Abstract
Central Pattern Generator (CPG) networks, which organize rhythmic movements, have long served as models for neural network organization. Modulatory inputs are essential components of CPG function: neuromodulators set the parameters of CPG neurons and synapses to render the networks functional. Each modulator acts on the network by many effects which may oppose one another; this may serve to stabilize the modulated state. Neuromodulators also determine the active neuronal composition in the CPG, which varies with state changes such as locomotor speed. The pattern of gene expression which determines the electrophysiological personality of each CPG neuron is also under modulatory control. It is not possible to model the function of neural networks without including the actions of neuromodulators.
Central Pattern Generators (CPGs) are limited neural networks that generate the timing, phasing and intensity cues to drive motoneuron output for simple rhythmic behaviors such as locomotion, mastication and respiration. They have long served as models for understanding neural network function in general, as their output can be directly observed both behaviorally and neurophysiologically. While CPG networks generate relatively simple behaviors, they are capable of considerable flexibility to adapt the behavior to changing environmental demands. Neuromodulatory inputs, which typically activate metabotropic receptors and modify the neuron’s biochemical state, contribute to this behavioral flexibility by modifying the CPG networks on the fly. In this review, I will summarize recent research on the roles of neuromodulators in shaping CPG network function and output. Other aspects of CPG function have been reviewed recently [1-6].
Neuromodulators: Optional or essential?
The traditional view of the role of neuromodulators in CPG networks is that they refine the basic motor pattern that is generated by fast synaptic mechanisms in the network [3]. In an insightful review, Jordan [7*] critiques this assumption, instead arguing that neuromodulatory actions are essential for normal network function. This could result from two separate mechanisms. First, neuromodulatory synapses may be intrinsic to the network itself. The prime example of this is the Tritonia swim CPG, where serotonergic neurons inside the network play a central role in enabling the swim pattern [8]. Given our increasing knowledge of metabotropic glutamate receptor activation at most excitatory synapses [9], intrinsic neuromodulation may be ubiquitous.
Second, modulatory inputs may be essential to set an inactive network in a functional state, which is either spontaneously active or can be rapidly activated by fast synaptic inputs. In the lobster stomatogastric ganglion, removal of modulatory inputs from other ganglia has long been known to abolish rhythmic activity from the 14-neuron pyloric network [10]. This results from the loss of intrinsic oscillatory properties of the prime network pacemakers, as well as changes in synaptic strength that regulate neuronal phasing.
In vertebrates, neuromodulators also appear to play essential roles to enable CPG networks to generate a rhythmic output. In the neonatal rodent spinal cord, serotonin appears to play an important role to enable locomotion [7,11]. Pharmacological blockade of 5HT2 or 5HT7 receptors abolishes brainstem-evoked fictive locomotion [12]. Genetic knockout of the 5HT7 receptor does not prevent spinal rhythm generation, but both inter- and intra-limb coordination are seriously disrupted [13*] In the normal adult mouse, administration of a 5HT7 antagonist severely disrupts treadmill locomotion; interestingly, sufficient homeostatic compensation occurs during development that the adult 5HT7 knockout mouse can again walk normally. Liu et al. [13*] propose that 5HT7 receptors normally regulate the excitability of inhibitory interneurons that coordinate alternation of limbs and intralimb muscles. However, serotonin is not essential for locomotor generation: mice lacking the transcription factor Lmx1b lose all central serotonergic neurons in embryonic life, but survive and can walk apparently normally as adults [14]. Detailed tests of locomotor coordination have not been carried out on these mice. While it is not clear how these animals walk in the absence of serotonin, it is possible that compensatory mechanisms are activated during development so that other modulatory inputs, for example using norepinephrine, dopamine or other transmitters, can help generate locomotion.
Serotonin and other neuromodulators also play critical roles in the neonatal mouse medullary respiratory CPG [15]. In vitro, antagonists of serotonin and/or Substance P receptors abolish the respiratory rhythm [16,17]. Neonatal mice genetically engineered to lack 5HT neurons show severe and prolonged episodes of apnea, and many of them die around birth, but can be rescued by 5HT2A agonists. Animals which survive the perinatal period recover normal respiration by P14 [17]. However, 5HT is not the only modulator to support neonatal respiration; the relative roles of the different modulators change with the state of the system. Doi and Ramirez [18*] studied the roles of endogenously released serotonin, norepinephrine and Substance P in vitro and in vivo. Substance P can accelerate respiratory rate, but only if the respiratory rate is low or there is low 5HT and NE activity. If these are elevated by stimulating the raphe nuclei or locus coeruleus, there is no additional effect of Substance P. Thus, the modulatory effects of these compounds depend critically upon the state of the system.
Opposing actions of a neuromodulator stabilize the modified state of the network
While a neuromodulator may alter a network’s output in a certain direction (for example strengthening the output or accelerating the rhythm), its cellular actions may not all be aimed in that direction, and some of them may be opposing (for example, to weaken the output or slow the rhythm). Given the somewhat non-specific second messenger mechanisms activated by a neuromodulator (ex., increases in cAMP can modify the properties of many proteins), opposing actions are expected, and may play important regulatory roles. Simple CPG networks provide a unique opportunity to study opposing actions of neuromodulators at the level of a single identified neuron or synapse. The cellular, ionic and synaptic mechanisms by which dopamine (DA) reconfigures the 14-neuron pyloric network have been systematically explored [19*]. At a number of sites, dopamine evokes responses that oppose its overall actions on pyloric neurons and synapses (Fig. 1). For example, DA hyperpolarizes and silences one neuron, but simultaneously enhances its hyperpolarization-activated inward current, Ih, which would depolarize the neuron to resume firing. DA increases spike frequency in several neurons but enhances a slowly activating potassium current that would reduce spike frequency. DA can also activate opposing effects at a single synapse. For example, it can enhance transmitter release from the pre-synaptic terminal by increasing calcium currents, but reduce post-synaptic responsiveness to the released transmitter. In the neonatal mouse spinal cord, mGluR1 agonists weaken motoneuron activity during fictive locomotion, and reduce spiking responses to depolarizing steps by reducing the fast sodium current. However, at the same time they depolarize motoneurons by enhancing a nonselective cation current, and hyperpolarize the spike threshold, actions which would enhance motoneuron firing [20*]. Levitan and colleagues showed that serotonin and the peptide egg-laying hormone (ELH) both activate opposing currents in the Aplysia burster neuron R15, which can either slow burst frequency or evoke bistable firing [21]. These actions are activated at different concentrations of the modulators, allowing a frequency-dependent switch in modulator activity regulating R15 bursting.
Figure 1. Positive and negative effects of neuromodulators in a CPG network.
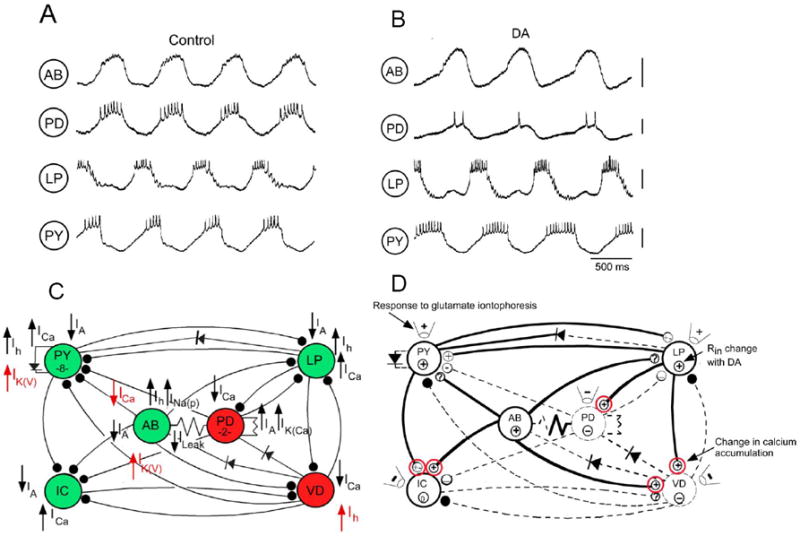
A: Simultaneous recordings from 4 neurons in the pyloric network of the lobster stomatogastric ganglion, showing the rhythmic pattern under normal conditions of modulatory inputs. AB: Anterior Burster; PD: Pyloric Dilator; LP: Lateral Pyloric; PY: Pyloric Constrictor. B: Changes in the pyloric motor pattern when dopamine (10-4M) is added. The AB, LP and PY neurons are all excited and fire more strongly while the PD is inhibited and fires weakly or not at all. There are also significant phasing changes. C: Wiring diagram of the pyloric network, showing the effects of dopamine on ionic currents. Inhibitory synapses are drawn with filled circles; non-rectifying electrical synapses are drawn with a resistor, while rectifying synapses are drawn with a diode symbol indicating the direction of preferred current flow. Neurons in green are excited by dopamine, while those in red are inhibited. Dopamine evokes different changes in ionic currents in each of the neurons. Modulated currents in red would cause opposite effects on neuronal firing than the overall effect of dopamine. D: Summary of dopamine’s effects on synaptic transmission in the pyloric network. Strengthened and weakened synapses are shown with thick and dashed lines, respectively. Some electrical synapses show opposite responses to dopamine depending on the direction of current flow, indicated by synapses that are partly bold and partly dashed. Plus and minus symbols in nerve terminals reflect changes in voltage-activated pre-synaptic calcium accumulation during dopamine. Pipette symbols with plus and minus symbols indicate changes in post-synaptic responsiveness to iontophoresed glutamate, the transmitter of most of the pyloric neurons. Circled plus and minus symbols in the cell bodies indicate dopamine’s effect on post-synaptic input resistance. Red-circled synapses are those where the pre-synaptic effects of dopamine are of opposing sign to its post-synaptic effects. Modified from Ref. 19.
We have considered four reasons why such opposing neuromodulatory effects have been retained during evolution [19]. First, they could reflect the nonspecific second messenger mechanisms activated by the neuromodulator; as long as the “preferred” effects predominate, the minor “non-preferred” effects may be ignored. Second, they could allow the sign of the modulator’s effect to reverse depending on the state of the system, for example, when other neuromodulators block one set of the modulator’s actions. Third, they may be activated with different concentration dependence or kinetics, allowing complex multi-component responses in the cell [21]. Finally, they could provide a system of checks and balances that constrain the degree of flexibility of the network, preventing it from going into a non-functional “over-modulated” state. The opposing actions could then serve as “brakes” to regulate the degree of modulation of the system. While the examples given here come primarily from CPG studies, opposing actions of neuromodulators could in theory stabilize neuronal activity in many kinds of circuits in the nervous system.
Regulation of CPG neuronal composition
It is often assumed that the neuronal composition of a CPG network is fixed, but the neurons participating in the network vary continuously with the state of the system. Different neuromodulators activate and inhibit unique subsets of STG pyloric neurons so that the active circuit varies with the modulatory state [22]. In rodents, the medullary respiratory circuit is dynamically reconfigured during normal respiration (eupnea), sighs, and gasping. Sighs and eupnea, for example, depend on different ionic currents expressed on different neurons, to provide the rhythmic drive for breathing. Sigh behavior depends critically on synaptic mechanisms driven by P/Q type calcium currents which innervate only a small subset of pre-Bötzinger complex neurons [23]. Eupnea does not depend strongly on these pathways, but on NMDA-dependent synaptic mechanisms. Thus, an overlapping but non-identical pool of neurons generates these different respiratory modes [15]. Gasping is evoked by hypoxia; as respiration slows and becomes stronger, many expiratory and inspiratory ventrolateral neurons fall silent. In the pre-Bötzinger complex, during eupnea, rhythm generation depends on neurons expressing both calcium-activated nonselective currents and persistent sodium currents [24,25*]. During gasping, the pattern depends most strongly on the subset of neurons that express INaP [26]. Neuromodulators play critical roles in all these transitions; their effects are complex and state-dependent, and redundancy has been built into this essential system to retain respiration under nearly all conditions [15].
In the vertebrate spinal cord, many interneurons are multifunctional, participating in different behaviors [27]. In the turtle spinal cord, some interneurons participate in both swimming and scratching, while others are active only during scratching and are inhibited during swimming. In the larval zebrafish, different components of the swim CPG are active during swimming, escape behavior and struggling. Different glycinergic commissural interneurons show variable multifunctionality during these behaviors [28]. The commissural bifurcating longitudinal neurons (CoBLs) and commissural secondary ascending interneurons (CoSAs)are multifunctional, being strongly active during swimming and most struggling episodes, and sometimes during escape swims. On the other hand, the commissural longitudinal ascending interneurons (CoLA) and commissural local interneurons (CoLos) are specialized, being active only during slow struggling movements and escape swims, respectively.
The neuronal composition of locomotor CPG networks also changes markedly with changes in locomotor speed. In the zebrafish, the circumferential descending interneurons (CiDs) show a dorsoventral gradient of activity at different speeds: ventral CiDs are active at lower frequencies but are inhibited at higher frequencies, while dorsal CiDs are silent at lower frequencies and are recruited at higher frequencies (Fig. 2) [29**]. In the mouse spinal cord, the V2a neurons express the same transcription factor as the CiDs (alx in zebrafish, Chx10 in mice). The V2a neurons also change their activity with speed, being less active at lower speeds and increasingly recruited and more active at higher speeds [30]. Genetic deletion of the V2a interneurons shows that they play a crucial role in regulating left-right limb alternation, but only at high speeds [31,32]. Clearly, the relative role of these neurons during locomotion varies as a function of speed from zebrafish to mouse.
Figure 2. Speed-dependent locomotor recruitment and inhibition of V2a interneurons in the zebrafish spinal cord.
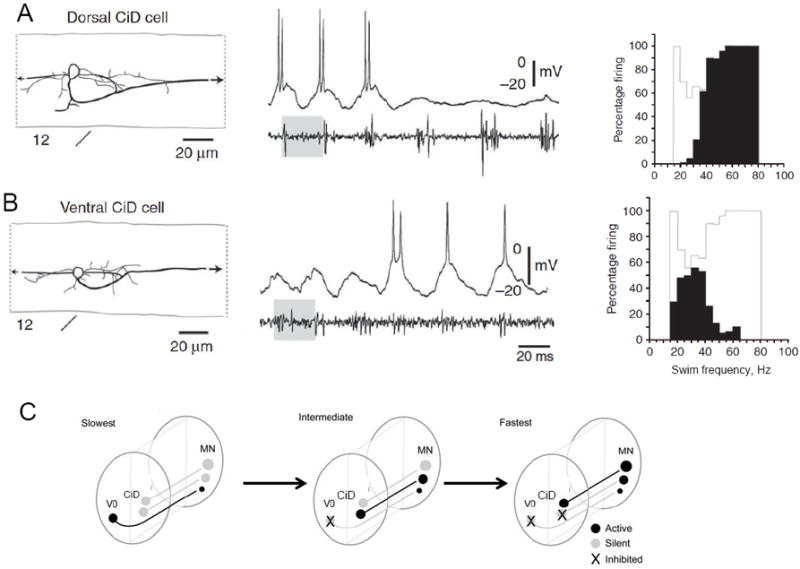
The CiD interneurons show a ventrodorsal gradient of activity at different speeds during locomotion. A: Dorsal CiD is activated at the beginning of a swim bout, when the cycle frequency is highest, and is inactive at lower frequencies later in the swim bout. The recordings in the middle show the intracellular recording from the CiD (top) and an extracellular recording from a motor nerve to monitor the swim frequency (bottom). The histogram at right shows the percentage of dorsal CiDs active at different swim frequencies. B: Ventral CiD is silent and actively inhibited at the highest frequencies at the beginning of the swim bout, but is activated at lower swim frequencies. Histogram at right shows that ventral CiDs are active at lower frequencies than the dorsal CiD neurons. Modified from Ref. 29. C: Summary diagram of recruitment of interneurons at different swim speeds in the larval zebrafish. Modified from Ref. 53.
The neural mechanisms encoding the selective recruitment and silencing of neurons at different speeds of locomotion are not well understood, but neuromodulatory inputs can play a role. In the mouse [20], Xenopus tadpole [33] and lamprey [34], group I metabotropic glutamate receptor agonists accelerate the locomotor rhythm from the isolated spinal cord, via combinations of synaptic and intrinsic neuronal mechanisms. Serotonin slows the ongoing fictive locomotor rhythm in lamprey [35] and mouse [36,37] spinal cords, but increases locomotor frequency in the Xenopus spinal cord [38]. These modulatory effects must act on CPG interneurons, but little is known about their interneuronal targets. In the neonatal mouse spinal cord, serotonin excites V2a interneurons, depolarizing them, increasing their input resistance and enhancing their f/I responses [39], excites commissural interneurons in part by reducing ICa and consequently IK(Ca) [40-42], and excites unidentified locomotor interneurons in part by enhancing Ih and persistent inward currents [43*,44].
Neuromodulator control of ionic current expression and neuronal identity
For any network to function, the component neurons must develop and maintain the specific electrical properties that define them as a particular neuron type. Electrical activity is determined by the pattern of expression of genes for ion channels, pumps and receptors. Recent work in the crustacean STG has addressed this issue. MacLean et al [45,46] showed that expression of the transient potassium current, IA and the inward current Ih is coregulated in a constant ratio in PD neurons, despite varying absolute levels of each current in different PD neurons. Modeling confirmed the surprising result that as long as the ratio of IA to Ih remains constant, the firing properties of the neurons remain constant. Schulz and colleagues [47] extended these data by showing that several pairs of ionic currents showed a constant ratio of gene expression in single STG neurons (Fig. 3). Neuron types differed both in which channel gene pairs showed a constant RNA ratio, and in the slopes of the relationships. Recent work showed that different neuron types also showed different patterns of alternative splicing of RNA for the voltage-dependent sodium channel, providing another path to neuron specification [52]. Thus, the electrophysiological identity of STG neurons is partially determined by their unique ratios and types of coregulated ion channel expression. Hudson and Prinz [48*] modeled these results by classifying the activity patterns in a large database of all possible neurons based on a generic STG neuron model with eight variable ionic conductances. When separated by class of firing properties (bursting, tonic, etc.), class-specific sets of correlations between pairs of ionic currents were seen. In general, when more constraints were placed on the neuron classification by requiring multiple parameters within limited ranges, the numbers and types of current correlations changed, sometimes in unexpected ways, but typically to reduce the total number of correlations (Fig. 3). Two kinds of correlations were found in the models which have not yet been observed experimentally: correlations involving calcium currents (which are difficult to measure in STG neurons), and negative correlations between currents, which have never been seen experimentally but represent 30% of the correlations in the model. This model will certainly focus future experimental research towards these two correlations.
Figure 3. Ionic current pair coregulation helps to define identities of neurons.
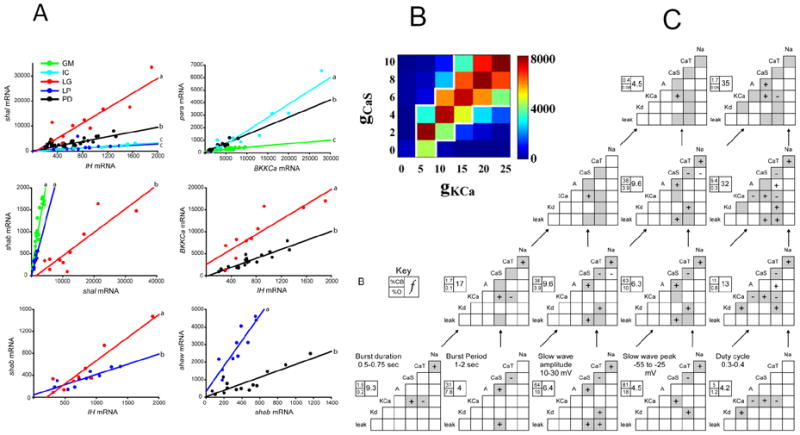
A: Variations in RNA expression levels in single identified neurons from the lobster stomatogastric ganglion. Each graph shows the relationship between the number of copies of RNA for two ion channel genes, measured in multiple copies of a number of different identified neurons. The lines show the average slope of the relationship. Note that no neuron type has a linear relationship between all the current pairs shown; different neurons show different subsets of relationships. Note also that the slope of the a current relationship can differ strongly between neurons. This helps define the electrophysiological properties of each neuron type. From Ref. 47. B: Modeling the relationship between levels of expression of the slow calcium current, gCaS and the calcium-activated potassium current, gKCa, in a database of bursting neuron models. Each current’s maximal conductance was allowed to vary over 6 values, shown on the axes. The database was polled to determine the current levels in many bursting models with duty cycles between 0.1 and 0.2. The color code shows the numbers of models with each correlation; clearly there is a linear relationship between these currents, similar to those found experimentally in A. C: Variation in the current correlations in bursting models as the criteria for inclusion are sequentially restricted. Bottom row: Grey boxes show the correlations between currents in the database models of bursting with the indicated parameters. Then pairs of parameters are combined to identify model neurons that combine both of the lower parameters. This process is repeated going up the figure. At each level, the correlations found in the parent pairs are shown in grey boxes, and if they are blank, there is no correlation at the next level. This shows that as the selected set becomes increasingly constrained by multiple parameters, the numbers of ion channel correlations changes in unexpected ways, but typically decreases. B and C from ref. 48.
This ion current coregulation depends in part on modulatory input to the STG. Khorkova and Golowasch [49] studied the changes in ionic currents following decentralization to remove all modulatory inputs to the STG. Pyloric neurons immediately stop oscillating, but over a period of days they resume oscillating [50]. Removal of descending modulatory input changed the levels of several ionic currents in the PD neuron, and eliminated most of the fixed current ratios. The peptide proctolin was able to prevent these changes and maintain the normal current ratios, even in the presence of TTX, showing that this was not due to an activity-dependent mechanism. Zhang et al [51] recently showed that activity and neuromodulation both contribute in the initial phase of recovery from removal of inputs, though in different ways. A model with two independent mechanisms for recovery after decentralization adequately reproduced these results. The activity-dependent component used an intracellular sensor regulated by intracellular calcium, with inverse effects on the membrane calcium conductance and the intracellular calcium pump activity. The activity-independent modulator component directly enhances calcium currents. With appropriate kinetics, these models reproduced the physiological results, resulting in a different set of currents driving the oscillations before and after recovery from decentralization [51].
Conclusion
Several major conclusions can be drawn from our analysis of flexibility in CPG networks. First, the idea that modulatory inputs are optional modifiers that fine-tune ongoing CPG function should be discarded. We predict that all CPG networks will require some modulatory input to coalesce into functional circuits, through a combination of intrinsic and extrinsic modulatory actions. Second, neuromodulators can exert both positive and negative effects on a particular neuron or synapse; such opposing actions could provide added flexibility and constraints to regulate CPG activity within its functional parameter space. Third, CPGs should be conceptualized as having a flexible composition: the neurons participating in the network and the strengths of their synaptic connections are highly variable; some neurons may only play a role in a particular state (developmental, speed, environmental). Finally neuromodulators play essential roles to maintain the ionic currents that determine the identity of CPG neurons and their synapses. Future modeling studies should take modulatory inputs and actions into account in developing new concepts for the generation of behavioral flexibility.
Highlights for R. Harris-Warrick, “Neuromodulation and Flexibility in Central Pattern Generator Networks”
Neuromodulators play essential roles to enable Central Pattern Generator networks to function
Neuromodulators can activate opposing actions on a network, which serve to stabilize it.
Neuromodulators can help select which neurons are active in a network in any state.
Neuromodulators regulate ion channel gene expression which determines neuronal function.
Acknowledgments
The work in our lab is supported by NIH grants NS17323 and NS057599, and NSF grant IOS-0749467
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dickinson PS. Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol. 2006;16:604–614. doi: 10.1016/j.conb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Grillner S, Jessell TM. Measured motion: Searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goulding M. Circuits controlling vertebrate locomotion: Moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts A, Li WC, Soffe SR. How neurons generate behavior in a hatchling amphibian tadpole: An outline. Front Behav Neurosci. 2010;4:16. doi: 10.3389/fnbeh.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selverston AI. Invertebrate central pattern generator circuits. Philos Trans R Soc Lond B Biol Sci. 2010;365:2329–2345. doi: 10.1098/rstb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Jordan LM, Slawinska U. Modulation of rhythmic movement: Control of coordination. Prog Brain Res. 2011;188:181–195. doi: 10.1016/B978-0-444-53825-3.00017-6.. An insightful review of the actions of neuromodulators in the CPGs controlling locomotion, respiration and mastication.
- 8.Katz PS. Intrinsic and extrinsic neuromodulation of motor circuits. Current Opinion in Neurobiology. 1995;5:799–808. doi: 10.1016/0959-4388(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 9.Knopfel T, Uusisaari M. Modulation of excitation by metabotropic glutamate receptors. Results Probl Cell Differ. 2008;44:163–175. doi: 10.1007/400_2007_035. [DOI] [PubMed] [Google Scholar]
- 10.Russell DF, Hartline DK. Bursting neural networks: A reexamination. Science. 1978;200:453–456. doi: 10.1126/science.644309. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- 12.Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: Potential targets for repair strategies? Prog Brain Res. 2002;137:125–139. doi: 10.1016/s0079-6123(02)37012-2. [DOI] [PubMed] [Google Scholar]
- *13.Liu J, Akay T, Hedlund PB, Pearson KG, Jordan LM. Spinal 5-HT7 receptors are critical for alternating activity during locomotion: In vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J Neurophysiol. 2009;102:337–348. doi: 10.1152/jn.91239.2008.. Shows that genetic deletion of 5-HT7 receptors disrupts left-right and unilateral flexor-extensor alternation while still allowing rhythmc generation. Suggests that thes receptors control activity of inhibitory interneurons responsible for limb and muscle coordination.
- 14.Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RWt, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia AJ, 3rd, Zanella S, Koch H, Doi A, Ramirez JM. Chapter 3--networks within networks: The neuronal control of breathing. Prog Brain Res. 2011;188:31–50. doi: 10.1016/B978-0-444-53825-3.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pena F, Ramirez JM. Endogenous activation of serotonin-2a receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010.. Studies the interactions between Substance P, serotonin and norepinephrine on the respiratory CPG; Substance P only affects respiratory frequency under conditions of low serotonin and norepinephrine activity.
- *19.Harris-Warrick RM, Johnson BR. Checks and balances in neuromodulation. Front Behav Neurosci. 2010;4:47. doi: 10.3389/fnbeh.2010.00047. Catalogs the multiple and sometimes opposing effects of dopamine on the pyloric network in the stomatogastric ganglion, and proposes possible uses of these opposing actions.
- *20.Iwagaki N, Miles GB. Activation of group i metabotropic glutamate receptors modulates locomotor-related motoneuron output in mice. J Neurophysiol. 2011 doi: 10.1152/jn.01037.2010. Epub ahead of print. Analyzes the multiple and opposing actions of mGluR1 receptor action on mouse spinal motoneurons.
- 21.Levitan ES, Kramer RH, Levitan IB. Augmentation of bursting pacemaker activity by egg-laying hormone in aplysia neuron r15 is mediated by a cyclic amp-dependent increase in ca2+ and k+ currents. Proc Natl Acad Sci U S A. 1987;84:6307–6311. doi: 10.1073/pnas.84.17.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 23.Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength. J Neurophysiol. 2006;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- *24.Dunmyre JR, Del Negro CA, Rubin JE. Interactions of persistent sodium and calcium-activated nonspecific cationic currents yield dynamically distinct bursting regimes in a model of respiratory neurons. J Comput Neurosci. 2011 doi: 10.1007/s10827-010-0311-y. Epub ahead of print. A model of pre-Bötzinger complex neurons shows that systematic variation of INaP and ICAN can generate a variety of firing intrinsic firing patterns similar to those seen in electrophysiological recordings.
- 25.Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- 27.Berkowitz A, Roberts A, Soffe SR. Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Front Behav Neurosci. 2010;4:36. doi: 10.3389/fnbeh.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao JC, Fetcho JR. Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish. J Neurosci. 2008;28:12982–12992. doi: 10.1523/JNEUROSCI.3330-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci. 2008;11:1419–1429. doi: 10.1038/nn.2225.. The CiD interneuons show a dorsoventral gradient in input resistance and activation during fictive locomotion. The higher resistance ventral CiDs are activated at low swim frequencies but inhibited at higher frequencies, whilc the lower resistance dorsal CiDs show the opposite pattern. This shows that the neuronal composition of the CPG varies with swim frequency.
- 30.Zhong G, Sharma K, Harris-Warrick RM. Frequency-dependent recruitment of v2a interneurons during fictive locomotion in the mouse spinal cord. Nature Comm. 2011 doi: 10.1038/ncomms1276. http://dx.doi.org/10.1038/ncomms1276. [DOI] [PMC free article] [PubMed]
- 31.Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O, Sharma K. Genetic ablation of v2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60:70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking v2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman RJ, Issberner JP, Sillar KT. Group i mglurs increase locomotor network excitability in xenopus tadpoles via presynaptic inhibition of glycinergic neurotransmission. Eur J Neurosci. 2008;28:903–913. doi: 10.1111/j.1460-9568.2008.06391.x. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakatos A, El Manira A. Long-term plasticity of the spinal locomotor circuitry mediated by endocannabinoid and nitric oxide signaling. J Neurosci. 2007;27:12664–12674. doi: 10.1523/JNEUROSCI.3174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris-Warrick RM, Cohen AH. Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. Journal of Experimental Biology. 1985;116:27–46. doi: 10.1242/jeb.116.1.27. [DOI] [PubMed] [Google Scholar]
- 36.Gordon IT, Whelan PJ. Monoaminergic control of cauda-equina-evoked locomotion in the neonatal mouse spinal cord. J Neurophysiol. 2006;96:3122–3129. doi: 10.1152/jn.00606.2006. [DOI] [PubMed] [Google Scholar]
- 37.Dunbar MJ, Tran MA, Whelan PJ. Endogenous extracellular serotonin modulates the spinal locomotor network of the neonatal mouse. J Physiol. 2010;588:139–156. doi: 10.1113/jphysiol.2009.177378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sillar KT, Reith CA, McDearmid JR. Development and aminergic neuromodulation of a spinal locomotor network controlling swimming in xenopus larvae. Ann N Y Acad Sci. 1998;860:318–332. doi: 10.1111/j.1749-6632.1998.tb09059.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhong G, Droho S, Crone SA, Dietz S, Kwan AC, Webb WW, Sharma K, Harris-Warrick RM. Electrophysiological characterization of v2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. J Neurosci. 2010;30:170–182. doi: 10.1523/JNEUROSCI.4849-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong G, Diaz-Rios ME, Harris-Warrick RM. Serotonin modulates the properties of ascending commissural interneurons in the neonatal mouse spinal cord. J Neurophysiol. 2006;95:1545–1555. doi: 10.1152/jn.01103.2005. [DOI] [PubMed] [Google Scholar]
- 41.Zhong G, Diaz-Rios M, Harris-Warrick RM. Intrinsic and functional differences among commissural interneurons during fictive locomotion and serotonergic modulation in the neonatal mouse. J Neurosci. 2006;26:6509–6517. doi: 10.1523/JNEUROSCI.1410-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlin KP, Dai Y, Jordan LM. Cholinergic and serotonergic excitation of ascending commissural neurons in the thoraco-lumbar spinal cord of the neonatal mouse. J Neurophysiol. 2006;95:1278–1284. doi: 10.1152/jn.00963.2005. [DOI] [PubMed] [Google Scholar]
- *43.Dai Y, Jordan LM. Multiple patterns and components of persistent inward current with serotonergic modulation in locomotor activity-related neurons in cfos-egfp mice. J Neurophysiol. 2010;103:1712–1727. doi: 10.1152/jn.01111.2009.. Using activity-dependent GFP labeling, this study shows that locomotor-activated interneurons possess both sodium- and calcium-mediated persistent inwards currents, and that these are enhanced in several ways by serotonin.
- 44.Dai Y, Jordan LM. Multiple effects of serotonin and acetylcholine on hyperpolarization-activated inward current in locomotor activity-related neurons in cfos-egfp mice. J Neurophysiol. 2010;104:366–381. doi: 10.1152/jn.01110.2009. [DOI] [PubMed] [Google Scholar]
- 45.MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Activity-independent homeostasis in rhythmically active neurons. Neuron. 2003;37:109–120. doi: 10.1016/s0896-6273(02)01104-2. [DOI] [PubMed] [Google Scholar]
- 46.MacLean JN, Zhang Y, Goeritz ML, Casey R, Oliva R, Guckenheimer J, Harris-Warrick RM. Activity-independent coregulation of ia and ih in rhythmically active neurons. J Neurophysiol. 2005;94:3601–3617. doi: 10.1152/jn.00281.2005. [DOI] [PubMed] [Google Scholar]
- 47.Schulz DJ, Goaillard JM, Marder EE. Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci U S A. 2007;104:13187–13191. doi: 10.1073/pnas.0705827104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *48.Hudson AE, Prinz AA. Conductance ratios and cellular identity. PLoS Comput Biol. 2010;6(7):e1000838. doi: 10.1371/journal.pcbi.1000838. Analysis of 1, 679,616 variants of a stomatogastric ganglion neuron model shows that correlations between expression of pairs of ionic currents are frequent within classes of firing patterns, especially when increasing constraints are placed on specification of the activity classes. These correlations vary by class. Both positive and negative correlations are seen in the model, though only positive correlations have been seen experimentally.
- 49.Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci. 2007;27:8709–8718. doi: 10.1523/JNEUROSCI.1274-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thoby-Brisson M, Simmers J. Neuromodulatory inputs maintain expression of a lobster motor pattern generating network in a modulation -dependent state: Evidence from long-term decentralization in vitro. J Neurosci. 1998;18:2212–2225. doi: 10.1523/JNEUROSCI.18-06-02212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Khorkova O, Rodriguez R, Golowasch J. Activity and neuromodulatory input contribute to the recovery of rhythmic output after decentralization in a central pattern generator. J Neurophysiol. 2009;101:372–386. doi: 10.1152/jn.01290.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai A, Temporal S, Shulz DJ. Cell-specific patterns of alternative splicing of voltage-gated ion channels in single identified neurons. Neuroscience. 2010;168:118–129. doi: 10.1016/j.neuroscience.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Fetcho JR, McLean DL. Some principles of organiation of spinal neurons underlying locomotion in zebrafish and their implications. Ann N Y Acad Sci. 2010;1198:94–104. doi: 10.1111/j.1749-6632.2010.05539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


