Abstract
Chronic neck pain is one of the most common musculoskeletal disorders in the US. Although biomechanical and clinical studies have implicated the facet joint as a primary source of neck pain, specific cellular mechanisms still remain speculative. The purpose of this study was to investigate whether a mediator (ATF4) of the integrated stress response (ISR) is involved in facet-mediated pain. Holtzman rats underwent C6/C7 facet joint loading that produces either painful (n=16) or nonpainful (n=8) responses. A sham group (n=9) was also included as surgical controls. Behavioral sensitivity was measured and the C6 DRGs were harvested on day 7 to evaluate the total and neuronal ATF4 expression. In separate groups, an intra-articular ketorolac injection was administered either immediately (D0 ketorolac) or 1 day (D1 ketorolac) after painful facet joint loading. Allodynia was measured at days 1 and 7 after injury to assess the effects on behavioral responses. ATF4 and BiP (an indicator of ISR activation) were separately quantified at day 7. Facet joint loading sufficient to elicit behavioral hypersensitivity produced a 3-fold increase in total and neuronal ATF4 expression in the DRG. After ketorolac treatment at the time of injury, ATF4 expression was significantly (p<0.01) reduced despite not producing any attenuation of behavioral responses. Interestingly, ketorolac treatment at day 1 significantly (p<0.001) alleviated behavioral sensitivity at day 7, but did not modify ATF4 expression. BiP expression was unchanged after either intervention time. Results suggest that ATF4-dependent activation of the ISR does not directly contribute to persistent pain, but may sensitize neurons responsible for pain initiation. These behavioral and immunohistochemical findings imply that facet-mediated pain may be sustained through other pathways of the ISR.
Keywords: ISR, ATF4, facet joint, pain, ketorolac, BiP
Neck pain affects up to 70% of individuals in their life span, and is one of the most commonly reported origins of musculoskeletal pain in the general population (Côté et al., 1998; Natvig et al., 2010). In particular, the facet joint is one of the most-common sources of pain in the cervical spine (Barnsley et al., 1995; Lord et al., 1996; Manchikanti 1999). The facet joint capsule contains not only mechanoreceptors for proprioception but also nociceptive fibers that provide a means for transmitting pain signals (Cavanaugh et al., 1996; Inami et al., 2001; McLain 1994). Mechanical loading of the facet joint, in particular stretch of its capsule, has been demonstrated as a primary mechanism of pain generation in biomechanical and in vivo studies (Cavanaugh et al., 1996; Cusick et al., 2001; Deng et al., 2000; Gore et al., 1987; Grauer et al., 1997; Ito et al., 2004; Lee et al., 2004a; Ono et al., 1997; Panjabi et al., 2004; Pearson et al., 2004; Siegmund et al., 2001). Indeed, local anesthetic blocks to the nerves of the facet joint can alleviate, or even abolish, pain in up to 62% of chronic pain cases from mechanical neck injury (Aprill and Bogduk 1992; Yoganandan et al., 1998). Despite the strong biomechanical and clinical data implicating the facet joint and its capsule’s involvement in pain, the cellular mechanisms related to pain from injury to this joint still remain speculative.
Inflammatory processes contribute to persistent pain through a variety of mediators (Kawakami and Weinstein 1986; McMahon et al., 2005; Millan 1999). Several different animal models of painful joint inflammation have reported cytokine upregulation and glial activation in the DRG and spinal cord (Fenzi et al., 2001; Lee et al, 2008; Miyagi et al., 2006). Glial activation can alter neuronal signaling and can also cause excessive glutamate release (Kawakami and Weinstein 1986). A number of in vitro and in situ studies have shown that the release of glutamate and cytokines can directly induce the integrated stress response (ISR) in neurons and other cells, which is critical for cell development and function (Cardozo et al., 2005; Kharroubi et al., 2004; Oyadomari et al., 2001; Shim et al., 2004). Despite mounting evidence linking inflammatory responses to activation of the ISR and the known role of inflammation in pain (Hartwig et al., 2003; Inglis et al., 2005; Lee et al., 2008; Markowitz et al., 2007), there is still very limited information on the role of the ISR in facet- or joint-mediated pain.
The integrated stress response, also known as the endoplasmic reticulum (ER) stress response, is a common cellular response to disruption of homeostasis in injury or disease states (Dong et al., 2008; Harding and Ron, 2002; Katayama et al., 2004; Rao and Bredesen, 2004). The ISR is a tripartite pathway initiated by three ER-localized proteins, PKR-like endoplasmic reticulum stress kinase (PERK), inositol requiring enzyme (IRE1a), and activating transtcription factor 6. Activation of the ISR culminates in increased expression of the ISR binding protein (BiP) which plays a major role in the repair of unfolded and mis-folded proteins (Schröder and Kaufman 2005). In addition to BiP, each pathway activates proteins that enhance protein folding, establish homeostasis and attenuate translation. The latter is a direct consequence of activation of the PERK pathway by phosphorylation and subsequent attenuation of eukaryotic initiation factor 2α (eIF2α), which promotes translation initiation. Interestingly, phosphorylation of eIF2a favors translation of activating transcription factor 4 (ATF4) which has been shown to promote apoptotic cell death via transactivation of C/EBP-homologous protein (CHOP) (Cherasse et al., 2007; Ohoka et al., 2005; Yamauchi et al., 2007). In neurons, ATF4 can contribute to long-term synaptic plasticity in mice (Chen et al., 2003). Sustained modification of spinal neurons has also been observed in our rat model of facet-mediated pain (Quinn et al., 2010). That model also exhibits spinal neuroinflammation and disrupted homeostasis in the injured afferents, as well as increased glutamate activity in the spinal cord and ISR activation in the DRG after facet injury in association with sustained behavioral sensitivity (Dong et al., 2008; Dong and Winkelstein 2010; Lee et al., 2008; Quinn et al., 2010). Although we have previously observed increases in neuronal BiP in the DRG after painful facet joint injury, no studies have investigated the extent and pathway of ISR activation in facet-mediated pain.
The objectives of this study were to investigate whether ATF4 in injured afferents is involved in behavioral hypersensitivity that develops after painful facet joint injury. As such, facet capsule stretch was applied in our rat model at magnitudes that do and do not produce sustained behavioral hypersensitivity in the forepaw (Dong et al., 2008; Dong and Winkelstein, 2010; Lee and Winkelstein, 2009) to characterize ATF4 expression in the affected DRG after joint injury and to determine if painful joint loading is associated with the up-regulation of ATF4 expression. In addition, to determine whether any changes in ATF4 are related to behavioral sensitivity, additional studies were performed assessing ATF4 and BiP expression after painful loading conditions with an NSAID treatment. Ketorolac is a non-steroidal anti-inflammatory drug (NSAID) that reduces pain by non-selectively inhibiting COX activity, which leads to diminished production of prostaglandins (Cassinelli et al., 2008; Dogan et al., 2004; Turner et al., 2011). Specifically, ketorolac injection reduces joint inflammation and postoperative pain in clinical and animal models (Convery et al., 1998; Ng et al., 2006; Swift et al., 1998). Therefore, in this study, ketorolac was administered to the injured joint either immediately after injury at day 0 or at 1 day after painful loading, in separate groups. In those studies, BiP expression was evaluated along with ATF4 to assess the effects of treatment on ISR activation.
Experimental Procedures
Animal Care & Surgical Procedures
All procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (Zimmerman 1983). Male Holtzman rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 350–425 grams were housed under USDA- and AAALAC-compliant conditions with free access to food and water and a 12-12 hour light-dark cycle.
All surgical procedures were performed under inhalation anesthesia (4% isoflurane for induction, 2.5% for maintenance) and have been previously described (Dong et al., 2008; Dong and Winkelstein, 2010; Lee et al., 2004b). Briefly, an incision was made from the base of the skull to the second thoracic vertebra and the bilateral C6/C7 facet joints were exposed by removing the surrounding soft tissue and musculature. The C6 and C7 laminae were rigidly attached to microforceps of a customized loading device that imposed a controlled joint injury by displacing the C6 vertebra rostrally, holding it for 30 seconds and returning to its initial unloading position, while the C7 vertebra remained stationary. Two different C6/C7 facet joint distractions were applied separately to either induce (0.5mm; painful n=16) or not induce (0.2mm; nonpainful n=8) behavioral hypersensitivity, based on previous studies (Dong et al., 2008; Dong and Winkelstein, 2010). Sham procedures were also performed as a surgical control with no applied joint distraction (0mm; sham n=9) but all other surgical procedures. The magnitude of the injury severity was measured by quantifying vertebral and joint capsule distractions during joint loading. Polystyrene particles were affixed to the C6 and C7 laminae and joint capsule for motion tracking. Joint distraction was defined as the maximum displacement of C6 laminae relative to C7, and the capsule distraction was defined as the average resultant displacement of the rostral edge of its capsule relative to the caudal edge. As an additional biomechanical measure of injury severity, maximum principal strain for the capsule was calculated using engineering software (LS-DYNA) (Dong and Winkelstein, 2010; Lee et al., 2004b; Weisshaar et al, 2010; Winkelstein et al., 2000). Joint displacements and capsule strains were compared between painful and nonpainful groups using an unpaired t-test.
A subset of rats from the painful group was randomly selected to receive intra-articular administration of ketorolac (12μg in 10μL saline) either immediately (D0 ketorolac; n=4) or at 1 day (D1 ketorolac; n=5) after painful joint loading. Joint injection procedures were performed under inhalation anesthesia (2.5% isoflurane) and ketorolac (Sigma-Aldrich; St. Louis, MO) was administered in the bilateral C6/C7 facet joints using a 10μL syringe with a 33G beveled needle (Hamilton; Reno, NA). The needle was gently inserted into the facet joint by piercing through its capsule in the dorsal medial region with the injection bolus delivered slowly.
Behavioral Assessment
Behavioral sensitivity was assessed by measuring bilateral mechanical hyperalgesia in the forepaws on days 1, 3, 5, and 7 after painful and nonpainful distraction injury or sham procedures. Prior to surgery, rats were also assessed for hyperalgesia to provide a baseline measurement to serve as an unoperated control response for each rat. Methods to measure hyperalgesia were adopted from Chaplan’s up/down method and have been previously reported and validated (Chaplan et al., 1994; Decosterd and Woolf, 2000; Hubbard and Winkelstein, 2005; Lee et al., 2008). The response threshold was measured using increasing strengths of von Frey filaments (Stoelting, Wood Dale, IL), ranging from 0.6 to 26g, to stimulate the forepaw. The lowest strength filament to provoke a positive withdrawal response was taken as the response threshold if a withdrawal response was also confirmed for application of the next higher filament. Each testing session consisted of 3 rounds of 5 stimulations to each forepaw, with at least a 10 minute rest-period separating each round. The left and right forepaw responses for each rat were averaged for each group on each testing day. A repeated measures ANOVA with Bonferroni correction compared temporal hyperalgesia between painful, nonpainful and sham groups. Additionally, hyperalgesia for each postoperative day was also compared across groups using a one-way ANOVA.
For the rats receiving ketorolac treatment, forepaw mechanical allodynia was measured at baseline, day 1 and day 7 to assess and determine if the onset of behavioral sensitivity was consistent with previous reports using the same distraction magnitude (Dong et al., 2008; Dong and Winkelstein, 2010; Lee et al., 2004), and to define the behavioral sensitivity on the day when DRG tissue was assayed (day 7). For each test session, forepaw mechanical allodynia was performed in 3 rounds for each paw. Each round consisted of 10 tactile stimulations to the plantar surface of each forepaw using a 4g von Frey filament (Stoelting) that has been shown to robustly detect allodynia after injury in the cervical spine (Dong and Winkelstein 2010; Hubbard and Winkelstein 2008; Lee et al., 2004; Rothman et al., 2005). For each session, the total number of paw withdrawals was counted for each paw of each rat and responses for the left and right paws were averaged for each group. Mechanical allodynia for D0 ketorolac and D1 ketorolac were compared using an ANOVA with repeated measures for the two time points.
DRG Harvest & Immunohistochemistry
The C6 DRGs on the left side were assayed on day 7 from all groups to assess ATF4 expression and its co-localization with neurons, using fluorescent confocal microscopy. Rats were deeply anesthetized and underwent transcardiac perfusion with 250ml of phosphate buffered saline (pH 7.4), followed by 300ml of 4% paraformaldehyde. DRGs were removed and post-fixed in 4% paraformaldehyde for 2-4 hours before being transferred to 50% ethanol for overnight incubation. DRG samples were dehydrated in a series of graded ethanol solutions and then paraffin-embedded. Thin (10μm) axial tissue sections were mounted on APES-coated slides and incubated at 55°C overnight before deparaffinization and rehydration, as previously described (Dong et al., 2008). Antigen retrieval was performed by incubating slides at 95°C in a water bath for 1 hour using target retrieval solution (Dako, Carpinteria, CA). Sections were washed 3 times in PBS for 10 minutes each and blocked using 10% normal goat serum (Chemicon International; Billerica, MA) with 0.3% triton X-100 for 2 hours at room temperature. Sections were incubated overnight in rabbit polyclonal ATF4/CREB2 (1:200; Santa Cruz Biotechnology; Santa Cruz, CA) and mouse microtubule associated protein (MAP2; 1:200; Covance Research Products; Princeton, NJ); MAP2 is a neuronal marker that stains the soma and dendrites of neuronal cells (Jalava et al., 2007; Matus et al., 1981). Sections were then washed with PBS and incubated in Alexa 488 goat anti-rabbit and Alexa 546 goat anti-mouse (1:1000; Invitrogen; Carlsbad, CA) for 2 hour in the dark. After 3 rounds of 5 minutes of washing, slides were mounted using anti-fade gel (BioMeda; Foster City, CA). Negative controls with no primary antibody as well as tissue sections from naïve un-operated rats were also included to verify staining methods.
Total and neuronal ATF4 expression in the DRG were separately analyzed using quantitative densitometry and compared between groups. Four tissue sections from each DRG were imaged at 40X magnification using a Zeiss LSM 510 confocal microscope (Carl Zeiss; Thornwood, NY), equipped with Argon, HeNe and Coherent Chameleon fs-pulsed NIR lasers. Total ATF4 expression in the DRG was measured as the percentage of pixels above a defined threshold, which was chosen based on expression in normal tissue (Lee et al., 2004; Rothman and Winkelstein, 2010). The colocalization of ATF4 with MAP2 was defined as areas in the DRG that were positive for both ATF4 and MAP2 staining above the defined threshold, chosen based on normal tissue; it was calculated as the percentage of pixels that were positive for both markers relative to the total number of pixels positive for ATF4 in each DRG sample. Each of the total and neuronal expression of ATF4 was averaged for all rats in each injury group and normalized to sham levels. A one-way ANOVA with post hoc Bonferroni correction was used to compare ATF4 immunoreactivity between groups (painful, nonpainful, D0 ketorolac, D1 ketorolac).
Both ATF4 and BiP immunoreactivity were evaluated at day 7 after injury using samples from the matched C6 DRGs from each rat in the D0 ketorolac and D1 ketorolac groups receiving ketorolac treatment. Tissue from the sham group was also included in the BiP assay as a control. Mouse monoclonal antibodies to BiP (clone 40, 1:10,000; BD BioSciences; San Diego, CA) and MAP2 (1:100; Covance Research Products) were used, followed by sequential secondary incubations of Alexa 488 F’ab goat anti-mouse and Alexa 568 F’ab goat anti-mouse (1:1000; Invitrogen). Using the same quantification methods as described above for ATF4, both total and neuronal ATF4 and BiP expression in the DRG for each group were quantified, averaged, and compared between D0 ketorolac, D1 ketorolac and sham using one-way ANOVA, for each marker separately.
Results
There was no visual or biomechanical evidence of rupture of the facet capsule for any rat in this study. The mean applied vertebral distraction was 0.49±0.09mm in the painful group and 0.19±0.03mm in the nonpainful group, with corresponding average capsule distractions of 0.34±0.08mm and 0.15±0.03mm. The painful and nonpainful groups underwent significantly different vertebral (p<0.001) and capsule (p<0.001) distractions. Similarly, the average maximum principal strain in the capsule for painful loading (31.2±14.0%) was significantly higher (p=0.047) than that for nonpainful loading (18.5±9.7%) of the joint. Although these biomechanical metrics indicate that the painful and nonpainful groups underwent significantly different injuries, there was no difference in any of biomechanical parameters defining the severity of joint loading for either of the injury groups receiving ketorolac treatment, supporting that both of the D0 ketorolac and D1 ketorolac treatment groups received similar injuries.
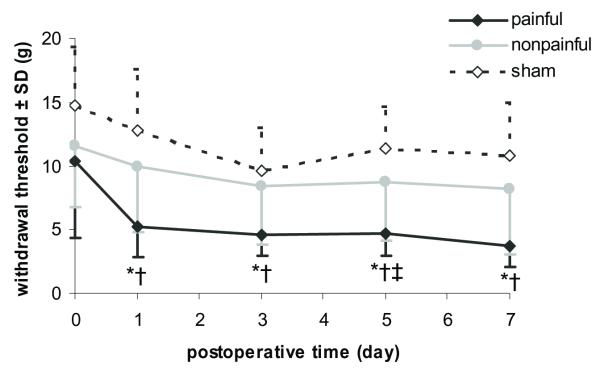
Tactile hypersensitivity in the forepaw was induced by facet joint distraction in the painful group only, with a significant (p<0.01) reduction in the threshold for paw withdrawal in that group compared to both nonpainful and sham groups as early as day 1 after injury (Fig. 1). Although hyperalgesia was immediate (day 1) after painful joint loading and the reduction in withdrawal threshold was sustained through the entire testing period until day 7, the threshold responses induced by sham and nonpainful joint distraction remained at baseline levels for all postoperative days (Fig. 1). The withdrawal threshold for nonpainful and sham was not significantly different from each other. Yet, the threshold response for both groups was significantly (p<0.03) higher than the painful group on all postoperative days (Fig. 1).
Fig. 1. Mechanical hyperalgesia as measured by the average response threshold to von Frey filament stimulation in the forepaw.
Increase sensitivity corresponds to a decreased response threshold. Painful distraction significantly reduced thresholds below nonpainful distraction (*p<0.002) and sham controls (†p<0.001) for all testing days. Nonpainful and sham responses were only significantly (‡p=0.02) different from each other on postoperative day 5.
For both groups of rats that underwent painful joint distraction and also received ketorolac treatment, mechanical allodynia was developed at day 1 regardless of the timing of treatment (Fig. 2). Both groups exhibited a greater than 4-fold increase in allodynia over responses of the sham controls at day 1, and there was no difference in allodynia between the D0 ketorolac and D1 ketorolac groups at that time point (Fig. 2). However, at day 7 after treatment, mechanical allodynia was significantly (p<0.001) reduced in the D1 ketorolac group only while remaining unchanged in the group receiving treatment at the time of injury (D0 ketorolac) (Fig. 2).
Fig. 2. Forepaw mechanical allodynia (MA) for D0 ketorolac and D1 ketorolac groups at days 1 and 7 after injury.
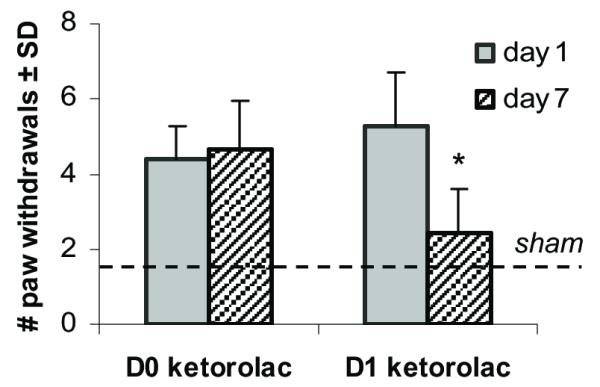
Increase sensitivity corresponds to a higher number of paw withdrawals. The dashed line indicates the average sham response. There was no significant difference between d1 and d7 MA for D0 ketorolac. However, following D1 ketorolac treatment, mechanical allodynia was significantly (*p=0.006) reduced to almost sham levels by day 7. The MA response for both treatment groups at day 1 was not significantly different from each other.
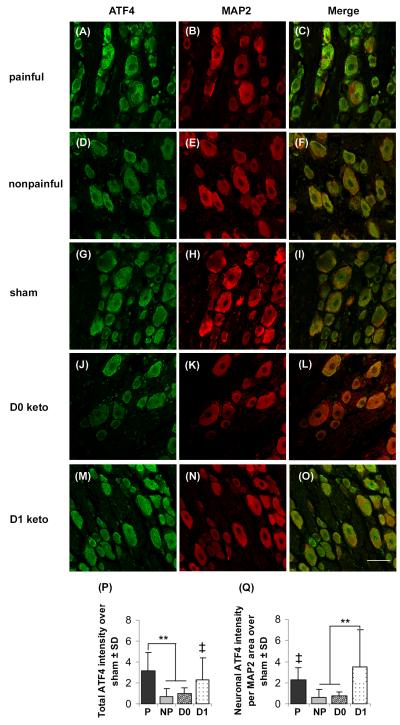
Mirroring the behavioral outcomes, ATF4 expression at day 7 in the DRG resulting from the nonpainful distraction was not different from sham, but ATF expression for painful joint distraction was significantly higher than both the nonpainful (p<0.05) and sham (p<0.01) responses (Fig. 3). Both total and neuronal ATF4 for painful group was more than twice that in the nonpainful group (Figs. 3P and 3Q). Unlike the behavioral outcomes, ketorolac treatment at the time of injury (D0 ketorolac) reduced total ATF4 expression but this was not the case for the later treatment time (D1 ketorolac). Total ATF4 expression in the D0 ketorolac group was significantly (p<0.001) lower than the painful distraction without any treatment and reduced to levels that were comparable to those after the nonpainful distraction (Fig. 3). However, total ATF4-immunoreactivity evident after D1 ketorolac treatment was comparable the ATF4 levels in the painful group, and was significantly (p=0.006) elevated above nonpainful responses (Fig. 3). Neuronal ATF4 expression in the DRG demonstrated similar responses as did the total ATF4 expression, with D0 ketorolac showing no difference from the nonpainful distraction but the D1 ketorolac group exhibiting a significant (p<0.001) 3-fold increase over nonpainful levels (Fig. 3).
Fig. 3. ATF4 expression (green) in neurons (red; MAP2) of the DRG.
ATF4 immunostaining was increased after painful distraction (A-C) compared to the nonpainful (D-F) and sham (G-I). Although D0 ketorolac (J-L) reversed such elevation, D1 ketorolac (M-O) showed no change in the ATF4 expression. Quantification of total ATF4 pixel intensity (P) showed a significantly higher amount of ATF4-immunoreactivity after painful distraction (**p<0.001); total ATF4 for D1 ketorolac was significantly higher than the nonpainful (‡p=0.006). Similarly, neuronal ATF4 intensity normalized to MAP2 area (Q) also quantified a significantly increased expression in painful compared to nonpainful (‡p=0.04). Neuronal ATF4 expression remained significantly elevated after D1 ketorolac (**p<0.001), but not after the D0 ketorolac treatment. Data are presented as fold increase over sham; the scale bar (50μm) applies to all panels.
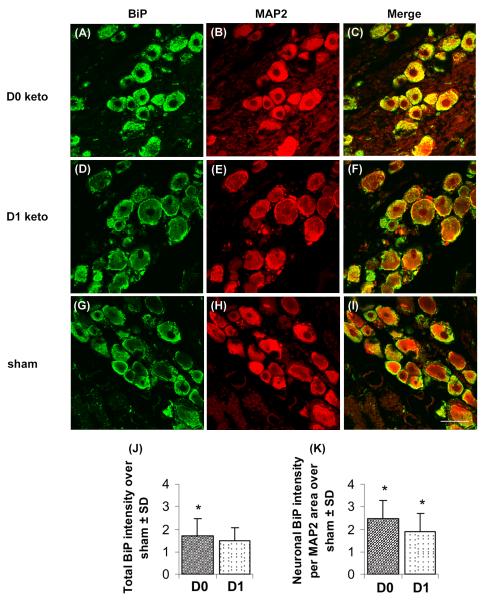
Despite differences in the behavioral responses and ATF4 expression at day 7, the amount of total and neuronal BiP expressed in the DRGs at day 7 was not different between the two treatment groups (D0 ketorolac, D1 ketorolac) (Fig. 4). Total BiP expression for D0 ketorolac was significantly greater (p=0.006) than the levels in sham, but was not different from those for D1 ketorolac (Fig. 4). However, neuronal BiP expression was significantly greater (p<0.05) for both D0 ketorolac and D1 ketorolac than sham (Fig. 4).
Fig. 4. BiP expression (green) in neurons (red; MAP2) of the DRG.
BiP immunoreactivity was more prominently expressed in D0 ketorolac (A-C) compared to D1 ketorolac (D-F) and sham controls (G-I). Quantification of total BiP intensity (J) and neuronal BiP intensity normalized to MAP2 area (K) showed no significant difference between the two treatment groups. Asterisk (*p<0.05) sign indicates significant elevation over sham; data are presented as fold increase over sham. The scale bar (50μm) applies to all panels.
Discussion
This study demonstrates that the differences in forepaw sensitivity produced by painful and nonpainful mechanical loading of the facet joint are also associated with similar graded changes in ATF4 expression in the DRG (Figs. 1 and 3). Previous studies with the same pain model have shown that the behavioral sensitivity measured in the forepaw is consistent with that which is also produced in the back of the neck (Lee et al., 2008, 2009). While whiplash and facet joint pain are most commonly reported to occur in the back of the neck along the “coat hanger” distribution, more than 60% of patients also report widespread sensitivity radiating to other parts of the body, including the trunk and upper extremity (Curatolo et al., 2001; Hincapié et al., 2010; Koelbaek Johansen et al., 1999; Mayou et al., 1996; Scott et al., 2005). Further, the behavioral and ATF4 responses were also modulated differentially by intra-articular ketorolac injection (Fig. 3). Intra-articular ketorolac administration was selected based on clinical evidence of its improved effectiveness in offering postoperative relief in knee arthroscopy surgery (Convery et al., 1998; Ng et al., 2006); yet, oral administration of NSAIDs is more common and should be the focus of future studies with this painful joint model.
The robust increase in total and neuronal ATF4 expression observed after painful loading compared to the nonpainful group (Fig. 3) suggests that behavioral hypersensitivity may be sustained, in part, through ATF4-mediated pathways of the ISR in the affected neurons of the C6/C7 facet joint. Induction of ATF4 in spinal neurons has been shown to play an important role in programmed cell death in a spinal cord ischemia model in the rabbit, and a number of in vitro studies found a direct relationship between upregulation of ATF4 and decreased neuronal and liver cell survival (Ohoka et al., 2005; Jousse et al., 2007; Magne et al., 2011; Yamauchi et al., 2007). Collectively, those studies point to the apoptotic characteristics of ATF4 activation. As such, findings from our study suggest that cell death in the DRG may be induced by prolonged activation of the ATF4 pathway of the integrated stress response after painful facet joint loading. However, ATF4 also activates several target genes of the ISR that promote cell survival (Harding et al., 2000, 2003; Lu et al., 2004), which highlights the need for future investigations to examine the specific cell fate for ATF4-positive neurons in this model. Previous studies with similar joint injury scenarios have shown that excessive stretch to this joint’s ligament can cause microstructural damage and axonal dysfunction in the capsule, activate and injure the innervating nociceptors, and also induces neuronal hyperexcitability in the spinal cord (Chen et al., 2006; Kallakuri et al., 2008; Markowitz et al., 2007; Quinn et al., 2007, 2010). Given that the cell bodies of the afferents of the facet joint reside in the DRG, it is not surprising to find that noxious facet joint loading sufficient to produce sustained behavioral hypersensitivity is associated with cellular responses in the DRG that could potentially alter their normal functioning via protein misfolding or even cell death.
Significant upregulation of BiP expression was observed in the DRG previously in the same model after painful joint injury (Dong et al., 2008), suggesting that the ISR is activated in the injured afferents of the facet joint. Findings from the current study confirm such activation, with a significant increase in both total and neuronal BiP expression after the D0 ketorolac treatment that failed to attenuate behavioral hypersensitivity (Figs. 2 and 4). Although treatment with ketorolac at day 1 (D1 ketorolac) did attenuate behavioral sensitivity and reduce it to nonpainful and sham levels (Fig. 2), the BiP expression at day 7 in that group was not different from that observed after D0 ketorolac treatment (Fig. 4). In contrast, ATF4-immunoreactivity was significantly reduced in that group (Fig. 3), suggesting that although ATF4 activation may have been blocked by the D0 ketorolac treatment, the cellular integrated stress response was still activated. Taken together with the observed maintenance of behavioral sensitivity in that group (Fig. 2), these data imply that a different arm of the ISR, one which is independent of ATF4, may be contributing to the maintenance of behavioral hypersensitivity. In an in vitro model mimicking endoplasmic reticulum stress in human chondrocytes, it was suggested that ATF4 is involved in joint diseases, such as osteoarthritis (Hamamura et al., 2009). However, results from current study indicate that ATF4 might not be directly responsible for the persistence of pain, despite being activated after painful joint loading. Since the downstream effectors of the ISR affect the broad aspects of cell fate (Lin et al., 2007; Pidoux and Armstrong 1993), in addition to the ATF4 response, additional work is needed to also probe the expression of the other pathways of the ISR, the IRE1α and ATF6 pathways, in order to understand which of them may be responsible for the maintenance of pain. An in vitro study using human embryonic kidney cells found that the stress response mediated by the PERK pathway persisted even after 30 hours after stress onset in culture, whereas IRE1 was quickly attenuated within 8 hours with ATF6 attenuation being slightly delayed (Lin et al., 2007). Since each of these signaling pathways varies remarkably with time, more studies are necessary to delineate the time profile of all three pathways to identify the differential contributions of various aspects of ISR to facet-mediated pain.
Our observations support activation of the ISR in facet mediated injury; however, dampening of the ISR pathways specifically following insult or injury has not been investigated. Interestingly, ATF4 can be activated and repressed by mechanisms independent of the ISR. Enhanced translation of ATF4 may also occur when eIF2α is phosphorylated by double-stranded RNA-activated protein kinase (PKR) and general control non-depressible-2 (GCN2). In addition to double-stranded RNA, PKR can be activated by interferon (Wek et al., 2006) which could be relevant in this model since interferon has been shown to sensitize nociceptive neurons and contribute to the generation of joint pain (Cuellar et al., 2009; Li et al., 2011; van Baarsen et al., 2010). GCN2 is activated under conditions of nutrient deprivation and is thought to induce apoptosis more readily than activation by PERK or PKR (Muaddi et al., 2010). Taking those findings in the literature together with the results of the current study, suggests it would be useful to determine the activity of these kinases in facet-mediated pain following both painful and nonpainful distractions. Finally, the ISR may be active while ATF4 is downregulated. One can envision that global translation inhibition may occur in the absence of enhanced ATF4 translation when amino acids are scarce. In addition, there is evidence that ATF4 is downregulated at the mRNA level by TRIF-mediated signaling through toll-like receptors 3 and 4 (Woo et al., 2009). This latter hypothesis may explain our observation of BiP remaining elevated suggestive of maintained ISR activation, while ATF4 levels are no longer elevated after D0 ketorolac treatment.
Biomechanical and behavioral outcomes from this study not only support that transient whiplash-like loading to the capsular ligament can elicit persistent pain behaviors, but also further indicate that the biomechanical threshold for activation of cellular responses might actually be between 20 and 30% strain. Consistent with the biomechanical data reported here, studies using human cadavers to simulate whiplash loading reported strains ranging from 19 to 40% at accelerations of 3.5g-8g at C6/C7 capsule, while the physiologic range was less than 11% (Kaneoka et al., 1999; Panjabi et al., 1998; Pearson et al., 2004; Yoganandan et al., 2002). Similarly, nociceptors innervating the facet capsule were reported to be activated at strains above 11% (Lu et al., 2005; Yoganandan et al., 1998). An excessive stretching of facet capsule beyond its physiologic range can result in altered axonal morphology in the nerve fibers in the facet capsule (Kallakuri et al., 2008). With an imposed strain magnitude more than twice the physiologic tolerance, it can be inferred that axonal damage might also be occurring in this study. A number of in vivo and in vitro studies reported that axonal injury can trigger the protective mechanisms of ISR and lead to changes in membrane potential and transient depolarization (Cardozo et al., 2005; Galbraith et al., 1993; Kharoubi et al., 2004; Penas et al., 2011). Taken together with the literature, findings from this study suggest that although ATF4 may not be directly responsible for the maintenance of pain, it may be a regulator for, or a potential contributor to, the enhanced neuronal excitability observed in the spinal cord (Quinn et al., 2010; Steiger et al., 2004). Certainly, additional work is necessary to investigate the relationship between ATF4 activation and neuronal excitation in the spinal cord.
Conclusions
The data presented here demonstrate that facet joint loading sufficient to induce heightened behavioral sensitivity can also trigger ATF4 activation in the DRG, but the persistence of pain may not be directly sustained by ATF4-dependent pathways of ISR. Although ketorolac treatment given at the time of joint injury did not attenuate behavioral sensitivity, it did reverse the ATF4 response in the DRG despite a lack of change in BiP expression. These findings suggest that pain may be maintained through other pathways of ISR, which mandates further investigation to fully outline the timing and extent ISR activation that may contribute to the initiation and maintenance of pain. Nevertheless, results from this study suggest a role for cellular stress response in the persistence of facet-mediated pain.
Highlights.
Activating transcription factor 4 (ATF4) is significantly upregulated in the DRG at day 7 after painful joint distraction
Intra-articular joint injection using an NSAID (ketorolac), given only at the time of injury (day 0), reversed the ATF4 upregulation but did not modify the behavioral hypersensitivity
Ketorolac, regardless of the timing of treatment, had no effect on BiP expression in the DRG, despite a significantly reduced ATF4 response after day 0 treatment
Acknowledgements
This work was funded in part by grants from the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (#AR056288) and the Catharine D. Sharpe Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aprill C, Bogduk N. The prevalence of cervical zygapophyseal joint pain. Spine. 1992;17:744–747. doi: 10.1097/00007632-199207000-00003. [DOI] [PubMed] [Google Scholar]
- Barnsley L, Lord S, Bogduk N. Comparative local anesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain. 1993;55:99–106. doi: 10.1016/0304-3959(93)90189-V. [DOI] [PubMed] [Google Scholar]
- Barnsley L, Lord SM, Wallis BJ, Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20(1):20–25. doi: 10.1097/00007632-199501000-00004. discussion 26. [DOI] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β–cells. Diabetes. 2005;54:452–461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- Cassinelli EH, Dean CL, Garcia RM, Furey CG, Bohlman HH. Ketorolac use for postoperative pain management following lumbar decompression surgery: a prospectice, randomized, double-blinded, placebo-controlled trial. Spine. 2008;33:1313–1317. doi: 10.1097/BRS.0b013e31817329bd. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JM, Ozaktay AC, Yamashita HT, King AI. Lumbar facet pain: biomechanics, neuroanatomy, and neurophysiology. J Biomech. 1996;29:1117–1129. doi: 10.1016/0021-9290(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen C, Lu Y, Kallakuri S, Patwardhan A, Cavanaugh JM. Distribution of A-delta and C-fiber receptors in the cervical facet joint capsule and their response to stretch. J Bone Joint Surg Am. 2006;88:1807–1816. doi: 10.2106/JBJS.E.00880. [DOI] [PubMed] [Google Scholar]
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, Yonan AL, Vronskaya S, Grody MB, Cepeda I, Gilliam TC, Kandel ER. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;239:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Cherasse Y, Maurin AC, Chaveroux C, Jousse E, Carraro V, Parry L, Deval C, Chambon C, Fafournoux P, Bruhat A. The p300/CBP-associated factor (PCAF) is a cofactor of ATF4 for amino acid-rgulated transcription of CHOP. Nuclic Acids Res. 2007;35:5954–5965. doi: 10.1093/nar/gkm642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convery PN, Milligan KR, Quinn P, Scott K, Clarke RC. Low-dose intra-articular ketorolac for pain relief following arthroscopy of the knee joint. Anaesthesia. 1998;53:1125–1129. doi: 10.1046/j.1365-2044.1998.00582.x. [DOI] [PubMed] [Google Scholar]
- Côté P, Cassidy J, Carroll L. The Saskatchewan health and back pain survey: the prevalence of neck pain and related disability in Saskatchewan adults. Spine. 1998;23:1689–1698. doi: 10.1097/00007632-199808010-00015. [DOI] [PubMed] [Google Scholar]
- Cuellar JM, Scuderi GJ, Cuellar VG, Golish SR, Yeomans DC. Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J Bone Joint Surg Am. 2009;91:2013–2020. doi: 10.2106/JBJS.H.00835. [DOI] [PubMed] [Google Scholar]
- Cusick JF, Pinta FA, Yoganandan N. Whiplash syndrome. Spine. 2001;26:1252–1258. doi: 10.1097/00007632-200106010-00015. [DOI] [PubMed] [Google Scholar]
- Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Giani C, Zbinden A, Radanov B. Central hypersensitivity in chronic pain after whiplash injury. Clin J Pain. 2001;17:306–315. doi: 10.1097/00002508-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuopathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Deng B, Begeman PC, Yang KH, Tashman S, King AI. Kinematics of human cadaver cervical spine during low speed rear-end impacts. Stapp Car Crash J. 2000;44:171–188. doi: 10.4271/2000-01-SC13. [DOI] [PubMed] [Google Scholar]
- Dogan N, Erdem AF, Gundogdu C, Kursad H, Kizilkaya M. The effects of ketorolac and morphine on articular cartilage and synovium in the rabbit knee joint. Can J Physiol Pharmacol. 2004;82:502–505. doi: 10.1139/y04-066. [DOI] [PubMed] [Google Scholar]
- Dong L, Odeleye AO, Jordan-Sciutto KL, Winkelsten BA. Painful facet injury induces neuronal stress activation in the DRG: implications for cellular mechanisms of pain. Neurosci Lett. 2008;443:90–94. doi: 10.1016/j.neulet.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Winkelstein BA. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord suggesting spinal plasticity is associated with painful dynamic cervical facet loading. J Neurotrauma. 2010;27:163–174. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenzi F, Benedetti MD, Moretto G, Rizzuto N. Glial cell and macrophage reactions in at spinal ganglion after peripheral nerve lesions: an immunocytochemical and morphometric study. Arch Ital Biol. 2001;139:357–365. [PubMed] [Google Scholar]
- Galbraith JA, Thibault LE, Matteson RA. Mechanical and electrical response of the squid giant axon to simple elongation. J Biomech Eng. 1993;115:13–22. doi: 10.1115/1.2895464. [DOI] [PubMed] [Google Scholar]
- Gore D, Sepic SB, Gardner GM, Murray MP. Neck pain: a long-term follow-up of 205 patients. Spine. 1987;12:1–5. doi: 10.1097/00007632-198701000-00001. [DOI] [PubMed] [Google Scholar]
- Grauer JN, Panjabi MM, Cholewicki J, Nibu K, Dvorak J. Whiplash produces an S-shaped curvature of the neck with hyperextension at lower levels. Spine. 1997;22:2489–2494. doi: 10.1097/00007632-199711010-00005. [DOI] [PubMed] [Google Scholar]
- Hamamura K, Goldring MB, Yokota H. Involvement of p38 MAPK in regulation of MMP13 mRNA in chrondrocytes in response to surviving stress to endoplasmic reticulum. Arch Oral Biol. 2009;54:279–286. doi: 10.1016/j.archoralbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa J, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes. 2002;51:S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response egulates amino acid metabolism and resistance to oxidative stress. Mol cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hartwig AC, Mathias SI, Law AS, Gebhart GF. Characterization and opioid modulation of inflammatory tempoomandibular joint pain in the rat. J Oral Maxillofac Surg. 2003;61:1302–1309. doi: 10.1016/s0278-2391(03)00732-8. [DOI] [PubMed] [Google Scholar]
- Hincapié CA, Cassidy JD, Côté P, Carroll LJ, Guzmán J. Whiplash injury is more than neck pain: a population-based study of pain localization after traffic injury. J Occup Environ Med. 2010;52:434–440. doi: 10.1097/JOM.0b013e3181bb806d. [DOI] [PubMed] [Google Scholar]
- Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine. 2005;30:1924–1932. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- Hubbard RD, Winkelstein BA. Dorsal root compression produces myelinated axonal degeneration near the biomechanical thresholds for mechanical behavioral hypersensitivity. Exp Neurol. 2008;212:482–489. doi: 10.1016/j.expneurol.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inami S, Shiga T, Tsujino A, Yabuki T, Okado N, Ochiai N. Immunohistochemical demonstration of nerve fibers in the synovial fold of the human cervical facet joint. J Ortho Res. 2001;19:593–596. doi: 10.1016/S0736-0266(00)00048-6. [DOI] [PubMed] [Google Scholar]
- Inglis JJ, Nissim A, Lees DM, Hunt SP, Chernajovsky Y, Kidd BL. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther. 2005;7:R807–816. doi: 10.1186/ar1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Ivancic PC, Panjabi MM, Cunningham BW. Soft tissue injury threshold during simulated whiplash. Spine. 2004;29:979–987. doi: 10.1097/00007632-200405010-00006. [DOI] [PubMed] [Google Scholar]
- Jalava NS, Lopez-Picon FR, Kukko-Lukjanov TK, Holopainen IE. Changes in microtubule-associated protein-2 (MAP2) expression during development and after status epilepticus in the immature rat hippocampus. Int J Dev Neurosci. 2007;25:121–131. doi: 10.1016/j.ijdevneu.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Jousse C, Deval C, Maurin AC, Parry L, Chérasse Y, Chaveroux C, Lefloch R, Lenormand P, Bruhat A, Fafournoux P. TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol Chem. 2007;282:5851–15861. doi: 10.1074/jbc.M611723200. [DOI] [PubMed] [Google Scholar]
- Kallakuri S, Singh A, Lu Y, Chen C, Patwardhan A, Cavanaugh JM. Tensile stretching of cervical facet joint capsule and related axonal changes. Eur Spine J. 2008;17:556–563. doi: 10.1007/s00586-007-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneoka K, Ono K, Inami S, Hayashi K. Motion analysis of cervical vertebrae during whiplash loading. Spine. 1999;24:763–770. doi: 10.1097/00007632-199904150-00006. [DOI] [PubMed] [Google Scholar]
- Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M. Induction of neuronal death by ER stress in Alzheimer’s disease. J Chem Neuroanat. 2004;28:67–78. doi: 10.1016/j.jchemneu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Weinstein J. Associated neurogenic and nonneurogenic pain mediators that probably are activated and responsible for nociceptive input. In: Weistein JN, Gordon SL, editors. Low back pain: A scientific and clinical overview. Amer Acad of Orthopeadic Surgeons; Rosemont, Illnois: 1986. pp. 265–273. [Google Scholar]
- Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokine induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinol. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- Koelbaek Johansen M, Graven-Nielsen T, Olesen A Schou, Arendt-Nielsen L. Generalized muscular hyperalgesia in chronic whiplash syndrome. Pain. 1999;83:229–234. doi: 10.1016/s0304-3959(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Lee KE, Davis MB, Mejilla RM, Winkelstein BA. In vivo cervical facet capsule distraction: mechanical implications for whiplash & neck pain. Stapp Car Crash J. 2004a;48:373–396. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J Neurotrauma. 2008;25:1383–1393. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- Lee KE, Thinnes JH, Gokhin DS, Winkelstein BA. A novel rodent neck pain model of facet-mediated behavioral hypersensitivity: implications for persistent pain and whiplash injury. J Neurosci Methods. 2004b;137:151–159. doi: 10.1016/j.jneumeth.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Lee KE, Winkelstein BA. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J Pain. 2009;10:436–445. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Li X, Kim JS, van Wijnen AJ, Im HJ. Osteoarthritic tissues modulate functional properties of sensory neurons associated with symptomatic OA pain. Mol Biol Rep. 2011 Feb 16; doi: 10.1007/s11033-011-0684-7. 2011. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, LaVail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine. 1996;21:1737–44. doi: 10.1097/00007632-199608010-00005. discussion 1744-1745. [DOI] [PubMed] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neural response of cervical facet joint capsule to stretch: a study of whiplash pain mechanism. Stapp Car Crash J. 2005;49:49–65. doi: 10.4271/2005-22-0003. [DOI] [PubMed] [Google Scholar]
- Magne L, Blanc E, Legrand B, Lucas D, Barouki R, Rouach H, Garlatti M. ATF4 and the integrated stress response are induced by ethanol and cytochrome P450 2E1 in human hepatocytes. J Hepatol. 2011;54:729–737. doi: 10.1016/j.jhep.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Manchikanti L. Facet joint pain and the role of neural blockade in its management. Curr Rev Pain. 1999;3:348–358. doi: 10.1007/s11916-999-0030-0. [DOI] [PubMed] [Google Scholar]
- Markowitz AJ, White MG, Kolson DL, Jordan-Sciutto KL. Cellular interplay between neurons and glia: toward a comprehensive mechanism for excitotoxic neuronal loss in neurodegeneration. Cellscience. 2007;4:111–146. [PMC free article] [PubMed] [Google Scholar]
- Matu A, Bernhardt R, Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dentritic microtubules in brain. Proc Natl Acad Sci USA. 1981;78:3010–3014. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayou R, Radanov B. Whiplash neck injury. J Psychosom Res. 1996;40:461–474. doi: 10.1016/0022-3999(95)00586-2. [DOI] [PubMed] [Google Scholar]
- McLain RF. Mechanoreceptor endings in human cervical facet joints. Spine. 1994;19:495–501. doi: 10.1097/00007632-199403000-00001. [DOI] [PubMed] [Google Scholar]
- McMahon S, Cafferty W, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Miyagi M, Ohtori S, Ishikawa T, Aoki Y, Ozawa T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of TNFalpha in DRG satellite cells following lumbar facet joint injury in rats. Eur Spine J. 2006;15:953–958. doi: 10.1007/s00586-005-1031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muaddi H, Majumder M, Peidis P, Papadakis AI, Holcik M, Scheuner D, Kaufman RJ, Hatzoglou M, Koromilas AE. Phosphorylation of eIF2α at Serine 51 Is an Important Determinant of Cell Survival and Adaptation to Glucose Deficiency. Mol Biol Cell. 2010;21:3220–3231. doi: 10.1091/mbc.E10-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natvig B, Ihlebaek C, Grotel M, Brage S, Bruusgaard D. Neck pain is often a part of widespread pain and is associated with reduced functioning. Spine. 2010;35:E1285–1289. doi: 10.1097/BRS.0b013e3181e38e73. [DOI] [PubMed] [Google Scholar]
- Ng HP, Nordstrom U, Axelsson K, Perniola AD, Gustav E, Pyttberg Lars, Gupta A. Efficacy of intra-articular bupivacaine, ropivacaine, or a combination of ropivacaine, morphine, and ketorolac on postoperative pain relief after ambulatory arthroscopic knee surgery: A randomized double-blind study. Reg Anesth Pain Med. 2006;31:26–33. doi: 10.1016/j.rapm.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathways and is involved in cell death. Embo J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Kaneoke K, Wittek A, Kajzer J. Cervical injury mechanism based on the analysis of human cervical vertebral motion and head-neck-torso kinematics during low speed rear impacts. Stapp Car Crash J. 1997;41:339–356. #973340. [Google Scholar]
- Oyadomari S, Takeda K, Takiquchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci. 2001;98:10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjabi MM, Cholewicki J, Nibu K, Babat LB, Dvorak J. Simulation of whiplash trauma using whole cervical spine specimens. Spine. 1998;23:17–24. doi: 10.1097/00007632-199801010-00005. [DOI] [PubMed] [Google Scholar]
- Panjabi MM, Pearson AM, Ito S, Ivancic P, Wang JL. Cervical spine curvature during simulated whiplash. Clin Biomech. 2004;19:1–9. doi: 10.1016/j.clinbiomech.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Pearson AM, Ivancic PC, Ito S, Panjabi MM. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine. 2004;2:390–397. doi: 10.1097/01.brs.0000090836.50508.f7. [DOI] [PubMed] [Google Scholar]
- Penas C, Font-Nieves M, Forés J, Petegnief V, Planas A, Navarro X, Casas C. Autophagy, and BiP level decrease are early key events in retrograde degeneration of motoneurons. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.24. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Armstrong J. The BiP protein and the endoplasmic reticulum of Schizosaccharomyces pombe: fate of the nuclear envelope during cell division. J Cell Sci. 1993;105:1115–1120. doi: 10.1242/jcs.105.4.1115. [DOI] [PubMed] [Google Scholar]
- Quinn KP, Dong L, Golder FJ, Winkelstein BA. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain. 2010;151:414–421. doi: 10.1016/j.pain.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KP, Lee KE, Ahaghotu CC, Winkelstein BA. Structural changes in the cervical facet capsular ligament: potential contributions to pain following subfailure loading. Stapp Car Crash J. 2007;51:169–187. doi: 10.4271/2007-22-0008. [DOI] [PubMed] [Google Scholar]
- Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16:653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Kreider RA, Winkelstein BA. Spinal neuropeptide responses in persistent and transient pain following cervical nerve root injury. Spine. 2005;30:2491–2496. doi: 10.1097/01.brs.0000186316.38111.4b. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Winkelstein BA. Cytokine antagonism reduces pain and modulates spinal astrocytic reactivity after cervical nerve root compression. Ann Biomed Eng. 2010;38:2563–2576. doi: 10.1007/s10439-010-0012-8. [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Scott D, Jull G, Sterling M. Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but not chronic idiopathic neck pain. Clin J Pain. 2005;21:175–181. doi: 10.1097/00002508-200503000-00009. [DOI] [PubMed] [Google Scholar]
- Shim J, Umemura T, Nothstein E, Rongo C. The unfolded protein response regulates glutamate receptor export from the endoplasmic reticulum. Mol Biol Cell. 2004;15:4818–4828. doi: 10.1091/mbc.E04-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund GP, Myes BS, Davis MB, Bohnet HF, Winkelstein BA. Mechanical evidence of cervical facet capsule injury during whiplash. Spine. 2001;26:2095–2101. doi: 10.1097/00007632-200110010-00010. [DOI] [PubMed] [Google Scholar]
- Steiger JL, Bandyopadhyay S, Farb DH, Russek SJ. cAMP response element-binding protein, activating transcription factor-4, and upstream stimulatory factor differentially control hippocampal GABABR1a and GABABR1b subunit gene expression through alternative promoters. J Neurosci. 2004;24:6115–6126. doi: 10.1523/JNEUROSCI.1200-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift JQ, Roszkowski MT, Alton T, Hargreaves KM. Effect of intra-articular versus systemic anti-inflammatory drugs in a rabbit model of temporomandibular joint inflammation. J Oral Maxillofac Surg. 1998;56:1288–1295. doi: 10.1016/s0278-2391(98)90611-5. [DOI] [PubMed] [Google Scholar]
- Turner CL, Eggleston GW, Luno S, Johnson N, Wiedmann TS, Bowles WR. Sniffing out endodontic pain: use of an intranasal analgesic in a randomized clinical trial. J Endod. 2011;37:439–444. doi: 10.1016/j.joen.2010.12.010. [DOI] [PubMed] [Google Scholar]
- van Baarsen LG, Bos WH, rustenburg F, van der Pouw Kraan TC, Wolbink GJ, Dijkmans BA, van Schaardenburg D, Verweij CL. Gene expression profiling in autoantibody-positive patients with arthralgia predicts development of arthritis. Arthritis Rheum. 2010;62:694–704. doi: 10.1002/art.27294. [DOI] [PubMed] [Google Scholar]
- Weisshaar CL, Dong L, Bowman AS, Perez FM, Guarino BB, Sweitzer SM, Winkelstein BA. Metabotropic glutamate receptor-5 and protein kinase C-Epsilon increase in dorsal root ganglion neurons and spinal glial activation in an adolescent rat model of painful neck injury. J Neurotrauma. 2010;27:2261–2271. doi: 10.1089/neu.2010.1460. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Winkelstein BA, Nightingale RW, Richardson WJ, Myers BS. The cervical facet capsule and its role in whiplash injury: a biomechanical investigation. Spine. 2000;25:1238–1246. doi: 10.1097/00007632-200005150-00007. [DOI] [PubMed] [Google Scholar]
- Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, Ron D, Tabas I. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Sakurai M, Abe K, Matsumiya G, Sawa Y. Impact of the endoplasmic reticulum stress response in spinal cord after transient ischemia. Brain Res. 2007;1169:24–33. doi: 10.1016/j.brainres.2007.06.093. [DOI] [PubMed] [Google Scholar]
- Yoganandan N, Pintar FA, Cusick JF. Biomechanical analyses of whiplash injuries using an experimental model. Accid Analy Prev. 2002;34:663–671. doi: 10.1016/s0001-4575(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Yoganandan N, Pinta PA, Klinenberger M. Cervical spine vertebral and facet joint kinematics under whiplash. J Biomech Eng. 1998;120:305–307. doi: 10.1115/1.2798318. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]