Abstract
OBJECTIVE
To prospectively investigate whether baseline findings on specific cardiovascular indices are predictive of subsequent rate of decline in Attention-Executive-Psychomotor function in a cohort of ambulatory older adults with cardiovascular diseases (CVDs).
METHODS
One hundred seventy-two older adults with CVD were administered a neuropsychological battery of executive functions tests at study entry, and at 12 and 36 months thereafter. At study entry, they also underwent vascular assessments including cardiac output (CO), ejection fraction, blood pressure (BP), brachial artery reactivity, and carotid intima media thickness. Random coefficient regressions were used to investigate the effect of these cardiac indices on rate of decline in Attention-Executive-Psychomotor function.
RESULTS
Cardiac output, systolic BP variability, and diastolic BP variability predicted decline in Attention-Executive-Psychomotor function. Specifically, lower CO, reduced variability in systolic BP, and increased variability in diastolic BP were associated with a faster rate of decline in Attention-Executive-Psychomotor function. Mean resting systolic and diastolic blood pressure did not predict decline in Attention-Executive-Psychomotor function.
CONCLUSION
Decline in frontal-subcortical cognitive functions among patients with CVDs appears to be mediated by systemic hypoperfusion and variability in blood pressure. The precise nature of these relationships, especially with regard to blood pressure variability, is complex and demands continued investigation.
Key words or phrases: Cardiovascular disease, cognition, executive function, cardiac output, systolic variability
Cardiovascular diseases (CVDs) affect more than 1 in 3 American adults, with incidence and prevalence increasing dramatically with advancing age.1 Health statistics going back to the start of the last century consistently show that CVDs are the principal cause of morbidity and mortality in the United States.2 Similar data from around the world suggest that CVDs represent an escalating public health problem in both industrialized and developing nations.3
Besides the obvious effects on physical health, there is now overwhelming evidence that chronic CVD impairs neurocognitive function even when clinically-manifest stroke is not evident,4 and that these impairments affect quality of life and functional status even when the severity does not meet criteria for a diagnosis of dementia.5,6 The characteristic constellation of CVD-associated neurocognitive disturbances include deficits in attention, executive functions, psychomotor speed, and information processing, particularly early in the disease process.4,7
Studies by our group and others have focused on delineating the pathophysiological mechanisms by which chronic CVD affects cognitive function. Two general factors, cardiac and systemic vascular dysfunction, were shown to underlie neurocognitive deficits among people with CVD.8 With respect to cardiac dysfunction, both cardiac output (CO)9 and ejection fraction (EF)10, 11 were associated with executive functioning and sustained attention. Variability in systolic blood pressure (BP),8,12 a putative indicator of cardiac reactivity, was also shown to be associated with cognitive performance. Among systemic vascular indices, correlates of cognitive function have included brachial artery reactivity (BAR)8 and carotid artery intima media thickness (IMT).13 Relationships were also found between reduced cerebral blood flow,14 the extent of white matter lesions on MRI15 and neurocognitive dysfunction. Taken together, these findings suggest linkages between the cardiac and systemic vascular abnormalities seen in CVD and the development of cerebral and cognitive dysfunction.
However, a review of the literature reveals that existing studies of the potential pathophysiological bases of cognitive disturbance in CVD have largely been cross-sectional. Perhaps, more importantly, the majority of these studies have examined these mechanisms in isolation as though each operated independently of the others. As a result, despite the extensive body of work in this field, the unique effect of these pathophysiological mechanisms on cognitive decline in CVD remains largely unknown.
In this study, we attempted to address this crucial knowledge gap by simultaneously investigating the effect of several important cardiac indices on the rate of decline in Attention-Executive-Psychomotor function in a cohort of ambulatory older adults with CVD. Our primary aim was to estimate the unique contribution that each index makes to the estimation of decline in Attention-Executive-Psychomotor function over the 3-year duration of the study.
METHODS
The sample consisted of 172 community-dwelling individuals enrolled in a prospective study examining cognitive function among older adults with CVD. They were recruited from local hospitals, cardiac rehabilitation programs, cardiology practices, and by advertisement. To be enrolled in the study, participants had to be between 55 to 85 years old, and have an established history of CVD including myocardial infarction, cardiac surgery, heart failure, atrial fibrillation, and hypertension. Exclusion criteria included history of neurological diseases (eg, dementia, seizures, stroke, traumatic brain injury with loss of consciousness exceeding 10 minutes), end-stage heart disease, or major psychiatric illness (eg, bipolar disorder, schizophrenia). Of note, all participants were receiving ongoing treatment by a cardiologist. Study visits occurred at baseline, and at 12 and 36 months thereafter. Written informed consent was obtained from all participants as part of this institutional review board-approved study. Table 1 details the characteristics of participants at baseline.
Table 1.
Characteristics of participants at baseline
| Characteristic | Value |
|---|---|
| Demographic | |
| Age, M (SD) | 69.18 (7.61) |
| Female, % | 38.0 |
| Ethnic minority, % | 7.6 |
| Years of education, M (SD) | 14.29 (2.74) |
| BDI-II, M (SD) | 5.34 (4.22) |
| MMSE, M (SD) | 28.47 (1.67) |
| DRS-2 Total score, M (SD) | 137.27 (5.27) |
| Vascular Indices, M (SD) | |
| Cardiac output, L/m | 4.55 (1.05) |
| Ejection fraction | .59 (.12) |
| Mean systolic blood pressure, mmHg | 129.52 (19.69) |
| Systolic blood pressure variability, mmHg | 8.31 (5.73) |
| Mean diastolic blood pressure, mmHg | 67.21 (9.94) |
| Diastolic blood pressure variability, mmHg | 5.83 (3.19) |
| IMT, mm | .88 (.13) |
| BAR-reactive hyperemia, % change | 5.93 (5.87) |
| Medical history, % | |
| Hypertension | 72.9 |
| Type 2 diabetes | 21.5 |
| Hypercholesterolemia | 41.4 |
| Coronary artery disease | 31.6 |
| Atrial fibrillation | 16.9 |
| Heart failure | 20.7 |
| Myocardial infarction | 45.9 |
| Heart valve surgery | 12.7 |
| Coronary artery bypass graft | 37.0 |
Abbreviations: BDI-II, Beck Depression Inventory II; MMSE, m Mini Mental Status Examination; DRS-2, Dementia Rating Scale, 2nd edition; IMT, carotid intima media thickness; BAR, brachial artery response.
Neuropsychological assessment
In addition to screening measures of global cognition (ie, Mini-Mental Status Examination (MMSE) and Dementia Rating Scale, 2nd edition [DRS-2]), participants completed several neuropsychological measures that tap abilities presumably mediated by frontal-subcortical systems.8,9,16. These included the Trail Making Test (parts A and B), the Letter Cancellation test, the Stroop test, the Controlled Oral Word Association (COWA) test, the Grooved Pegboard test, the Digit Span subtest of the Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III), and the Digit Symbol subtest of the WAIS-III. All tests were administered and scored by trained technicians, using standardized procedures and under the supervision of a licensed clinical neuropsychologist. Participant test scores at baseline are summarized in Table 2.
Table 2.
Neuropsychological test scores at baseline
| Test | M (SD) |
|---|---|
| Trails A, time | 39.01 (12.92) |
| Trails B, time | 100.96 (52.99) |
| Letter search, time | 92.86 (25.79) |
| Letter search, errors | 1.56 (2.36) |
| Stroop test, interference trial, time | 30.88 (9.47) |
| COWA, number correct | 40.18 (12.66) |
| Grooved pegboard, dominant hand, time | 93.91 (25.05) |
| WAIS-III, Digit span, number correct | 17.46 (3.72) |
| WAIS-III, Digit symbol, number correct | 54.94 (14.46) |
Abbreviation: COWA, Controlled Oral Word Association test; WAIS-III, Wechsler Adult Intelligence Scale, 3rd edition.
Cardiovascular assessment
Participants were asked to refrain from taking vasoactive medications (e.g., calcium channel blockers, ACE inhibitors, and beta-blockers), drinking caffeinated beverages, exercising, and smoking for 6 hours before the vascular assessment. Furthermore, all participants fasted for 6 hours prior to their cardiac assessment. Prior to the assessment, participants remained supine for 15 minutes in a quiet room.
Echocardiogram
A complete, transthoracic echocardiogram was obtained from each participant according to guidelines set forth by the American Society of Echocardiography. From the echocardiogram, we derived 2 cardiac indices: CO and EF. Stroke volume was calculated as the mean velocity of blood flow leaving the left ventricle multiplied by the area of left ventricle outflow tract measured from the 2D echo image (ie, CO = (TVI × CSA)×HR, where TVI = time velocity integral, CSA = cross-sectional area, and HR = heart rate). EF was calculated based upon biplane volumes using the formula, EF = (EDV – ESV)÷EDV, where EDV = end-diastolic volume and ESV = end-systolic volume. The biplane method of computing EF is independent of 5 preconceived ventricular shape and is less sensitive to geometric distortions. Therefore, it is the recommended approach for patients with coronary artery disease and regional wall motion abnormalities.
Blood pressure
Using an automatic monitor (Press-Mate 8800, Colin Medical Instruments Corp, San Antonio TX), BP was measured in the left arm at 10-minute intervals for 2 hours. This approach minimizes the error associated with a single BP reading without the higher cost of a 24-hour monitoring. Several BP indices were generated (for both systolic BP and diastolic BP), including mean resting pressure and variability in resting pressure across the serial measurements. For the analyses reported here, we only used the indices of variability because prior studies from our group and other groups17–20 have shown that they are likely more closely related to cognition and cerebrovascular abnormalities than mean resting pressure.
Carotid artery intima media thickness
High-resolution B-mode carotid ultrasonography was performed using a 7.5 mHz transducer and an Agilent 5500 machine (Agilent, Andover, MA). Intima media thickness (IMT) was calculated using scans of the far wall of the left common carotid artery approximately 1 cm proximal to the carotid bulb. IMT was defined as the distance between the luminal-endothelial interface and the junction between the media and the adventitia. Automated, objective, edge-detection software was developed to measure IMT thickness based on a validated technique.21
Flow-mediated brachial artery reactivity
High-frequency B-mode ultrasound was used to visualize the brachial vessel. A Hewlett Packard 5500 ultrasound system, equipped with a 7.5 MHz linear array vascular transducer was used to acquire 2D and Doppler flows of each participant’s left arm. Images were obtained in longitudinal orientation approximately 5 cm above the antecubital fossa. Straight segments ≥0 mm were targeted for optimal assessments. Blood pressure was measured with an automated Datascope accutor 3SAT (Paramus, NJ) in the contralateral arm.
To assess flow-mediated brachial artery reactivity (BAR), first, baseline images of brachial artery diameter and blood flow velocity were recorded for 1minute (sequential images, captured and digitized on each R-wave). Then, a 4 cm cuff positioned on the mid-forearm was inflated to 40 mg above the baseline systolic blood pressure for 5 minutes so that mechanical ischemia was induced. Thereafter, the cuff was deflated and the same brachial segment was interrogated for 3 minutes, the period of hyperemic flow. Flow-mediated vasodilation was calculated as the percentage change in arterial diameter from baseline to the maximum diameter induced by reactive hyperemia. A blinded investigator analyzed the images using a validated software that automatically calculates the average diameter over the selected segment.22
Data analyses
Participant scores on each of the neuropsychological measures were z-transformed (using the cohort’s means and standard deviations at respective time points), summed, and averaged to create a composite variable representing Attention-Executive-Psychomotor function. To ensure that higher scores on the composite indicated better performance, z scores for neuropsychological tests whose unit of measurement is completion time (eg, Trail Making Test) were multiplied by -1 prior to the creation of the Attention-Executive-Psychomotor composite, to correct for the fact that shorter completion time reflects better performance. A similar procedure has been used in prior studies.23, 24
We used random coefficient regression to examine the potential effects of our cardiac indices on rate of change in Attention-Executive-Psychomotor function. Also known as individual growth curve modeling, random coefficient regression provides a flexible approach to analyzing longitudinal data. It easily accommodates variability in the number and spacing of assessment points across study participants.23,25 In addition, because it assumes that each individual’s observed scores are a random sample of data from their underlying true growth curve, it facilitates analyses that include all participants, even those with only one wave of data.25
Our regression model included terms for age, baseline score on the Attention-Executive-Psychomotor composite, year, the cardiac indices, and interactions between each cardiac index and year. The primary terms of interest were the interactions between the cardiac indices and year because they would indicate whether rate of change in Attention-Executive-Psychomotor function varied as a function of the cardiac indices. All analyses were performed using SPSS 16.0 (SPSS Inc, Chicago, IL). Only findings with a two-tailed P value ≤ .05 were considered significant.
RESULTS
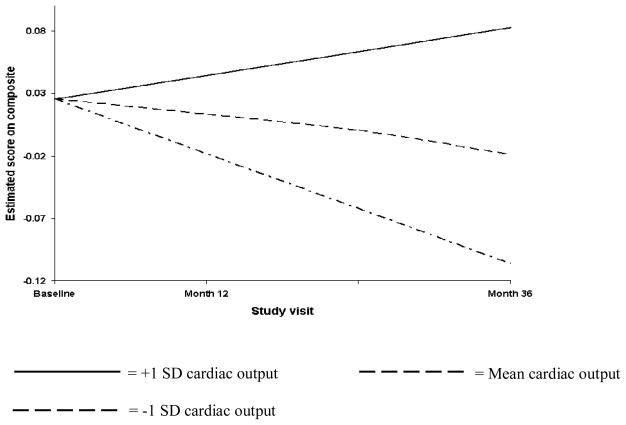
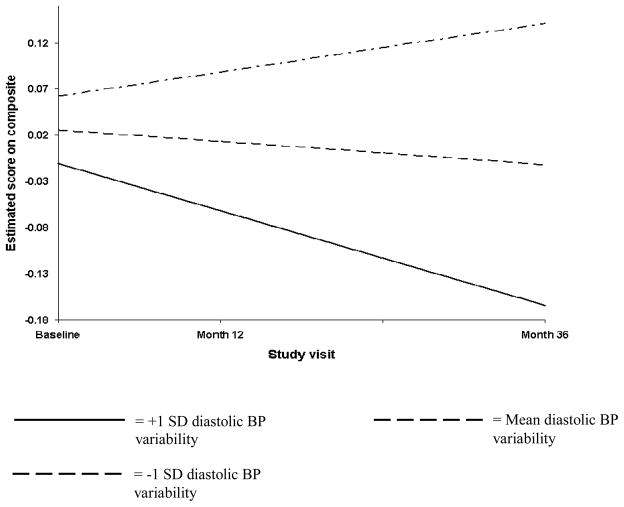
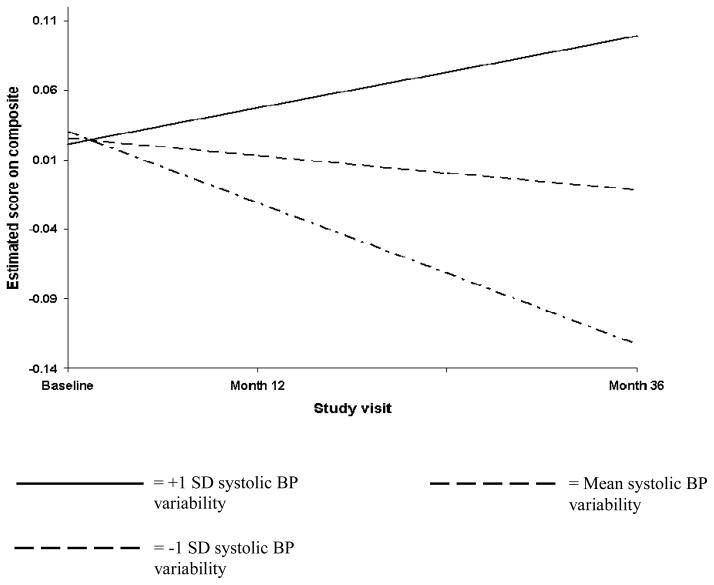
Table 3 details the result of the random coefficient regression that examined the effect of the cardiac indices on rate of change in Attention-Executive-Psychomotor function. The only indices that significantly predicted decline in Attention-Executive-Psychomotor function were CO, systolic BP variability, and diastolic BP variability. Specifically, we found that lower CO was associated with a faster rate of decline in Attention-Executive-Psychomotor function; that reduced variability in systolic BP was similarly associated with a faster rate of decline in Attention-Executive-Psychomotor function; and that increased variability in diastolic BP was associated with a faster rate of decline in Attention-Executive-Psychomotor function. These findings are respectively illustrated in Figures 1–3. The figures depict the Attention-Executive-Psychomotor function growth trajectories for 3 prototypical groups of participants: those with the mean score on the cardiac index (ie, CO, systolic BP variability, and diastolic BP variability respectively), those with scores that are 1 SD below the mean on the cardiac index, and those with scores that are 1 SD above the mean on the cardiac index.
Table 3.
Effect of vascular indices on rate of change in Attention-Executive-Psychomotor function.
| Term | Estimate | S.E. | Pvalue |
|---|---|---|---|
| Time | −.14 | .11 | .212 |
| Cardiac outputa | −.00 | .02 | .991 |
| Ejection fractiona | .15 | .18 | .426 |
| SBP variabilitya | −.00 | .01 | .887 |
| DBP variabilitya | −.01 | .01 | .162 |
| IMTa | −.18 | .17 | .311 |
| BAR-reactive hyperemiaa | −.00 | .00 | .465 |
| Cardiac output x yearb | .03 | .01 | .012 |
| Ejection fraction x yearb | −.17 | .11 | .122 |
| SBP variability x yearb | .01 | .00 | .031 |
| DBP variability x yearb | −.01 | .01 | .021 |
| IMT x yearb | .13 | .10 | .210 |
| BAR-reactive hyperemia x yearb | −.00 | .00 | .570 |
The analysis adjusted for age and baseline score on the Attention-Executive-Psychomotor composite.
Abbreviation: SBP, systolic blood pressure; DBP, diastolic blood pressure; IMT, carotid intima media thickness; BAR, brachial artery response.
Time represents the mean estimated rate of change in Attention-Executive-Psychomotor function in the cohort, when all vascular indices are set to zero.
Represents the estimated difference in baseline scores on Attention-Executive-Psychomotor function for each unit increase in the cardiovascular index, adjusted for the other vascular indices.
Represents the estimated difference in rate of change on Attention-Executive-Psychomotor function for each unit increase in the vascular index, adjusted for the other vascular indices.
Figure 1.
Lower cardiac output is associated with faster rate of decline in Attention-Executive-Psychomotor function.
Figure 3.
Increased variability in diastolic blood pressure is associated with faster rate of decline in Attention-Executive-Psychomotor function.
Although we chose to only include measures of BP variability (as opposed to mean BP) in our analyses based on past findings from our group and other groups,8,12,17,20, we acknowledge that there remains considerable interest in mean resting BP as a predictor of cognitive change. For this reason, we repeated the analyses reported in Table 3 while including mean resting BP (systolic and diastolic) in the model. Mean systolic BP and mean diastolic BP were not significantly associated with decline in Attention-Executive-Psychomotor function in the refitted model (P values of .890 and .637 respectively). In addition, the refitted model, assessed using −2 log likelihood, the Akaike information criteria, and the Bayesian information criteria, had a worse fit compared to the original model fitted with only the measures of variability.
DISCUSSION
Although a variety of pathophysiological mechanisms have been postulated to account for the impact of CVD on cognitive function, investigations of these mechanisms have largely been cross-sectional and focused on individual mechanisms in isolation. Therefore, in this study, we undertook a concurrent examination of the contributions of several cardiac indices to longitudinal change in Attention-Executive-Psychomotor function among a cohort of geriatric patients with CVD. We found that lower CO, reduced variability in systolic BP, and increased variability in diastolic BP were the only predictors of prospective decline in Attention-Executive-Psychomotor function.
Our finding that patients with lower CO experienced a faster rate of decline in Attention-Executive-Psychomotor function than those with higher CO is consistent with prior cross-sectional studies that showed that circulatory insufficiency is associated with poorer cognitive function. For example, in a previous cross-sectional study, we found that ambulatory CVD patients with low CO exhibited selective impairments in cognitive measures of processing speed, cognitive flexibility, and problem solving.,9 It is possible that systemic hypoperfusion affects cognitive function because it engenders inadequate cerebral perfusion, cerebral hypoxia, and the disruption of vital white matter tracts.26–29 Indeed, there is indication that subcortical brain structures are particularly vulnerable to systemic and cerebral hypoperfusion, hence the characteristic “subcortical” cognitive profile seen in vascular disorders.30
Historically, studies of the relationship between BP and cognitive function in CVD have focused on mean BP perhaps because of the known association between hypertension and impaired cognition.31 However, there is growing evidence that while mean resting BP is an important factor, failure to consider other BP indices such as variability is a critical knowledge gap.17 Rothwell et al12 showed that measures of variability in systolic BP were strong predictors of stroke in large epidemiological cohorts independent of mean systolic BP, which was itself a weak predictor. Other studies by our research group have also shown that greater variability in systolic BP is associated with better cognitive function.8,20. Although the finding of an association between reduced variability in systolic BP and decline in Attention-Executive-Psychomotor function might appear counterintuitive, it has plausible conceptual underpinnings.
There is an extensive literature suggesting that the capacity for autonomic reactivity is conducive to brain function. For instance, Cohen and Waters32 demonstrated that heart rate and skin conductance response during a levels-of-processing memory task was associated with the allocation of attentional effort during the tasks and also subsequent incidental recall. Other studies have shown that greater heart rate variability is indicative of greater vagal tone and is associated with better performance on measures of attention and working memory.33,34 Similar findings have been reported using measures of pupil dilation and skin conductance.35,36 Other studies have shown that older adults with restricted diurnal variation in BP exhibit cognitive deficits.37,38 Taken together, these findings suggest that variability in systolic BP might be beneficial, just as brachial artery reactivity to mechanical ischemia indicates healthy vascular endothelium. It is noteworthy that Rothwell et al12 showed that increased systolic BP variability was deleterious whereas we found it to be protective. Although the reasons for this discrepancy are not entirely clear, a few possibilities exist. For instance, their cohort was composed of hypertensive patients with a history of transient ischemic attack (TIA) or stroke, but no history of coronary artery disease. In contrast, our cohort consisted of older adults with a heterogeneous mix of CVD, including coronary artery disease. Furthermore, we explicitly excluded persons with history of stroke or TIA. It is plausible that the effect of systolic BP variability varies across patient populations, and outcome measures (eg, cognition, stroke, white matter lesions).12,19,20
In contrast to our finding with respect to variability in systolic BP, we found that increased variability in diastolic BP was predictive of decline in Attention-Executive-Psychomotor function. To our knowledge, this is the first time such a finding has been reported. While interesting, and perhaps in line with those studies that suggest that higher variability in BP is detrimental,12 we do not fully understand this finding. Nor is it clear why the effect of variability in diastolic BP is in the reverse direction of the effect of variability in systolic BP. Clearly, there is need for additional well-designed studies in this area.
It is worth noting that the participants in our study were all under the active care of a cardiologist, especially with respect to hypertension. It is plausible that a different pattern of findings might have emerged in a cohort with poorly controlled CVD. In addition, as already noted, our cohort consisted of a heterogeneous mix of older adults with various CVD. Although this makes our study more ecological valid (and, thus, potentially more generalizable) as cardiac patients rarely have isolated vascular diseases, it also limits the ability to understand how the effects of the cardiac indices we studied might differ across specific patient populations.
In summary, this prospective study examined the multivariable effects of several pathophysiological mechanisms on rate of decline in Attention-Executive-Psychomotor function among a cohort of ambulatory geriatric cardiac patients. We found that lower CO, reduced systolic BP variability, and increased diastolic BP variability at baseline were significant predictors of decline in Attention-Executive-Psychomotor function, pointing to the potential importance of cardiac function and systemic perfusion as determinants of CVD-associated cognitive dysfunction. Further studies would be needed to elucidate certain aspects of our findings, especially with respect to variability in diastolic BP.
Figure 2.
Reduced variability in systolic blood pressure is associated with faster rate of decline in Attention-Executive-Psychomotor function.
Acknowledgments
Funding: This research was supported by grant R01 AG017975 from the National Institute on Aging (Cohen, PI).
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. [Accessed September 3, 2010];Health Data Interactive. 2010 Available at www.cdc.gov/nchs/hdi.htm.
- 3. [Accessed September 3, 2010];The global burden of disease: 2004 update. 2004 Available at www.who.int/healthinfo/global_burden_disease/2004_report_update.
- 4.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurology. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 5.Leblanc GG, Meschia JF, Stuss DT, Hachinski V. Genetics of vascular cognitive impairment: The opportunity and the challenges. Stroke. 2006;37:248–255. doi: 10.1161/01.STR.0000195177.61184.49. [DOI] [PubMed] [Google Scholar]
- 6.Cohen RA, Moser DJ, Clark MM, et al. Neurocognitive functioning and improvement in quality of life following participation in cardiac rehabilitation. Am J Cardiol. 1999;83:1374–1378. doi: 10.1016/s0002-9149(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 7.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 8.Cohen RA, Poppas A, Forman DE, et al. Vascular and cognitive functions associated with cardiovascular disease in the elderly. J Clinical Experimental Neuropsychol. 2008;31:96–110. doi: 10.1080/13803390802014594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottesman RF, Grega MA, Bailey MM, et al. Association between hypotension, low ejection fraction and cognitive performance in cardiac patients. Behavioural Neurol. 2009;22:63–71. doi: 10.3233/BEN-2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerskey BA, Cohen RA, Jefferson AL, et al. Sustained attention is associated with left ventricular ejection fraction in older adults with heart disease. J Int Neuropsychol Soc. 2009;15:137–141. doi: 10.1017/S1355617708090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 13.Haley AP, Forman DE, Poppas A, et al. Carotid artery intima-media thickness and cognition in cardiovascular disease. Int J Cardiol. 2007;121:148–154. doi: 10.1016/j.ijcard.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitagawa K. Cerebral blood flow measurement by PET in hypertensive subjects as a marker of cognitive decline. J Alzheimer's Dis. 2010;20:855–859. doi: 10.3233/JAD-2010-091324. [DOI] [PubMed] [Google Scholar]
- 15.Tate DF, Jefferson AL, Brickman AM, et al. Regional white matter signal abnormalities and cognitive correlates among geriatric patients with treated cardiovascular disease. Brain Imaging Behavior. 2008;2:200–206. doi: 10.1007/s11682-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoth KF, Haley AP, Gunstad J, et al. Elevated C-reactive protein is related to cognitive decline in older adults with cardiovascular disease. J Am Geriatr Soc. 2008;56:1898–1903. doi: 10.1111/j.1532-5415.2008.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 18.Keary TA, Gunstad J, Poppas A, et al. Blood pressure variability and dementia rating scale performance in older adults with cardiovascular disease. Cognitive Behavioral Neurol. 2007;20:73–77. doi: 10.1097/WNN.0b013e3180335f9f. [DOI] [PubMed] [Google Scholar]
- 19.Gunstad J, Cohen RA, Tate DF, et al. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood Press. 2005;14:353–358. doi: 10.1080/08037050500364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunstad J, Keary TA, Spitznagel MB, et al. Blood pressure and cognitive function in older adults with cardiovascular disease. Int J Neurosci. 2009;119:2228–2242. doi: 10.3109/00207450903139713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadler RW, Karl WC, Lees RS. New methods for arterial diameter measurement from B-mode images. Ultrasound Med Biol. 1996;22:25–34. doi: 10.1016/0301-5629(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi H, Fukuda H, Oyanagi C. Significance of white matter high intensity lesions as a predictor of stroke from arteriolosclerosis. J Neurol Neurosurg Psychiatry. 2002;72:576–582. doi: 10.1136/jnnp.72.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selnes OA, Grega MA, Bailey MM, et al. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63:581–590. doi: 10.1002/ana.21382. [DOI] [PubMed] [Google Scholar]
- 24.Okonkwo OC, Cohen RA, Gunstad J, Tremont G, Alosco ML, Poppas A. Longitudinal trajectories of cognitive decline among older adults with cardiovascular disease. Cerebrovasc Dis. 2010;30:362–373. doi: 10.1159/000319564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer JD, Willett JB. Applied longitudinal data analysis. New York: Oxford University Press; 2003. [Google Scholar]
- 26.Bennett SJ, Sauve MJ, Shaw RM. A conceptual model of cognitive deficits in chronic heart failure. J Nursing Scholarship. 2005;37:222–228. doi: 10.1111/j.1547-5069.2005.00039.x. [DOI] [PubMed] [Google Scholar]
- 27.Roman GC. Brain hypoperfusion: A critical factor in vascular dementia. Neurol Res. 2004;26:454–458. doi: 10.1179/016164104225017686. [DOI] [PubMed] [Google Scholar]
- 28.Jefferson AL, Tate DF, Poppas A, et al. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc. 2007;55:1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maule S, Caserta M, Bertello C, et al. Cognitive decline and low blood pressure: the other side of the coin. Clin Exp Hypertens. 2008 Nov;30:711–719. doi: 10.1080/10641960802573344. [DOI] [PubMed] [Google Scholar]
- 30.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR American Journal of Neuroradiology. 1990 May;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 31.Paglieri C, Bisbocci D, Caserta M, et al. Hypertension and cognitive function. Clin Exp Hypertens. 2008;30:701–710. doi: 10.1080/10641960802563584. [DOI] [PubMed] [Google Scholar]
- 32.Cohen RA, Waters WF. Psychophysiological correlates of levels and states of cognitive processing. Neuropsychologia. 1985;23:243–256. doi: 10.1016/0028-3932(85)90108-3. [DOI] [PubMed] [Google Scholar]
- 33.Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 34.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda K, Stern A, Brown TB, Russo MB. Cognition, blinks, eye-movements, and pupillary movements during performance of a running memory task. Aviat Space Environ Med. 2005;76:C75–85. [PubMed] [Google Scholar]
- 36.Hillman CH, Snook EM, Jerome GJ. Acute cardiovascular exercise and executive control function. Int J Psychophysiol. 2003;48:307–314. doi: 10.1016/s0167-8760(03)00080-1. [DOI] [PubMed] [Google Scholar]
- 37.van Boxtel MP, Henskens LH, Kroon AA, et al. Ambulatory blood pressure, asymptomatic cerebrovascular damage and cognitive function in essential hypertension. J Hum Hypertens. 2006;20:5–13. doi: 10.1038/sj.jhh.1001934. [DOI] [PubMed] [Google Scholar]
- 38.Ohya Y, Ohtsubo T, Tsuchihashi T, et al. Altered diurnal variation of blood pressure in elderly subjects with decreased activity of daily living and impaired cognitive function. Hypertens Res. 2001;24:655–661. doi: 10.1291/hypres.24.655. [DOI] [PubMed] [Google Scholar]