Abstract
The “oldest-old” comprise the fastest growing segment of the population in much of the world. Rates of dementia are extremely high in this age group and will present a major public health burden as the numbers of these individuals quadruple by the middle of the century. Studies in this age group are rare and frequently have small numbers of participants. In research studies and the clinic, the diagnosis of dementia and determination of the etiology of the disorder are challenging. In this review, we include some of our experiences in a population-based longitudinal investigation, The 90+ Study. Oldest-old individuals are more likely to suffer from medical comorbidities and have high rates of sensory loss, psychoactive medication usage, frailty and fatigue. Moreover, social and cultural expectations affect the reporting and interpretation of behavioral changes. These and other factors make it difficult to determine the relative contributions of cognitive losses and non-cognitive losses in the development of functional disability. Contributing further to the complexities of diagnosis, current research suggests that dementia in the oldest-old, compared to younger people, is more likely to be related to mixed disease pathologies. Frequent cerebral neuropathologies include Alzheimer’s disease neurodegeneration, small and large vessel vascular disease, and hippocampal sclerosis. More research is necessary in the oldest-old to better understand the etiologies of dementia in this age group, and factors that may affect the expression of disease as we age.
Keywords: Dementia, Oldest-Old, Diagnosis, Alzheimer’s Disease
1. Introduction
Variously described as those over 85 years old, 90 years or even 95+, the “oldest old” comprise the fastest growing segment of the population in much of the world. There are approximately 2 million people 90 years and older in the United States and this number is projected to increase 5-fold by the middle of the century presenting an unprecedented public health challenge (1–2). With extraordinarily high rates of dementia in this group, there will be more people aged 90 and older with dementia in the coming decades than we currently have at all ages combined. Surprisingly, however, we know little about the causes and expression of dementia in our oldest citizens. Studies in this age group are limited and the generally small number of subjects may not reflect the general population. There are many challenges and difficulties when investigating dementia in this age group, beginning with making the diagnosis.
2. How Common is Dementia in the Oldest Old?
2.1 Prevalence and Incidence
Measuring prevalence and incidence is dependent on accurate diagnosis in population-based studies. A modest number of epidemiological studies have published rates of dementia and Alzheimer’s Disease (AD) in oldest individuals, but the numbers of subjects tend to be small and the confidence intervals wide with rates varying more than 8-fold. Thus, it has been difficult to determine if the risk of dementia goes up, down or levels off after age 85. See Figure.
Figure.
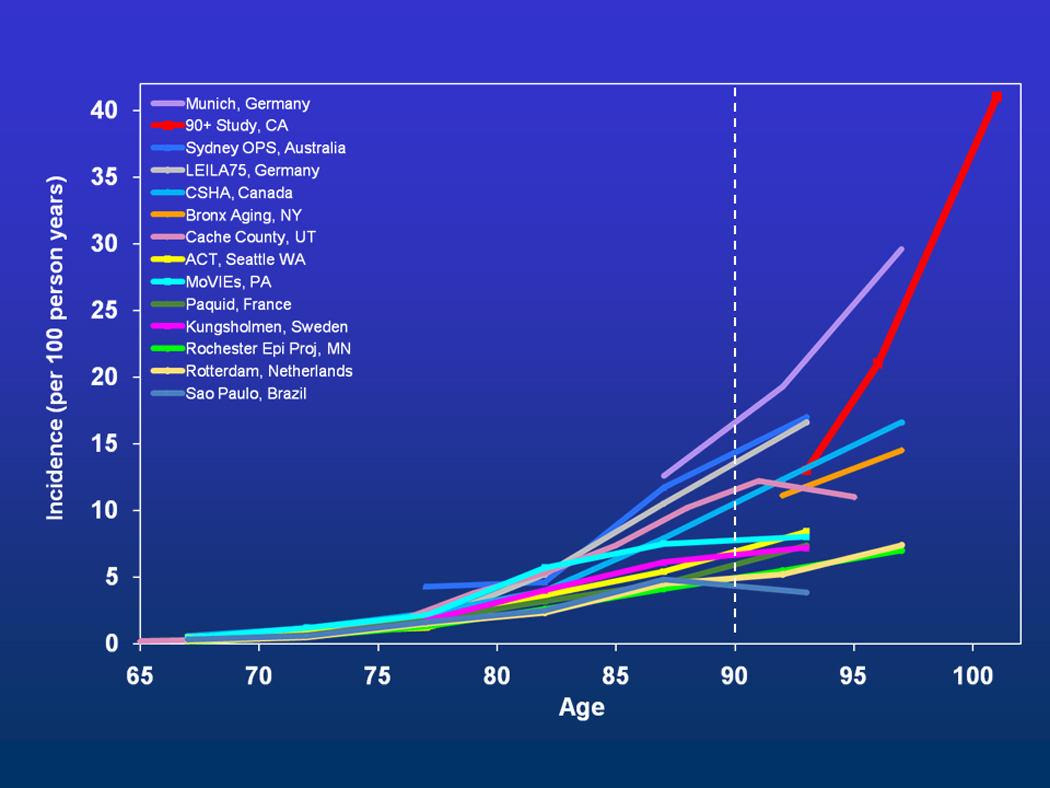
Age-Specific Incidence of Dementia in Studies with Subjects Aged 90+
Note: Munich, Germany(44), 90+ Study, CA(6), Sydney OPS, Australia(45), LEILA75, Germany(46), CSHA, Canada(47), Bronx Aging, NY(48), Cache County, UT(49), ACT, Seattle, WA(50), MoVIEs, PA(51), Paquid, France(52), Kungsholmen, Sweden(53), Rochester Epi Proj, MN(54), Rotterdam, Netherlands(55), Sao Paulo, Brazil(56)
2.2 The 90+ Study
The 90+ Study is a population-based sample of more than 1600 individuals, aged 90 – 108 years. Initiated in 2003, 90+ participants are predominantly white, female (76%) and well educated (63% >high school). As part of the study procedures described more fully elsewhere (3–5), participants receive neuropsychological evaluations and neurological examinations every 6 months. They provide medical records and a designated informant for additional data collection. Additional studies include protocols for DNA and brain donation, amyloid PET scans, and structural and functional neuroimaging. Dementia and etiological diagnoses are assigned in a multi-disciplinary consensus conference where all available information is reviewed. The diagnosis of dementia in these individuals has proven to be a challenging task as we discuss below.
Shown in Figure, the age-specific incidence rates from The 90+ Study (6) fell in the middle of the published estimates, but it did not go down or level off. Dementia risk continued to double with every 5.5 years of age past age 100. The incidence rate of all-cause dementia for centenarians in The 90+ Study was approximately 40% annually. The risk of developing dementia was similar for the sexes, although dementia prevalence was higher for women presumably related to greater longevity (7). Although there was poor correlation between cognition and cerebral pathology, there were high rates of pathological changes in the brain at autopsy. These included changes associated with Alzheimer’s disease (amyloid plaques, neurofibrillary tangles, amyloid angiopathy), vascular and white matter disease, Lewy bodies and hippocampal sclerosis. Moreover, multiple pathologies were more likely to be associated with clinical dementia.
3. Challenges to Diagnosing Dementia in the Oldest Old
According to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (8), a diagnosis of dementia requires two main components: 1) loss of memory and at least one other cognitive domain, and 2) decline in social or occupational functioning due to cognitive impairment. In the oldest old, however, it can be extremely difficult to determine the contribution of cognitive loss to the loss of functional abilities. The extreme elderly comprise a population with high rates of sensory losses, medical comorbidities, physical disability, frailty, and fatigue. These factors all contribute to loss of abilities, such as driving, managing financial matters and other functional losses associated with dementia in younger elderly. Here we discuss some of the challenges encountered in The 90+ Study, a population-based investigation of the oldest old in Laguna Woods, California, and highlight methods that can be applied in the clinic as well as in research studies.
3.1 Sensory Loss
Although most people are aware that the oldest-old suffer from sensory difficulties, many may be surprised about the magnitude of the problem. In The 90+ Study, the majority of participants (72%) had significant hearing loss, vision loss, or both (9). One population-based study found that 90% of people aged 80–92 had some level of hearing loss (10). Similarly, visual losses frequently occur in this age group. Of the different types of vision loss, age-related macular degeneration is one of the most prevalent and debilitating. A meta-analysis of population-based studies showed that macular degeneration increases greatly with age and 16% of white women aged 80 and older have the condition (11).
The very high prevalence of visual and hearing loss in the oldest-old make evaluations for cognitive impairment difficult and may lead to a misclassification bias where dementia is diagnosed more frequently in people with sensory losses. However, some studies have suggested that the sensory loss itself may be indicative of neurodegeneration and, thus, related to cognitive decline. For example, a case-control study of 100 AD patients and 100 matched controls found that hearing loss was associated with the severity of cognitive dysfunction in both the demented patients and normal controls after controlling for relevant variables (12). A prospective cohort study found that vision impairment and combined vision and hearing impairment predicted both cognitive and functional decline in a sample of older women (13). Although there is some evidence that sensory losses may be related to neurodegenerative processes associated with cognitive decline, in order to properly assess the oldest-old, various methods to compensate for vision and hearing loss should be implemented.
Our experience in The 90+ Study led to standardized changes in our administration of all tests and the design of forms. Participants with hearing difficulties are provided with amplifiers. To maximize visibility, stimuli are presented in size 90 font for all subjects. Additionally, we have modified several common neuropsychological tests. For example, when administering the Mini-Mental State Examination (14) and the California Verbal Learning Test-II Short Form (15), the words for recall are spoken in a loud, clear voice and simultaneously shown to the participant on a card with large typeface. This type of multi-modality presentation promotes the ability of the participant to register the appropriate word despite sensory limitations. These compensations for sensory losses are crucial for valid assessment of the cognitive abilities in the oldest old.
3.2 Comorbidities
In the oldest-old, high comorbidity rates make it difficult to parse out impairments related to neurodegenerative disorders such as AD from impairments related to medical illness. In a study of participants from The 90+ Study without functional disability (average age=94), almost all participants had at least one major medical illness or cardiovascular risk factor (cerebrovascular disease, cardiac disease, arthritis, diabetes, thyroid disease, hypertension, or high cholesterol) and 62% had two or more (16). A population-based study of Danish centenarians reported that the average centenarian (demented or cognitively normal) had more than four chronic conditions or diseases (17). These studies demonstrate the high levels of comorbidities in oldest old individuals and further complicate the interpretation of their neuropsychological results and losses in daily function.
It has been hypothesized that with the extension of lifespan and advances in medical science, the health of our elderly population may be improving. However, a recent Swedish survey of people aged 77 and older showed that between 1992 and 2002 the prevalence of various medical conditions increased (18). Although people are living longer, they are not necessarily living healthier. Moreover, higher rates of medical illnesses result in greater medication use and these medications can affect cognitive performance through a number of mechanisms. A large, Finnish study examined medication usage in elderly people (aged 64 years and older) in the early and late 1990s (19). In less than a decade, the average number of medications taken daily per person increased from 3.1 to 3.8. In people aged 85 and older, 97% took at least one prescription medication, with the average man in this age category taking more than five medications and the average woman, seven. These medical illnesses and medications frequently lead to delirium, which is probably under-recognized, especially in the oldest-old and those with dementia (20).
3.3 Lack of Normative Data
Ideally we would like to measure if an individual’s cognitive performance has declined from a previous level. However in practice, it is typically necessary to compare performance to standardized age-specific norms to determine if a person has cognitive loss. Hence, appropriate normative data must be available. Very few normative studies have included large numbers of people aged 90 and older (21–22) and several have excluded the oldest-old entirely (23–24). For example, a paper written with the purpose of providing normative data over age 80 for the California Verbal Learning Test included only 39 people aged 77–94 (25). Age-associated cognitive losses accelerate in the ninth and tenth decades of life. A study examining nearly 200 non-demented participants aged 85 and older found that neuropsychological test scores were significantly affected by age, education and gender. The authors concluded that using normative data from younger populations could potentially result in over-diagnosing cognitive impairment in older age groups (26).
With data from The 90+ Study, we recently published normative data for several commonly used neuropsychological tests. In this publication, the norms from 339 non-demented participants (average age=94) are presented with age-specific means, standard deviations, and percentiles (3). These data are from a well educated, mostly Caucasian sample, reflecting the composition of our population. While only a first step, these normative values may be useful to clinicians and researchers. Future normative studies encompassing more diverse populations of oldest-old are necessary.
3.4 Disability
Functional disability is very prevalent in the oldest-old. One study of people aged 84–90 found that only 23% had mild disability or were free from disability entirely (27). The 90+ Study has examined the prevalence and incidence of disability. Difficulty in Activities of Daily Living (28) (difficulty with or needing help with feeding, dressing, bathing, toileting, transferring, and walking indoors) was present in 71% of 90–94 year olds, 89% of 95–99 year olds, and 97% of centenarians (29). In people aged 90–94 with no disability, more than 8% became disabled each year; in people aged 95 and older, the incidence of disability increased to 26% per year (16).
Functional disability can be due to physical impairment, cognitive impairment, or both. As a dementia diagnosis requires functional loss specifically due to cognitive impairment, it is essential for the researcher or care provider to probe extensively to accurately determine the cause(s) of the disability. Moreover, unlike traditional disability assessments, the information about functional abilities must be obtained from a variety of sources as well as the individual being evaluated. Reports may vary considerably when obtained by self-report, the individual’s family, friends, or care providers. In many cases, clinical judgment must be used in addition to the information gathered, particularly when determining the contribution of cognitive loss to functional disability.
3.5 Fatigue and Frailty
Frailty has been characterized as a syndrome of weakness, fatigue, and decline in physical activity (30), and is associated with many negative health outcomes (31). It is very common in the extreme elderly. In the Cardiovascular Health Study, frailty was present in 7% of elderly overall, but in almost a quarter (23%) of participants aged 90 and older (32). The concept of frailty is highly interrelated with disability and comorbidity (31). A component of frailty frequently present in the elderly is extreme fatigue, a factor we greatly underestimated before conducting research in this population. Both fatigue and frailty can be due to comorbidities, medication usage, sensory problems, or a variety of other, even unknown, factors. Not surprisingly, fatigue has effects on cognitive performance and functional abilities that are difficult to separate from cognitive impairment due to a neurodegenerative disorder.
The most obvious initial step for accommodating frailty and fatigue is to shorten neuropsychological and other tests given to the oldest-old. For neuropsychological testing in The 90+ Study, we shortened versions of several common tests and omitted some tests that were considered to be too tiring for the participants. The battery can be completed in approximately forty-five minutes (for a detailed explanation of the battery, see (3)). However, even though the utmost care has been taken to design the battery to be as short as possible while still collecting the needed data, approximately 20% of people still omit at least one test because of fatigue (3). Another strategy for acquiring the necessary data from participants who are frail or fatigued is to complete the testing in multiple short visits. This strategy can cause difficulties, however, as the individual’s cognition and health can change between visits, or they may be unable to schedule additional assessments. In the end, flexibility is crucial and the limitations of each participant or patient must be taken into consideration.
3.6 Cultural and Environmental Expectations
Key to the diagnosis of the clinical syndrome of dementia is report from family, friends or co-workers who document a loss of cognitive or functional abilities. An informant is so crucial for obtaining information that most dementia research includes a designated informant for additional data gathering. “He’s good for his age” is a red flag when obtaining information about an oldest old individual. Social and cultural expectations contribute to age adjustments in our expectations and evaluations of abilities. Individuals 90 or 100 years old who are reported by family “to have a memory that is as sharp as a tack” may remember things well from long ago but not be able to recall the morning breakfast conversation or names of grandchildren. Repetitiveness and other signs of forgetfulness may be regarded as endearing. Finally, expectations for occupational and social functioning are typically modest, and may be further attenuated by medical illnesses, pain, sensory losses, or loss of mobility. In The 90+ Study structured phone interviews were conducted with informants multiple times during the length of the study. During these interviews, trained interviewers probed extensively regarding the participants’ cognitive and functional abilities to try to elucidate whether diminished expectations are in fact due to cognitive losses.
4. Challenges to Determining Dementia Etiology
Once the clinical syndrome of dementia is diagnosed in an oldest old individual, determination of the dementia etiology is the next challenge. Multiple studies have shown that the correlation between neuropathology, particularly AD neuropathology, and clinical dementia declines with age (33). In part, the loss of association reflects increasing prevalence of cerebral pathologies in control subjects as they age (34–35). Interestingly, approximately half of clinically demented oldest-old have insufficient neuropathology to account for their dementia (36–37) while approximately half of the individuals without dementia meet the neuropathological criteria for AD (35, 37–38). Results from The 90+ Study confirmed these investigations. Approximately half of all non-demented participants (49%) and just over half of clinically demented participants (57%) met pathological criteria for AD. Other types of neuropathology (hippocampal sclerosis, vascular dementia, and diffuse Lewy body disease) accounted for some of the demented participants without AD, but about a quarter (22%) did not have enough neuropathology of any kind to explain their cognitive loss (39). These studies all suggest a poor relationship between clinical dementia and neuropathological observations in the oldest-old(40–41).
In contrast, a recent study from the Baltimore Longitudinal Study of Aging with over 200 autopsies (approximately half were non-demented) found that plaques and tangles, hallmark pathologies of AD, were significant predictors of dementia independent of age (42). This study also noted that intracranial atherosclerosis predicted dementia in people with low levels of AD neuropathology. Vascular disease may promote the expression of AD or be simply additive. Multiple pathologies are more likely to co-exist in the oldest old. A study of more than 1,100 autopsies of demented patients found that mixed AD and vascular dementia was the most common pathological finding in the oldest-old (43).
Complicating the etiological diagnosis of dementia, and possibly related to it, is delirium. Delirium can result in poor cognitive performance that may or may not be related to a person’s actual level of cognition when not acutely ill. Symptoms suggesting delirium accompany the high number of medical illnesses and medications in this group, but can also be symptomatic of Lewy Body dementia, where individuals may have fluctuating levels of attention and cognitive performance (20).
5. Conclusion
The oldest old are the fastest growing segment of the population and have the highest rates of dementia. The diagnosis of dementia and AD in this age group is complicated by sensory losses, medical co-morbidities, medication use, frailty and fatigue. Subclinical delirium is probably more common than recognized. To overcome these challenges, modifications to test materials, flexibility and allowances in procedures, and obtaining information from a variety of sources are all necessary to adequately study dementia in the oldest old. At present, the best evidence suggests that the etiology of dementia in the oldest-old is complex, and likely to be multifactorial more often than in younger elderly. Alzheimer pathologies, vascular disease and other pathologies may all contribute to the expression of dementia in this age group. More clinical pathological research in large samples of population-based oldest-old will be necessary to better understand the causes of dementia in this extreme age group.
Acknowledgements
This study was supported by the National Institute on Aging, Grant R01-AG21055; R01-AG21055-2S1; The Hillblom Foundation Fellowship Grant 2008-A-008-FEL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carrie Brumback-Peltz, Email: cpeltz@uci.edu.
Archana B. Balasubramanian, Email: archanab@uci.edu.
María M. Corrada, Email: mcorrada@uci.edu.
Claudia H. Kawas, Email: ckawas@uci.edu.
References
- 1.Kawas CH, Corrada MM. Alzheimer's and dementia in the oldest-old: a century of challenges. Curr Alzheimer Res. 2006;3(5):411–419. doi: 10.2174/156720506779025233. 12/2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whittle C, Corrada MM, Dick M, Ziegler R, Kahle-Wrobleski K, Paganini-Hill A, et al. Neuropsychological data in nondemented oldest-old: The 90+ Study. J Clin Exp Neuropsychol. 2007;29(3):290–299. doi: 10.1080/13803390600678038. 4/2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71(5):337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. 7/2008. [DOI] [PubMed] [Google Scholar]
- 5.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest-old: The 90+ Study. Ann Neurol. 2010 doi: 10.1002/ana.21915. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010 Jan;67(1):114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrada M, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas C. Prevalence of dementia after age 90: Results from The 90+ Study. Neurology. 2008 doi: 10.1212/01.wnl.0000310773.65918.cd. In Press. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 9.Kahle-Wrobleski K, Corrada M, Kawas C. Dementia and cognition in the oldest-old. In: Miller BL, Boeve BF, editors. The Behavioral Neurology of Dementia. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 10.Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998 Nov 1;148(9):879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- 11.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004 Apr;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 12.Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989 Apr 7;261(13):1916–1919. [PubMed] [Google Scholar]
- 13.Lin MY, Gutierrez PR, Stone KL, Yaffe K, Ensrud KE, Fink HA, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004 Dec;52(12):1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2nd ed. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 16.Berlau DJ, Corrada MM, Peltz CB, Kawas CH. Disability in the Oldest-Old: Incidence and Risk Factors in The 90+ Study. Am J Geriatr Psychiatry. 2011 doi: 10.1097/JGP.0b013e31820d9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen-Ranberg K, Schroll M, Jeune B. Healthy centenarians do not exist, but autonomous centenarians do: a population-based study of morbidity among Danish centenarians. J Am Geriatr Soc. 2001 Jul;49(7):900–908. doi: 10.1046/j.1532-5415.2001.49180.x. [DOI] [PubMed] [Google Scholar]
- 18.Parker MG, Ahacic K, Thorslund M. Health changes among Swedish oldest old: prevalence rates from 1992 and 2002 show increasing health problems. J Gerontol A Biol Sci Med Sci. 2005 Oct;60(10):1351–1355. doi: 10.1093/gerona/60.10.1351. [DOI] [PubMed] [Google Scholar]
- 19.Linjakumpu T, Hartikainen S, Klaukka T, Veijola J, Kivela SL, Isoaho R. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol. 2002 Aug;55(8):809–817. doi: 10.1016/s0895-4356(02)00411-0. [DOI] [PubMed] [Google Scholar]
- 20.Rahkonen T, Eloniemi-Sulkava U, Halonen P, Verkkoniemi A, Niinisto L, Notkola IL, et al. Delirium in the non-demented oldest old in the general population: risk factors and prognosis. Int J Geriatr Psychiatry. 2001;16(4):415–421. doi: 10.1002/gps.356. [DOI] [PubMed] [Google Scholar]
- 21.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests' norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. Clin Neuropsychol. 1996;10(3):262–278. [Google Scholar]
- 22.Kozora E, Cullum CM. Generative naming in normal aging: Total output of qualitative changes using phonemic and semantic constraints. Clin Neuropsychol. 1995;9(4):313–320. [Google Scholar]
- 23.Heaton RK, Grant I, Matthews C. Comprehensive norms for an expanded Halstead-Reitan Neuropsychological Battery: Demographic corrections, research findings, and clinical applications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 24.Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation - Harcourt Brace & Company; 1997. [Google Scholar]
- 25.Paolo AM, Troster AI, Ryan JJ. California Verbal Learning Test: normative data for the elderly. J Clin Exp Neuropsychol. 1997 Apr;19(2):220–234. doi: 10.1080/01688639708403853. [DOI] [PubMed] [Google Scholar]
- 26.Beeri MS, Schmeidler J, Sano M, Wang J, Lally R, Grossman H, et al. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology. 2006 Sep 26;67(6):1006–1010. doi: 10.1212/01.wnl.0000237548.15734.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarit SH, Johansson B, Berg S. Functional Impairment and Co-Disability in the Oldest Old: A Multidimensional Approach. J Aging Health. 1993;5(3):291–305. [Google Scholar]
- 28.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 29.Berlau DJ, Corrada MM, Kawas C. The prevalence of disability in the oldest-old is high and continues to increase with age: findings from The 90+ Study. Int J Geriatr Psychiatry. 2009 doi: 10.1002/gps.2248. 3/3/2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Studenski S, Hayes RP, Leibowitz RQ, Bode R, Lavery L, Walston J, et al. Clinical Global Impression of Change in Physical Frailty: development of a measure based on clinical judgment. J Am Geriatr Soc. 2004 Sep;52(9):1560–1566. doi: 10.1111/j.1532-5415.2004.52423.x. [DOI] [PubMed] [Google Scholar]
- 31.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004 Mar;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 32.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 33.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009 May 28;360(22):2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 34.Imhof A, Kovari E, von Gunten A, Gold G, Rivara CB, Herrmann FR, et al. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci. 2007 Jun 15;257(1–2):72–79. doi: 10.1016/j.jns.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 35.Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, et al. Clinicopathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology. 1988;38(11):1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 36.Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of "dementia of unknown etiology" increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000 May;57(5):713–719. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- 37.Polvikoski T, Sulkava R, Myllykangas L, Notkola IL, Niinisto L, Verkkoniemi A, et al. Prevalence of Alzheimer's disease in very elderly people: a prospective neuropathological study. Neurology. 2001 Jun 26;56(12):1690–1696. doi: 10.1212/wnl.56.12.1690. [DOI] [PubMed] [Google Scholar]
- 38.Katzman R, Terry RD, DeTeresa R, Brown T, Davies P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 39.Corrada MM, Berlau DJ, Kawas CH. A Population-Based Clinicopathological Study in the Oldest-Old: The 90+ Study. Curr Alzheimer Res. doi: 10.2174/156720512801322537. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72(9):829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. 3/4/2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berlau DJ, Kahle-Wrobleski K, Head E, Goodus M, Kim R, Kawas C. Dissociation of neuropathologic findings and cognition: case report of an apolipoprotein E epsilon2/epsilon2 genotype. Arch Neurol. 2007 Aug;64(8):1193–1196. doi: 10.1001/archneur.64.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O'Brien RJ. Age, Alzheimer's disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010 Aug;133(Pt 8):2225–2231. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010 Apr;119(4):421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- 44.Fichter MM, Schroppel H, Meller I. Incidence of dementia in a Munich community sample of the oldest old. Eur Arch Psychiatry Clin Neurosci. 1996;246(6):320–328. doi: 10.1007/BF02189026. [DOI] [PubMed] [Google Scholar]
- 45.Waite LM, Broe GA, Grayson DA, Creasey H. The incidence of dementia in an Australian community population: the Sydney Older Persons Study. Int J Geriatr Psychiatry. 2001 Jul;16(7):680–689. doi: 10.1002/gps.404. [DOI] [PubMed] [Google Scholar]
- 46.Riedel-Heller SG, Busse A, Aurich C, Matschinger H, Angermeyer MC. Incidence of dementia according to DSM-III-R and ICD-10: results of the Leipzig Longitudinal Study of the Aged (LEILA75+), Part 2. British Journal of Psychiatry. 2001;179:255–260. doi: 10.1192/bjp.179.3.255. [DOI] [PubMed] [Google Scholar]
- 47.The incidence of dementia in Canada. The Canadian Study of Health and Aging Working Group. Neurology. 2000;55(1):66–73. [PubMed] [Google Scholar]
- 48.Hall CB, Verghese J, Sliwinski M, Chen Z, Katz M, Derby C, et al. Dementia incidence may increase more slowly after age 90: results from the Bronx Aging Study. Neurology. 2005 Sep 27;65(6):882–886. doi: 10.1212/01.wnl.0000176053.98907.3f. [DOI] [PubMed] [Google Scholar]
- 49.Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women. The Cache County Study. Neurology. 2002;58(2):209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 50.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, et al. Dementia and Alzheimer disease incidence: A prospective cohort study. Archives of Neurology. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 51.Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: the MoVIES Project. Neurology. 2000;54(5):1109–1116. doi: 10.1212/wnl.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 52.Letenneur L, Commenges D, Dartigues JF, Barberger-Gateau P. Incidence of dementia and Alzheimer's disease in elderly community residents of south-western France. International Journal of Epidemiology. 1994;23(6):1256–1261. doi: 10.1093/ije/23.6.1256. [DOI] [PubMed] [Google Scholar]
- 53.Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer's disease: incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997 Jan;48(1):132–138. doi: 10.1212/wnl.48.1.132. [DOI] [PubMed] [Google Scholar]
- 54.Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Archives of Neurology. 2002;59(10):1589–1593. doi: 10.1001/archneur.59.10.1589. [DOI] [PubMed] [Google Scholar]
- 55.Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MM. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22(4):575–580. doi: 10.1016/s0197-4580(01)00231-7. [DOI] [PubMed] [Google Scholar]
- 56.Nitrini R, Caramelli P, Herrera E, Jr, Bahia VS, Caixeta LF, Radanovic M, et al. Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2004 Oct-Dec;18(4):241–246. [PubMed] [Google Scholar]


