Abstract
Background
To prevent further degeneration, it is desirable to fill a meniscal defect with a supportive scaffold that mimics the mechanics of native tissue. Degradable porous scaffolds have been used, but it is unclear whether the tissue that fills the site of implantation is mechanically adequate, particularly with respect to frictional performance.
Questions/purposes
We therefore determined the frictional behavior of native and engineered meniscal replacement tissue from in vivo implantation over time.
Methods
We evaluated boundary and mixed-mode friction coefficients of tissue generated in porous polyurethane scaffolds used to augment the repair of the meniscus of 13 skeletally mature sheep after partial meniscectomy. Implants were removed for evaluation at 3, 6, and 12 months. The friction coefficient, aggregate modulus, and hydraulic permeability were evaluated for tissue harvested from native meniscus adjacent to the implants, native meniscus from the intact contralateral knee, and repair tissue from the site of the scaffold implantation. The equilibrium friction coefficient (μeq) was measured in the presence of a lubricant bath of either phosphate-buffered saline (PBS) or equine synovial fluid (ESF).
Results
Boundary μeq in PBS of engineered meniscus improved with time and was similar to native tissue after 6 months. ESF enhanced lubrication for all samples at nearly all time points demonstrating the efficacy of ESF as a joint lubricant for repair tissue as well as native meniscus. Modulus increased and permeability decreased with implantation, likely as a result of tissue ingrowth.
Conclusions
Promoting tissue ingrowth into porous scaffolds is a potential strategy for improving friction performance in meniscal repair.
Introduction
Menisci play important mechanical roles in the knee, including load transmission, energy dissipation, and joint congruity and stability [1]. Partial meniscectomy is a common surgical treatment among the over one million meniscal procedures performed each year [23]; however, loss of even part of the meniscus can destabilize the knee and predispose the joint to long-term degenerative changes and osteoarthritis [4, 8, 28].
The degree of joint degeneration after meniscectomy is reportedly related to the amount of meniscus removed [9], making it beneficial to save as much tissue as possible. Replacing resected tissue with a support material may further improve outcome; contact area is increased and contact pressure decreased with a replacement scaffold in place when compared with a partially meniscectomized knee [3].
Various replacement materials currently in use attempt to mimic the mechanical function of cartilaginous tissues and allow the patient improved use of the joint. Specifically, polymeric materials provide a favorable cellular environment for tissue ingrowth [32] in addition to distributing load [3]. Porous polyurethane foams in particular are of interest as a meniscal replacement because the porosity of the foam scaffold facilitates tissue ingrowth [32]. Previous studies suggest tissue regenerates in meniscal lesions in dogs using a degradable porous polymer scaffold [17, 31].
For both native meniscus and engineered meniscal replacements, frictional properties are particularly important because the meniscus continually slides against articular cartilage during the gait cycle [26]. The resulting coefficient of friction (μ) can vary widely from 0.015 to 0.28 for native cartilage [5, 13], partly as a result of the tissue’s biphasic behavior. Biphasic time-dependent response to loading is a characteristic of cartilaginous tissues [12, 13]; when compressed, the load is initially supported by hydrostatic pressure but as fluid pressurization diminishes, support is increasingly carried by the solid surfaces [25]. The frictional behavior observed at this point depends on several factors, including fluid viscosity, relative speed, and normal force [13]. When slow speeds and high contact forces exist, the result is boundary mode friction, in which μ and thus potential for damage and wear are highest. Boundary mode contrasts to mixed mode in which a fluid layer is partially supported between the solid surfaces and helps to carry the load, diminishing overall μ.
Acting as a load-bearing surface is an important role for polyurethane foam replacements, as it is for native tissue. Meniscus μ has been examined as part of whole-joint friction investigations [26], but the frictional behavior of meniscal tissue has not been widely studied to date. The frictional properties of porous polyurethane foam/cartilage articulating surfaces have recently been investigated [14], but the mechanical behavior of the resultant meniscal tissues after in vivo integration is unknown.
We used an ovine partial meniscectomy model to assess the mechanical behavior of meniscal repair tissue. Specifically, the goals of this study were (1) to determine whether the equilibrium friction properties of native meniscus are well described by Stribeck behavior across a range of articulating conditions (2) to determine if synovial fluid effectively lubricates repair meniscal tissue; (3) to determine the effect of implantation time on frictional behavior; and (4) to determine the effect on mechanical properties resulting from tissue ingrowth into a porous polyurethane foam.
Materials and Methods
The right lateral menisci of 13 healthy, skeletally mature Columbia X Rambouillet sheep were subjected to partial resection (Fig. 1). We performed an arthrotomy to expose the meniscus and a wedge of meniscus to within 1 mm of the capsule was removed by a longitudinal cut connecting two radial cuts in the midanterior and posterior horns. The removed meniscal tissue was used as a template to size the replacement polyurethane material. We replaced the defect site with fitted 80% biodegradable aliphatic polyurethane with pore sizes of less than 400 μm (ActifitTM; Orteq Ltd, Cambridge, UK) [14, 31]. The scaffold used to fill the defect had a C-shaped geometry with a cross-sectional wedge shape and a peripheral thickness of 8 mm thinning to a 2-mm central thickness. The implant was approximately 2 mm thicker than the native meniscus to better mimic the mechanical characteristics of the native tissue. The implant was sutured to adjacent meniscal tissue and capsule in a horizontal mattress fashion using one cranial, lateral, and caudal suture with No. 3-0 Ethibond sutures (Ethicon, Somerville, NJ). The lateral collateral ligament and bone block were reattached with a stainless steel bone screw. Right knees only were implanted and left knees were left intact as age-matched controls. Animals were not immobilized after surgery.
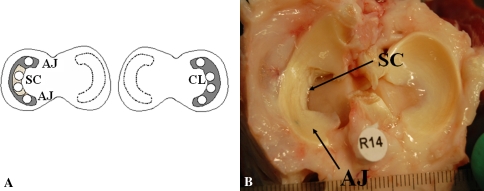
Fig. 1A–B.
(A) Diagram of explant/implant locations in right and left knees: SC = scaffold region; AJ = native meniscal tissue adjacent to the implanted scaffold; CL = native meniscus from the contralateral intact knee. Lightly shaded region denotes region of scaffold placement. Open circles are explanted tissue locations taken from menisci with dark shading. (B) Twelve-month operated knee before explant removal. AJ and SC sites indicated with SC region showing integration with surrounding tissue.
In this pilot study, six animals were euthanized after 3 months, three after 6 months, and four after 12 months. Sheep were anesthetized through intravenous injection of 3.3 mg/kg ketamine and 0.1 mg/kg valium. Postoperative pain was managed by intramuscular injection of 0.3 mg/kg buprenorphine administered twice per day for 3 days. At the termination of the study, animals were euthanized by overdose of 390 mg/kg sodium pentobarbital with 50 mg/ml phenytoin. All animal studies were approved by the Institutional Animal Care and Use Committees at the Hospital for Special Surgery and Colorado State University.
We removed both knees at the midfemur and midtibia. Knees were dissected down to the joint capsule, both menisci of both knees removed, and 13 4-mm cores taken with a biopsy punch. We divided samples into three groups based on location: meniscal samples cored from the regenerated tissue of the scaffold region (SC), from native meniscal tissue from the anterior and posterior horns adjacent to the implanted scaffold region of the right knee (AJ), and native meniscus from the contralateral intact knee (CL) (Fig. 1). Cores were flash-frozen in liquid nitrogen and stored at −20ºC until testing. Immediately before testing, we thawed samples in a hydrating bath of phosphate-buffered saline (PBS) (Invitrogen, Carlsbad, CA) supplemented with a protease inhibitor cocktail (complete protease inhibitor cocktail; Roche Applied Science, Indianapolis, IN) and cut to 2-mm thickness.
During testing, we submerged tissue samples in a lubricant bath of either PBS with protease inhibitor or equine synovial fluid (ESF). Bovine and equine synovial fluid lubricate bovine cartilage similarly [13] and the relative abundance of synovial fluid in the equine joint (several millimeters compared with trace amounts in ovine and bovine joints) make it particularly attractive as a lubricant. ESF was aspirated from the leg joints of four skeletally mature, healthy horses immediately after euthanasia (College of Veterinary Medicine, Cornell University, Ithaca, NY). Contaminant and blood-free aspirates were pooled and stored at −20ºC. Before friction testing, we thawed ESF aliquots in a water bath at 37°C.
The scaffold constructs and native tissue controls were tested in a custom friction apparatus previously described [14]. Briefly, the linearly oscillating friction apparatus placed a normal strain on the tissue and regulated the relative speed between samples and a counterface. In this study, we used smooth polished glass as a counterface. A custom biaxial load cell simultaneously measured the normal and frictional shear loads on the sample. The resulting equilibrium friction coefficient (μeq, the ratio of the normal load to the shear load when the engineered sample has fully relaxed from the applied normal strain) was calculated through a custom MATLAB (Natick, MA) code.
We created a Stribeck surface [13] to determine the entraining speeds and normal strains that produce boundary and mixed lubrication modes for native meniscus. Because lubricant greatly affects the transitions between lubrication modes, this was performed as follows for meniscus both lubricated with PBS and with ESF. Samples were loaded in the apparatus while submerged in a PBS bath. We applied a normal strain of 10% and the samples were allowed to equilibrate for 40 minutes. This time was to permit the normal stress to come to equilibrium and was determined from preliminary studies. We then oscillated the meniscal samples against the counterface glass in a series of 10 entraining speeds. A further step increase of 10% strain was applied and the equilibration-oscillation process repeated up to a maximum of 40% strain. The resulting region of speed/strain space that yielded maximal μ was considered boundary lubrication.
For all explants, we measured μeq over a range of speeds from 0.2 mm/s to 30 mm/s and normal strains from 10% to 40%. Samples were removed from the apparatus and allowed to relax for 40 minutes while submerged in PBS. We then repeated the strain and oscillation sequence with the samples in an ESF bath.
We determined boundary and mixed mode μeq relative to native meniscus. From the Stribeck curve for native meniscus in PBS, a representative value for boundary mode was selected at 40% strain, 0.2-mm/s entraining speed, and mixed mode at 10% strain and a speed of 10 mm/s. All samples were measured at these conditions. The equilibrium stress relaxation profile resulting from the unconfined compression in 10% strain step increments was fit to a poroelastic model [24] and used to calculate equilibrium modulus (HA) and permeability (κ) as described previously [15] from the normal loads recorded during the unconfined compression equilibrium relaxation period. For comparison purposes, lubrication properties of the unimplanted scaffold are included; their measurement has been previously described [14].
All data are presented as mean ± SD. We determined the effect of lubricant, location, and implantation time on μeq using a series of two-factor analysis of variance with Tukey’s honestly significant difference post hoc test. We compared HA and κ using a one-factor analysis of variance. All analyses were carried out using SigmaStat (SPSS Inc, Chicago, IL).
Results
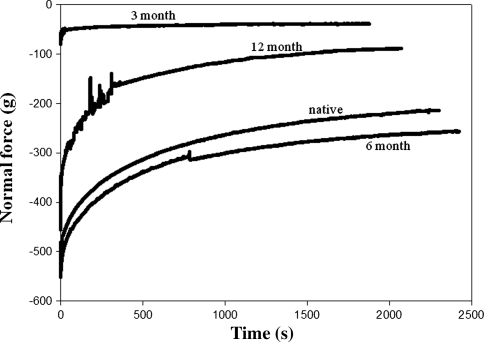
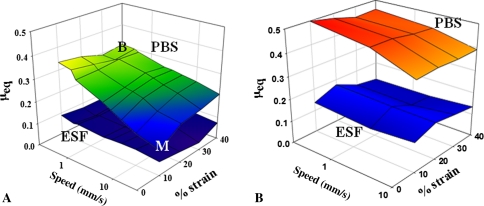
Native meniscus tissue lubricated by PBS and by ESF exhibited frictional behavior well described by Stribeck surfaces. Normal force exerted by both native and engineered tissue changed with time in typical stress-relaxation behavior (Fig. 2); native tissue and 6-month explants showed similar characteristics with similar final load, whereas 3-month explants reached equilibrium much faster than native values. The boundary mode regime is visible in the small region of maximum μeq and the larger mixed lubrication mode where μeq is varying (Fig. 3A). Comparatively, μeq for explanted SC were overall higher both in PBS and ESF (Fig. 3B). The zone of maximal μeq indicating boundary lubrication mode extended over a much larger strain-speed space than the native meniscus.
Fig. 2.
Normal force as a function of time for 3-, 6-, and 12-month explants and native meniscus. Repair tissue shows cartilage relaxation behavior with much higher equilibrium values and longer time to equilibrium values for explanted tissue.
Fig. 3A–B.
Stribeck curves in phosphate-buffered saline (PBS) (upper surface) and equine synovial fluid (ESF) (lower surface) for (A) native meniscus and (B) 3-month scaffold explants. B indicates speed/strain used to compare boundary mode and M mixed mode. Native and engineered meniscus show Stribeck behavior with higher μeq for PBS compared with ESF and transitions from boundary to mixed mode.
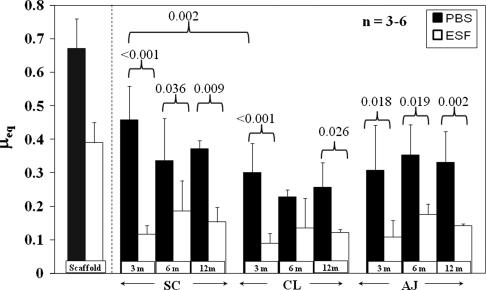
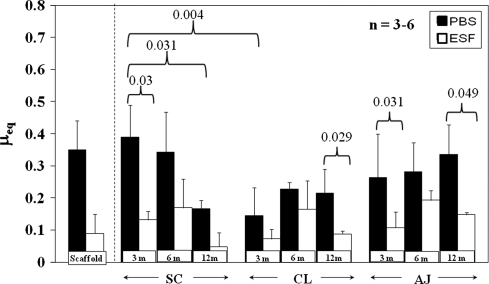
μeq measured in boundary mode conditions for almost all samples and time points was lower in ESF compared with PBS (Fig. 4). Native meniscus was more effectively lubricated by ESF with a boundary mode μeq approximately 30% of its PBS μeq at 3 months. For native meniscus mixed-mode lubricating conditions (Fig. 5), μeq showed similar but less pronounced trends than in boundary mode conditions. μeq in PBS was higher than in ESF for several times and locations, eg, SC at 3 months (p = 0.03) and CL at 12 months (p = 0.029).
Fig. 4.
μeq for native and engineered cartilage explants at all time points measured at ε = 40%, v = 0.2 mm/s, equivalent to boundary mode lubrication in native meniscus. Mean ± SD. Scaffold refers to unimplanted scaffold taken from Gleghorn et al. [14]. PBS = phosphate-buffered saline; ESF = equine synovial fluid; SC = scaffold region; AJ = native meniscal tissue adjacent to the implanted scaffold; CL = native meniscus from the contralateral intact knee. Boundary μeq changes with time and is lower for ESF compared with PBS.
Fig. 5.
μeq for native and engineered cartilage explants at all time points measured at ε = 10%, v = 10 mm/s, equivalent to mixed mode lubrication in native meniscus. Mean ± SD. Scaffold refers to unimplanted scaffold, taken from Gleghorn et al. [14]. PBS = phosphate-buffered saline; ESF = equine synovial fluid; SC = scaffold region; AJ = native meniscal tissue adjacent to the implanted scaffold; CL = native meniscus from the contralateral intact knee. Mixed μeq is generally lower for ESF compared with PBS.
μeq also changed with implantation time. For SC explants in PBS, boundary μeq decreased (p = 0.006) with time, dropping to 0.46 ± 0.06 at 3 months and subsequently to 0.34 ± 0.13 at 6 months (Fig. 4). At both 6 and 12 months, μeq of SC implants was approximately 0.35 in PBS. By 3 months, μeq had decreased (p = 0.007) to 0.12 ± 0.03 in ESF, a 69% drop from 0.39 at 0 months. At 6 and 12 months, μeq of SC implants was approximately 0.15 in ESF. Starting at 6 months, there was no difference (p = 0.877) between SC μeq values in either ESF or PBS for any subsequent time point nor were these two values different (p < 0.001) from either native tissue location. In mixed mode, μeq of SC samples in PBS dropped from 3 to 12 months (p = 0.031) and was also higher than for CL 3-month samples (p = 0.004) (Fig. 5). All native CL and AJ tissue was not different.
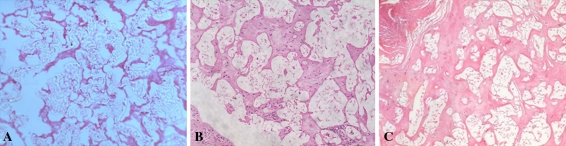
Tissue infiltrated the replacement scaffold as evidenced histologically by hematoxylin eosin staining (Fig. 6). At 2 weeks postoperatively, little extracellular matrix is visible (the animal died from unrelated causes), whereas at 3 and 12 months, substantial staining is apparent. At 3 months, HA was 132 ± 25 kPa, changing to 387 ± 260 kPa at 6 months and 192 ± 90 kPa at 12 months (Table 1). Native meniscus was 466 ± 191 kPa, measured in CL samples. Permeability trend was higher for SC compared with CL samples, 1.01 × 10−14 to 2.56 × 10−14 m4/Ns for SC at 40% strain compared with 2.71 × 10−15 m4/Ns in native meniscus; however, as a result of high variability in these samples, we observed no statistically significant differences. Relaxation times (τeq) varied between 21 and 334 seconds for SC samples with strains of 10% to 40%.
Fig. 6A–B.
Hematoxylin and eosin staining of SC samples at (A) 2 weeks, (B) 3 months, and 12 months. Progressive tissue ingrowth occurs over the course of 1 year in vivo.
Table 1.
Compressive modulus (HA) and hydraulic permeability (k) of meniscal repair tissue at 3, 6, and 12 months compared to native meniscal tissue from the contralateral knee
| Mechanical property | 3 month (n = 4) | 6 month (n = 3) | 12 month (n = 4) | Native (n = 9) |
|---|---|---|---|---|
| HA (kPa) | 132 ± 25 | 387 ± 260 | 192 ± 90 | 466 ± 191 |
| k (10% strain) | 3.38E−14 ± 1.52E−14 | 2.66E−14 ± 1.83E−15 | 1.20E−13 ± 3.87E−14 | 3.05E−14 ± 2.86E−15 |
| k (20% strain) | 1.91E−14 ± 8.92E−15 | 1.44E−14 ± 1.17E−14 | 5.43E−14 ± 4.31E−14 | 8.83E−15 ± 6.26E−15 |
| k (30% strain) | 1.50E−14 ± 4.36E−15 | 1.50E−14 ± 1.27E−14 | 4.29E−14 ± 3.58E−14 | 3.66E−15 ± 2.37E−15 |
| k (40% strain) | 1.42E−14 ± 1.45E−14 | 1.01E−14 ± 7.61E−15 | 2.56E−14 ± 2.46E−14 | 2.71E−15 ± 2.29E−15 |
Discussion
A complete knowledge of the mechanical behavior of a scaffold when implanted into a defect is essential in predicting its ability to protect the surrounding tissues from further degradation. With meniscal replacement design, understanding the tribologic behavior of the meniscus and meniscal replacements is necessary to effectively design scaffolds for implantation. We therefore sought to quantify the friction behavior of both native and engineered meniscal repair tissue that resulted from implantation in vivo. The goals of this study were to determine if Stribeck surface analysis can characterize the frictional behavior of native meniscus, assess if replacement material can be lubricated by synovial fluid, and determine if friction and mechanical characteristics of the engineered scaffold change with time and tissue ingrowth into scaffolds implanted in meniscal defects in sheep over the course of 1 year.
We caution readers of limitations of our study. First is the low power used in this small number large-animal study. As a result, we may be underreporting the changes that may occur in the meniscal implants in vivo. Second, although there are significant changes in the frictional behavior of meniscal repair tissue over the course of the first year in vivo, the relative importance of these frictional properties in determining the long-term durability of this tissue is still unclear.
Native meniscus tissue lubricated by PBS and by ESF exhibited frictional behavior well described by Stribeck surfaces, which have been used to visualize cartilage lubrication modes in native cartilage [13] and porous materials used in cartilage replacement [14]. In the case of solid tribologic materials, a relationship between porosity and friction has previously been reported [16]. Similarly, in this study, the frictional properties of the resultant tissues change with the porosity and permeability changes caused by ingrowth into the meniscal scaffold. The higher porosity and pore size of polyurethane foams compared with cartilaginous tissues presumably limit its ability to maintain a pressurized fluid film at the meniscal surface. This is shown in the region of maximal μeq boundary mode friction that is broader for SC samples at 3 months than native tissue (Fig. 3B). Three-month SC Stribeck surfaces are different from both unimplanted foam [14] and native meniscus (Fig. 3). This may indicate that the early repair tissue has an intermediate permeability and can sustain a lubricating fluid film over different tribologic conditions. Tissue explanted at 3 months showed a response to loading that was different from both later time points or native meniscus (Fig. 2), reaching equilibrium much more quickly. This is in accordance with the high permeability and the higher μeq in both conditions corresponding to native mixed (Fig. 5) and boundary (Fig. 4) modes at this time point.
Several studies show synovial fluid is a more effective lubricant than saline on cartilage [5, 6, 11, 12, 29, 30] and for some engineered tissues [15]. Our data are in agreement: ESF enhanced lubrication for nearly all tissues at all times. Lower μeq in ESF than PBS indicates the efficacy of ESF as a boundary lubricant for repair tissue as well as native meniscus. Partial meniscal replacement with a porous polyurethane scaffold reportedly restores mean contact pressures to those of the intact knee and improves peak contact pressures and mean contact area relative to a partially meniscectomized knee while under physiological loading [3]. The similarity of μeq of CL and AJ samples in this study is in agreement with this; the frictional behavior of the native meniscus adjacent to the implants was not adversely affected by scaffold placement.
In this study, the μeq of native meniscus boundary mode conditions of engineered meniscus improved with time and was similar to native tissue after 6 months (Fig. 4). Tissue ingrowth into the scaffold may also have caused a drop in μeq as a result of the extracellular matrix produced by infiltrating cells that may enable binding and localization of lubricating biomolecules. Interaction between matrix and synovial fluid components would account for the precipitous drop in boundary μeq in ESF after 3 months. A likely candidate is lubricin, a glycoprotein found in the synovial fluid that binds to the surface of cartilage in articular joints [18, 21, 29] and is the best predictor of lubricating function in engineered cartilage [15]. A number of authors propose that lubricin serves as the primary boundary lubricant in articular joint lubrication and possibly acts synergistically with hyaluronic acid [2, 7, 10, 19, 20, 29]. Trends in mixed-mode frictional properties of both native and replacement meniscal tissue were generally similar to their boundary mode behavior. Overall mixed μeq was lower than boundary μeq, as expected.
In addition to affecting the frictional behavior, the mechanical properties of the repair tissue were likely influenced by the tissue ingrowth that occurs in this material [27, 31, 32]. This could affect the properties in at least two ways. First, infiltration of the artificial scaffold would have the effect of decreasing porosity and pore size, which would result in decreasing permeability. This is supported by the change in the characteristic high permeability and fast relaxation times (τeq less than 30 seconds up to 40% strain [14]) of unimplanted polyurethane foams that were absent in the implanted scaffolds; at 6 months, relaxation times were 95, 125, 184, and 323 seconds at 10%, 20%, 30%, and 40% strains, respectively. The values we recorded well match previously reported κ values for sheep [22], and this corresponds to decreases in κ of up to 28%. Tissue ingrowth is also indicated by changes in aggregate modulus; at 6 months, HA had increased to 387 MPa and was 192 at 12 months, which, although lower than 6 months, was still an improvement over the value at 3 months (Table 1). With implantation, scaffold properties (Table 1) and structure (Fig. 6) had changed to become more cartilage-like. The decrease in κ with increasing strain in the engineered menisci is consistent with patterns of poroelastic behavior exhibited by various types of cartilage.
This study of a year-long in vivo meniscal implant has provided a more complete understanding of the frictional response of meniscal repair tissue. The high friction coefficient of polyurethane scaffolds changed with implantation, decreasing to near native values after 6 to 12 months in vivo. Our observations suggest that by promoting tissue ingrowth into porous scaffolds, meniscal repair tissue can attain similar frictional properties to native meniscus.
Acknowledgments
We thank Simon Tuner, DVM, Colorado State University, for care of research animals.
Footnotes
One or more of the authors (NKG, JPG, SR, RW, LJB) received funding from Orteq Bioengineering, Inc, NSERC (NKG), FQRNT (NKG), and NASA (JPG).
Each author certifies that appropriate approval for the animal protocol for this investigation was obtained at all institutions involved and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Cornell University, Ithaca, NY, USA and Hospital for Special Surgery, New York, NY, USA.
References
- 1.Ahmed A, Burke D. In-vitro measurement of static pressure distribution in synovial joints—Part I: Tibial surface of the knee. J Biomech Eng. 1983;105:216–225. doi: 10.1115/1.3138409. [DOI] [PubMed] [Google Scholar]
- 2.Bell CJ, Ingham E, Fisher J. Influence of hyaluronic acid on the time-dependent friction response of articular cartilage under different conditions. Proc Inst Mech Eng H. 2006;220:23–31. doi: 10.1243/095441105X69060. [DOI] [PubMed] [Google Scholar]
- 3.Brophy RH, Cottrell J, Rodeo SA, Wright TM, Warren RF, Maher SA. Implantation of a synthetic meniscal scaffold improves joint contact mechanics in a partial meniscectomy cadaver model. J Biomed Mater Res A. 2010;92:1154–1161. doi: 10.1002/jbm.a.32384. [DOI] [PubMed] [Google Scholar]
- 4.Burks RT, Metcalf MH, Metcalf RW. Fifteen-year follow-up of arthroscopic partial meniscectomy. Arthroscopy. 1997;13:673–679. doi: 10.1016/S0749-8063(97)90000-1. [DOI] [PubMed] [Google Scholar]
- 5.Caligaris M, Ateshian GA. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthritis Cartilage. 2008;16:1220–1227. doi: 10.1016/j.joca.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caligaris M, Canal CE, Ahmad CS, Gardner TR, Ateshian GA. Investigation of the frictional response of osteoarthritic human tibiofemoral joints and the potential beneficial tribological effect of healthy synovial fluid. Osteoarthritis Cartilage. 2009;17:1327–1332. doi: 10.1016/j.joca.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang DP, Abu-Lail NI, Guilak F, Jay GD, Zauscher S. Conformational mechanics, adsorption, and normal force interactions of lubricin and hyaluronic acid on model surfaces. Langmuir. 2008;24:1183–1193. doi: 10.1021/la702366t. [DOI] [PubMed] [Google Scholar]
- 8.Christoforakis J, Pradhan R, Sanchez-Ballester J, Hunt N, Strachan RK. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy. 2005;21:1366–1369. doi: 10.1016/j.arthro.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Cox JS, Nye CE, Schaefer WW, Woodstein IJ. The degenerative effects of partial and total resection of the medial meniscus in dogs’ knees. Clin Orthop Relat Res. 1975;109:178–183. doi: 10.1097/00003086-197506000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Forsey RW, Fisher J, Thompson J, Stone MH, Bell C, Ingham E. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials. 2006;27:4581–4590. doi: 10.1016/j.biomaterials.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Forster H, Fisher J. The influence of loading time and lubricant on the friction of articular cartilage. Proc Inst Mech Eng H. 1996;210:109–119. doi: 10.1243/PIME_PROC_1996_210_399_02. [DOI] [PubMed] [Google Scholar]
- 12.Forster H, Fisher J. The influence of continuous sliding and subsequent surface wear on the friction of articular cartilage. Proc Inst Mech Eng H. 1999;213:329–345. doi: 10.1243/0954411991535167. [DOI] [PubMed] [Google Scholar]
- 13.Gleghorn JP, Bonassar LJ. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J Biomech. 2008;41:1910–1918. doi: 10.1016/j.jbiomech.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Gleghorn JP, Doty SB, Warren RF, Wright TM, Maher SA, Bonassar LJ. Analysis of frictional behavior and changes in morphology resulting from cartilage articulation with porous polyurethane foams. J Orthop Res. 2010;28:1292–1299. doi: 10.1002/jor.21136. [DOI] [PubMed] [Google Scholar]
- 15.Gleghorn JP, Jones AR, Flannery CR, Bonassar LJ. Boundary mode frictional properties of engineered cartilaginous tissues. Eur Cell Mater. 2007;14:20–28. doi: 10.22203/ecm.v014a02. [DOI] [PubMed] [Google Scholar]
- 16.He Y, Winnubst L, Burggraaf AJ, Verweij H, Varst PGTh, With B. Influence of porosity on friction and wear of tetragonal zirconia polycrystal. J Amer Ceram Soc. 1997;80:377–380. doi: 10.1111/j.1151-2916.1997.tb02841.x. [DOI] [Google Scholar]
- 17.Heijkants RGJC, Calck RV, Groot JH, Pennings AJ, Schouten AJ. Design, synthesis and properties of a degradable polyurethane scaffold for meniscus regeneration. J Mater Sci Mater Med. 2004;15:423–427. doi: 10.1023/B:JMSM.0000021114.39595.1e. [DOI] [PubMed] [Google Scholar]
- 18.Jay GD. Lubricin and surfacing of articular joints. Curr Opin Orthop. 2004;15:355–359. doi: 10.1097/01.bco.0000136127.00043.a8. [DOI] [Google Scholar]
- 19.Jay GD, Haberstroh K, Cha CJ. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and Healon. J Biomed Mater Res. 1998;40:414–418. doi: 10.1002/(SICI)1097-4636(19980605)40:3<414::AID-JBM11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 20.Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci USA. 2007;104:6194–6199. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones AR, Gleghorn JP, Hughes CE, Fitz LJ, Zollner R, Wainwright SD, Caterson B, Morris EA, Bonassar LJ, Flannery CR. Binding and localization of recombinant lubricin to articular cartilage surfaces. J Orthop Res. 2007;25:283–292. doi: 10.1002/jor.20325. [DOI] [PubMed] [Google Scholar]
- 22.Joshi MD, Suh JK, Marui T, Woo SL. Interspecies variation of compressive biomechanical properties of the meniscus. J Biomed Mater Res. 1995;29:823–828. doi: 10.1002/jbm.820290706. [DOI] [PubMed] [Google Scholar]
- 23.Khetia EA, McKeon BP. Meniscal allografts: biomechanics and techniques. Sports Med Arthrosc. 2007;15:114–120. doi: 10.1097/JSA.0b013e3180dca217. [DOI] [PubMed] [Google Scholar]
- 24.Kim YJ, Bonassar LJ, Grodzinsky AJ. The role of cartilage streaming potential, fluid flow and pressure in the stimulation of chondrocyte biosynthesis during dynamic compression. J Biomech. 1995;28:1055–1066. doi: 10.1016/0021-9290(94)00159-2. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCann L, Ingham E, Jin Z, Fisher J. Influence of the meniscus on friction and degradation of cartilage in the natural knee joint. Osteoarthritis Cartilage. 2009;17:995–1000. doi: 10.1016/j.joca.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Ramrattan NN, Heijkants RGJC, Tienen TGv, Schouten AJ, Veth RPH, Buma P. Assessment of tissue ingrowth rates in polyurethane scaffolds for tissue engineering. Tissue Eng. 2005;11:1212–1223. doi: 10.1089/ten.2005.11.1212. [DOI] [PubMed] [Google Scholar]
- 28.Roos H, Laurbn M, Adalberth T, Roos EM, Jonsson K, Lohmander S. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 2007;15:35–47. doi: 10.1016/j.joca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Tienen TG, Heijkants RGJC, Groot JH, Schouten AJ, Pennings AJ, Veth RPH, Buma P. Meniscal replacement in dogs. Tissue regeneration in two different materials with similar properties. J Biomed Mater Res B Appl Biomater. 2006;76:389–396. doi: 10.1002/jbm.b.30406. [DOI] [PubMed] [Google Scholar]
- 32.Tienen TGv, Heijkants RGJC, Buma P, Groot JHd, Pennings AJ, Veth RPH. Tissue ingrowth and degradation of two biodegradable porous polymers with different porosities and pore sizes. Biomaterials. 2002;23:1731–1738. doi: 10.1016/S0142-9612(01)00280-0. [DOI] [PubMed] [Google Scholar]