Abstract
Purpose
To develop fenretinide oral mucoadhesive patch formulations and evaluate their in vitro and in vivo release performance for future site-specific chemoprevention of oral cancer.
Methods
Solubilization of fenretinide in simulated saliva (SS) was studied by incorporating nonionic surfactants (Tween® 20 and 80, and Brij® 35 and 98), bile salts (sodium salt of cholic, taurocholic, glycocholic, and deoxycholic acids), phospholipid (lecithin), and novel polymeric solubilizer (Souplus®). Adhesive (polycarbophil: hydroxypropyl methylcellulose 4KM) and drug release (Fenretinide/Eudragit® RL PO with or without solubilizers) layers were prepared by solvent casting. Oral mucoadhesive patches were formed by attaching drug and adhesive layers onto backing layer (Tegaderm™ film). Physical state of drug in Eudragit® films was examined by X-ray diffraction (XRD). Evaluation of in vitro and in vivo fenretinide release from the patch was conducted in SS containing 5%w/v sodium deoxycholate and rabbits, respectively. Fenretinide was quantified by HPLC.
Results
Tween® 20 and 80, Brij® 98, and sodium deoxycholate exhibited the highest fenretinide solubilization potential among the solubilizers. Drug loading efficiency in Eudragit® films was 90%–97%. XRD suggested fenretinide was amorphous in solubilizer-free and solubilizer-loaded films. Solubilizer-free patch exhibited poor in vitro and in vivo controlled drug release behavior. Increases in drug loading (5–10 wt%) or changes in polymeric matrix permeability did not provide continuous drug release. Co-incorporation of either single or mixed solubilizers in fenretinide/Eudragit® patches, (20 wt% Tween® 20, Tween® 80 and sodium deoxycholate or 20 wt% Tween® 80 + 40 wt% sodium deoxycholate solubilizers) led to significantly improved continuous in vitro/in vivo fenretinide release.
Conclusion
Fenretinide/Eudragit® RL PO patches with 20 wt% Tween® 80 + 40 wt% sodium deoxycholate solubilizers exhibit excellent release behavior for further preclinical and/or clinical evaluation in oral cancer chemoprevention.
Keywords: controlled release, Eudragit®, fenretinide, intraoral drug delivery, cancer chemoprevention, mucoadhesive patch, solubilization
INTRODUCTION
Oral squamous cell carcinoma (OSCC) will affect over 36,000 Americans this year, resulting in over 7,000 deaths (1). Notably, the overall prognosis for persons diagnosed with oral cancer is among the lowest of any solid tumors (2). Even patients fortunate enough to obtain a surgical cure face major functional and esthetic sequelae due to loss of tissues essential for esthetics and function (2). Furthermore, OSCC does not arise de novo, but rather from visibly accessible precursor lesions known as oral epithelial dysplasia (OED). Clearly, early detection of premalignant OED lesions in conjunction with effective chemopreventive strategies could help alleviate the morbidity and mortality associated with OSCC.
An underlying problem in OED lesions is inappropriate growth and maturation of lesional epithelial cells resulting in failure to undergo terminal differentiation and, if warranted, entrance into apoptosis (3). Notably, fenretinide (4-hydroxyphenylretinamide), which is a synthetic derivative of vitamin A, is a superlative inducer of epithelial differentiation (at lower doses) as well as apoptosis (at higher doses) in vitro (4,5). As a result of these positive in vitro attributes, numerous oral cancer clinical trials have evaluated fenretinide (6,7). Complications such as low bioavailability and rapid drug elimination from the body (8,9), along with toxicity (e.g., mucositis and hyperlipidemia) (10,11), originating respectively after oral and intravenous administration, have impeded its use in chemoprevention for oral and other cancers.
Local drug delivery, on the other hand, has proven to be highly effective in providing therapeutic drug levels directly at the site of numerous cancers, thereby improving the therapeutic efficacy of the drug and patient compliance (12–14). Due to anatomic considerations, which include capacity for direct visualization (which enables monitoring of therapy and direct placement of drug delivery system), oral mucosa is more amenable to the use of local drug delivery strategies than either primary chemoprevention (suppressing progression of premalignant lesions to cancer) or secondary chemoprevention (inhibition of cancer recurrence) (15). Previous studies in our labs have demonstrated that oral site-specific delivery from mucoadhesive gels (16–18) or nanoparticles (19) could sustain localized therapeutic levels of hydrophilic chemopreventive agents. Very high hydrophobicity (log P = 8.03) and extremely low water solubility (below detection limit) of fenretinide, however, complicate the development of oral mucoadhesive formulations with optimal drug release behavior (20). Hence, we sought to develop oral mucoadhesive patches of fenretinide and evaluated in vitro and in vivo for the purpose of providing continuous drug release.
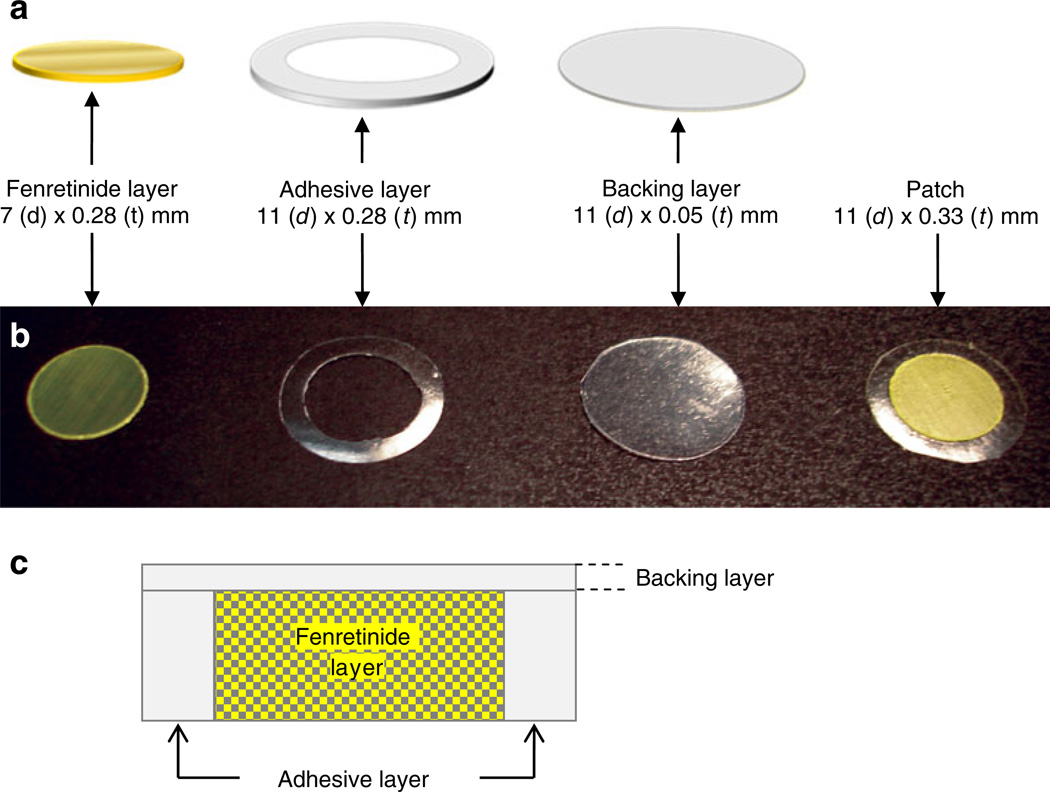
Eudragit® copolymers are derived from esters of acrylic and methacrylic acid and widely used in numerous drug delivery applications (21,22). Eudragit® RL PO and RS PO provide a highly flexible polymeric matrix for manipulation of drug release behavior of both hydrophilic and hydrophobic drugs (22). Eudragit® RL PO/RS PO, polycarbophil, and hydroxypropyl methylcellulose (HPMC) are well-known to exhibit excellent oral mucoadhesive properties (23,24). Hence, Eudragit® RL PO/RS PO-based mucoadhesive patches for delivery of fenretinide with and without drug solubilizers were developed (as shown Fig. 1) and evaluated for future oral cancer chemoprevention therapy.
Fig. 1.
Schematic diagram (a), photographic image (b), and schematic cross-sectional diagram (c) of a mucoadhesive patch comprising drug (fenretinide/Eudragit® RL PO with or without solubilizers), adhesive (HPMC 4KM: polycarbophil (3:1), and backing (Tegaderm™ adhesive film) layers.
MATERIALS AND METHODS
Materials
Fenretinide was received as a gift sample from Merck & Co., Inc. (Whitehouse Station, NJ, USA), Fidia Fharmaceuti s.p.a. (Abano Terme, Italy), and National Cancer Institute (USA). Noveon®AA-1 polycarbophil (PC), Soluplus® (Polyvinyl caprolactum-polyvinyl acetate-polyethylene glycol graft copolymer), Hydroxypropyl methylcellulose (HPMC) 4KM, and Eudragit® RS PO and RL PO were all gifts from Lubrizol Corp. (Wickliffe, OH, USA), BASF (Limburgerhof, Germany), Colorcon®, Inc. (West Point, PA, USA), and Evonik Degussa Corp. (Piscataway, NJ, USA), respectively. Brij® 98, Brij® 35, Tween® 80, Tween® 20, sodium deoxycholate, sodium taurocholate, and sodium cholate were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Triethyl citrate (TEC) and sodium glycocholate were purchased from Acros Organics (New Jersey, USA). Propylene glycol was purchased from MP Biomedicals (Solon, Ohio, USA). Pyrex® petri dishes (60 × 15 and 150 × 20 mm) were purchased from Fisher-Scientific (Pittsburgh, PA, USA). Teflon® overlay was purchased from Scientific Commodities, Inc. (Lake Havasu City, AZ, USA). Amber color ampoules were purchased from Wheaton (Millville, NJ, USA). Tegaderm™ roll was purchased from 3M Health Care (St. Paul, MN, USA).
Fenretinide HPLC Assay
All HPLC assays were performed on a Waters 2695 alliance system (Milford, MA, USA) consisting of a 2996 Photodiode array detector and a personal computer with Empower 2 Software. A symmetry C18 column (4 µm, 150 mm × 4.6 mm) was used. Isocratic elution with acetonitrile: 0.1% (v/v) phosphoric acid (67:33 v/v) was employed at a flow rate of 1.0 mL/min, and detection wavelength was set at 365 nm. Standard curves of fenretinide were established from a solvent mixture of acetonitrile and ethanol (1:1), and concentrations of unknown samples were calculated from the standard curve.
Solubilization of Fenretinide in Simulated Saliva (pH 6.8)
Solubilizers for fenretinide included nonionic surfactants (Tween® 20 and 80, and Brij 35 and 98), bile salts (sodium salt of cholic, taurocholic, glycocholic, and deoxycholic acids), phospholipid (lecithin), and/or a novel amphiphilic polymer (Soluplus®). Briefly, an excess amount of fenretinide was added into separate amber color ampoules containing 1 mL 0.5, 1, 2, and 5%w/v solutions of solubilizers (prepared using N2-purged simulated saliva) and sealed under vacuum in order remove the oxygen from the head-space. The ampoules were then placed in an incubator maintained at 37°C and shaken at 240 RPM for 72 h. This duration was found to be sufficient to reach equilibrium, as indicated by insignificant increase in fenretinide solubility beyond 72 h incubation duration (data not shown). Then, the ampoules were broken, and the mixture was passed through a 0.45 µm PVDF filter unit (Millipore, USA) and diluted suitably with respective solubilizer solution, and the amount of fenretinide solubilized in simulated saliva was determined by HPLC.
Preparation of Oral Mucoadhesive Patches for Delivery of Fenretinide
Preparation of Adhesive Layer
An adhesive layer based on the blend of HPMC 4KM and PC at a weight ratio of 3:1 was prepared by a casting method. Briefly, 1.5% polymer solution was prepared in ddH2O containing required amount (20 wt% based on polymer mass) of propylene glycol by stirring the polymer/water mixture overnight. About 50 mL of polymer solution was then casted onto glass petri dish (150 × 20 mm) and incubated at 50°C for 48 h. Then, the polymer film was cut into required size and stored in a desiccator at room temperature until further use.
Preparation of Fenretinide Layer
Preparation of fenretinide films was performed under protection from light. Required quantity of plasticizer (triethyl citrate), solubilizer (Tween® 20, Tween® 80, Brij® 98, sodium deoxycholate), and Eudragit® (RS PO or RL PO) were weighed in 15 mL polypropylene tubes to which 8 mL of a 50:50 (v/v) acetone-ethanol mixture was added. The quantity of plasticizer or solubilizer added was calculated based on the mass of polymer. The resulting mixture was vortexed until all ingredients were dissolved. The required quantity (5 or 10 wt% based on the total mass of polymer + excipients) of fenretinide was then added to above-prepared polymer-plasticizer or polymer-solubilizer solution and vortexed again, and the volume was adjusted to 10 mL with the same solvent mixture. Five milliliter of fenretinide-polymer solution was added onto Teflon (Scientific Commodities, Inc., Lake Havasu City, AZ, USA) overlaid glass petri dish (60 × 15 mm) and incubated at 38°C for 48 h. After sufficient drying, fenretinide-loaded polymer film was cut into required size (7 mm diameter), packed in aluminum foil, and stored in a desiccator at −20°C until further use.
Assembly of Oral Mucoadhesive Patches of Fenretinide
An annular adhesive layer with 11 (outer diameter) and 7 (inner diameter) mm dimensions was formed by cutting the film with 11 and 7 mm cork borers, respectively. The adhesive layer was then placed onto adhesive side of the Tegaderm™ film (backing layer), followed by insertion of previously cut 7 mm fenretinide/Eudragit® layer into open region of adhesive layer to obtain oral mucoadhesive patch of fenretinide (see Fig. 1).
Determination of Fenretinide Loading
Seven-millimeter fenretinide/Eudragit® films were digested in acetonitrile: ethanol (50:50), passed through 0.45 µm PVDF filter units, and analyzed by HPLC after suitable dilution. The fenretinide loading was calculated as the percentage of the amount of fenretinide versus the total weight of the film mixture (i.e., fenretinide, Eudragit®, and other excipients).
X-ray Diffraction (XRD)
The physical state of fenretinide in Eudragit® films was examined by measuring the XRD pattern of fenretinide, blank, and fenretinide/Eudragit® films with and without solubilizers using a Scintag powder X-ray diffractometer (Scintag, CA, USA). The X-ray source was copper Ka (40 kV, 30 mA), and the scanning speed was 2 deg/min.
Evaluation of In Vitro Release of Fenretinide from Oral Mucoadhesive Patch
Simulated saliva was respectively comprised of 14.4, 16.1, 1.3, 0.55, and 2 mM sodium chloride, potassium chloride, calcium chloride dihydrate, magnesium chloride hexahydrate, and dibasic potassium phosphate, with the pH adjusted to 6.8. In vitro release studies were conducted in simulated saliva containing 5% (w/v) sodium deoxycholate under perfect sink conditions. Oral mucoadhesive patches were placed in 50 mL tubes (separate tubes for each sampling interval), and 40 mL release medium was added to each tube. The tubes were placed in an incubator maintained at 37°C and shaken at 100 RPM. After every 2 h of incubation, the release medium was replaced with fresh buffer. At predetermined time intervals (1, 2, 4, 6, and 8 h), three tubes were taken out, and the patches were freeze-dried. Determination of remaining drug content in the patch was performed according to the drug loading assay. The cumulative amount of released fenretinide was determined by subtracting the fraction remaining in the patches from the initial drug content.
Eudragit® Polymeric Matrices Hydration/Swelling
Fenretinide-Eudragit® films (7 mm) were weighed (initial weight) in 50 mL tubes (separate samples for predetermined periods of time), and 40 mL of simulated saliva (pH 6.8) containing 5% (w/v) sodium deoxycholate was added. The tubes were then placed in an incubator maintained at 37°C and shaken at 100 RPM. At predetermined time intervals (1, 2, 4, 6, and 8 h), three tubes were taken out, and films were wiped off from the excess surface water using filter paper and weighed (hydrated weight). Experiments were performed in triplicate (n = 3), and the swelling (%) of fenretinide-Eudragit® matrices was calculated as follows:
Evaluation of In Vivo Fenretinide Release from Oral Mucoadhesive Patch
Animal studies were approved by the Ohio State University Institutional Animal Care and Use Committee and adhered to National Institute of Health guidelines. Female New Zealand white rabbits (12 weeks old and weighing about 2.78 kg) were anesthetized with isoflurane (5% v/v in oxygen) via inhalation for patch placement and removal. Six fenretinide oral mucoadhesive patches/time point were placed on the buccal mucosa of individual rabbits’ (three patches each on the left and right buccal mucosa) oral cavities (drug + adhesive layers facing the mucosa). Slight pressure was applied to the backing layer of the patch for 1 min to establish mucoadhesion with the rabbit buccal mucosa. After different attachment times (1, 4, and 8 h), the patches were carefully removed, and remaining drug content in the patch was determined as per the method described in drug loading assay. The cumulative amount of released fenretinide was determined by subtracting the fraction remaining in the patches from the initial drug content.
Statistical Analysis
The results are expressed as mean ± SE (n = 3 (in vitro) or 6 (in vivo)). An unpaired Student’s t-test was used to compare the means of in vitro, in vivo, and in vitro-in vivo drug release at the different release duration among various patch formulations and assess statistical significance. Results were considered statistically significant if p < 0.05.
RESULTS AND DISCUSSION
Oral Mucoadhesive Patch of Fenretinide—Challenges and Formulation Strategies
Fenretinide is a highly lipophilic chemopreventive compound with extremely low water solubility (below detection limit) (20). Clinical trials conducted with an oral gelatin capsule containing fenretinide in corn oil and polysorbate 80 demonstrated poor bioavailability due to its minimal intestinal membrane permeability resulting from very high log P value (8.03) (8,9,25–27). In addition, fenretinide was rapidly eliminated from the body after intravenous injection (8,9). These issues emphasize the necessity for a suitable drug delivery carrier and strategy to improve its efficacy in cancer chemoprevention.
Local drug delivery implants, patches, liposomes, microparticles, nanoparticles, gels, or ointments have demonstrated the strong ability to provide high, localized doses of drug directly at the site of tumor or tumor resection (14–16,19,28–30). Importantly, the ability of fenretinide to accumulate in cell lipid bilayers (9) makes it an ideal drug candidate for the development of intraoral site-specific drug delivery carriers in oral cancer chemoprevention. However, highly hydrophobic drugs often exhibit poor release performance from both hydrophilic and hydrophobic polymeric carriers (8,20). Hence, it is necessary to adopt an effective drug solubilization strategy to obtain continuous drug release from the polymeric carriers. In order to achieve this objective, we initially studied fenretinide solubilization by employing nonionic surfactants, bile salts, a phospholipid, and a novel amphiphilic polymer (as described below) to determine the most effective solubilizers.
Significant Solubilization of Fenretinide in Simulated Saliva by Co-incorporation of Nonionic Surfactants, Bile Salts, Phospholipid, and Novel Amphiphilic Polymer
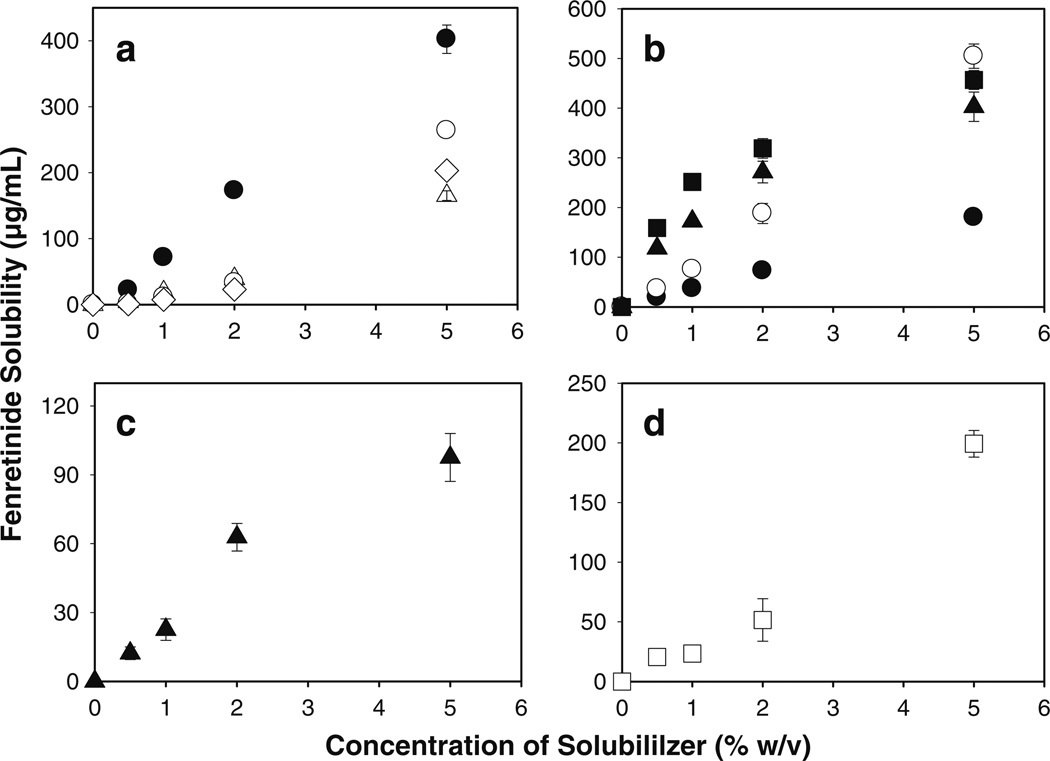
To determine suitable solubilizers, we studied the enhancement of fenretinide solubility in simulated saliva (pH 6.8) by 0.5–5%w/v nonionic surfactants (Tween® 20 and 80, and Brij® 35 and 98), bile salts (sodium salt of cholic, taurocholic, glycocholic, and deoxycholic acids), phospholipid (lecithin), and a novel amphiphilic polymer (Soluplus®) (see Fig. 2). The solubility of fenretinide in simulated saliva was significantly enhanced by all the solubilizers studied (e.g., below detection limit to 98–505 µg/mL at 5%w/v concentration). The relationship between solubility of fenretinide and the concentration of bile salts is shown in Fig. 2a. With an increase in concentration of bile salt from 0.5 to 5% (w/v), the solubility of fenretinide increased either proportionally (in case of sodium deoxycholate) or gradually until 2% (w/v) bile salt and then in a more pronounced manner (in case of other bile salts), indicating micellar solubilization of fenretinide. Solubilization capacity of bile salts, however, decreases as follows: sodium deoxycholate > sodium cholate > sodium taurocholate > sodium cholate. For example, the solubility of fenretinide with 5% w/v sodium deoxycholate, sodium cholate, sodium taurocholate, and sodium glycocholate was 403, 264, 203, and 166 µg/mL, respectively. Similar solubilization behavior by these bile salts has been observed before for steroid hormones (31). The effect of nonionic surfactants (Tween® 20 and 80, and Brij® 35 and 98) on fenretinide solubility is shown in Fig. 2b. Tween® 20 and 80 and Brij® 98 exhibited higher solubilization potential compared to all other kinds of solubilizers studied in the current study. For example, the solubility of fenretinide with 5% w/v Brij® 98, Tween® 20, and Tween® 80 was 505, 404, and 457 µg/mL, respectively. Intermediate solubility enhancement was observed with Soluplus® (see Fig. 2c) and lecithin (see Fig. 2d) (solubility of fenretinide with 5%w/v lecithin and Soluplus® was 200 and 98 µg/mL, respectively). Significant fenretinide solubility enhancement achieved by employing bile salts (32,33), nonionic surfactants (34,35), phospholipid (36), and polymeric solubilizer (37) can be attributed to their excellent ability to exhibit micellar solubilization for hydrophobic molecules.
Fig. 2.
Significant enhancement of fenretinide solubility in simulated saliva (pH 6.8) by non-ionic surfactants, bile salts, phospholipid, and novel amphiphilic polymer. Effect of co-incorporation of 0.5–5%w/v bile salts (a: sodium deoxycholate (●), sodium cholate (○), sodium glycocholate (△), and sodium taurocholate (◊)), nonionic surfactants (b: Brij® 35 (●), Brij® 98 (○), Tween® 20 (▲), and Tween® 80 (■)), novel polymeric solubilizer (c: Soluplus®), and phospholipid (d: lecithin) on the solubility of fenretinide in simulated saliva. Studies were conducted in amber color ampoules under evacuated conditions at 37°C and symbols represent mean ± SE, n = 3.
Design, Development, and Characteristics of Fenretinide Oral Mucoadhesive Patch Formulations
Numerous kinds of devices, such as tablets, films, patches, disks, strips, ointments, and gels, have been studied for oral transmucosal drug delivery (38,39). Among them, mucoadhesive patches are highly flexible and better tolerated by patients than tablet formulations (39). In addition, patches are more efficient in providing accurate dosing and effective localized delivery of drugs compared to gels and ointments (40). Ideally, oral mucoadhesive systems should consist of swellable polymeric matrix layer to release the drug in a controlled manner, good mucoadhesive strength, and impermeable backing layer to prevent the drug release/loss from back surface (41). In the current study, we designed and successfully developed an oral mucoadhesive patch comprising drug release, mucoadhesion, and backing layers (see Fig. 1).
The concentration of polymer, volume of polymer solution, and size of the petri dishes that are required to form fenretinide and adhesive layers of approximately equivalent thickness was initially optimized and listed in the “Materials and Methods” section. Fenretinide-loaded Eudragit® RL PO or RS PO layers were prepared by a solvent casting method with excellent drug loading efficiency of 90%–97% (see Table I). The thickness of fenretinide and adhesive layers, and the Tegaderm™ adhesive film were measured to be ~0.28, 0.28, and 0.05 mm, respectively. After assembling drug and adhesive layers onto backing layer, the total thickness of the patch was measured to be ~0.33 mm.
Table I.
Evaluation of Microencapsulation of Fenretinide in Solubilizer-Free and Solubilizer-Loaded Eudragit® RS PO/RL-PO Films
| Patch formulation | Fenretinide loading (wt%) | Loading efficiency (%)a | |
|---|---|---|---|
| Theoreticalb | Actuala | ||
| Eudragit® RS-PO | 5.0 | 4.5 ± 0.1 | 90.0 ± 1.2 |
| Solubilizer-free Eudragit® RL-PO | 5.0 | 4.6 ± 0.1 | 92.1 ± 1.0 |
| 10.0 | 9.2 ± 0.2 | 92.0 ± 2.0 | |
| Solubilizer-loaded Eudragit® RL-PO | |||
| 20 wt% Tween® 20 | 5.6 | 5.4 ± 0.1 | 96.3 ± 1.5 |
| 20 wt% Tween® 80 | 5.1 | 4.9 ± 0.2 | 95.1 ± 1.6 |
| 20 wt% Brij® 98 | 5.2 | 4.8 ± 0.1 | 92.3 ± 1.5 |
| 20 wt% Sodium deoxycholate | 5.6 | 5.5 ± 0.2 | 97.0 ± 1.0 |
| 40 wt% Sodium deoxycholate | 5.0 | 4.6 ± 0.1 | 91.4 ± 1.0 |
| 20 wt% Tween® 80 + 40 wt% Sodium deoxycholate | 4.9 | 4.5 ± 0.2 | 92.0 ± 2.1 |
Mean ± SE, n = 3
Based on polymer + excipients weight
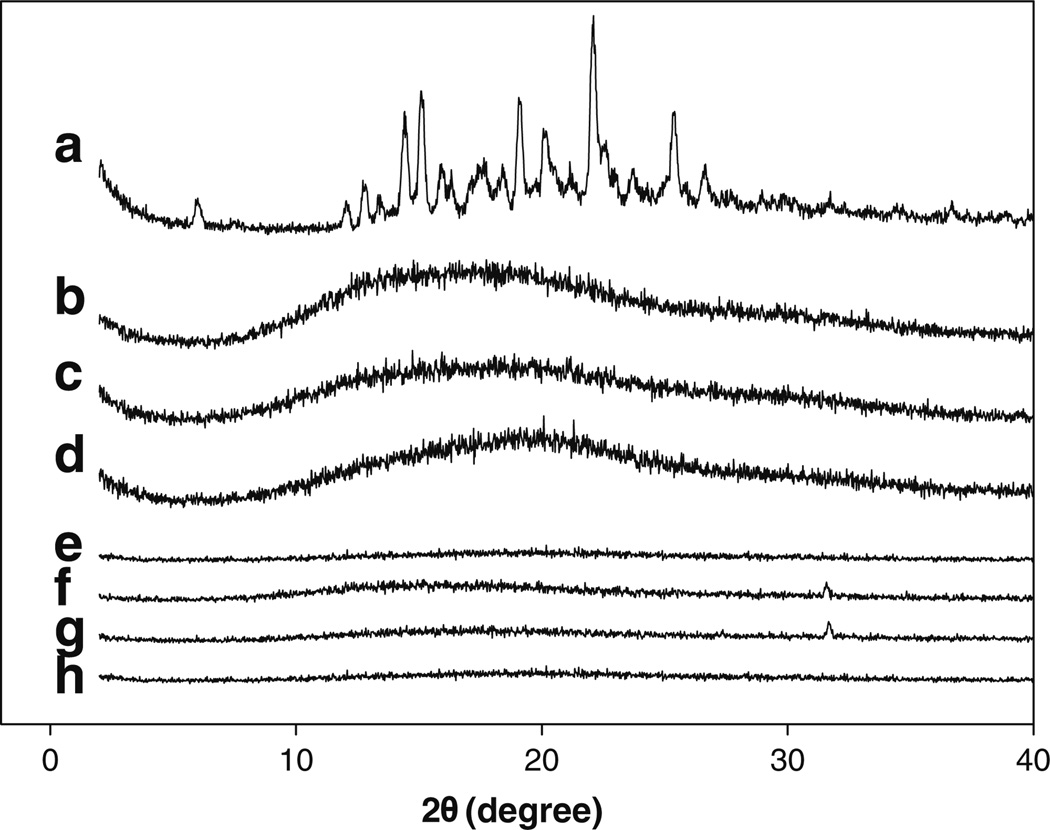
The physical state/distribution of fenretinide in Eudragit® films was examined byXRD. TheXRD pattern of fenretinide and fenretinide/Eudragit® layers with and without solubilizers are displayed in Fig. 3. As shown in Fig. 3a, fenretinide displayed numerous peaks corresponding to its crystal form, whereas Eudragit® RL PO polymer (Fig. 3b) was amorphous in nature. The major diffraction peaks of fenretinide crystals were observed at 2θ values 5.9, 12, 12.7, 14.4, 15, 16, 19, 20.2, 22.1, 25.3, and 26.6. The diffraction peaks of fenretinide were absent in all the fenretinide/Eudragit® layers (Fig. 3c–h), and only the XRD pattern of Eudragit® polymer was observed. This finding strongly suggests that drug was distributed in an amorphous form (molecular level distribution) in the polymeric matrices.
Fig. 3.
Miscibility of fenretinide with Eudragit® RL PO. Effect of drug loading and co-incorporation of 20 wt% solubilizers on the miscibility of fenretinide with Eudragit® RL PO polymer. X-ray diffraction patterns of fenretinide (a), blank Eudragit® RL PO film (b), 5 (c) and 10 (d) wt% fenretinide, and 20 wt% Tween® 20 (e), sodium deoxycholate (f), Brij® 98 (g), and Tween® 80 (h) loaded Eudragit® RL PO films. Fenretinide loading in solubilizer-loaded Eudragit® RL PO films was 5 wt%.
Effect of Formulation Parameters on In Vitro and In Vivo Release of Fenretinide from Eudragit® Oral Mucoadhesive Patch
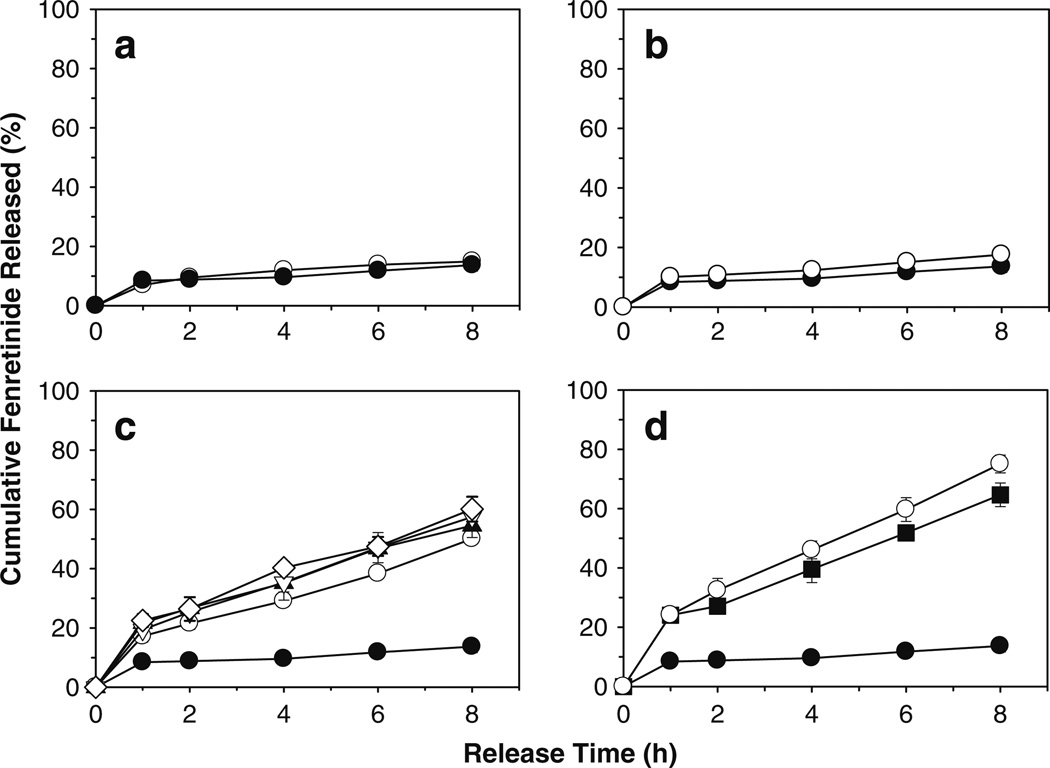
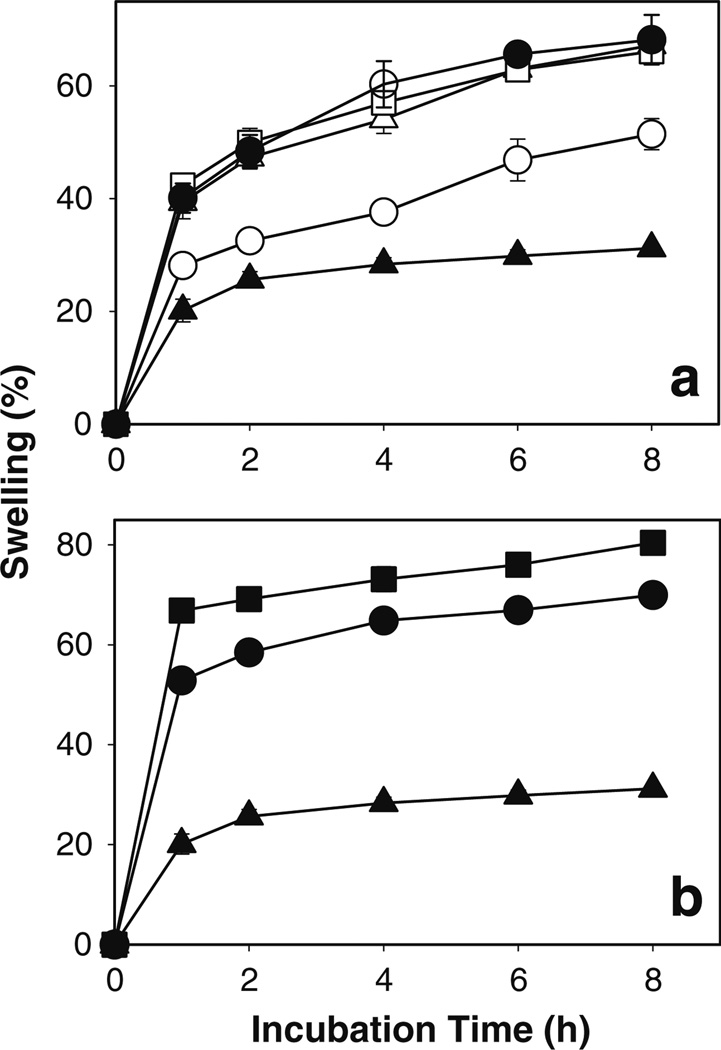
Since the solubility of fenretinide in water is very low (below detection limit), 5%w/v sodium deoxycholate was incorporated in release medium to maintain the sink condition. The effect of drug loading, polymeric matrix permeability of Eudragit®, and co-incorporation single (20 wt% Tween® 20 and 80, and Brij® 98, and sodium deoxycholate) or mixed (40 wt% sodium deoxycholate + 20 wt% Tween® 80) solubilizers on in vitro fenretinide release is shown in Fig. 4. Use of a highly permeable polymer, Eudragit® RL PO in place of Eudragit® RS PO (see Fig. 4a), or increasing drug loading (see Fig. 4b) did not provide continuous release of fenretinide over a period of 8 h (i.e., 7–10, 10–13, and 14–18% drug release after 1, 4, and 8 h, respectively). Co-incorporation of single (see Fig. 4c) or mixed (see Fig. 4d) solubilizers in the highly permeable Eudragit® RL PO patch provided significantly (p < 0.05) improved continuous in vitro fenretinide release (i.e., 17–24, 10–46, and 50–75% drug release after 1, 4, and 8 h, respectively). This finding was in good agreement with water uptake/polymer hydration results, where solubilizer-loaded patches exhibited significantly higher water uptake/polymer hydration than the solubilizer-free patch (see Fig. 5).
Fig. 4.
Co-incorporation of single or mixed solubilizers provides significantly improved continuous in vitro release of fenretinide from Eudragit® RL PO mucoadhesive patches. Effect of polymeric matrix permeability of Eudragit® (a: low permeability RS PO (○) and high permeability RL PO (●)), drug loading (b: 5 (●) and 10 (○) wt%), and co-incorporation of single (c: 0 (●) and 20 wt% Tween® 20 (▲), Tween® 80 (▽), Brij® 98 (◊), sodium deoxycholate (○)) or mixed (d: 0 (●), 40 wt% sodium deoxycholate (■), and 40 wt% sodium deoxycholate + 20 wt% Tween® 80 (○)) solubilizers on in vitro release of fenretinide. Fenretinide loading (theoretical) in a, c, and d formulations was 5 wt%. Eudragit® RL PO or RS PO mucoadhesive patch formulations with 5 and 10 wt% fenretinide, and 20 wt% sodium deoxycholate were prepared using triethyl citrate (20 wt%) as a plasticizer. Patch formulations loaded with 20 wt% surfactants (Tween® 20 and 80, and Brij® 98) were prepared without triethyl citrate. Studies were conducted in simulated saliva (pH 6.8) containing 5%w/v sodium deoxycholate under perfect sink condition (concentration of fenretinide was <10% of drug solubility in release medium) at 37°C and symbols represent mean ± SE, n = 3.
Fig. 5.
Co-incorporation of single or mixed solubilizers significantly improved water uptake/polymer hydration characteristics of Eudragit® RL PO films. Effect of co-incorporation of single (a: 0 (▲) and 20 wt% Tween® 20 (●), Tween® 80 (□), Brij® 98 (△), sodium deoxycholate (○)) or mixed (b: 0 (▲), 40 wt% sodium deoxycholate (●), and 40 wt% sodium deoxycholate + 20 wt% Tween® 80 (■)) solubilizers on polymer hydration/swelling. Fenretinide loading (theoretical) in all the film formulations was 5 wt%. Films containing 20 wt% sodium deoxycholate were prepared using triethyl citrate (20 wt%) as a plasticizer. Films containing 20 wt% surfactants (Tween® 20 and 80, and Brij® 98) were prepared without triethyl citrate. Studies were conducted in simulated saliva (pH 6.8) containing 5%w/v sodium deoxycholate at 37°C and symbols represent mean ± SE, n = 3.
To further understand the kinetics of fenretinide release from Eudragit® RL PO patch, the release data were analyzed using Higuchi and Korsmeyer-Peppas kinetic equations (42). Solubilizer-free Eudragit® RL PO patches exhibited a biphasic drug release pattern with an initial burst release phase followed by a lag-phase and then steady controlled drug-release phase. Similar biphasic drug-release behavior was also observed from dicyclomine/Eudragit® S-100 matrices (43). When all the data of fenretinide release vs. time were considered for the Higuchi plots, poor fits (R2 < 0.9) were observed for the solubilizer-free patch formulations, and only the latter phase was used to assess the release mechanism (see Fig. S1). By contrast, with solubilizer-loaded patches, fenretinide release was monophasic, and the entire data set was analyzed. Best fits (R2 = 0.98 to 1) (see Fig. S1 and Table SI) observed with the Higuchi equation and square-root time dependence strongly suggest a drug diffusion mechanism driven by a limited solubility driving force. Fenretinide release data was further analyzed by Korsmeyer-Peppas equation. As shown in Table SII, the value of n was 0.447–0.52 with correlation co-efficient value of 0.949 to 1, further supporting Fickian diffusion. Microencapsulation of nonionic surfactants in Eudragit® RL PO/RS PO matrices have been shown to reduce the Tg of the polymer considerably (44,45), leading to improved permeability and hydration of the polymeric matrix (45,46). It is important to emphasize that swelling was substantially greater upon incorporation of solubilizer in the patch (see Fig. 5), and the lag time for diffusion from solubilizer-free patches may reasonably be related to diffusion time of the solubilizer from the simulated saliva release medium to enter the patch and solubilize the drug. Hence, improved continuous release of fenretinide from solubilizer-loaded patches relative to solubilizer-free patches can be reasonably explained by increased water in the hydrogel, micellar solubilization of fenretinide (32,47), and diffusion of the drug-associated micelles (48).
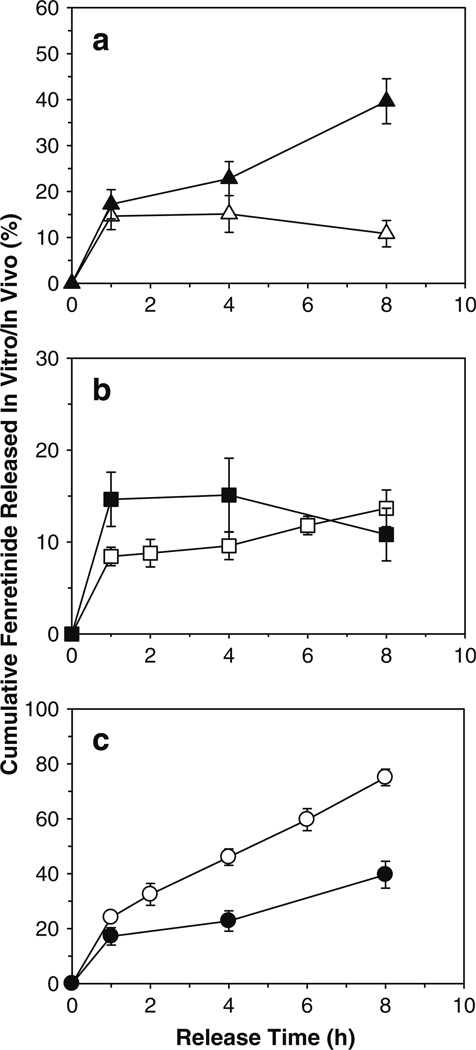
The potential of incorporating appropriate solubilizers in Eudragit® patches to provide continuous in vivo fenretinide release was evaluated by conducting the drug release study in rabbits. The effect of co-incorporation of appropriate solubilizers (20 wt% Tween® 80 + 40 wt% sodium deoxycholate with respect to Eudragit®) on in vivo fenretinide release and comparison of in vitro and in vivo fenretinide release from solubilizer-free and solubilizer-loaded Eudragit® RL PO patches are shown in Fig. 6. Poor in vivo controlled fenretinide release behavior was observed with the solubilizer-free patch. For example, solubilizer-free patch exhibited about 11–15% fenretinide release after 1–8 h of mucosal attachment, respectively (see Fig. 6a). This finding can be attributed to its extremely low water solubility and/or permeability across biological membranes (9). In contrast, solubilizer-loaded patches exhibited 17, 23, and 40% in vivo fenretinide release, respectively, after 1, 4, and 8 h of mucosal attachment (see Fig. 6a), indicating high effectiveness of solubilizers to provide continuous in vivo release of fenretinide from Eudragit® polymeric matrices. A variety of solubilizing and permeation enhancing agents have been shown to facilitate buccal mucosal permeation and provide tissue localization for hydrophobic drugs (49–51). It is noted that there was no significant difference (p>0.05) between in vitro and in vivo fenretinide release from solubilizer-free patches (see Fig. 6b). There was a significant difference (p<0.05), however, between in vitro and in vivo fenretinide release from solubilizer-loaded patches (see Fig. 6c), although the continuous release trend was the same. The latter difference can be attributed to dissimilarity in test conditions (e.g., in vitro drug release in simulated saliva vs. in vivo drug release followed by permeation across buccal mucosal membrane). The finding of the current study was in good agreement with the results of Junginger et al. (52), where significant differences between in vitro and in vivo permeation of FITC-labeled dextran across pig buccal mucosa was observed. It should be noted, however, that similar trends were observed in both experiments, where FITC-dextran permeated easily under in vitro and in vivo conditions, and the permeability of this compound increased in the presence of a permeation enhancer, sodium glycodeoxycholate.
Fig. 6.
Co-incorporation of solubilizers provides significantly improved continuous release of fenretinide from Eudragit® RL PO mucoadhesive patches after buccal administration in rabbits. a Effect of co-incorporation of mixed solubilizers (0 (△) and 20 wt% Tween® 80 + 40 wt% sodium deoxycholate (▲)) on in vivo release of fenretinide. b Comparison of in vitro (□) and in vivo (■) fenretinide release from solubilizers-free patches. c Comparison of in vitro (○) and in vivo (●) fenretinide release from 20 wt% Tween® 80 + 40 wt% sodium deoxycholate-loaded patches. In vitro release studies were conducted in simulated saliva (pH 6.8) containing 5% w/v sodium deoxycholate at 37°C. Fenretinide loading (theoretical) in all patch formulations was 5 wt%. Solubilizer-free patch formulation was prepared using triethyl citrate (20 wt%) as a plasticizer. Solubilizer-loaded patches were prepared without triethyl citrate. Symbols represent mean ± SE, n = 3 (in vitro) or 6 (in vivo).
Stability of Fenretinide
Fenretinide is a light- and oxygen-sensitive drug. From control studies (data not shown), it was found that fenretinide in aqueous solution is relatively more sensitive to ambient light than oxygen (~5 and 41% drug loss after 7 days of exposure of fenretinide/phosphate-buffered saline + 0.1% Tween® 20 solution to oxygen and light, respectively). Therefore, fenretinide solutions were protected from exposure to light and analyzed immediately by HPLC. In contrast, we observed excellent fenretinide stability in biodegradable polymers during in vitro drug release over a period of 28 days (20). In the current study, there was no observable degradation of fenretinide in Eudragit® polymer during in vitro and in vivo drug release (see Fig. S2). Finally, preliminary studies with solubilizer-loaded patches, which had been stored for 6 months at −20°C and then incubated in saliva at 37°C, did not show any evidence of drug degradation in the LC-MS analysis of saliva samples (data not shown). These data collectively suggest good stability of fenretinide in the formulations described here for intraoral administration.
CONCLUSIONS
This study demonstrates significant solubilization of fenretinide in aqueous medium by a variety of solubilizers. Tween® 20 and 80, Brij® 98, and sodium deoxycholate exhibited the highest fenretinide solubilization potential among the solubilizers studied. Oral mucoadhesive patches for delivery of fenretinide were developed and evaluated to optimize important formulation variables, including drug loading, polymeric matrix permeability of Eudragit®, and suitable solubilizers, and to obtain continuous release of fenretinide. Fenretinide/Eudragit® RL PO patches with 20 wt% Tween® 80 + 40 wt% sodium deoxycholate solubilizers appear to be an optimal continuous-release oral mucoadhesive patch formulation for future preclinical and clinical evaluation of intraoral site-specific continuous release of highly hydrophobic fenretinide.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Fanconi Anemia Research Fund (GRT 00016074), NCI R01 CA129609, and F30 DE020992. The project described was also supported by Award Number UL1RR025755 from the National Center for Research Resources. We thank Dr. Vernon Steele, National Cancer Institute, for providing us a gift sample of fenretinide. We also thank Merck, Evonik Degussa Corp., BASF., Lubrizol Corp., and Colorcon®, Inc., for the gift samples of fenretinide, Eudragit®, Soluplus®, Carbopol®, and HPMC polymers, respectively.
Footnotes
Electronic Supplementary Material The online version of this article (doi:10.1007/s11095-011-0489-3) contains supplementary material, which is available to authorized users.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Contributor Information
Kashappa-Goud H. Desai, Department of Pharmaceutical Sciences, University of Michigan, 428 Church St., Ann Arbor, Michigan, USA
Susan R. Mallery, Department of Oral Maxillofacial Surgery and Pathology, College of Dentistry and the Comprehensive Cancer Center and Solove Research Institute, The Ohio State University Columbus, Ohio, USA
Andrew S. Holpuch, Department of Oral Maxillofacial Surgery and Pathology, College of Dentistry and the Comprehensive Cancer Center and Solove Research Institute, The Ohio State University Columbus, Ohio, USA
Steven P. Schwendeman, Email: schwende@umich.edu, Department of Pharmaceutical Sciences, University of Michigan, 428 Church St., Ann Arbor, Michigan, USA.
REFERENCES
- 1. http://seer.cancer.gov/statfacts/html/oralcav.html.
- 2.Lefebvre JL. Current clinical outcomes demand new treatment options for SCCHN. Ann Oncol. 2005;16:VI7–VI12. doi: 10.1093/annonc/mdi452. [DOI] [PubMed] [Google Scholar]
- 3.Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 2008;87:14–32. doi: 10.1177/154405910808700104. [DOI] [PubMed] [Google Scholar]
- 4.Chen SY, Samuel W, Fariss RN, Duncan T, Kutty RK, Wiggert B. Differentiation of human retinal pigment epithelial cells into neuronal phenotype by N-(4-hydroxyphenyl)retinamide. J Neurochem. 2003;84:972–981. doi: 10.1046/j.1471-4159.2003.01608.x. [DOI] [PubMed] [Google Scholar]
- 5.Hail N, Kim HJ, Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11:1677–1694. doi: 10.1007/s10495-006-9289-3. [DOI] [PubMed] [Google Scholar]
- 6.Lippman SM, Lee JJ, Martin JW, El-Naggar AK, Xu XC, Shin DM, et al. Fenretinide activity in retinoid-resistant oral leukoplakia. Clin Cancer Res. 2006;12:3109–3114. doi: 10.1158/1078-0432.CCR-05-2636. [DOI] [PubMed] [Google Scholar]
- 7.Chiesa F, Tradati N, Grigolato R, Boracchi P, Biganzoli E, Crose N, et al. Randomized trial of fenretinide (4-HPR) to prevent recurrences, new localizations and carcinomas in patients operated on for oral leukoplakia: long-term results. Int J Cancer. 2005;115:625–629. doi: 10.1002/ijc.20923. [DOI] [PubMed] [Google Scholar]
- 8.Okuda T, Kawakami S, Higuchi Y, Satoh T, Oka Y, Yokoyama M, et al. Enhanced in vivo antitumor efficacy of fenretinide encapsulated in polymeric micelles. Int J Pharm. 2009;373:100–106. doi: 10.1016/j.ijpharm.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Kokate A, Li XL, Jasti B. Transport of a novel anti-cancer agent, fenretinide across Caco-2 monolayers. Invest New Drug. 2007;25:197–203. doi: 10.1007/s10637-006-9026-3. [DOI] [PubMed] [Google Scholar]
- 10.William WN, Lee JJ, Lippman SM, Martin JW, Chakravarti N, Tran HT, et al. High-dose fenretinide in oral leukoplakia. Cancer Prev Res. 2009;2:22–26. doi: 10.1158/1940-6207.CAPR-08-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conley B, O’Shaughnessy J, Prindiville S, Lawrence J, Chow C, Jones E, et al. Pilot trial of the safety, tolerability, and retinoid levels of N-(4-hydroxyphenyl) retinamide in combination with tamoxifen in patients at high risk for developing invasive breast cancer. J Clin Oncol. 2000;18:275–283. doi: 10.1200/JCO.2000.18.2.275. [DOI] [PubMed] [Google Scholar]
- 12.Reddy LH. Drug delivery to tumours: recent strategies. J Pharm Pharmacol. 2005;57:1231–1242. doi: 10.1211/jpp.57.10.0001. [DOI] [PubMed] [Google Scholar]
- 13.GuhaSarkar S, Banerjee R. Intravesical drug delivery: challenges, current status, opportunities and novel strategies. J Control Release. 2010;148:147–159. doi: 10.1016/j.jconrel.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg BD, Ai H, Blanco E, Anderson JM, Gao JM. Antitumor efficacy and local distribution of doxorubicin via intratumoral delivery from polymer millirods. J Biomed Mater Res A. 2007;81A:161–170. doi: 10.1002/jbm.a.30914. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Russell D, Conway BR, Batchelor H. Strategies and therapeutic opportunities for the delivery of drugs to the esophagus. Crit Rev Ther Drug. 2008;25:259–304. doi: 10.1615/critrevtherdrugcarriersyst.v25.i3.20. [DOI] [PubMed] [Google Scholar]
- 16.Mallery SR, Zwick JC, Pei P, Tong M, Larsen PE, Shumway BS, et al. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008;68:4945–4957. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ugalde CM, Liu ZF, Ren C, Chan KK, Rodrigo KA, Ling Y, et al. Distribution of anthocyanins delivered from a bioadhesive black raspberry gel following topical intraoral application in normal healthy volunteers. Pharm Res. 2009;26:977–986. doi: 10.1007/s11095-008-9806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shumway BS, Kresty LA, Larsen PE, Zwick JC, Lu B, Field HW, et al. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin Cancer Res. 2008;14:2421–2430. doi: 10.1158/1078-0432.CCR-07-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holpuch AS, Hummel GJ, Tong M, Seghi GA, Pei P, Ma P, et al. Nanoparticles for local drug delivery to the oral mucosa: proof of principle studies. Pharm Res. 2010;27:1224–1236. doi: 10.1007/s11095-010-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wischke C, Zhang Y, Mittal S, Schwendeman SP. Development of PLGA-based injectable delivery systems for hydrophobic fenretinide. Pharm Res. 2010;27:2063–2074. doi: 10.1007/s11095-010-0202-y. [DOI] [PubMed] [Google Scholar]
- 21.Dittgen M, Durrani M, Lehmann K. Acrylic polymers—a review of pharmaceutical applications. STP Pharm Sci. 1997;7:403–437. [Google Scholar]
- 22.Hombreiro-Perez M, Siepmann J, Zinutti C, Lamprecht A, Ubrich N, Hoffman M, et al. Non-degradable microparticles containing a hydrophilic and/or a lipophilic drug: preparation, characterization and drug release modeling. J Control Release. 2003;88:413–428. doi: 10.1016/s0168-3659(03)00030-0. [DOI] [PubMed] [Google Scholar]
- 23.Perioli L, Ambrogi A, Angelici F, Ricci M, Giovagnoli S, Capuccella M, et al. Development of mucoadhesive patches for buccal administration of ibuprofen. J Control Release. 2004;99:73–82. doi: 10.1016/j.jconrel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Wong CF, Yuen KH, Peh KK. An in-vitro method for buccal adhesion studies: importance of instrument variables. Int J Pharm. 1999;180:47–57. doi: 10.1016/s0378-5173(98)00402-5. [DOI] [PubMed] [Google Scholar]
- 25.Formelli F, Cavadini E, Luksch R, Garaventa A, Villani MG, Appierto V, et al. Pharmacokinetics of oral fenretinide in neuroblastoma patients: indications for optimal dose and dosing schedule also with respect to the active metabolite 4-oxo-fenretinide. Cancer Chemother Pharmacol. 2008;62:655–665. doi: 10.1007/s00280-007-0649-7. [DOI] [PubMed] [Google Scholar]
- 26.Formelli F, Luksch R, Cavadini E, Ponzoni M, Montaldo PG, Fossati Bellani F, et al. Phase I and pharmacokinetic evaluation of fenretinide in children with advanced neuroblastoma. Proc Am Assoc Cancer Res. 1998;39:322. [Google Scholar]
- 27.Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR, et al. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res. 2003;9:2032–2039. [PubMed] [Google Scholar]
- 28.Arai T, Benny O, Joki T, Menon LG, Machluf M, Abe T, et al. Novel local drug delivery system using thermoreversible gel in combination with polymeric microspheres or liposomes. Anticancer Res. 2010;30:1057–1064. [PubMed] [Google Scholar]
- 29.Petelin M, Sentjurc M, Stolic Z, Skaleric U. EPR study of mucoadhesive ointments for delivery of liposomes into the oral mucosa. Int J Pharm. 1998;173:193–202. [Google Scholar]
- 30.Shemer A, Amichai B, Trau H, Nathansohn N, Mizrahi B, Domb AJ. Efficacy of a mucoadhesive patch compared with an oral solution for treatment of aphthous stomatitis. Drugs R&D. 2008;9:29–35. doi: 10.2165/00126839-200809010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakkar AL. Solubilization of some steroid hormones in aqueous solutions of bile salts. J Pharm Sci. 1970;59:1499–1501. doi: 10.1002/jps.2600591030. [DOI] [PubMed] [Google Scholar]
- 32.Mithani SD, Bakatselou V, TenHoor CN, Dressman JB. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm Res. 1996;13:163–167. doi: 10.1023/a:1016062224568. [DOI] [PubMed] [Google Scholar]
- 33.Wiedmann TS, Kamel L. Examination of the solubilization of drugs by bile salt micelles. J Pharm Sci. 2002;91:1743–1764. doi: 10.1002/jps.10158. [DOI] [PubMed] [Google Scholar]
- 34.Samaha MW, Naggar VF. Micellar properties of non-ionic surfactants in relation to their solubility parameters. Int J Pharm. 1988;42:1–9. [Google Scholar]
- 35.Dutt GB. Rotational diffusion of hydrophobic probes in Brij-35 micelles: effect of temperature on micellar internal environment. J Phys Chem B. 2003;107:10546–10551. [Google Scholar]
- 36.Magee GA, French J, Gibbon B, Luscombe C. Bile salt/lecithin mixed micelles optimized for the solubilization of a poorly soluble steroid molecule using statistical experimental design. Drug Dev Ind Pharm. 2003;29:441–450. doi: 10.1081/ddc-120018379. [DOI] [PubMed] [Google Scholar]
- 37.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 38.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Smart JD. Drug delivery using buccal-adhesive systems. Adv Drug Deliv Rev. 1993;11:253–270. [Google Scholar]
- 40.De Araujo DR, Padula C, Cereda CMS, Tofoli GR, Brito RB, De Paula E, et al. Bioadhesive films containing benzocaine: correlation between in vitro permeation and in vivo local anesthetic effect. Pharm Res. 2010;27:1677–1686. doi: 10.1007/s11095-010-0151-5. [DOI] [PubMed] [Google Scholar]
- 41.Desai KGH, Kumar TMP. Preparation and evaluation of a novel buccal adhesive system. AAPS PharmSciTech. 2004;5:9. doi: 10.1208/pt050335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliv Rev. 2001;48:139–157. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 43.Jain V, Jain D, Singh R. Factors effecting the morphology of eudragit S-100 based microsponges bearing dicyclomine for colonic delivery. J Pharm Sci. 2011;100:1545–1552. doi: 10.1002/jps.22360. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Mehta KA, McGinity JW. Influence of plasticizer level on the drug release from sustained release film coated and hot-melt extruded dosage forms. Pharm Dev Technol. 2006;11:285–294. doi: 10.1080/10409230600767551. [DOI] [PubMed] [Google Scholar]
- 45.Sawant PD, Luu D, Ye R, Buchta R. Drug release from hydroethanolic gels. Effect of drug’s lipophilicity (log P), polymer-drug interactions and solvent lipophilicity. Int J Pharm. 2010;396:45–52. doi: 10.1016/j.ijpharm.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Acartürk F, Encan A. Investigation of the effect of different adjuvants on felodipine release kinetics from sustained release monolithic films. Int J Pharm. 1996;131:183–189. [Google Scholar]
- 47.Li CY, Zimmerman CL, Wiedmann TS. Solubilization of retinoids by bile salt/phospholipid aggregates. Pharm Res. 1996;13:907–913. doi: 10.1023/a:1016013414457. [DOI] [PubMed] [Google Scholar]
- 48.Cussler EL. Diffusion: mass transfer in fluid systems. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 49.Shin SC, Kim JY. Enhanced permeation of triamcinolone acetonide through the buccal mucosa. Eur J Pharm Biopharm. 2000;50:217–220. doi: 10.1016/s0939-6411(00)00101-6. [DOI] [PubMed] [Google Scholar]
- 50.Nicolazzo JA, Reed BL, Finnin BC. Buccal penetration enhancers—how do they really work? J Control Release. 2005;105:1–15. doi: 10.1016/j.jconrel.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Nicolazzo JA, Reed BL, Finnin BC. Enhanced buccal mucosal retention and reduced buccal permeability of estradiol in the presence of padimate O and Azone®: a mechanistic study. J Pharm Sci. 2005;94:873–882. doi: 10.1002/jps.20240. [DOI] [PubMed] [Google Scholar]
- 52.Junginger HE, Hoogstraate JA, Verhoef JC. Recent advances in buccal drug delivery and absorption—in vitro and in vivo studies. J Control Release. 1999;62:149–159. doi: 10.1016/s0168-3659(99)00032-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.