Abstract
In a continuing study of our clinical candidate 5 (VN/124-1 or TOK-001) and analogs as potential agents for prostate cancer therapy, putative metabolites (10, 15 and 18) of compound 5 were rationally designed and synthesized. However, none of these agents were as efficacious as 5 in several in vitro studies. Using western blot analysis, we have generated a preliminary structure-activity relationship (SAR) of 5 and related analogs as androgen receptor ablative agents (ARAAs). In vivo using the androgen-dependent LAPC-4 prostate cancer xenograft model, we demonstrated for the first time that 5 is more efficacious than the 17-lyase inhibitor 3 (abiraterone)/4 (abiraterone acetate) that is currently in phase III clinical trials. In our desire to optimize the potency of 5, compounds 6 (3ξ-fluoro-) and 9 (3β-sulfamate-) designed to increase the stability and oral bioavailability of 5, respectively were evaluated in vivo. We showed, that on equimolar basis, compound 6 was ~2-fold more efficacious versus LAPC-4 xenografts than 5, but the toxicity observed with 6 is of concern. These studies further demonstrate the efficacy of 5 in a clinically relevant prostate cancer model and justify its current clinical development as a potential treatment of prostate cancer.
Keywords: Androgen receptor, androgen receptor ablative agents CYP17, CYP17 inhibitors, prostate cancer therapy, VN/124-1 (TOK-001), abiraterone, abiraterone acetate, potent anti-prostate cancer agents
1. Introduction
Prostate cancer (PCA) is the most common malignancy, and second leading cause of cancer related deaths in men in the western world. For the year 2009, the American Cancer Society estimated that 192,280 new cases would be diagnosed and 27,360 patients would die from the disease in the United States alone [1]. Despite advances in screening and treatment of localized disease, advanced prostate cancer remains incurable [2]. Prostate cancer that is resistant to all currently available endocrine therapies remains for the most part driven by ligand-dependent or ligand-independent activation of androgen receptor signaling. Although strategies such as surgical or chemical castrations, which can effectively reduce serum testosterone levels have remained the most effective therapy for PCA for over 65 years [3], recent studies clearly show that prostate cancers may generate intracrine androgenic steroids and also become hypersensitive to low steroidal levels through AR gene mutations and/or amplification supporting continued tumor growth [4–6]. Indeed, the limited prognosis of patients with castration-resistant prostate cancer (CRPC) on all current types of therapies calls for the urgent need for new and innovative drugs.
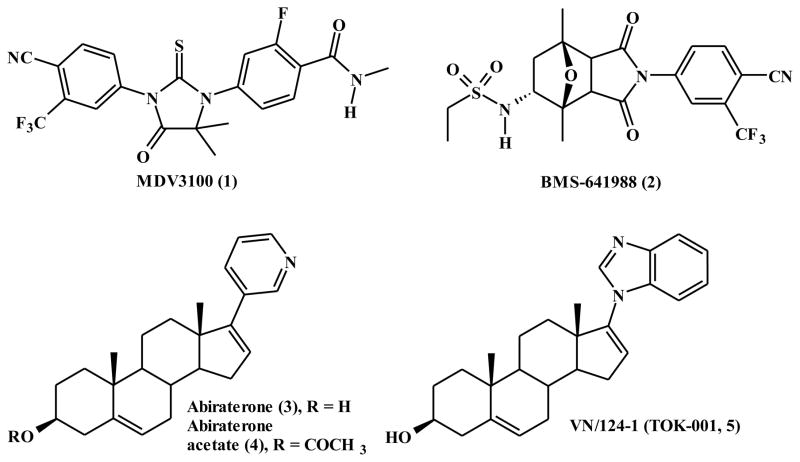
The efficacy of androgen receptor blockade in the treatment of prostate cancer [7] and the observation that the androgen receptor activation is maintained and vital for disease progression has prompted current interest in the development of new therapies to maximally suppress this vital androgenic pathway [8]. Current investigations in both preclinical and clinical arena are focused on discovery and development potent antiandrogens, CYP17 inhibitors and androgen receptor ablative agents [9]. In these regard, there are two antiandrogens (MDV3100 (1) and BMS641988 (2)) [10, 11], one CYP17 inhibitor (abiraterone/abiraterone acetate (3/4)) [12] and the exciting novel multi-active agent VN/124-1 (5, 3β-hydroxy-17-(1H-benzimidazole)androsta-5,16-diene, now called TOK-001) [13, 14] with antiandrogenic, CYP17 inhibitory and AR ablative activities in various phases of clinical trials (Figure 1). Because of promising results in clinical trials with abiraterone, Hartmann and colleagues have designed, synthesized and evaluated several non-steroidal abiraterone analogs with promising androgen biosynthesis inhibitory activities [15–18].
Figure 1.
Chemical structures of MDV3100 (1), BMS-641988 (2), abiraterone (3), abiraterone acetate (4) and VN/124-1 (TOK-001, 5).
We first reported the CYP17 inhibitory, antiandrogenic and antitumor properties of 5 in 2005 [13] and recently we discovered that the agent is also a potent AR ablative agent in several human prostate cancer in vitro and in vivo models [14, 19]. In addition, the compound caused marked reduction of circulating testosterone levels in the male mouse, androgen-dependent organ weights, anti-tumor efficacy and it is superior to castration or the clinically used anti-androgen, bicalutamide. These promising anti-prostate cancer activities justified its selection for clinical evaluation.
On the basis of previous pharmacokinetic studies of compound 5 in mice which showed extensive metabolism of 5 [13], we have now synthesized and evaluated putative metabolically stable analogs of the compound and have for the first time conducted a head to head evaluation of 5 and the CYP17 inhibitor abiraterone (3/4) that is currently undergoing phase III clinical trials in prostate cancer patients [20, 21]. These studies are the subject of this report. A preliminary account of part of this work has been reported [22] and patents to protect these novel and related compounds in the United States and many countries are pending.
2. Experimental
2.1. Chemistry
2.1.1. General
General procedures and techniques were identical with those previously reported.[13] 1H NMR spectra were recorded in CDCl3 at 500 MHz with Me4Si as an internal standard using a Varian Inova 500 MHz spectrometer. High-resolution mass spectra (HRMS) were determined on a Bruker 12Tesla APEX-Qe FTICR-MS by positive ion ESI mode by Ms. Susan A Hatcher, Facility Director, College of Sciences Major Instrumentation Cluster, Old Dominion University, Norfolk, VA. 3β-Hydroxy-5α-androstan-17-one (trans-androsterone) was purchased from Aldrich. Compound 5 was synthesized in our laboratory as we previously described.[13] All other reagents were purchased from Sigma-Aldrich. All compounds were stored in an atmosphere of argon and in the cold (0–8 °C).
2.1.2. 3β-Hydroxy-17-(3-pyridyl)androsta-5,16-dien (3) and 3β-Acetoxy-17-(3-pyridyl)androsta-5,16-dien (4)
These compounds also known as abiraterone and abiraterone acetate, prepared by a literature method [23], provided spectral and analytical data identical with those reported [23, 24]. However, we developed a new purification procedure that resulted in improved overall yield compared to the reported procedures.
2.1.3. 3β-Hydroxy-17-(1H-benzimidazol-1-yl)androsta-5,16-dien (5)
This compound, prepared as we have previously reported, provided spectral and analytical as described [13].
2.1.4. 3ξ-Fluoro-17-(1H-benzimidazol-1-yl)androsta-5,16-dien (6)
Compound 5 (776 mg, 2 mmol) was dissolved in dichloromethane (30 ml) and to this added diethylaminosulfur trifluoride (DAST, 483 mg, 396 μl, 1.5 eq.) at room temperature. Reaction mixture was stirred for 1 hour and was filtered through basic alumina, washed (H2O × 3), dried and evaporated. The crude product was purified by FCC (dichloromethane:ethanol, 9:1) to afford 530 mg (68 %) of white solid: mp 140–142 °C. 1H NMR (400 MHz, CDCl3): δ 1.05 (s, 3H, 18-CH3), 1.10 (s, 3H, 19-CH3), 4.11–4.23 (d, 1/2H, JHF = 48 Hz, 3α-H), 4.37–450 (d, 1/2H, JHF = 50 Hz, 3β-H), 5.46 (s, 1H, 6-H), 6.00 (s, 1H, 16-H), 7.32 (s, 1H, aromatic-H), 7.51 (s, 1H, aromatic-H). 7.83 (s, 1H, aromatic-H), 7.98 (s, 1H, aromatic-H). HRMS calcd 391.2544 (C26H30FN2 [M + H]+), found 391.2535.
2.1.5. 3β-O-Mesyl-17-(1H-benzimidazol-1-yl)androsta-5,16-dien (7)
A stirring cold solution of 3β-hydroxy-17-(1H-benzimidazol-1-yl)androsta-5,16-diene (5) (0.388 gm, 1 mmol) in pyridne (5 ml) at 0 °C was treated with methane sulfonyl chloride solution (1.2 ml, 1.2 mmol,) and was maintained at 0 °C for 30 minutes. The reaction mixture was maintained at 0–8 °C for 16 hours and then poured over ice-water mixture (50 ml) with stirring. The crude product precipitate was filtered and purified by FCC, [petroleum ether/EtOAc (9:1)] to give the titled compound 7 (0.37 gm, 82.11 %): mp149–150 °C. 1H NMR (500 MHz, CDCl3): δ 1.03 (s, 3H, 19-CH3), 1.04 (s, 3H, 18-CH3), 2.92 (s, 3H, 3β-SO2CH3), 4.30 (m, 1H, 3α-H), 5.44 (s, 1H, 6-H), 5.74 (s, 1H, 16-H), 7.15 (s, 1H, aromatic-H), 7.71 (s, 1H, aromatic-H). 8.31(s, 1H, aromatic-H).
2.1.6. 3α-Azido-17-(1H-benzimidazol-1-yl)androsta-5,16-dien (8)
Compound 7 (0.3 gm, 0.77 mmol) was dissolved in DMF (5ml) and to this added sodium azide (0.1 gm, 1.53 mmol) and heated at 80 °C for 48 hours. The reaction mixture was diluted with ice-water mixture (50 ml), extracted with ethyl acetate (3 × 25 ml) and solvent was evaporated to obtain crude compound that was purified by FCC [petroleum ether/EtOAc (7:3)] to give compound 8 (0.18 gm, 65.7 %): mp120–122 °C. 1H NMR (400 MHz, CDCl3): δ 1.03 (s, 3H, 18-CH3), 1.07 (s, 3H, 19-CH3), 3.92 (m, 1H, 3β-H), 5.47 (s, 1H, 6-H), 6.00 (s, 1H, 16-H), 7.31 (m, 2H, aromatic-H), 7.39 (s, 1H, aromatic-H). 7.84 (s, 1H, aromatic-H), 8.05 (s, 1H, aromatic-H). HRMS calcd 436.2471 (C26H31N5•Na+), found 436.2469.
2.1.7. 3β-O-Sulfamoyl-17-(1H-benzimidazol-1-yl)androsta-5,16-dien (9)
A stirring cold solution of 3β-hydroxy-17-(1H-benzimidazol-1-yl)androsta-5,16-diene (5, 0.388 gm, 1 mmol) in DMF (5 ml) at 0 °C was treated with potassium tert-butoxide solution (1.2 ml, 1.2 mmol, 1M in THF) and was maintained at 0 °C for 30 minutes. Sulfamoyl chloride (5 ml, 5 mmol, 1M in toluene) was added drop wise over 30 minutes. The reaction mixture is allowed to attain room temperature over 2 hours, and was cooled to 0 °C, and then quenched by sequential addition of saturated solution of ammonium chloride (5 ml) and then water (30 ml). Following extraction with ethyl acetate (3 × 25 ml), the organic layers were washed with brine (3 × 25 ml), dried over anhydrous sodium sulfate and then evaporated to give pure compound 9 as white solid (0.34 gm, 72.2 %): mp 159–160 °C. 1H NMR (500 MHz, CDCl3): δ 1.02 (s, 3H, 18-CH3), 1.03 (s, 3H, 19-CH3), 4.25 (m, 1H, 3α-H), 5.44 (s, 1H, 6-H), 5.74 (s, 1H, 16-H), 7.15 (s, 1H, aromatic-H), 7.71 (s, 1H, aromatic-H). 8.31 (s, 1H, aromatic-H). HRMS calcd 490.2134 (C26H33N3O3S•Na+), found 490.2124.
2.1.8. 3β-Hydroxy-17-(1H-benzimidazol-1-yl)androsta-5-ene (10)
To a solution of 5 (0.388 g, 1 mmol) in ethanol (25 mL) was added hydrazine hydrate (1.6 ml, 5 mmol) and acetic acid (1 ml) and reaction mixture was heated at 80°C while a stream of air was passed through the solution. The reaction mixture was cooled to room temperature after 18 hours, concentrated to approximately 5 ml under vacuum and poured onto ice cold water. Treatment of this solution with drop-wise addition of saturated aqueous solution of sodium bicarbonate resulted in a crude product precipitate. The crude product was purified by FCC [dichloromethane/MeOH (9: 1)] to give pure compound 10 (0.280 g, 71.9%): mp 143–145 °C; 1H NMR (500 MHz, CDCl3) δ 0.67 (s, 3H,18-CH3), 0.81 (s, 3H, 19-CH3), 4.69 (m,1H, 3α-H), 7.29 (m, 2H, aromatic-Hs), 7.43 (s, 1H, aromatic-H), 7.79 (s, 1H, aromatic-H), 8.09 (s, 1H, 21-H). HRMS calcd 391.2743 (C26H33N2O [M + H+]), found 391.2741.
2.1.9. 3β-Acetoxy-17-chloro-16-formyl-5α-androstan-16-ene (12)
First, 3β-acetoxy-5α-androstan-17-one was synthesized from trans androstane. Thus, trans-androstane (11, 5.8 gm, 20 mmole) was dissolved in 15 ml of pyridine and cooled to 0 °C and to this added acetic anhydride (7.2 gm, 6.6 ml, 3 eq.) over period of 30 minutes. Reaction mixture was kept in refrigerator for 16 hours and then poured over ice-water (50 ml) under stirring. The precipitated white product was filtered and dried under vacuum to give 5.5 gm of desired 3β-acetate: mp 113–115 °C. 1H NMR (500 MHz, CDCl3): δ 0.851 (3H, s, 18-CH3), 0.857 (3H, s, 19-CH3), 2.02 (3H, s, 3β-OAc), 4.68 (1H, m, 3α-H). A solution of 3β-acetoxy-5α-androst-17-one (2 g, 6.6 mmol) in dry chloroform (40 mL) was added drop wise to a cold and stirred solution of phosphorus oxychloride (10 mL) and dimethylformamide (10 mL). The mixture was allowed to attain room temperature and then refluxed under argon for 5 h. It was then concentrated under reduced pressure, poured onto ice and then extraction with a mixture of ether and EtOAc (8:2, v/v). The combined extracts were washed with brine and dried (anhydrous Na2SO4), and solvent was removed under vacuum to give a white solid (2.3 g). Purification by flash column chromatography [FCC, petroleum ether/EtOAc (15:1)] gave the title compound 12 (1.75 g, 77%): mp 155–157 °C; 1H NMR (500 MHz, CDCl3) δ 0.862 (3H, s, 18-CH3), 0.956 (3H, s, 19-CH3), 2.02 (3H, s, 3β-OAc), 4.69 (1H, m,3α-H), 9.98 (1H, s, 16-CHO).
2.1.10. 3β-Acetoxy-17-(1H-benzimidazol-1-yl)-16-formyl-5α-androstan-16-ene (13)
A mixture of 3β-acetoxy-17-chloro-16-formyl-5α-androstan-16-ene (12, 2.5 g, 6.65 mmol), benzimidazole (2.35 g, 19.9 mmol) and K2CO3 (2.76 g, 23.9 mmol) in dry DMF (20 mL) was stirred at approx. 80 °C under argon for 1.5 h. After cooling to room temperature, the reaction mixture was poured onto ice-cold water (250 mL) and the resulting precipitate was filtered, washed with water and dried to give a crude white solid (ca. 2.9 g). Purification by FCC [petroleum ether/EtOAc/Et3N (6:4:0.3)] gave 2.7 g (88.7%) of pure compound 13: mp 217–218 °C; IR (CHCl3) 3691, 3024, 2951, 2359, 1725, 1670, 1604, 1491, 1452, 1375, 1253, 1032, 897, 852, 818, 700, 657, 618, 576, 565, 550, 529, 511, 476 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.88 (s, 6H, 18- and 19-CH3), 2.02 (s, 3H, 3β-OCH3), 4.70 (m, 1H, 3α-H), 7.35 (br. s, 2H, aromatic-Hs), 7.85 (s, 1H, aromatic-H), 7.87 (s, 1H, aromatic-H), 7.97 (s, 1H, 21-H) and 9.58 (s, 1H, 16-CHO).
2.1.11. 3β-Acetoxy-17-(1H-benzimidazol-1-yl)-5α-androsta-16-ene (14)
A solution of 3β-Acetoxy-17-(1H-benzimidazol-1-yl)-16-formyl-5α-androsta-16-ene (13, 2.04 g, 4.45 mmol) in dry benzonitrile (10 mL) was refluxed in the presence of 10% palladium on activated charcoal (1.02 g, i.e., 50% weight of 3) for 5 h. After cooling to room temperature, the catalyst was removed by filtration through a Celite pad. The filtrate was evaporated, and the residue was purified by FCC petroleum ether/EtOAc/Et3N (7.5:3:0.5)] to give 1.41 g (73.8%) of pure compound 14: mp 159–160 °C; IR (CHCl3) 3687, 2947, 2854, 2358, 2340, 1725, 1633, 1609, 1557, 1489, 1454, 1373, 1291,1253, 1195, 1136, 1031, 985, 910, 839, 735, 665, 590, 544, 533,513, 502, 488 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.876 (s, 3H, 18-CH3), 0.974 (s, 3H, 19-CH3), 2.02 (s, 3H, 3β-OCH3), 4.70 (m,1H, 3α-H), 5.95 (s, 1H, 16-H), 7.30 (m, 2H, aromatic-Hs), 7.49 (s, 1H, aromatic-H), 7.81 (s, 1H, aromatic-H), and 7.94 (s, 1H, aromatic-H).
2.1.12. 3β-Hydroxy-17-(1H-benzimidazol-1-yl)-5α-androsta-16-ene (15)
The acetate 14 (0.432 gm 1 mmol) was dissolved in methanol (10 mL) under an inert argon atmosphere, and the resulting solution was treated with 10% methanolic KOH (4 mL). The mixture was stirred at room temperature for 1.5 h and then concentrated under reduced pressure at approximately 40 °C to a volume of 5 mL. This solution was poured into ice water (50 mL), and the resulting white precipitate was filtered, washed with water and dried to obtain 15 (0.310 gm, 79.48%): mp 189–190 °C. 1H NMR (500 MHz, CDCl3) δ 0.675 (s, 3H, 18-CH3), 0.805 (s, 3H, 19-CH3), 3.61 (m, 1H, 3α-H), 7.30 (m, 2H, aromatic-Hs), 7.42 (s, 1H, aromatic-H), 7.78 (s, 1H, aromatic-H), and 8.08 (s, 1H, aromatic-H). HRMS calcd 391.2743 (C26H33N2O [M + H]+), found 391.2742.
2.1.13. 3β-Acetoxy-17-(1H-benzimidazol-1-yl)androstane (16)
To a solution of 14 (0.432 g, 1 mmol) in ethanol (30 mL) was added hydrazine hydrate (1.6 ml, 5 mmol) and acetic acid (1ml) and the reaction mixture was heated at 80°C while a stream of air was passed through the solution. The reaction mixture was cooled to room temperature after 18 hours, concentrated to 5 ml under vacuum and poured onto ice cold water. Treatment of this solution with drop-wise addition of saturated aqueous solution of sodium bicarbonate resulted in a crude product precipitate. The crude product was purified by FCC [dichloromethane/MeOH (9.5: 0.5)] to give 0.350 g (73.8%) of pure compound 14: mp 159–160 °C; IR (CHCl3) 3687, 2947, 2854, 2358, 2340, 1725, 1633, 1609, 1557, 1489, 1454, 1373, 1291,1253, 1195, 1136, 1031, 985, 910, 839, 735, 665, 590, 544, 533,513, 502, 488 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.67 (s, 3H,18-CH3), 0.81 (s, 3H, 19-CH3), 2.02 (s, 3H, 3β-OCH3), 4.69 (m,1H, 3α-H), 7.29 (m, 2H, aromatic-Hs), 7.43 (s, 1H, aromatic-H), 7.79 (s, 1H, aromatic-H), and 8.09 (s, 1H, aromatic-H).
2.1.14. 3β-Hydroxy-17-(1H-benzimidazol-1-yl)-5α-androstane (17)
The acetate 16 (1.3 g 3.02 mmol) was dissolved in methanol (20 mL) under an inert argon atmosphere, and the resulting solution was treated with 10% methanolic KOH (8 mL). The mixture was stirred at room temperature for 1.5 h and then concentrated under reduced pressure at approximately 40 °C to a volume of 10 mL. This solution was poured into ice water (300 mL), and the resulting white precipitate was filtered, washed with water and dried. Crystallization from EtOAc/MeOH gave 15 (1.10 g, 94%), mp 189–190 °C; IR (CDCl3) 2934, 2339, 1609, 1490, 1453, 1291, 1040, 837, 808, 705, 663, 608, 578, 550, 517 cm−1; 1H NMR (300 MHz, CDCl3) δ 0.675 (s, 3H, 18-CH3), 0.805 (s, 3H, 19-CH3), 3.61(m, 1H, 3α-H), 7.30 (m, 2H, aromatic- Hs), 7.42 (s, 1H, aromatic-H), 7.78 (s, 1H, aromatic-H), and 8.08 (s, 1H, aromatic-H). HRMS calcd 415.2719 (C26H36ON2•Na+), found 415.2714.
2.1.15. 17-(1H-Benzimidazol-1-yl)androsta-4,16-dien-3-one (18)
To a mixture of compound 17 (660 mg, 1.70 mmol), N-methylmorpholine-N-oxide (NMO) (2.5 mL) and dichloromethane (40 mL), was added tetrapropylammonium perruthenate (TPAP) (521 mg, 2.55 mmol) and molecular sieves (0.5 gm), and the mixture was stirred under argon for 3 h. The mixture was filtered and diluted with EtOAc (50 mL), washed successively with 5% aqueous NaHCO3 (×3) and brine (×2) and then dried (Na2SO4). The solvent was evaporated, and the crude product was purified by FCC [CH2Cl2/EtOH (25:1)] to give the title compound 16 (544 mg, 82%): mp 215–216 °C; IR (CHCl3) 2946, 2858, 1622, 1611, 1490, 1453, 1376, 1291,1270, 1228, 1189, 893, 850, 837, 722, 662, 615, 568, 553, 537,519 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.84 (s, 3H, 18-CH3),1.02 (s, 3H, 19-CH3), 7.27(m, 2H, aromatic-Hs), 7.41 (m, 1H, aromatic-H), 7.79 (s, 1H, aromatic-H), and 8.09 (s, 1H, aromatic-H). HRMS calcd 391.2743 (C26H33ON2 [M + H]+), found 391.2735.
2.1.16. HPLC analysis for relative retention time of putative metabolites
Chromatographic separations and identification of the putative metabolites were achieved by a reverse phase HPLC method on a Waters Symmetry R C18 Column (4.6 × 75 mm). Briefly, the HPLC system used in this study consisted of Waters Alliance System coupled with 2695 Separation Module and Waters 2998 PDA detector operated at 254 nm. The mobile phase composition was Water/MeOH/CH3CN (35:35:30, v/v/v + 200 μL of Et3N and 0.77 g of NH4OAc per 1000 mL of mobile phase) at a flow rate of 1.0 mL/min. The HPLC analysis was performed at ambient temperature, and data acquisition and management were achieved with a Waters millennium chromatography manager.
2.2. Biology
2.2.1. General
The human prostate cancer cell line LNCaP was obtained from the American Type Culture Collection (Rockville, MD). 293T cells were the gift of Dr. Yun Qiu (University of Maryland, Baltimore), and LAPC4 cells were kindly provided by Dr. Charles L. Sawyers of UCLA School of Medicine. The RPMI 1640 medium, Dulbeccos Modified Eagle Medium (DMEM), trypsin/EDTA (0.25%/0.02%), penicillin/streptomycin (P/S), geneticin (G418), and Lipofectamine were purchased from Gibco-BRL (Grand Island, NY). Phenol red free IMEM and trypsin/versene were purchased from Biofluids Inc. (Rockville, MD). Fetal Bovine Serum (FBS) and steroid free FBS were obtained from Biofluids Inc. (Rockville, MD). Calcium Phosphate transfection kit was purchased from Invitrogen (Carlsbad, California).
The 293T and 293T-CYP17 cells were routinely maintained in DMEM supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin solution. LNCaP cells were grown in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin solution. LAPC4 cells were grown in IMEM supplemented with 15% FBS, 1% penicillin/streptomycin solution, and 10 nM DHT.
2.2.2. Transfection
Calcium Phosphate transfections were carried out as described by the manufacturers protocol (Promega Profection Mammalian Transfection System). Briefly, 100 mm plates were coated with poly-L-lysine for 30 minutes, rinsed twice with distilled water and allowed to dry for two hours. The 293T cells were plated in a 100 mm plate the day before transfection at a density sufficient for approximately 60% confluency on the day of transfection. Three hours prior to transfection the media was replaced with fresh growth media. The pCDNA3Hmod17His(4) (10 μg) was added to sterile, deionized water, vortexed briefly, and then 62 μl 2M CaCl2 was added to bring the final volume to 500 μl. This solution was added drop-wise to 500 μl HBS solution, and incubated at room temperature for 30 minutes. The solution was vortexed again, and distributed evenly across the 293T cell monolayer. Media was changed 18 hours later, and enzyme activity was assayed as described below 48 hours after transfection. LNCaP-ARR2-Luc transfections were carried out utilizing LipofectAMINE 2000 transfection reagent (Invitrogen) according to the manufacturer’s protocol.
2.2.3. Acetic acid releasing assay for CYP17 activity
The 293T cells were transfected with the human CYP17 (293T-CYP17) as described in transfections. The 293T-CYP17 cells were grown in a 100 mm dish for 24 hours and then evenly divided into 6 well tissue culture plates and incubated for 24 hours. The cells were washed with Dulbecco’s Phosphate Buffered Saline (DPBS), and incubated with steroid-free DMEM (1ml) containing 0.5–20 μM of [21-3H]17α-hydroxypregnenolone. This substrate is converted to DHEA and [3H]acetic acid is released during cleavage of the C-21 side chain. Cold 17α-hydroxypregnenolone (1mM) was added to control wells for detection of non-specific substrate conversion. After an 18 hour incubation at 37 °C (16 hours for LNCaP-CYP17) medium was collected and the steroids were extracted by shaking with 2ml of chloroform at 4 °C. After 30 minutes, the aqueous phase, which contains the [3H]acetic acid, was collected, and a charcoal suspension (2.5% final concentration) was added to it. Following a 30 minute incubation at 4 °C, 1 mL of the supernatant was removed and radioactivity measured by liquid scintillation counting. After determining the saturating concentration of [21-3H]17α-hydroxypregnenolone (6.8 M), compounds were evaluated for IC50 values by incubation with medium (1 ml) containing the saturating concentration of [21-3H]17α-hydroxypregnenolone (6.8 M) and different concentrations (1–1000 nM) of the inhibitors. After an18 hour incubation, the medium was analyzed as described above and IC50 values were determined with Graphpad Prism software (GraphPad Software, Inc, San Diego, CA).
2.2.4. Androgen receptor competitive binding assay
Competitive binding assays were performed with the synthetic androgen methyltrienolone [3H]R1881 essentially as described by Wong et al. and Yarbrough et al. [25, 26]. Wells in 24-well multiwell dishes were coated with poly-l-lysine (0.05 mg/ml) for 30 minutes, rinsed with sterilized, distilled water, and dried for 2 hours. To determine the kinetics of [3H]R1881 binding to the LNCaP AR and the wild-type AR, LNCaP and LAPC4 cells were plated (2–3 × 105 cells/well) in 24 well multiwell dishes in steroid-free medium and allowed to attach. The following day the medium was replaced with serum-free, steroid free RPMI supplemented with 0.1 % BSA and containing [3H]R1881 (0.01–10 nM) in the presence or absence of a 200 fold excess of cold DHT, to determine nonspecific binding, and 1μM triamcinolone acetonide to saturate progesterone and glucocorticoid receptors. Following a 2 hour incubation period at 37 °C, cells were washed twice with ice-cold DPBS and solubilized in DPBS containing 0.5 % SDS and 20 % glycerol. Extracts were removed and cell associated radioactivity counted in a scintillation counter. The data was analyzed, including Kd and Bmax determination, by nonlinear regression using Graphpad Prism software (GraphPad Software, Inc, San Diego, CA). When the concentration of[3H]R1881 required to almost saturate AR in both cell lines was established (5.0 nM), the ability of the test compounds (1 nM–10 μM) to displace [3H]R1881 (5.0 nM) from the receptors was determined as described above. The IC50 of each compound was determined by nonlinear regression with Graphpad Prism software (GraphPad Software, Inc, San Diego, CA).
2.2.5. Transcriptional activation - luciferase assay
LNCaP cells were transferred to steroid-free medium 3 days before the start of the experiment, and plated at 1 X 105 cells/well in steroid-free medium. The cells were dual transfected with ARR2-Luc and the Renilla luciferase reporting vector pRL-null with LipofectAMINE 2000 transfection reagent (Invitrogen, Carlsbad, California) according to the manufacturer’s protocol. After a 24 hour incubation period at 37 °C, the cells were incubated with fresh phenol-red free serum-free RPMI 1640 medium and treated with DHT, ethanol vehicle and/or the selected compounds in triplicate. After an 18 hour treatment period the cells were washed twice with ice-cold DPBS and assayed using the Dual Luciferase kit (Promega) according to the manufacturer’s protocol. Briefly, cells were lysed with 100 μl of luciferase lysing buffer, collected in a microcentrifuge tube, and pelleted by centrifugation. Supernatants (100 μl aliquots) were transferred to corresponding wells of opaque 96-well multiwell plates. Luciferin was added to each well, and the light produced during the luciferase reaction was measured in a Victor 1420 scanning multi-well spectrophotometer (Wallac, Inc., Gaithersburg, MD). After measurement, Stop and Glo reagent (Promega) was added to quench the firefly luciferase signal and initiate the Renilla luciferase luminescence. Renilla luciferase luminescence was also measured in the Victor 1420. The results are presented as the fold induction, that is, the relative luciferase activity of the treated cells divided by that of the control, normalized to that of the Renilla.
2.2.6. Cell proliferation tetrazolium salt reduction
LNCaP cells were grown in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin solution. LAPC4 cells were grown in IMEM supplemented with 15% FBS, 1% penicillin/streptomycin solution, and 10 nM DHT. To determine the effect of steroids and novel compounds on cell proliferation, each cell type was transferred into steroid-free medium three days prior to the start of the experiments (steroid-free medium consisted of phenol red free RPMI supplemented with 5 % dextran-coated, charcoal treated serum, and 1% penicillin/streptomycin solution). Growth studies were then performed by plating cells (1.5 × 104 cells/well) in 24-well multiwell dishes (Corning, Inc. Corning, NY). After a 24 hour attachment period, the medium was aspirated and replaced with steroid-free medium containing vehicle or the indicated concentrations of androgens and novel compounds (1 nM–5 μM). Control wells were treated with vehicle (ethanol). The medium was changed every three days and the cell viability was compared by XTT assay on the seventh day per the manufacturers protocol (Invitrogen, Carlsbad, California). All results represent the average of a minimum of three wells. Plates were read at 450 nM with a Victor 1420 scanning multi-well spectrophotometer.
2.2.7. Protein expression - gel electrophoresis and western blotting
Equal amounts of total protein (50–100 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE 60 V, 3 hr) and transferred (90 V, 1 hr) to nitrocellulose membranes (Hybond ECL, Amersham). Immunodetections were performed using mouse monoclonal antibodies against human androgen receptor (SC-7305 Santa Cruz Biotechnologies, Inc, Santa Cruz, CA). Immunoreactive bands were visualized using the enhanced chemiluminescence detection reagents (Amersham Corp., Arlington Heights, IL) according to the manufacturer’s instructions and quantified by densitometry using Bio-Rad software (Quantity One ).™
2.2.8. Mouse Xenograft Studies
All animal studies were performed according to the guidelines and approval of the Animal Care Committee of the University Of Maryland School of Medicine. Male SCID mice 4–6 weeks of age were obtained from the National Cancer Institute-Frederick Cancer Research Center (Frederick, MD). The mice were housed in a pathogen-free environment under controlled conditions of light and humidity and received food and water ad libitum. Sub-confluent cells were scraped into DPBS, collected by centrifugation and re-suspended in Matrigel (10 mg/ml) at 2.0 × 107 cells/mL. Each mouse received subcutaneous inoculations in 1 site per flank with 100 μL of cell suspension. Once tumors formed in enough mice and had an average tumor volume of at least 90 mm3, mice were grouped (10–12 mice per group) with equal average tumor volumes. Doses were administered as outlined with all compounds formulated in 0.3 % hydroxypropyl cellulose (HPC) in 0.9 % saline. Tumors were measured twice weekly with calipers and tumor volume was calculated by the formula 4/3 r12 × r2, where r1 is the smaller radius and r2 is the larger radius. Animals were also weighed weekly and monitored for general health status and signs of possible toxicity due to treatment. At the end of the treatment period, the animals were sacrificed under isoflurane anesthesia; tumors were excised and weighed. For in-vivo comparison of the effects of 5 and 3, testis, seminal vesicles, and prostates were removed and weighed upon sacrifice as well.
2.2.9. Statistical Analysis
Western blots were analyzed with a Kruskal-Wallis and Dunn’s multiple comparison post-hoc. For the LAPC-4 xenograft study comparing 5 to 3 and 4, statistical analysis was carried out as follows. The tumor volume data were log transformed due to the exponential growth of tumors in mice. The mixed-effects linear models were used to model the data. The resulting multilevel regression model included random effects associated with tumor side nested within mouse. The regression model had random effects for intercept and slope (day) at mouse level.
The tumor growth rate was estimated for each treatment group. Average tumor growth rate was also compared between the following groups: compound 5 and control, 5 and castration, 5 and 3, 5 and 3, 4 and control. Treatment means (average tumor volumes) were also compared across groups on day 0 and day 31. As intended, there were no differences in groups’ means on day 0, p = 0.96.
3. Results and Discussion
3.1. Chemistry
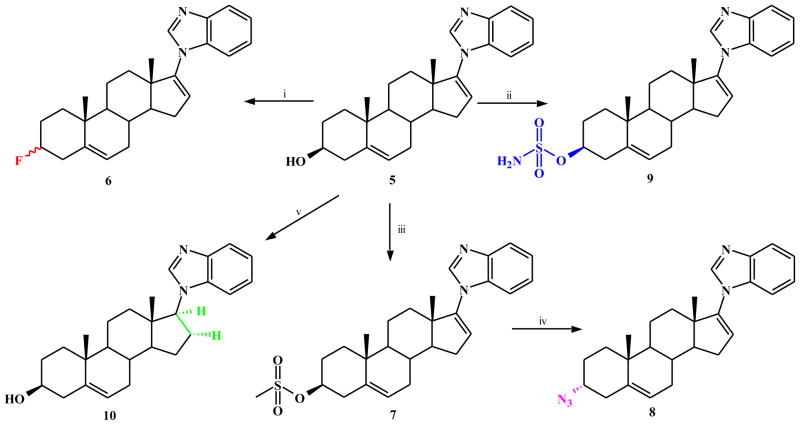
On the basis of the molecular mass (m/z 391 = M + H+) of the metabolite of 5 in the mouse, we had tentatively identified it as 17-(1H-benzimidazol-1-yl)androst-3-one.[13]. We reasoned that the metabolite may have been formed from 5 via oxidation of the 3β-OH → 3-oxo, followed by reduction (reductases?) of both Δ5 and Δ16 double bonds. Consequently, our studies focused on generation analogs with modification of the 3β-OH of compound 5, via bioisostere replacement with known metabolically stable functional groups, with specifically fluoro (F) or azido group (N3) to afford compounds 6 and 8 respectively (Scheme 1). Both functional groups are known to act as hydrogen bond acceptors just as the hydroxyl group and are present in some approved drugs.
Scheme 1.
Synthesis of compound 5 analogs (6, 8, 9 and 10).
a Reagents and conditions: (i) DAST, DMC, rt, 1h; (ii) t-BuOK, NH2SO2Cl, 0 °C - rt, 2h; (iii) MeSO2Cl, py, 0–8 °C, 16h; (iv) NaN3, DMF, 80 °C, 48h; (v) H2NNH2.H2O, AcOH, EtOH, air, reflux, 18h.
3ξ-Fluoro-17-(1H-benzimidazole)androsta-5,16-diene (6) was readily synthesized in excellent yield from 5 following treatment with diethylaminosulfur trifluoride (DAST) at room temperature [27]. This compound was clearly shown by 1H-NMR (integration of 3-H signals) to be a stereoisomers mixture, consistent with reported NMR data for related 3α- and 3β-fluoro steroidal compounds [28]. Several attempts to separate the two stereoisomers were unsuccessful, and may constitute future studies. The 3α-azido compound 8 was synthesized by reaction of 5 with methane sulfonyl chloride to give the corresponding mesylate (7) that was then treated with sodium azide in dimethylformamide (DMF) at 80 °C [29]. This reaction sequence is reported to give the final azide with complete inversion of configuration [30], consistent with its 1H-NMR data. Finally, because of improved oral bioavailability reported for several steroidal compounds with sulfamate substitutions at C3 [31, 32], we consider it a worthy endeavor to synthesized and test the 3β-sulfamate analog of compound 5. Thus, 5 was sulfamoylated to afford 3β-O-sulfamoyl-17-(1H-benzimidazole)androsta-5,16-diene (9) (Scheme 1) using tert-butoxide and sulfamoyl chloride in DMF [32].
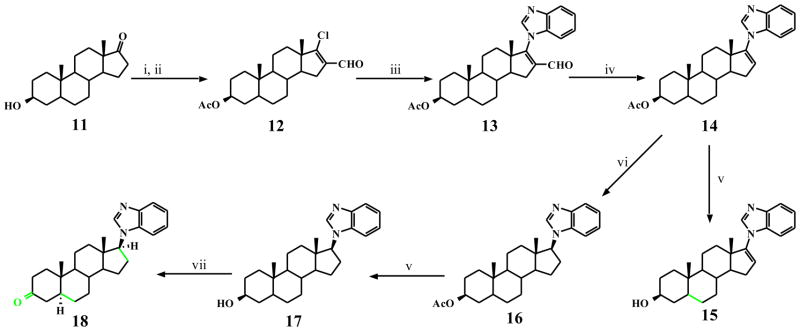
It was also of interest to synthesize possible metabolites of compound 5. The rationale for the three compounds (10, 15 and 18) synthesized was based on the fact that the metabolite previously isolated had a molecular mass of 390 amu [13]. This together with the known metabolic transformations of steroidal molecules led us to synthesize compounds with: i) reduced Δ16 double bond (10), ii) reduced Δ 5 double bond (15) and iii) reduced Δ5 and Δ16 and oxidation of 3β-OH → 3-oxo (18) as outlined in Schemes 1 and 2.
Scheme 2.
Synthesis of compounds 15 and 18.
a Reagents and conditions: (i) Ac2O, py, 0–8 °C, 16h; (ii) POCl3-DMF, CHCl3, Ar, reflux; (iii) benzimidazole, K2CO3, DMF, Ar, 80 °C; (iv) 10% Pd on activated charcoal, PhCN, reflux; (v) 10% methanolic KOH, Ar, rt; (vi) H2NNH2.H2O, EtOH/AcOH, air, 80 °C, 18h; (vii) TPAP, NMO, DMC, rt, 3h.
The saturated D-ring analog, 10 was readily prepared from 5 by reduction with diimide as we have previously reported for closely related 17-imidazole [33]. Compounds 15 and 18 (Scheme 2) were also readily synthesized from the commercially available trans-androsterone (11). The key intermediate in our synthesis of these two putative metabolites, 3β-acetoxy-17-chloro-16-formyl-5α-androstan-16-ene (12) was prepared by our routine procedure as previously described [13, 33]. Treatment of 12 with benzimidazole in the presence of K2CO3 in DMF at ~80 °C gave the desired 3β-acetoxy-17-1H-benzimidazole 13 in near quantitative yield. Compound 13 was smoothly deformylated with 10% palladium on activated charcoal in refluxing benzonitrile to give 14 in excellent yield, from which hydrolysis gave the required 3β-hydroxy-Δ5-dihydro 17-benzimidazole 15. Compound 14 was also subjected to D-ring saturation as described above for 10, from which hydrolysis gave 3β-hydroxy-Δ5,16-tetrahydro 17-benzimidazole 17. Finally, oxidation of 17 with tertapropylammonium perruthenate (TPAP) in CH2Cl2 in the presence of N-methylmorpholine N-oxide (NMO) as co-oxidant[34] gave the desired 3-oxo-Δ5,16-tetrahydro 17-benzimidazole 18 (Scheme 2).
Interestingly, we observed that these three putative mouse metabolites (10, 15 and 18) of compound 5, with the same molecular mass (390 amu) as the mouse metabolite of 5 exhibited different reverse phase HPLC chromatographic profiles with different retention times (tR) of 10.88, 22.67 and 15.02, minutes, for 10, 15 and 18, respectively (tR for 5 = 18.79 min) (Figure 2). Of these three compounds, 15 showed identical retention time as the metabolite of compound 5, reported in our previous study. Clearly, further studies are required to obtain adequate amounts of the metabolite for rigorous identification by proton nuclear magnetic resonance (1H-NMR) spectroscopy and high resolution mass spectroscopy (HRMS).
Figure 2.
HPLC chromatogram of 5 and its putative synthesized metabolites 10, 15 and 18. The retention times (tR) for 10, 18, 5 and 15 are 10.88, 15.02, 18.79 and 22.67 minutes, respectively.
3.2. Inhibition of CYP17 and androgen receptor (AR) binding studies
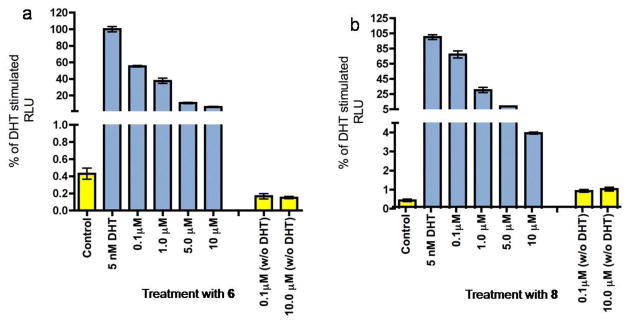
These studies were conducted as we have previously reported [13, 14]. The new analogs of compound 5 still afforded strong CYP17 lyase inhibition, with IC50 values of 150 and 120 nM for 6 and 8, respectively (Table 1). However, they were less potent than 5 which has an IC50 of 47 nM [35], which suggest that the 3β-OH is important for CYP7 inhibition. In sharp contrast, one of the putative metabolites of 5, that is, 18 exhibited weak inhibition of CYP17 (less than 30% inhibition at 500 nM). In competitive binding studies, the ability to displace [3H]R1881 from both the wild-type AR (in LAPC-4 cells) and the mutant T877A AR (in LNCaP cells) occurred in a dose-dependent manner (figure not shown). Compounds 6 and 18 with EC50 values of 934 and 1100 nM versus the wild-type AR were moderately reduced (2.3- and 2.7-fold), respectively compared to 5. However, binding of both compound to the mutant AR were not significantly different from binding of 5. In contrast the binding affinities of compound 8 to both receptors were greatly reduced (Table 1). As shown in (Figure 3) both compounds 6 and 8 were found to be pure AR antagonists in transcriptional activation studies. However, neither was as potent as 5 at inhibiting androgen-induced AR transcription.
Table 1.
CYP17, growth inhibitory activities and androgen receptor binding of 5 and analogs.
| Compound | CYP17 IC50 (nM)a | AR binding EC50 (nM)b | Cell growth inhibition GI50 (μM)c | ||
|---|---|---|---|---|---|
| Wild-type (LAPC-4) | Mutant (T877A, LNCaP) | LNCaP | LAPC-4 | ||
| 5 | 47 | 405 | 1240 | 6.0 | 3.2 |
| 6 | 150 | 934 | 1600 | 8.0 | 16.0 |
| 8 | 120 | 2460 | 4340 | 7.0 | 19.0 |
| 15 | - | - | - | 17.6 | - |
| 18 | * | 1100 | 1280 | - | - |
| For comparison: | |||||
| Casodex | - | 4300 | 971 | - | - |
IC50 is the concentration of inhibitor required to inhibit the CYP17 enzyme activity by 50%, each in duplicate.
EC50 is the concentration of compound required for a 50% displacement of [3H]R1881 from the androgen receptor.
GI50 is the concentration of compound required to cause 50% growth inhibition of LNCaP or LAPC-4 cells.
Less than 30% inhibition at 500 nM.
= not determined.
Figure 3.
Effect of compounds 6 and 8 on DHT stimulated transcription. LNCaP Cells were transfected with the ARR-2 reporter construct and treated with novel compounds in the presence or absence of DHT. Blue bars represent treatment with DHT and the indicated concentration of compound. Yellow bars represent treatment with compound only.
3.2. Inhibition of prostate cancer cell proliferation
In cell viability assays, compounds 6 and 8 inhibited LNCaP proliferation by 85% and 60% at 10 μM, respectively, which also did not equal the effectiveness of 5. In DHT stimulated LNCaP cells, 6 had an IC50 of 8 μM, while 8 was less effective with an IC50 of 16 μM. Both compounds exhibited a similar pattern in DHT stimulated LAPC4 proliferation, with IC50 values of 7 μM and 19 μM for 6 and 8, respectively (Table 1).
3.3. Effects of agents on AR Down-regulation
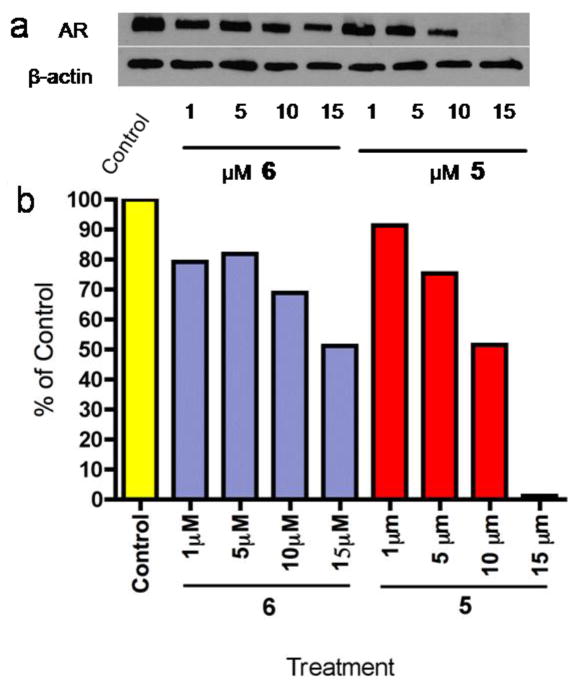
Because we had previously demonstrated that some of our novel CYP inhibitors/AR antagonists are also strong AR down-regulating agents [14], we assessed the abilities of the new analogs to cause AR down-regulation in LNCaP cells. Compound 5 was still the most effective compound in vitro with nearly complete (>95%) AR down-regulation at 15 μM. Of the three new compounds tested in this study, 6 was able to down regulate AR expression by 50% at 15 μM, the same effect was achieved with 10 μM VN/124-1 in LNCaP cells (Figure 4). Compounds 8, 10, 15 and 18 did not cause down-regulation of AR expression even at the highest concentration (15 μM) tested (data not presented). On the basis of our previous and present studies, we have derived a preliminary in vitro structure activity relationship (SAR) of our CYP17 inhibitors/AR antagonists as androgen receptor ablative agents (ARAAs), summarized in Figure 5. There is no clear correlation of agents AR binding affinities to AR down-regulating activities. Modifications of the A- or B-ring resulted in significant decrease in AR down-regulating activity, suggesting that 3β-OH-Δ5 moiety may be critical for activity. While isosteric replacement of β-OH group at C-3 with fluorine (F) significantly reduced activity, replacement with an azido (N3) group completely abolished activity versus AR down-regulation. Other modifications that also resulted in drastic reduction in AR down-regulation include, saturation of either or both Δ5 and Δ16 double bonds, introduction of 16-methyl alcohol or replacement of 17-benzimidazole with substituted and unsubstituted purines (data not shown).
Figure 4.
Densitometry analysis of AR protein expression in treated LNCaP Cells. Cells were treated with test compounds for 24 hours at the indicated concentrations. Cell extracts were prepared and probed with anti-AR and anti-βactin antibodies. Densitometry quantification was performed utilizing ImageQuant 5 software
Figure 5.
In vitro SAR of CYP17 inhibitors/antiandrogens as androgen receptor ablative agents (ARAAs).
3.4. Evaluation of in vivo efficacy of compounds against LAPC4 xenografts
We have previously demonstrated the exceptional anti-tumor efficacy of our lead compound 5 in several prostate cancer xenograft models.[13, 14] In the present study, we wanted to compare the anti-tumor efficacy of 5 versus its putative metabolically stable analog, 6, prodrug sulfamate 9 and abiraterone (3) (head-to-head experiment between 5 and 3/4), a CYP17 inhibitor that is currently undergoing phase III clinical trials n prostate cancer patients [20, 21]. However, for the sake of clarity, these experiments are presented as three in vivo studies.
In the first in vivo study, the relative efficacies of 5 and 3 were compared in vivo in an LAPC-4 xenograft model. Mice inoculated with LAPC-4 tumors were treated subcutaneously with 0.15 mmol/kg of 3 or 5 twice daily. Because compound 3 is administered clinically orally as the acetate pro-drug, mice were also treated with either 3-acetate (4) or 5-acetate 0.15mmol/kg/o.d. p.o. At day 24, oral dosing of 3 and 4 was changed to twice daily because neither compound demonstrated any significant efficacy.
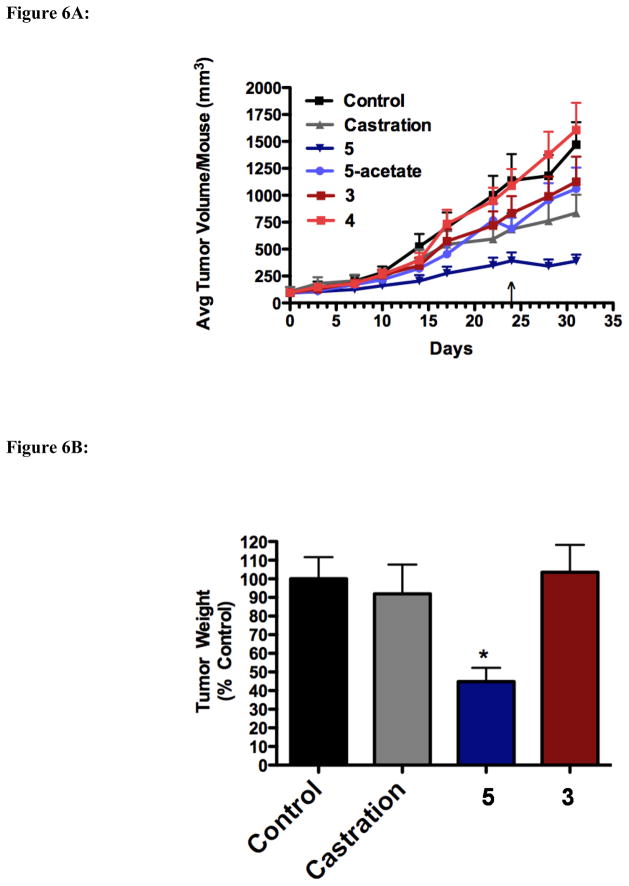
As shown in Figure 6A, 5 s.c. b.i.d. was the most effective therapy in inhibiting the growth of LAPC-4 tumors. Surprisingly, even tumors in the castration group grew rapidly suggesting the LAPC-4 cells used in the inoculation may have acquired some resistance to androgen-deprivation therapy. Such acquired androgen independence has been shown to occur after long-term culture of LNCaP cells [19], and it is possible a similar effect has occurred here. Nonetheless, mice treated with 5 had smaller average tumor volume on day 31 when compared to control (p = 0.0001), 3-acetate (4) (p = 0.005), 3 (p = 0.053, of marginal statistical significance), and 5-acetate (p = 0.006). Compound 5 treatment also significantly reduced the growth rate of tumor growth compared to control, 3 and 4 (p < 0.0001, p = 0.025, p < 0.0001, respectively). Upon excision, final tumor weights were also significantly reduced in animals treated with compound 5 compared to animals treated with control, 3, and castration (p < 0.05; Figure 6B).
Figure 6.
Figure 6A. Effect of 5 and 3/4 on the growth of LAPC-4 tumors in-vivo. Male SCID mice were inoculated with LAPC-4 cells and treated with either vehicle (0.3% HPC), 0.15mmol/kg of 5 (s.c. b.i.d.), 3 (s.c. b.i.d.), 5-acetate (p.o. o.d.⇒b.i.d), or 4 (p.o., o.d. ⇒b.i.d.). 5 b.i.d. treated tumors had a significantly reduced growth rate over control, 3 and 4 (p < 0.0001, p = 0.025, p < 0.0001, respectively). ↓ = marks day at which p.o. dosing of 4 and 5-acetate were increased to b.i.d.
Figure 6B. Effect of 5 and 3 on LAPC-4 tumor weights. Excised LAPC-4 tumors were weighed following 31 days of treatment. *p<0.05 compared every other group.
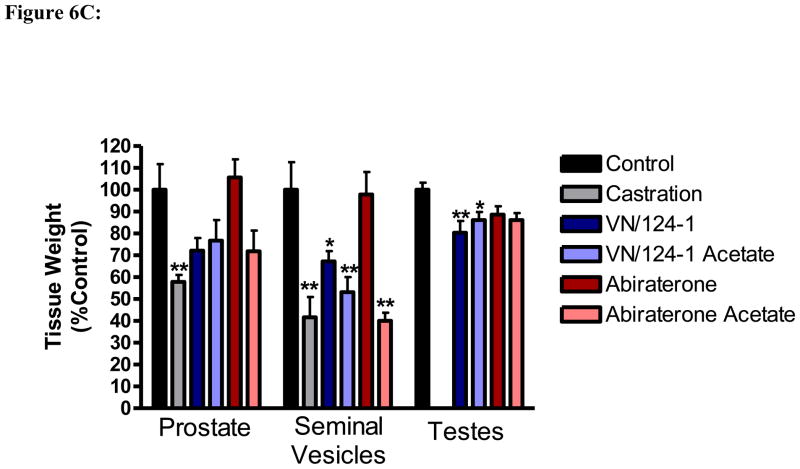
Figure 6C. Effect of 3, 4, 5 and 5-acetate on urogenital tissue weights. Following 31 days of treatment, urogenital tissues from mice in each treatment group were excised and weighed.*p < 0.05, **p < 0.01 compared to control.
The effect of each treatment on the weights of the prostate, seminal vesicles, and the testes was also measured (Figure 6C). As these tissues are sensitive to androgens, a reduction in their weight is a biomarker for androgen deprivation. Compounds 5 and 5-acetate significantly reduced the size of the seminal vesicles and testes, and also reduced the size of the prostate, although the reduction was not quite significant. Compound 3 failed to significantly reduce the size of any of the organs, while 4 significantly reduced the size of the seminal vesicles. None of the treatments resulted in any noticeable toxicity, as exemplified by no significant changes in body weight (data not shown).
The effect of both 5 and 3 on AR expression within tumors was also compared (Figure 7). Consistent with previous reports [14], 5 treatment resulted in an average 2-fold reduction in AR protein levels with the majority of tumors analyzed not expressing any detectable levels of AR. Compound 3, on the other hand, had no significant effect on AR levels with an average expression found to be slightly higher than that found in control tumors. The difference in the effect of each molecule on AR levels is striking and represents a major distinction of compound 5’s therapy. It should be noted that the in vivo efficacy of targeting AR expression to block tumor growth and delaying tumor progression was first demonstrated in 2006 by using short hairpin RNA (shRNA)-mediated AR knockdown in an LNCaP hormone-dependent tumor xenograft model [36]. Subsequently, the same group recently (2009) demonstrated for the first time that this strategy is also a viable therapeutic strategy for prostate C4-2 tumors that have progressed to the castration-resistant state [37]. These important findings provide strong proof-of-principle that inhibition of AR expression represents a therapeutically relevant strategy for prostate cancer treatment.
Figure 7.
Effect of abiraterone (3) abiraterone acetate (4) and VN/124-1 (5) on AR protein levels in-vivo. Tumors from each treatment group were excised and analyzed by western blot for relative AR expression: A) AR expression of representative tumors. B) Average AR expression of at least 3 tumors from each treatment determined by densitometry * p < 0.05.
In preclinical studies, we found that compound 5 is not orally active. Because oral formulations of drugs are preferred clinically, we synthesized a sulfamate prodrug analog of 5, that is, compound 9, with the hope that it would be orally active. Our motivation was based on previous reported of improved oral bioavailability for several steroidal compounds with sulfamate substitutions at C3 [31, 32]. However, studies with 9 administered orally as described for 5 above did not show any significant effect on LAPC-4 tumor growth (data not presented). It should be noted that formulated (micronized) 5 is administered orally to patients in ongoing phase I/II clinical trials.
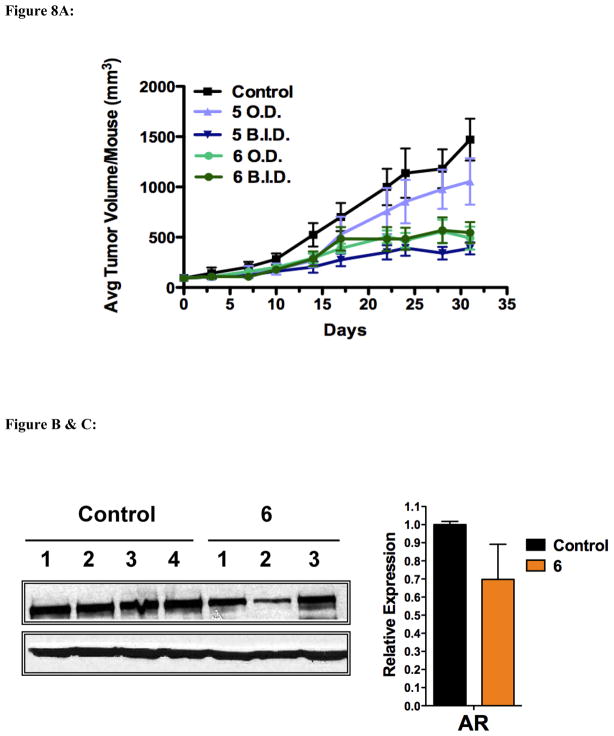
In the third in vivo experiment, we tested the anti-tumor efficacy of 5 versus its putative stable 3-fluoro analog, compound 6. SCID mice were inoculated as before with the LAPC-4, and tumors were allowed to form for 6 weeks until the average tumor volume was ~90 mm3. Mice were then grouped: 10 mice per group and treated with once or twice daily doses of 0.15 mmol/kg 5 or 6.
As shown in Figure 8A, once daily administration of 6 was as effective in inhibiting LAPC-4 tumor growth as twice daily administration of 5. Once daily administration of 5 did not have a significant effect on tumor growth. Unfortunately, treatment with 6 resulted in the death of five mice in the once daily administration group and two mice in the twice daily administration group. The mice began to die around day 25, with two mice dying before the final measurement on day 31. The remaining mice died following the last measurement but before necropsy (2 days later). It is likely the drug accumulated overtime resulting in the toxicities after extended treatment. Large deposits of compound 6 were discovered under the skin of the mice during necropsy. Despite the apparent toxicity, no changes in mouse weights were observed (data not shown) and no overt signs toxicity were seen during the internal examination of the mice. Clearly, additional studies are warranted with this novel compound.
Figure 8.
Figure 8A. Effect of 5 and 6 on LAPC-4 tumor growth in-vivo. Male SCID mice were inoculated with LAPC-4 cells and treated with either vehicle (0.3% HPC), 0.15 mmol/kg/b.i.d of 5 s.c., 0.15 mmol/kg/o.d. of 5 s.c., 0.15 mmol/kg/o.d. of 6 s.c.., or 0.15 mmol/kg/b.i.d. of 6 s.c. Tumors were measured with calipers as described in the materials and methods.
Figure 8B & C. Effect of 6 on AR levels in vivo. (B) Tumors from each treatment group were excised and analyzed by western blot for relative expression of the AR. (C) Average expression as determined by densitometry.
Tumor samples treated with 6 were analyzed to determine the molecules effect on AR protein levels. As the AR was markedly reduced with treatment with 5, it was expected that twice daily administration of 6 would also have an effect. However, as shown in Figures 8B & C, 6 had only a minor and statistically insignificant effect on AR protein levels.
4. Conclusions
The superior in vivo anti-tumor efficacy (prevention of tumor formation and inhibition of tumor growth) of our investigational new drug (IND) 5 in LAPC-4 tumor xenografts that express AR versus the PC-3 xenografts that lack AR, reinforces our previous findings [14] that AR ablation is an important anti-cancer property of compound 5. The present studies also provide compelling evidence that 5 is a potent inhibitor of human prostate cancer tumor growth and is remarkably more effective than castration or compound 3 (4) that is currently undergoing phase III clinical trials in prostate cancer patients. We also show that neither of the plausible metabolites of 5 is as effective as the parent compound which suggests that it may be possible to improve on its in vivo efficacy if the site of metabolism is unequivocally identified and blocked. In addition, because we strongly believe that the unique AR ablative property of 5 is what distinguishes it from other closely related compounds; our efforts are currently directed to develop new analogs of compound 5 with nanomolar AR ablative activities. We also showed, that on equimolar basis, compound 6 was ~2-fold more efficacious versus LAPC-4 xenografts than 5, but the toxicity observed with 6 is of concern. Overall, these results strongly support the ongoing clinical evaluation of compound 5 and we eagerly await the phase I/II trial findings.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R21 CA11799-01 (Njar, VCO), and R01 CA027440-28 (Brodie, AMH) and funds from Tokai Pharmaceutical Inc., (Brodie, AMH and Njar VCO). Bruno, RD was supported in part by National Institute of Environmental Health Safety training grant T32 ES007263-16A. Vasaitis, TS was supported in part by US National Institutes of Health training grant T32 AG000219.
Abbreviations
- AIPC
androgen-independent prostate cancer
- AR
androgen receptor
- ARAAs
androgen receptor ablative agents
- CRPC
castration-resistant prostate cancer
- DAST
diethylaminosulfur trifluoride
- DHT
dihydrotestosterone
- PCA
prostate cancer
- PK
pharmacokinetics
- PSA
prostate specific antigen
- SAR
structure activity relationship
- SCID
severe combined immunodeficient
Appendix A: Supplementary data
HPLC chromatograms and high resolution mass spectral data for compounds 6, 8–10, 15, and 18.
Footnotes
Disclosure of Potential Conflict of Interest
Vincent C. O. Njar and Angela M. H Brodie hold an ownership interest in the VN/124-1 patents and technologies thereof. The other authors declare no potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Harzstark AL, Ryan CJ. Novel therapeutic strategies in development for prostate cancer. Expert Opin Investig Drugs. 2008;17:13–22. doi: 10.1517/13543784.17.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–96. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 5.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–33. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 7.Labrie F. Multiple intracrine hormonal targets in the prostate: opportunities and challenges. BJU Int. 2007;100 (Suppl 2):48–51. doi: 10.1111/j.1464-410X.2007.06955.x. [DOI] [PubMed] [Google Scholar]
- 8.Mellado B, Codony J, Ribal MJ, Visa L, Gascon P. Molecular biology of androgen-independent prostate cancer: the role of the androgen receptor pathway. Clin Transl Oncol. 2009;11:5–10. doi: 10.1007/s12094-009-0304-3. [DOI] [PubMed] [Google Scholar]
- 9.Vis AN, Schroder FH. Key targets of hormonal treatment of prostate cancer. Part 1: the androgen receptor and steroidogenic pathways. BJU Int. 2009;104:438–48. doi: 10.1111/j.1464-410X.2009.08695.x. [DOI] [PubMed] [Google Scholar]
- 10.Attar RM, Jure-Kunkel M, Balog A, Cvijic ME, Dell-John J, Rizzo CA, et al. Discovery of BMS-641988, a novel and potent inhibitor of androgen receptor signaling for the treatment of prostate cancer. Cancer Res. 2009;69:6522–30. doi: 10.1158/0008-5472.CAN-09-1111. [DOI] [PubMed] [Google Scholar]
- 11.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ang JE, Olmos D, de Bono JS. CYP17 blockade by abiraterone: further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009;100:671–5. doi: 10.1038/sj.bjc.6604904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handratta VD, Vasaitis TS, Njar VC, Gediya LK, Kataria R, Chopra P, et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem. 2005;48:2972–84. doi: 10.1021/jm040202w. [DOI] [PubMed] [Google Scholar]
- 14.Vasaitis T, Belosay A, Schayowitz A, Khandelwal A, Chopra P, Gediya LK, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7:2348–57. doi: 10.1158/1535-7163.MCT-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Q, Jagusch C, Hille UE, Haupenthal J, Hartmann RW. Replacement of imidazolyl by pyridyl in biphenylmethylenes results in selective CYP17 and dual CYP17/CYP11B1 inhibitors for the treatment of prostate cancer. J Med Chem. 2010;53:5749–58. doi: 10.1021/jm100317b. [DOI] [PubMed] [Google Scholar]
- 16.Hu Q, Yin L, Jagusch C, Hille UE, Hartmann RW. Isopropylidene substitution increases activity and selectivity of biphenylmethylene 4-pyridine type CYP17 inhibitors. J Med Chem. 2010;53:5049–53. doi: 10.1021/jm100400a. [DOI] [PubMed] [Google Scholar]
- 17.Jagusch C, Negri M, Hille UE, Hu Q, Bartels M, Jahn-Hoffmann K, et al. Synthesis, biological evaluation and molecular modelling studies of methyleneimidazole substituted biaryls as inhibitors of human 17alpha-hydroxylase-17,20-lyase (CYP17). Part I: Heterocyclic modifications of the core structure. Bioorg Med Chem. 2008;16:1992–2010. doi: 10.1016/j.bmc.2007.10.094. [DOI] [PubMed] [Google Scholar]
- 18.Pinto-Bazurco Mendieta MA, Negri M, Jagusch C, Muller-Vieira U, Lauterbach T, Hartmann RW. Synthesis, biological evaluation, and molecular modeling of abiraterone analogues: novel CYP17 inhibitors for the treatment of prostate cancer. J Med Chem. 2008;51:5009–18. doi: 10.1021/jm800355c. [DOI] [PubMed] [Google Scholar]
- 19.Schayowitz A, Sabnis G, Njar VC, Brodie AM. Synergistic effect of a novel antiandrogen, VN/124-1, and signal transduction inhibitors in prostate cancer progression to hormone independence in vitro. Mol Cancer Ther. 2008;7:121–32. doi: 10.1158/1535-7163.MCT-07-0581. [DOI] [PubMed] [Google Scholar]
- 20.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69:4937–40. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- 22.Bruno RD, Gediya LK, Purushottamachar P, Brodie AMK, Njar VCO. Lyase inhibitor/androgen receptor modulator, VN/124-1 (TOK-001), shows improved efficacy over abiraterone in a LAPC-4 xenograft model. Amereican Association for Cancer Reserach; Denver: p. CO2009. [Google Scholar]
- 23.Potter GA, Hardcastle IR, Jarman M. A Convenient, Large-Scale Synthesis of Abiraterone Acetate [3 Beta-acetoxy-17(3-pyridyl)-Androsta-5, 16-diene], a Potential New Drug for the Treatment of Prostate Cancer. Organic Preparations and Procedures International. 1997;29:123–8. [Google Scholar]
- 24.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–71. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 25.Wong C, Kelce WR, Sar M, Wilson EM. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J Biol Chem. 1995;270:19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- 26.Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, et al. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990;265:8893–900. [PubMed] [Google Scholar]
- 27.Liu A, Carlson KE, Katzenellenbogen JA. Synthesis of high affinity fluorine-substituted ligands for the androgen receptor. Potential agents for imaging prostatic cancer by positron emission tomography. J Med Chem. 1992;35:2113–29. doi: 10.1021/jm00089a024. [DOI] [PubMed] [Google Scholar]
- 28.Marson CMDRA, Smith KE. Preparation of 3alpha-fluoro- and 3beta-fluoro derivatives of 5alpha-androst-16-enes and androsta-5,16-dienes. Synthetic Communications. 2002;32:2125–35. [Google Scholar]
- 29.Reddy PVR, LVR, Kumar B, Kumar R, Maulik PR, Shaw AK. A general and efficient stereoselective synthesis of gama-azido-tetrahydrofuran carboxylic acids from glycols. Tetrahedron. 2008;64:2153–9. [Google Scholar]
- 30.Santaniello E, Caspi E. Reduction of certain steroidal 19-sulfonic esters with metal hydrides. J Steroid Biochem. 1976;7:223–7. doi: 10.1016/0022-4731(76)90205-3. [DOI] [PubMed] [Google Scholar]
- 31.Bubert C, Leese MP, Mahon MF, Ferrandis E, Regis-Lydi S, Kasprzyk PG, et al. 3,17-disubstituted 2-alkylestra-1,3,5(10)-trien-3-ol derivatives: synthesis, in vitro and in vivo anticancer activity. J Med Chem. 2007;50:4431–43. doi: 10.1021/jm070405v. [DOI] [PubMed] [Google Scholar]
- 32.Leese MP, Leblond B, Smith A, Newman SP, Di Fiore A, De Simone G, et al. 2-substituted estradiol bis-sulfamates, multitargeted antitumor agents: synthesis, in vitro SAR, protein crystallography, and in vivo activity. J Med Chem. 2006;49:7683–96. doi: 10.1021/jm060705x. [DOI] [PubMed] [Google Scholar]
- 33.Njar VC, Kato K, Nnane IP, Grigoryev DN, Long BJ, Brodie AM. Novel 17-azolyl steroids, potent inhibitors of human cytochrome 17 alpha-hydroxylase-C17,20-lyase (P450(17) alpha): potential agents for the treatment of prostate cancer. J Med Chem. 1998;41:902–12. doi: 10.1021/jm970568r. [DOI] [PubMed] [Google Scholar]
- 34.Njar VCSE, Silverton JV, Robinson CH. Novel 10beta-aziridinyl steroids; inhibitors of aromatase. J Chem Soc Perkin Trans. 1993;1:1161–8. [Google Scholar]
- 35.Vasaitis TS. PhD Thesis. University of Maryland School of Medicine; Baltimore, USA: 2007. Multi-mechanistic inhibitors of androgen action for the treatment of prostate cancer; pp. 1–193. [Google Scholar]
- 36.Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 2006;66:10613–20. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- 37.Snoek R, Cheng H, Margiotti K, Wafa LA, Wong CA, Wong EC, et al. In vivo knockdown of the androgen receptor results in growth inhibition and regression of well-established, castration-resistant prostate tumors. Clin Cancer Res. 2009;15:39–47. doi: 10.1158/1078-0432.CCR-08-1726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.