Abstract
In this methods paper, we describe collection and storage of clinically acquired blood and adipose samples for transcript analysis in an ongoing study exploring obesity in renal transplant recipients. Total ribonucleic acid (RNA) was isolated from whole blood using the LeukoLOCK™ Total RNA Isolation System (n=4), and comparisons between fresh and frozen samples were made. Abdominal subcutaneous adipose samples (n=4) were obtained during kidney transplantation, flash frozen, and stored at −80°C. Adipose RNA was extracted using either the STAT-60 method modified for lipids or Trizol plus RNeasy extraction. Affymetrix HG-U133 plus 2.0 arrays and Affymetrix Human Gene 1.0 ST arrays were used for both blood and adipose transcriptome analysis. Purity, quality and quantity of RNA were high with comparable results using both array platforms.
Keywords: genetics, transplant, obesity, adulthood
Human subject accrual for clinical translational research, unlike subject accrual in animal studies, can take years. Long-term storage of tissue samples is therefore required for later analysis using expression profiling tools, such as microarrays and real-time quantitative polymerase chain reaction (RT-PCR). Well-defined and vetted protocols and procedures on the best collection methods for tissue samples in clinical settings are emerging, yet collection methods may not have been developed for specific clinical research protocols.
The clinical setting presents challenges to collecting tissue samples for research. For example, we have found that equipment (i.e., centrifuges) and reagents may not be as readily available within surgical suites as they are within laboratory settings. In addition, ribonucleic acid (RNA) is more sensitive to enzymatic degradation than deoxyribonucleic acid (DNA), and RNases are common in clinical settings. It is, therefore, important to carefully design tissue collection protocols for later RNA extraction. In this way investigators can be assured the protocols will provide quality RNA and sufficient quantity RNA, especially for gene expression studies using microarrays that need higher quality and quantity RNA than RT-PCR.
Human ribosomes contain four different ribosomal RNA (rRNA) molecules: 5S, 5.8S, 18S and 28S. The S is an abbreviation for “Svedberg,” a unit of molecular size. Typically, more than 80% of an RNA sample will be composed of rRNA, mostly in the form of 18S and 28S species. The remaining 20% of the sample contains messenger RNA (mRNA), micro RNA, and 5S and 5.8S rRNA species. Messenger RNA makes up only 1–3% of total RNA and is not easily detected.
There are numerous methods to collect blood samples. What is unknown, however, are which ones work best within a clinical setting while maintaining integrity of the sample for RNA analysis. We chose to use the LeukoLock system rather than other systems because a centrifuge is not required. The LeukoLock system can therefore be easily used in a clinical setting to quickly isolate leukocytes (without a percoll gradient), which are stabilized on a filter and can be frozen. In addition, a later globin reduction step is not needed.
Another consideration is that once samples are collected, the effects of freezing and thawing could further compromise sample integrity (Botling et al., 2009; Mutter et al., 2004). Botling et al. recommended assessing RNA integrity for all frozen samples (Botling et al., 2009). Pure high quality RNA has been extracted from human blood cells after up to 15 months of freezing (Marteau, Mohr, Pfister, & Visvikis-Siest, 2005). We have not found reports of RNA integrity after long term storage of frozen adipose samples. Adipose sample storage is further confounded with issues related to storage and processing of a tissue sample containing relatively high concentrations of lipids.
Currently, in our program of research we explore predictors and mechanisms of weight gain. Consequently, we need to store over 80 adipose samples for up to 5 years for later analysis. Conducting this analysis can be expensive, therefore considering the most cost effective approach is appropriate.
In this article, we describe the methodological process that we used to determine how best to obtain, store, and process our samples. Our purposes were to (a) determine a feasible method for collecting and storing of whole blood and adipose tissue for later RNA transcript analysis, (b) determine a feasible method for RNA extraction from clinically acquired whole blood and human adipose specimens, and (c) compare the performance of Affymetrix Human Gene (HG) U133 plus 2.0 to the significantly less expensive Human Gene 1.0 ST array.
Methods
A cross-sectional, descriptive design was used. Whole blood was collected in 2009 from two healthy human volunteers. In addition, abdominal subcutaneous adipose tissue was obtained from two transplant recipients at the time of kidney transplantation in a southeast regional transplant center. Institutional Review Board approval for this project was obtained. Informed consent was obtained from subjects providing adipose tissue samples, and a waiver of informed consent was granted for the two subjects providing blood samples. RNase precautions included the use of RNase Away ™, wearing gloves and mask, and the use of RNase-free containers and solutions while obtaining and processing the samples. All tissues were considered as infectious materials, and personnel implemented universal precautions at all times.
Sample Collection
Blood samples
Whole blood was collected from two healthy human volunteers (mean age 52.7 ± 0.7, 100% female, 100% Caucasian) by venipuncture using standard ethylenediaminetetraacetic acid (EDTA) Vacutainer® Tubes. A total of four whole blood samples (9 milliliter [ml] each) were obtained: two samples from each subject. The LeukoLOCK™ Total RNA Isolation System (Ambion, Austin, TX) was used to isolate leukocytes and stabilize RNA samples. Two LeukoLOCK™ filters (one from each subject) were stored for approximately 1week at −20°C for RNA isolation. The other two (one from each subject) were stored briefly at room temperature for immediate RNA isolation.
The LeukoLOCK™ Total RNA Isolation System uses a vented transfer spike that is inserted into the EDTA tube and attached to the filter device. An empty 10 ml evacuated tube is then attached which draws blood through the filter without the need to open the tubes. This filtering process generally takes less than 2 minutes. Residual red blood cells are then removed from the filter by flushing it with phosphate buffered saline (PBS). Total RNA, within the remaining intact leukocytes, is stabilized on the filter by the addition of RNAlater®. Centrifugation, percoll gradients, or other time-intensive steps that could disturb RNA expression profiles are not necessary. The RNAlater®-treated filters can be stored up to 72 hours at room temperature or up to 6 months if frozen at −20°C or −80°C. Longer storage times may be possible, but they have not been systematically studied.
Adipose tissue samples
As part of a larger study, abdominal subcutaneous adipose tissue was collected intra-operatively from a standardized site in kidney transplant recipients at the time of kidney transplantation. The transplant surgeon used a scalpel to obtain adipose samples from the abdominal incision. Samples were immediately placed in a Petri dish on ice, moved outside the operating room, cut into 10 samples, placed into individual cryo vials, and then flash frozen in liquid nitrogen. The time from collection until freezing was less than 2 minutes. Specimens flash frozen in liquid nitrogen were then transported from the hospital to the university laboratory and maintained at −80°C for long term storage.
RNA Isolation
Blood samples
Total RNA was isolated for each sample using the LeukoLOCK™ Total RNA Isolation System (Ambion, Austin, TX) according to the manufactures’ protocol. Fresh filters were processed immediately. The frozen filters containing captured leukocytes were thawed at room temperature for 5 minutes prior to RNA isolation. Briefly, the filters were treated to remove the residual RNAlater® by flushing the filters with a pH adjusted lysis/binding solution; the lysate was collected in a 15 ml tube. Nuclease-free water and Proteinase K were then added and the tube was shaken for 5 minutes. RNA binding beads were added, along with 100% isopropopal alcohol, the sample was incubated for 5 minutes. The binding beads were recovered, then washed three times and air dried. Finally, RNA was eluted with an elution solution (Ambion, 2008). RNA was aliquoted prior to storage at −80°C so that the RNA would not undergo repeat freeze and thaw cycles, which can degrade the RNA.
Adipose tissue samples
A total of four subcutaneous adipose samples were obtained from the biobank. There were two samples from two subjects (mean age 38.9 ± 2.9, 100% Caucasian, 100% male). A laboratory technician with no knowledge of any clinical aspect or personal identity of the subjects was asked to choose two subjects and then within each subject to choose two samples. Samples had been frozen for 7.5 ± 1.3 days prior to RNA isolation.
Total RNA was isolated from aliquots of each sample by standardardized methods, using the STAT-60 method (a 60 minute isolation reagent) or the Trizol plus RNeasy extraction method. The STAT-60 method was modified for lipids (Keller, Keller, Marshal, & Pedersen, 2003). Briefly, frozen tissue was weighed and added directly to STAT-60 (two ml of STAT-60 per 100 mg tissue) and homogenized using a Polytron (Brinkman, Westbury, NY) for 30 seconds. The phase was separated by centrifugation at 500 × g for 5 minutes at room temperature. The lipid layer was removed with a sterile pipette. Chroform:isoamyl alcohol (24:1), 600 μl per one ml STAT-60, was added, mixed by vortexing, allowed to stand at room temperature for 30 minutes and centrifuged for 30 minutes at 3000 × g.
For the Trizol plus RNeasy extraction (Vohl et al., 2004), total RNA was isolated as recommended for the Trizol reagent. The total RNA was then applied to an RNeasy spin column (RNeasy Lipid Midi Kit, Qiagen, Germantown, MD) and processed according to the manufacturer’s instructions. Both the STAT-60 and Trizol reagents are commercial versions of the original single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction method (Chomczynski & Sacchi, 2006).
Purity, Quality, and Quantity of RNA
A NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE) was used to quantify RNA in each sample by determining the ratio of absorbance at 260 and 280 nanometers (nm). In addition, an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) was used to determine the condition or integrity of the RNA in the samples by calculating the RNA integrity numbers (RIN) and measuring 28S to 18S rRNA ratio in each sample. RIN numbers range from 1–10; a higher RIN indicates more intact RNA (Schroeder et al., 2006).
Microarray Processing
For whole blood transcriptome analysis, we compared two types of Affymetrix Human Genechips—the Affymetrix HG-U133 plus 2.0 array (two of the four samples; one fresh and one frozen) and the Affymetrix Human Gene 1.0 ST array (all 4 samples). In Table 1 we show a comparison of these arrays. The HG-U133 plus 2.0 array has a 3′ bias in that the probes are designed to interrogate primarily at the beginning of the gene. The newer 1.0 ST array interrogates throughout the gene thus providing some information about alternative splicing.
Table 1.
Comparison of Two Affymetrix Arrays
| Variables | Human GeneChip® | Human GeneChip® |
|---|---|---|
| ST 1.0 | U133 Plus 2.0 | |
| Number of probes | 764,885 | >54,000 |
| Probe design | 26 probes per gene on average with at least 1 probe per exon | 11 probes are designed to the first 600–800bp of a gene |
| Number of transcripts | 19,734 | >47,000 |
| Genes well annotated | >28,869 | >38,500 |
| Alternative splicing | some data available | not available |
| Hybridization cocktail volume | 80 μl | 200 μl |
| Sample required | 25 ng/μl | 50 ng/μl |
| Cost | lower | higher |
Note. Annotations provided are the most current brochure information. Frequently updated annotation files are available to users on the Affymetrix website. ST denotes sense target.
We performed both arrays according to manufacturer’s instructions. Briefly, for the HG-U133 plus 2.0 arrays, we processed 3μg of total RNA using the ENZO (ENZOR Life Sciences, Plymouth Meeting, PA) labeling kit for in vitro transcription synthesis according to the manufacturer. The Biotin-Labeled complementary RNA (cRNA) was purified using an Affymetrix clean-up module kit; 20 μL of labeled cRNA was fragmented and hybridized to the GeneChip overnight at 45 degrees for 16 hours. For the Gene 1.0 ST arrays, whole transcript (WT) cRNA was synthesized from 100 ng of total RNA to eliminate the need for ribosomal RNA reduction. The hydrolysis of cRNA to single stranded DNA was performed using the WT cDNA Amplification kit and the Genechip clean-up module (Affymetrix, Santa Clara, CA). Amplified single stranded DNA was fragmented and labeled using the Genechip WT Terminal labeling kit (Affymetrix, Santa Clara, CA). Labeled samples (5.5 μg) were hybridized to a human Gene 1.0 array overnight for 16 hours at 45 degrees. The arrays were washed and stained on GeneChip Fluidics Station 450 (Affymetrix, Santa Clara, CA), followed by scanning using the GeneChip Scanner 3000.
GeneChip Normalization and Summary Statistics
The Affymetrix raw image files (known as .CEL or cell files) were background-corrected using Robust MultiArray Analysis (RMA; Irizarry et al., 2003), then base two logarithm-transformed. The transformed data were quintile-normalized. Figures 1 and 2 include pre and post normalized distributions. A similar median indicates that experimental variation is small enough to be handled by normalization tools. The intensity value for each gene was summarized using the median polish method implemented in JMP Genomics (a special statistical and graphics software package for visualizing and exploring genetic data; SAS Institute Inc., Cary, NC). We accessed the quality of these chips by performing the distribution analysis and correlation analysis using JMP Genomics (SAS Institute Inc., Cary, NC).
Figure 1.
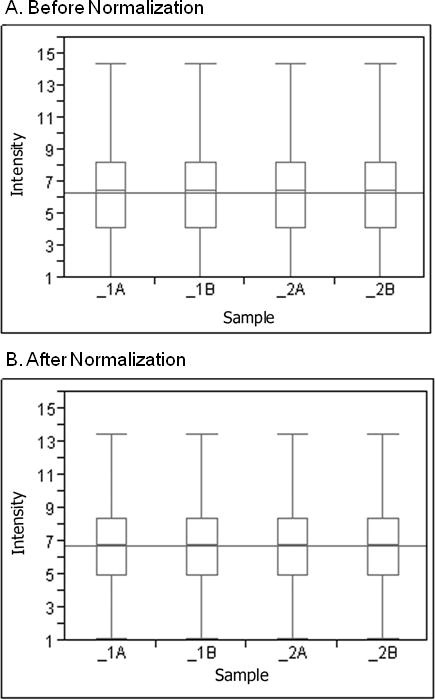
Robust multiarray analysis of pre and post normalized expression of whole blood. The box and whisker plot of each fresh sample (marked as 1A and 2A) and frozen sample (marked as 1B and 2B) illustrates the distribution of log 2 intensity values for transcript expression levels before (A) and after (B) normalization. The top and bottom of the box represent the 25th and 75th percentile, respectively. The band within each box is the median and the band line extending through all boxes is the grand mean of all arrays. The ends of the whiskers represent the 10th and the 90th percentile.
Figure 2.
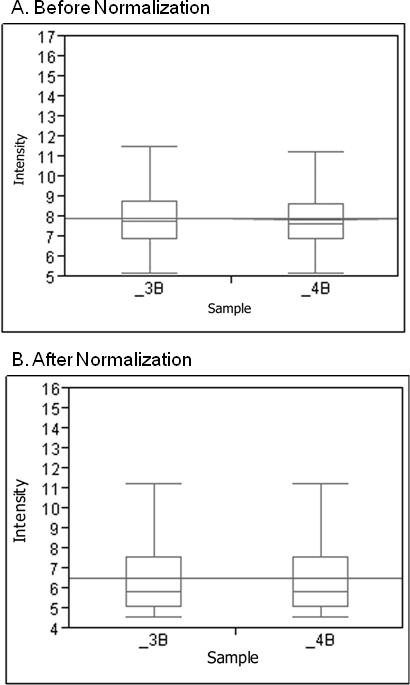
Robust multiarray analysis of pre and post normalized expression of adipose tissue. The box and whisker plot of each sample illustrates the distribution of log 2 intensity values for transcript expression levels before (A) and after (B) normalization. The top and bottom of the box represent the 25th and 75th percentile, respectively. The band within each box is the median and the band line extending through all boxes is the grand mean of all arrays. The ends of the whiskers represent the 10th and the 90th percentile. These box plots were done prior to normalization.
Results
RNA Quality from Blood Samples
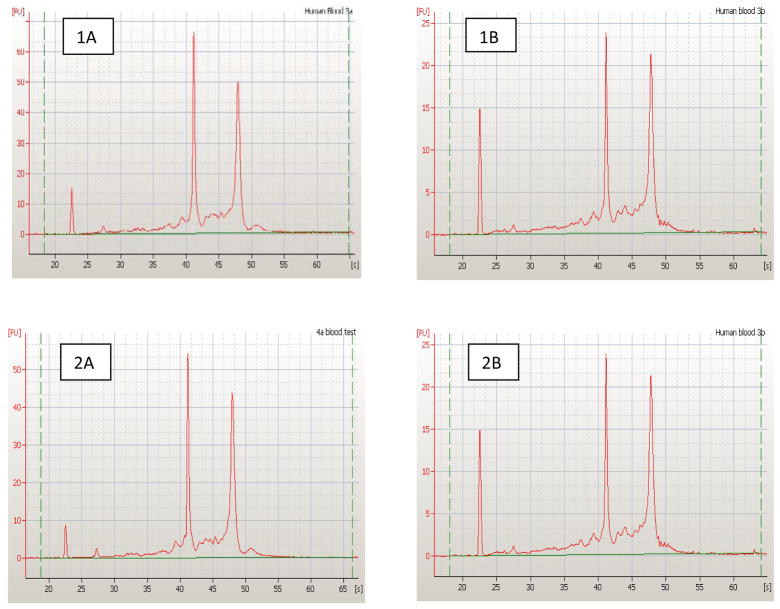
RNA yield and quantity were assessed from the fresh and frozen samples and compared. Using the NanoDrop® to determine quantity, yields ranged from 3.3–14.4 μg from samples isolated from 9 ml blood (Table 2). One sample (2A) contained a smaller RNA yield because less blood passed through the LeukoLOCK filter. This was related to our technique as we were learning to use the system. We found little difference in the RIN between fresh and frozen samples (RIN ranged from 8.9–9.0). The electropherogram (a graphical display of RNA sample integrity) results from the Agilent 2100 Bioanalyzer confirmed the high quality of the RNA in both fresh and frozen samples of total RNA (Figure 3). The 18s ribosomal peak (at ~ 42 seconds) and the 28s ribosomal peak (at ~ 48 seconds) indicate high quality total RNA. RNA yield and quality were consistent with the literature and adequate for future analysis (i.e., real-time PCR, microarray).
Table 2.
RNA Quantity and Quality from Fresh and Frozen Whole Blood Samples
| Samples | 260/280 | RNA Yield (μg) | RIN |
|---|---|---|---|
| Fresh Samples | |||
| 1A | 2.00 | 14.4 | 8.9 |
| 2A | 1.99 | 3.3* | 9.0 |
| Frozen Samples | |||
| 1B | 2.11 | 9.0 | 9.0 |
| 2B | 2.09 | 8.0 | 9.0 |
Note. RNA denotes ribonucleic acid. RIN indicates ribonucleaic acid integrity number. The sample indicated with an asterisk had less whole blood due to less blood vacuuming through the filter due to novice use. This resulted in a smaller yield; however, the RIN number quantity was sufficient for microarray processing. The ratio between light absorbance at 260 and 280 nm (260/280) indicates the quality of RNA.
Figure 3.
Assessment of total riboneucleic acid (RNA) quality for whole blood. Fresh samples (marked as 1A and 2A) and frozen samples (marked as 1B and 2B) samples of total RNA were analyzed by capillary electrophoresis (Agilent Bioanalyzer). Shown for each sample is the electropherogram indicating florescent intensity on y-axis vs. time (sec) on x-axis. The 2 major peaks correspond to the 18s (at ~ 42 sec) and the 28s (at ~ 48 sec) ribosomal peaks and indicate high quality total RNA.
Transcript Analysis Results From Blood Samples
The two blood samples processed on the HG-U133 plus 2.0 arrays (one fresh and one frozen) gave present calls (positive probe binding detection) of 26,399 (48.3 % of the probe sets) with an average signal of 1337 and 25,506 (46.7%) with an average signal of 1395, respectively. Background average (signal intensity caused by autoflorescence or nonspecific binding of the target) was 66.13 and 81.64, respectively. Housekeeping and spike controls are used for monitoring target sample quality and hybridization, respectively. These contols were within Affymetrix recommendations. When all four samples (two fresh and two frozen) were processed on Gene 1.0 ST arrays, every transcript had a value. The number of transcripts detected was similar to the HG-U133. In addition, the samples processed on the Gene 1.0 ST arrays showed similar median intensity values and distributions pre and post normalization (Figure 1). After normalization, the grand mean was 6.76 with log 2 expression values ranging from 1.2 to 15.2. The raw chip data was highly consistent to normalized data. There was a wide distribution of gene expression and adequacy of normalization process. The chips were not skewed. The small difference that we detected is evidence of good chips and high quality RNA obtained using the LeukoLOCK system.
RNA Quality from Adipose Tissue
We obtained comparable amounts of total RNA (0.012 μg per mg of starting tissue weight) from two subcutaneous adipose samples using the Stat-60 lipid protocol. Although we obtained acceptable 260/280 ratios averaging 1.97 we were not able to obtain RIN numbers using this protocol (Table 3, 3A and 4A). Based on the lack of RIN numbers, we could not use these samples.
Table 3.
Comparison of Two Methods of RNA Extraction from Adipose Tissue Samples
| Methods and Samples | Tissue weight mg | RNA Yields
|
||
|---|---|---|---|---|
| 260/280 | (μg/100 mg) | RIN | ||
| Modified STAT-60 Lipids (Tel-Test) | ||||
| 3A | 500 | 1.97 | 1.8 | NA |
| 4A | 300 | 1.96 | 1.8 | NA |
| Trizol plus RNeasy Lipid Kit | ||||
| 3B | 500 | 2.10 | 1.7 | 8.0 |
| 4B | 300 | 2.10 | 2.3 | 8.0 |
Note. NA indicates not available. RNA denotes ribonucleic acid. RIN indicates ribonucleic acid integrity number. The ratio between light absorbance at 260 and 280 nm (260/280) indicates the quality of RNA.
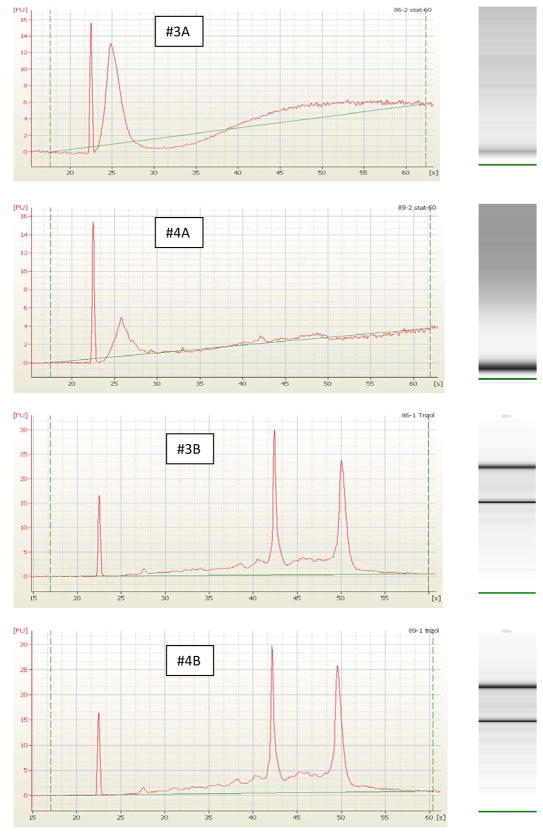
The second subcutaneous adipose sample from these subjects produced higher quality RNA using RNeasy Lipid kit. The 260/280 ratios for these samples using this method were 2.10 and the RIN numbers were 8.0 for each sample (Table 3; 3B and 4B). The electropherogram results from the Agilent 2100 Bioanalyzer indicate degradation of RNA as shown by the lack of the 18s and 28s peaks in 3A and 4A. Likewise, the computer generated gel from the electropherogram lacked a band for the 18s and 28s peaks (Figure 4). In addition, the high quality of the RNA extracted using Trizol plus RNeasy (Figure 4; 3B and 4B) was confirmed based on the 18s and 28s peaks. Also note the sharp bands on computer generated gel corresponding to the 18s and 28s peaks (Figure 4; 3B and 4B).
Figure 4.
Assessment of total riboneucleic acid (RNA) quality for adipose tissue. Total RNA from adipose tissue extracted using Stat-60 (3A and 4A) and Trizol plus RNeasy Lipid Kit (3B and 4B) were analyzed by capillary electrophoresis (Agilent 2100 Bioanalyzer). Shown for each sample is the electropherogram indicating florescent intensity (y-axis) vs. time (x-axis). In samples 3B and 4B the 2 major peaks correspond to the 18s (at ~ 42 sec) and the 28s (at ~ 48 sec). Lack of the 18s and 28s peaks in samples 3A and 4A indicate degradation of RNA. The corresponding computer generated gel from the electropherogram is shown for each sample. The two dark bands correspond to the 18s and 28s rRNA.
Transcript Analysis Results From Adipose Tissue
We processed the RNA that had been extracted from adipose tissue by the Trizol plus RNeasy Lipid kit method on Gene 1.0 ST array. The box and whisker plots in Figure 2 summarize our results showing similar median intensity values and distributions across arrays pre and post normalization. The grand mean intensity was 6.59 with a range of 4.6 to 15.2. The correlation between the two samples was .95. We identified high expression levels for genes that are involved in lipid metabolism and are usually expressed by adipose tissue including leptin receptor (LEPR), peroxisome proliferator-activated receptor gamma (PPARG), fatty acid synthase (FASN) and uncoupling protein 2 (UCP2; Dolinkova et al., 2008). These findings illustrate that our microarray process documented the expected genes within our samples.
Discussion
Feasibility of Sample Collection, Processing, and Storage
We determined that it was feasible for our transplant center and laboratory to collect and store filter-captured lymphocytes and abdominal subcutaneous adipose tissue collected from a clinical setting for later RNA extraction and analysis. Our process included using the LeukoLock system. We successfully dealt with numerous clinical challenges. These included institutional restrictions preventing the use of liquid nitrogen in the operating room, training surgeons and technicians to properly obtain and handle adipose samples to prevent RNA degradation (i.e., using scalpels/scissors instead of bovis to obtain adipose samples), timing the collection of the blood samples so they were obtained prior to initiating immunosuppression, determining the individual adipose sample size and number needed to obtain sufficient quantity of RNA, and “clean” processing techniques needed to prevent contamination and ensure optimum quality of RNA. In addition, protocols needed for the biobank were identified and instituted, such as the use of a continuous alarm system, carbon dioxide backup, sample bar coding, and creation of a computer database system for sample retrieval.
Often the focus in preserving quality RNA is the protection of the specimens from RNase to prevent degradation. RNase is found on hands and other surfaces. It is important, therefore, to wear gloves and clean surfaces with RNase Away® or a similar product. In working with tissue samples, we have observed that the timing from specimen collection until flash freezing and storage is an important factor in preserving RNA quality, because RNA can be easily and quickly degraded. We found that it is also important that specimens be gently handled—both in resection of the sample and placement in the Petri dish while in the operation room. The samples must be obtained using a sterile scalpel or scissors as opposed to using a bovi. We found that the bovi caused tissue damage, resulting in RNA degradation. In earlier work we were not able to extract RNA from samples collected using the bovi. We were able to collect sufficient quantity and quality of RNA in our samples when using these current techniques.
Determining Sample Integrity, Quality, and Quantity
We used the Agilent®2100 Bioanalyzer to measure 28S to 18S rRNA ratio in each of our samples (Figures 3 and 4) to determine RNA integrity, quality, and quantity. The first peak on each electropherogram graph is the 18S followed by the 28S rRNA, which is generally larger with a greater area under the curve. Although the 28S peak area should be greater, it typically is the first to degrade and its state can be used to assess the intactness of RNA (Schroeder et al., 2006). The 2:1 ratio of 28S:18S rRNA molecules has been used as a benchmark to assess the integrity of RNA. It is inconsistent and subjective, however.
The NanoDrop® ND-1000 Spectrophotometer provides accurate and reproducible measurements within the 220–750 nm spectral range and requires only one μl of sample. The NanoDrop® can be used to measure highly concentrated samples without dilution. In addition to a small sample size, another advantage of this method is the risk of potential contamination by use of a cuvette is eliminated. The purity of RNA can be assessed by the ratio of absorbance at 260 and 280 nm. A 260/280 ratio of 2.00 is generally considered pure for total RNA. Our ratios ranged from 1.96 to 2.10. Lower ratios can indicate protein or other contamination in the sample. RNA with absorbance ratios outside this range, however, may still function well for future applications. The yield of RNA is calculated by applying Beer’s Law based on the absorbance at 260 nm (NanoDrop Technologies, 2005).
The NanoDrop ® provides a measure of the purity of the samples, but it does not evaluate the condition or integrity of RNA in the sample. To evaluate the integrity of RNA, Agilent Technologies has developed a new software algorithm. The algorithm extracts information about the RNA sample integrity from a bioanalyzer electrophoretic trace called an electropherogram. This algorithm generates an RNA integrity number (RIN) from one to ten which removes individual interpretation of the RNA quality. A higher RIN number indicates more intact RNA (Schroeder, et al., 2006). The integrity of RNA is a major concern for gene expression studies. The RIN algorithm was devised to overcome the inconsistencies with the 28s to 18s rRNA ratio. The RIN contributes information about the RNA integrity to provide a more objective and robust universal measure (Meuller, Lightfoot, & Schroeder, 2004). NanoDrop® results may not, however, always be consistent with the Agilent 2100 Bioanalyzer results. Although there are limitations to current methods to determine quantity and quality of RNA, results that reflect the use of both methods are commonly reported. Our blood RIN numbers ranged from 8.9–9.0 and our adipose RIN number was 8.0 for both samples, indicating high quality RNA.
The LeukoLOCK™ system is an efficient system for collecting and storing RNA from blood samples. Based on our experience, we are now successfully obtaining our whole blood samples from the recipient early in the renal transplant operation. Because our study results demonstrates a high RIN number with no significant difference between fresh and frozen samples, we are more confident about long-term storage of our LeukoLOCK™ filters. An additional advantage of using the LeukoLOCK™ system is that a globin reduction step is not needed. This is because the innovative system only collects leukocytes, and the absence of red blood cells results in a depletion of globin mRNA. Additionally, we observed the number of transcripts present using this method was higher than previous work done using a method requiring globin reduction step (Driscoll et al., 2006).
In previous work Driscoll et al. (2006) also used the Affymetrix HG-U133 plus 2.0 array, and the comparison was based on array data. This results in improved sample utility for expression profiling and other applications (Ambion, 2008). Likewise, our methods for RNA extraction from lipids were successful. Initially, we failed to extract adequate quality RNA (noted by lack of RIN numbers or 18s and 28s peaks and by lack of 18s or 28s bands on computer generated gel) using the modified STAT-60 lipid method. We later obtained adequate quality RNA from these same subjects when using Trizol plus RNeasy Lipid kits (Qiagen, Germantown, MD). We chose this latter and successful method for subsequent extractions. Tissue with a high fat concentration may require additional handling during the homogenization step. RNeasy Lipids kits allowed us to optimize the adipose tissue lysis conditions.
Gene Expression
The goal in assessing the quality, quantity, and purity of the RNA is to determine sample integrity for downstream gene expression studies such as microarray and real time-PCR analysis. We selected the Affymetrix platform (proprietary framework) because it was readily available to our core facilities (university laboratory housing major shared equipment) although other platforms such as Illumina exist. Illumina also offers thorough genome coverage using bead based chips with chips capable of detecting 1.1 million SNPs and probes and up to 1.2 million genetic markers per samples. Both Affymetrix and Illumina offer focused panels for specific markers.
If both platforms are available within a researcher’s core facility, the selection of which platform to use can be based on several factors. Factors usually include the research team’s past experience and current comfort level with the platform, which platform was used to collect previous data (specifically if planning to compare or compile results), literature reviews, and determining the most current annotation files for each platform and array type.
Affymetrix was one of the earliest commercial platforms. The use of Affymetrix is therefore, prevalent and well described in the literature. The Affymetrix Human U133 plus 2.0 Array provides the most comprehensive coverage for analysis of gene expression profiles for the whole genome. It requires a larger amount of total RNA in contrast to Affymetrix Gene 1.0 ST array, which uses less sample and provides whole transcript gene expression profile at a reduced cost. The Affymetrix HG-U133 plus 2.0 array is more expensive and required more sample, yet it can be tailored to focus on subsets of well-characterized genes if needed. We obtained useful data on more than 20,000 well annotated transcripts using either array platform, so cost and the required amount of total RNA may be the deciding factors in future studies. Technology is continually evolving, and newer arrays are now available such as Affymetrix’ Human Exon 1.0 ST array which includes 1.4 million probes and provides more comprehensive alternative splicing information with4 probes per exon and 40 probes per gene.
Conclusions
The LeukoLOCK™ system allows sample storage and quality RNA isolation without the need for globin reduction, and is suitable for either type of Affymetrix expression array. Data comparison between fresh and frozen blood samples showed no significant difference in total RNA quantity or quality. Thus, leukocytes captured on LeukoLOCK™ filters provide a useful method for prospective clinical studies using human whole blood. For subcutaneous adipose specimens, Trizol plus RNeasy Lipid Kit was most effective in obtaining high quality and sufficient quantities of RNA. Our current protocol for adipose collected during kidney transplantation (less than two minutes from extraction to flash freeze in liquid nitrogen), processing, and storage prior to RNA extraction is adequate. Although kits to do this work are commonplace and commercially available, it was important to test them in each research setting. The choice of array depends on research needs. Overall, we found the less expensive Affymetrix Human Gene 1.0 ST array to be effective and optimal for our research needs given the data it provided to allow for later transcript analysis to explore mechanisms of weight gain. The Gene 1.0 ST array is well described in the literature, providing a strong body of evidence for comparison in future studies.
Acknowledgments
Funding
This project was funded by the National Institutes of Health, National Institute of Nursing Research (NR-009270).
Footnotes
Parts of this manuscript have been presented at the International Society for Nurses in Genetics annual meeting.
This project was conducted by team members from The University of Tennessee Health Science Center (Deborah Gibson, Robin Bloodworth), Methodist University Hospital Transplant Institute (James Eason), and the University of Memphis W. Harry Feinstone Center for Genomic Research (Quynh Tran).
Contributor Information
Ann K. Cashion, Email: acashion@uthsc.edu.
Reba A. Umberger, Email: rumberge@uthsc.edu.
Shirlean B. Goodwin, Email: sgoodwin@memphis.edu.
Thomas R. Sutter, Email: tsutter@memphis.edu.
References
- Ambion. LeukoLOCK(TM) Total RNA Isolation System Protocol. 2008:1–23. Retrieved from http://www.ambion.com/
- Botling J, Edlund K, Segersten U, Tahmasebpoor S, Engstrom M, Sundstrom M, Micke P. Impact of thawing on RNA integrity and gene expression analysis in fresh frozen tissue. Diagnostic Molecular Pathology. 2009;18:44–52. doi: 10.1097/PDM.0b013e3181857e92. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nature Protocols. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- Dolinkova M, Dostalova I, Lacinova Z, Michalsky D, Haluzikova D, Mraz M, Haluzik M. The endocrine profile of subcutaneous and visceral adipose tissue of obese patients. Molecular and Cellular Endocrinology. 2008;291:63–70. doi: 10.1016/j.mce.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Driscoll CJ, Cashion AK, Hathaway DK, Thompson C, Conley Y, Riely C, Homayouni R. Blood gene expression profiling in liver transplant recipients with hepatitis C virus and posttransplantation diabetes mellitus. Transplant Proceedings. 2006;38:3646–3648. doi: 10.1016/j.transproceed.2006.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Keller C, Keller P, Marshal S, Pedersen BK. IL-6 gene expression in human adipose tissue in response to exercise--effect of carbohydrate ingestion. The Journal of Physiology. 2003;550:927–931. doi: 10.1113/jphysiol.2003.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau JB, Mohr S, Pfister M, Visvikis-Siest S. Collection and storage of human blood cells for mRNA expression profiling: a 15-month stability study. Clinical Chemistry. 2005;51:1250–1252. doi: 10.1373/clinchem.2005.048546. [DOI] [PubMed] [Google Scholar]
- Meuller O, Lightfoot S, Schroeder A. RNA Integrity Number (RIN)-Standardization of RNA Quality Control. Waldbronn, Germany: Agilent Technologies; 2004. [Google Scholar]
- Mutter GL, Zahrieh D, Liu C, Neuberg D, Finkelstein D, Baker HE, Warrington JA. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics. 2004;5:88. doi: 10.1186/1471-2164-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NanoDrop Technologies, I. NanoDrop, ND-1000 Spectrophotometer, V3.2. User’s manual. 2005 Retrieved from www.nanodrop.com.
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Ragg T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Molecular Biology. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, Tchernof A. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obesity Research. 2004;12:1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]