers who work at our hospital.
Implications and Future Directions
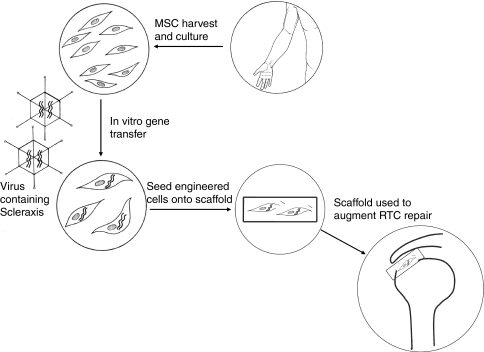
The ultimate goal of this research is to develop a cell-based therapy that can improve rotator cuff repairs and limit the rates of anatomic failures (Fig. 1). This should improve the outcomes for thousands of patients each year who undergo rotator cuff repair surgery. If our hypothesis is correct, we then will develop ways to deliver these cells to the repair site. One possibility is the development of a cell-seeded scaffold that contains these genetically modified cells. We hope to develop a technique whereby stem cells are harvested from patients and grown in culture in the laboratory. They then will be altered genetically to overexpress Scx and then seeded on a scaffold. This scaffold then can be implanted between the bone and the repaired tendon to augment healing.
Fig. 1.
The schematic shows how this technology may be used to augment rotator cuff repairs in the future. MSC = mesenchymal stem cells; RTC - rotator cuff.
Acknowledgments
We thank the following people for input in this study design: Chisa Hidaka MD, Christina Cheng BS, Matthew Cunningham MD, PhD, and Steven Swendeman PhD. We also thank Ronen Schweitzer PhD, for generously supplying the mouse Scx cDNA.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Laboratory for Soft Tissue Research, Hospital for Special Surgery, New York, NY, USA.
References
- 1.American Academy of Orthopaedic Surgeons. Research statistics on rotator cuff repairs, national ambulatory medical care survey, 1998–2004. US Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. Available at: http://www.aaos.org/Research/stats/patientstats.asp. Accessed Nov 5, 2010.
- 2.Asou Y, Nifuji A, Tsuji K, Shinomiya K, Olson EN, Koopman P, Noda M. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. J Orthop Res. 2002;20:827–833. doi: 10.1016/S0736-0266(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 3.Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51–54. doi: 10.3109/17453679909000958. [DOI] [PubMed] [Google Scholar]
- 4.Brown D, Wagner D, Li X, Richardson JA, Olson EN. Dual role of the basic helix-loop-helix transcription factor scleraxis in mesoderm formation and chondrogenesis during mouse embryogenesis. Development. 1999;126:4317–4329. doi: 10.1242/dev.126.19.4317. [DOI] [PubMed] [Google Scholar]
- 5.Burgess R, Cserjesi P, Ligon KL, Olson EN. Paraxis: a basic helix-loop-helix protein expressed in paraxial mesoderm and developing somites. Dev Biol. 1995;168:296–306. doi: 10.1006/dbio.1995.1081. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. doi: 10.1016/S1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 7.Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH, Lim BH. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg Am. 2007;89:74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- 8.Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;3r:362–369. doi: 10.1177/0363546505280428. [DOI] [PubMed] [Google Scholar]
- 9.Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005;23:84–92. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Dines JS, Grande DA, Dines DM. Tissue engineering and rotator cuff tendon healing. J Shoulder Elbow Surg. 2007;16(5 suppl):S204–S207. doi: 10.1016/j.jse.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 13.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gulotta LV, Kovacevic D, Ehteshami J, Dahger E, Packer JD, Rodeo SA. Application of bone-marrow derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37:2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 15.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff: correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982–989. [PubMed] [Google Scholar]
- 16.Kim HM, Galatz LM, Das R, Havlioglu N, Rothermich SY, Thomopoulos S. The role of transforming growth factor beta isoforms in tendon-to-bone healing. Connect Tissue Res. 2010 Jul 8. [Epub ahead of print]. [DOI] [PubMed]
- 17.Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, Okada K, Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15:371–377. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89:1533–1541. doi: 10.2106/JBJS.F.00305. [DOI] [PubMed] [Google Scholar]
- 19.Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899–910. doi: 10.1016/S0749-8063(04)00653-X. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Watanabe H, Nifuji A, Yamada Y, Olson EN, Noda M. Overexpression of a single helix-loop-helix-type transcription factor, scleraxis, enhances aggrecan gene expression in osteoblastic osteosarcoma ROS17/2.8 cells. J Biol Chem. 1997;272:29880–29885. doi: 10.1074/jbc.272.47.29880. [DOI] [PubMed] [Google Scholar]
- 21.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang HW, Goh JC, Lee EH. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med. 2004;32:321–327. doi: 10.1177/0095399703258682. [DOI] [PubMed] [Google Scholar]
- 23.Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236:1677–1682. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- 24.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 26.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 27.Seeherman HJ, Archambault JM, Rodeo SA, Turner AS, Zekas L, D’Augusta D, Li XJ, Smith E, Wozney JM. rhBMP-12 accelerates rotator cuff healing in a sheep model. J Bone Joint Surg Am. 2008;90:2206–2219. doi: 10.2106/JBJS.G.00742. [DOI] [PubMed] [Google Scholar]
- 28.Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair: a prospective outcome study. J Bone Joint Surg Am. 2007;89:953–960. doi: 10.2106/JBJS.F.00512. [DOI] [PubMed] [Google Scholar]
- 29.Wurgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg. 2007;16(5 suppl):S198–S203. doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]